Abstract
Background
Fetal aortic valvuloplasty (FAV) may prevent progression of mid-gestation aortic stenosis (AS) to hypoplastic left heart syndrome (HLHS). However, FAV has well-established risks, and its survival benefit remains unknown. Our primary aim was to determine if FAV for mid-gestation AS increases survival from fetal diagnosis to age 6.
Methods and Results
We performed a retrospective analysis of 143 fetuses who underwent FAV from 2000-2017 and a secondary analysis of the Pediatric Heart Network Single Ventricle Reconstruction trial. Using these results, we developed a decision model to estimate probability of transplant-free survival from fetal diagnosis to age 6 and postnatal restricted mean transplant-free survival time (RMST). FAV was technically successful in 84% of 143 fetuses with fetal demise in 8%. Biventricular circulation was achieved in 50% of 111 liveborn infants with successful FAV but in only 16% of the 19 patients with unsuccessful FAV. The model projected overlapping probabilities of transplant-free survival to age 6 at 75% (95% CI 67% - 82%) with FAV versus 72% (95% CI 61% - 82%) with expectant fetal management, resulting in a RMST benefit of 1.2 months. When limiting analyses to the improved FAV experience since 2009 to reflect current practice, probability of technical success (94%), fetal demise (4%), and biventricular circulation (66%), the model projected that FAV increased the probability of survival to age 6 to 82% (95% CI 73% - 89%). Expectant management is favored if risk of fetal demise exceeded 12% or probability of biventricular circulation fell below 26%, but FAV remained favored over plausible recent range of technical success.
Conclusions
Our model suggests that FAV provides a modest, medium-term survival benefit over expectant fetal management. Appropriate patient selection and low risk of fetal demise with FAV are critical factors for obtaining a survival benefit.
Hypoplastic left heart syndrome (HLHS) comprises a spectrum of cardiac malformations characterized by significant underdevelopment of left heart structures and implies a left heart unable to support systemic circulation. Mid-gestation fetuses with severe aortic stenosis (AS) and a normal sized or dilated left ventricle with systolic dysfunction frequently develop HLHS by birth.1–3 Characteristics on fetal echocardiogram that predict progression of mid-gestation fetal AS to HLHS include at least moderate left ventricular dysfunction, retrograde flow in the transverse aortic arch, and left-to-right flow through the patent foramen ovale.1–6 First performed in the early 1990, fetal aortic balloon valvuloplasty (FAV) has shown promise in averting in utero progression of AS to HLHS.7–12 Selection criteria for FAV and postnatal outcomes have previously been published and demonstrate lower mortality in the biventricular group compared to the HLHS group.13
However, the potential benefit of FAV must be weighed against the known risk of fetal demise and the possibilities of a technically unsuccessful procedure, and/or postnatal single ventricle circulation despite a technically successful FAV. A randomized trial of FAV versus expectant fetal management of AS with evolving HLHS is impractical given the relative rarity of the condition, likely enrollment difficulties, and potential strong patient and physician preferences. Decision analysis provides an alternative approach to comparing two treatments. In the presence of uncertainty, decision analysis quantifies the expected value of alternative treatment strategies and identifies the key parameters that drive potential benefit. By varying the model parameters, such as probability of fetal demise or a biventricular circulation after a technically successful procedure, decision analysis can identify thresholds above or below which the procedure loses expected benefit. These data could inform patient selection for FAV, enhance pre-procedural counseling, and focus research and clinical attention on factors that improve the expected benefit of FAV.
The primary aim of this study was to determine the optimal fetal management strategy for mid-gestation fetal AS with evolving HLHS to maximize the probability of survival to age 6 years using decision analysis. Secondary study aims included determining the treatment strategy that maximizes restricted mean transplant-free survival time at 3 and 6 years, and identifying thresholds for technical success, fetal demise, and probability of biventricular circulation at which the expected benefit of FAV is lost.
Methods
Patient Population
The decision model considers all mid-gestation fetuses with aortic stenosis and evolving HLHS who are deemed eligible for FAV. Our selection criteria for FAV have evolved over time and have previously been published.12,14–16 Patients were excluded if the indication for FAV was either a) severe mitral regurgitation with intact atrial septum and giant left atrium or b) intact atrial septum, or if they were still in utero as of January 2018. To inform parameter estimates, we performed a retrospective review of all available records of fetuses who underwent FAV for evolving HLHS at our institution from the initiation of the program in March 2000 to December 2017 to determine the following outcomes: technical success of FAV, fetal demise, postnatal circulation type (biventricular versus single ventricular), and survival at ages 3 and 6 years.
Due to the limited number of single ventricle (SV) patients in the FAV cohort with long-term follow-up, we derived SV survival estimates from a secondary analysis of the mitral stenosis/aortic stenosis (MS/AS) subgroup of the Pediatric Heart Network Single Ventricle Reconstruction (SVR) trial.17 Analysis was restricted to MS/AS because this subtype of HLHS is most physiologically similar to fetal AS patients with evolving HLHS and has better outcomes than the mitral or aortic atresia subtypes.18,19 We excluded MS/AS patients who had undergone FAV or biventricular conversion. The inclusion and exclusion criteria and follow-up protocol for the SVR trial have been previously published.17,18 Because 3-year and 6-year follow-up outcomes did not differ by right ventricle to pulmonary artery versus modified Blalock-Taussig shunt, we did not stratify by shunt-type.18,19
Decision Analytic Model
For fetuses with mid-gestation aortic stenosis with evolving HLHS who are eligible for FAV, as defined above, the decision tree considered the strategies of FAV or expectant management (Figure 1). For those who undergo FAV, there is a probability of technical success (vs. technically unsuccessful procedure), subsequent fetal demise, achievement of postnatal biventricular circulation (vs. SV circulation), and transplant-free survival to ages 3 and 6 years. For those who do not undergo FAV, there is a probability of fetal demise and postnatal biventricular circulation, and transplant-free survival to ages 3 and 6 years. The model assumes that the prognosis of a fetus who underwent a technically unsuccessful procedure, which is often the result of inability to properly position the fetus, is equivalent to the outcome of an eligible fetus who did not undergo the procedure. As such, the probability of biventricular circulation after a technically unsuccessful procedure is derived from our cohort and assumed to be the probability of biventricular circulation for an eligible patient who did not undergo the procedure (i.e. probability of biventricular circulation is 16% in both groups). Because no significant maternal complications related to FAV have occurred in our cohort, the model did not consider FAV-related maternal risk (15). The model also assumes that patients who had a technically successful FAV but with postnatal SV circulation had the same 3- and 6-year transplant-free survival as SV patients who either did not undergo or had a technically unsuccessful procedure. The primary outcome is probability of transplant-free survival from fetal diagnosis to age 6 years for FAV versus expectant management. Secondary outcomes include probability of transplant-free survival to age 3 years and restricted mean transplant-free survival time (RMST) up to age 3 and 6 years, i.e., the area under the transplant-free survival curve (or mean life expectancy) up to ages 3 and 6.20
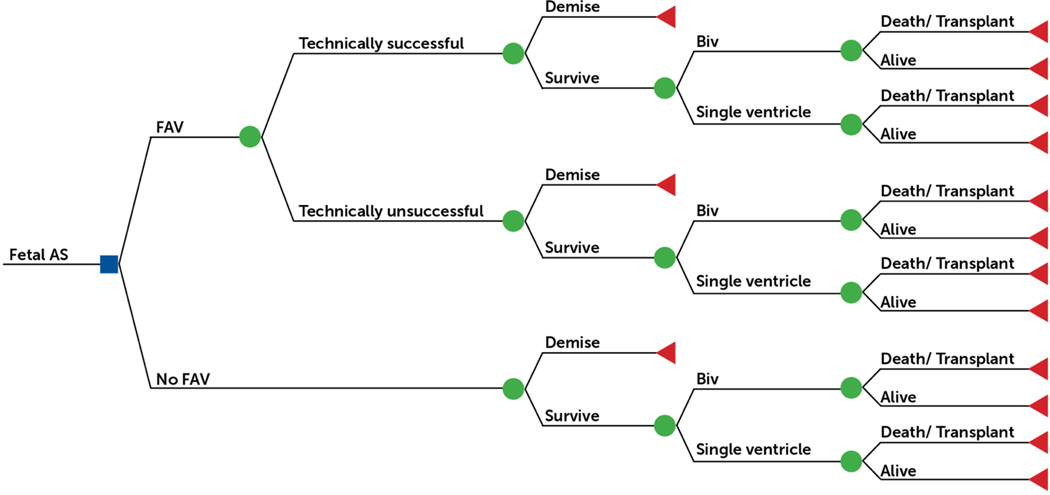
Figure 1: Decision Tree for the Management of Mid-gestation Aortic Stenosis with Evolving Hypoplastic Left Heart Syndrome.
Decision tree with the square representing a decision node for the choice between fetal aortic valvuloplasty versus expectant management for the management of mid-gestation aortic stenosis with evolving hypoplastic left heart syndrome. The circles represent chance nodes, and the triangles represent terminal nodes (dead or alive).
All analyses were performed using TreeAge Pro 2018 (Williamstown, MA), SAS version 9.4 (SAS Institute, Cary, NC), R version 3.6.1, and SPSS version 24 (IBM Corp. Armonk, NY).
Data
Table 1 presents the model parameter probabilities.
Table 1.
Parameter Probabilities, Sensitivity Analysis Ranges, and Sources
| Parameter | Probability | #/N | 95% CI | Range | Distribution | Source |
|---|---|---|---|---|---|---|
| All Years | ||||||
| Technical success | 0.84 | 120/143 | 0.77–0.89 | 0.70–1.0 | Beta | Primary Data |
| Post FAV fetal demise | 0.08 | 12/143 | 0.04–0.13 | 0.04–0.30 | Beta | Primary Data |
| No FAV fetal demise | 0.06 | 4/72 | 0.02–0.12 | 0.00–0.20 | Beta | Beroukhim et al.23 |
| TS: Biv outcome | 0.50 | 56/111 | 0.41–0.60 | 0.10–0.70 | Beta | Primary Data |
| TU: Biv outcome | 0.16 | 3/19 | 0.04–0.35 | 0.00–0.50 | Beta | Primary Data |
| 2009–2017 | ||||||
| Technical success | 0.94 | 67/71 | 0.88–0.98 | 0.70–1.00 | Beta | Primary Data |
| Post FAV fetal demise | 0.04 | 3/71 | 0.01–0.10 | 0.01–0.20 | Beta | Primary Data |
| TS: Biv outcome | 0.66 | 42/64 | 0.54–0.77 | 0.10–0.70 | Beta | Primary Data |
| 3-year Outcomes | ||||||
| Survival | ||||||
| Post TS FAV: Biv | 0.96 | 0.90–1.0 | Beta | Primary Data | ||
| SV (MS/AS) | 0.77 | 0.64–0.86 | Beta | SVR18 | ||
| Postnatal LE (months) | ||||||
| Post TS FAV: BiV | 35.4 | 34.2–36.5 | Normal | Primary Data | ||
| SV (MS/AS) | 31.0 | 28.3–33.7 | Normal | SVR18 | ||
| 6-year Outcomes | ||||||
| Survival | ||||||
| Post TS FAV: Biv | 0.92 | 0.83–1.0 | Beta | Primary Data | ||
| SV (MS/AS) | 0.72 | 0.58–0.82 | Beta | SVR19 | ||
| Postnatal LE (months) | ||||||
| Post TS FAV: Biv | 69.4 | 66.2–72.5 | Normal | Primary Data | ||
| SV (MS/AS) | 57.0 | 52.6–64.7 | Normal | SVR19 | ||
Biv = biventricular circulation; FAV = fetal aortic valvuloplasty; MS/AS = mitral stenosis/aortic stenosis; SVR = Single Ventricle Reconstruction trial secondary analysis; TS = technically successful; TU = technically unsuccessful
Technical Success
The technique for FAV used at our institution has been previously described.12,21,22 A technically successful FAV was defined as one in which the aortic valve was crossed and a balloon inflated, with clear evidence of increased flow across the valve and/or new aortic regurgitation (AR). There have been no significant changes in FAV technique from the description published by Marshall et al.21 For the two fetuses who underwent more than one FAV procedures during this study period, only the technical success of the first procedure was considered.
Fetal Demise
FAV may result in fetal demise. We determined the probability of fetal demise by combining the demise rate in all fetuses who underwent FAV, both technically successful and unsuccessful procedures, and assumed that rate applied to all fetuses regardless of technical success or not. In the absence of FAV, Beroukhim et al. reported the probability of fetal demise for prenatally diagnosed standard risk HLHS, after excluding cases of elective termination.23
Postnatal Circulation
Postnatal management of fetuses who underwent FAV varied based on provider and postnatal institution (all fetuses had FAV at our institution but many were born and managed postnatally elsewhere). Circulation type (single versus biventricular) was classified based on circulation at the time of discharge from the neonatal hospital stay. Biventricular circulation was defined as left ventricle being the only source of systemic cardiac output with no intracardiac shunt apart from an atrial communication. Any patient who required a Stage 1 or hybrid palliation was classified as SV circulation. In this analysis, the single to biventricular conversion patients (n=6) were classified in the SV group. One live born patient was excluded from the analysis due to premature birth at 32 weeks and postnatal comfort care. It is assumed that the probability of biventricular circulation for a fetus who did not have FAV is equivalent to that of a fetus who had a technically unsuccessful procedure.
Survival
We plotted Kaplan-Meier curves over 6 years for patients who underwent FAV and achieved a biventricular circulation and for SV patients with aortic and mitral stenosis enrolled in the SVR trial (Figure 2). Restricted mean survival time (RMST), which is the average transplant-free survival time, was calculated as the area under the survival curves to ages 3 and 6 years using Riemann sums for each day of follow-up.24 RMST is interpretable as ‘life expectancy’ to the specified time point and is an alternative measure of treatment effect that remains valid under any distribution of time to event, such as when the proportional hazards assumption is breached.25 The time horizon for RMST and probability of transplant-free survival at ages 3 and 6 were selected to correspond to previously published outcome data from the SVR trial.
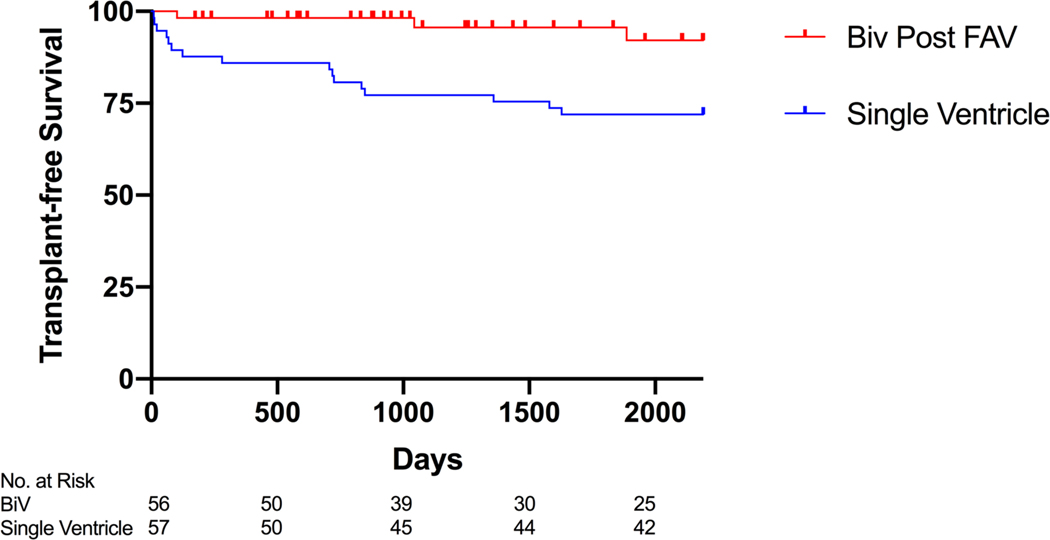
Figure 2. Kaplan-Meier Postnatal Survival Analysis.
Kaplan-Meier curve demonstrating the transplant-free survival from live birth to age 6 for patients with biventricular circulation (Biv) status post fetal aortic valvuloplasty (FAV) and with single ventricle (mitral stenosis/aortic stenosis) circulation enrolled in the Single Ventricle Reconstruction (SVR) Trial, excluding those who underwent FAV or biventricular conversion.
Sensitivity Analyses
A probabilistic sensitivity analysis (PSA) with Monte Carlo simulation was performed with 10,000 simulations to estimate the mean expected values and 95% CIs. The PSA incorporates uncertainty in the precision of the parameters (second order uncertainty) by sampling the value for each model probability parameter from beta distributions. RMST was calculated as the area under the transplant-free survival curves to ages 3 and 6 years using the method described by Zhao et al.26 The mean value and 95% CI of the 10,000 simulations are presented, as well as the frequency of simulations in which FAV was the favored strategy to maximize transplant-free survival or RMST. The delta RMST value for each of the 10,000 simulations (FAV – no FAV) was plotted as a frequency distribution.
One- and two-way sensitivity analyses were performed using the deterministic model (i.e. fixed probabilities, not sampled from a distribution). The value of each parameter was varied over a clinically plausible range based on clinical experience and previously published estimates to determine if the preferred strategy changed. If the preferred strategy changed with variation, we determined the threshold value for that parameter. Table 1 lists the baseline values and the plausible range of values over which we varied that parameter. Ranges were based on previously published values and expert consensus.
Because of modification of FAV selection criteria in 2009 and improved outcomes, we performed an additional analysis reflecting our current practice. In the post-2009 analysis, we included parameters obtained only from patients who underwent FAV from January 2009 to December 2017 except for probability of biventricular circulation after a technically unsuccessful procedure, which was derived from the entire cohort (2000-2017) as there were insufficient technically unsuccessful procedures after 2009 to produce a stable estimate.
The International Fetal Cardiac Intervention Registry (IFCIR), which includes 18 institutions, previously reported the outcomes of 86 FAVs (Table 2).11 They excluded from their cohort the 100 previously reported FAV cases from our institution. We performed a secondary analysis substituting probabilities for fetal demise, technical success, and biventricular circulation from their previously published data to determine impact on preferred management strategy.
Table 2.
Parameter Probabilities from the International Fetal Cardiac Intervention Registry (IFCIR)11
| Parameter | Probability | #/N | Distribution | Source |
|---|---|---|---|---|
| No FAV*: fetal demise | 0.15 | 4/27 | Beta | IFCIR |
| No FAV: Biv outcome | 0.22 | 5/23 | Beta | IFCIR |
| Technical success | 0.81 | 70/86 | Beta | IFCIR |
| Post FAV fetal demise | 0.20 | 16/80 | Beta | IFCIR |
| TS: Biv outcome | 0.43 | 24/56 | Beta | IFCIR |
Eligible for FAV but not performed due to maternal contraindications, not offered at center, or family declined. Loss to follow-up and terminations were excluded from the denominator of fetal demise. Biv = biventricular circulation; FAV = fetal aortic valvuloplasty; TS = technically successful
This study was conducted with the approval from the Committee for Clinical Investigation at Children’s Hospital Boston and the Institutional Review Board at Brigham and Women’s Hospital.
Results
In the 143 fetuses who underwent FAV from March 2000 to December 2017 at our institution, technical success occurred in 120 (84%). Fetal demise occurred in 12 (8%), of whom 9 fetal losses were attributed directly to FAV. Biventricular circulation after technically successful FAV was achieved in 56/111 (50%). Biventricular circulation was achieved in 3 of 19 (16%) patients who had a technically unsuccessful FAV. Since 2009 (n=71), all outcomes have improved including probability of technical success (94%), fetal demise (4%), and biventricular circulation (66%) (Table 1).
In the group of patients who underwent FAV and achieved biventricular circulation, there were 4 deaths (3 cardiac deaths and 1 car accident) and 1 orthotopic heart transplant over a median follow-up of 5.0 years (0.3-17.3 years). One of the deaths was following transplant. Transplant-free survival at 6 years was 92% (95% CI 83% - 100%) (Table 1 and Figure 2). In the SVR trial, there were 57 patients with MS/AS who had not undergone FAV or biventricular repair. Transplant-free survival at 6 years was 72% (95% CI 58% - 82%) (Table 1 and Figure 2).
Baseline Analysis
FAV for management of mid-gestation AS with evolving HLHS increased the probability of survival from fetal diagnosis to age 3 from 75% (95% CI 64% - 84%) to 78% (95% CI 70% - 84%) and to age 6 from 72% (95% CI 61% - 82%) to 75% (95% CI 67% - 82%). FAV increased the restricted mean transplant-free survival time by 0.5 months [29.9 months (95% CI 27.7-31.9) versus 29.4 months (95% CI 26.5-32.2)] at age 3 and by 1.2 months [58.2 months (95% CI 53.5-62.5) versus 57.0 months (95% CI 50.8-62.8)] at age 6.
When limiting our analysis to contemporary estimates of fetal demise, technical success, and biventricular circulation from patients who underwent FAV from January 2009 to December 2017 (Table 1), FAV increased the probability of survival to age 6 by from 72% (95% CI 61% - 81%) to 82% (95% CI 73% - 89%) and RMST by 5.5 months at age 6 [62.6 months (95% CI 57.8 – 66.6) versus 57.1 years (95% CI 50.8-62.9)].
Sensitivity Analyses
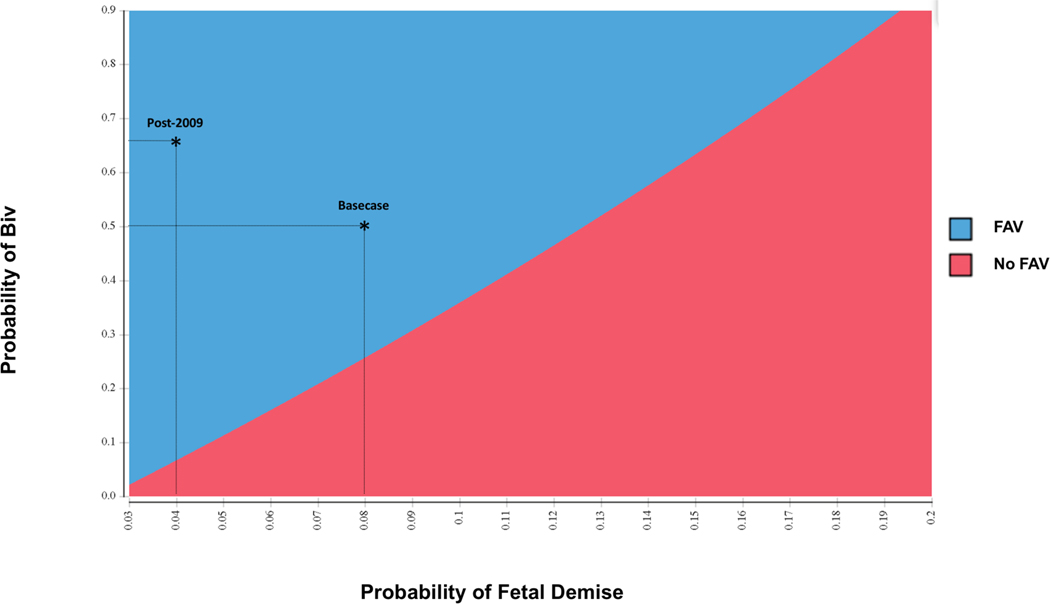
Univariate sensitivity analyses tested the stability of the model results to alternative assumptions. When varying the probability of technical success over the clinically plausible range (70%−100%), FAV remained the preferred strategy. However, FAV was no longer preferable when probability of technical success fell below 24%. If the risk of fetal demise after FAV exceeded 12% or the probability of a biventricular circulation following a technically successful FAV fell below 26%, expectant fetal management was the preferred strategy. A two-way sensitivity analysis (Figure 3) illustrates the joint effect of the probabilities of fetal demise and biventricular circulation after FAV on the preferred strategy that maximizes probability of survival to age 6. In the post-2009 analysis, a lower probability of achieving a biventricular circulation after technically successful procedure can be tolerated (threshold 8%) due to reduced risk of fetal demise and increased probability of technical success.
Figure 3. Two-way Sensitivity Analysis.
Two-way sensitivity analysis of optimal strategy to maximize 6-year survival for management of mid-gestation aortic stenosis over a range of probabilities of fetal demise (x axis) and postnatal biventricular (Biv) circulation after technically successful fetal aortic valvuloplasty (FAV) (y axis). Values for the two parameters that fall in the shaded blue area favor FAV, and those that fall in the shaded red area favor no FAV. The asterisks represent the base case and post-2009 values, both of which are in the blue area.
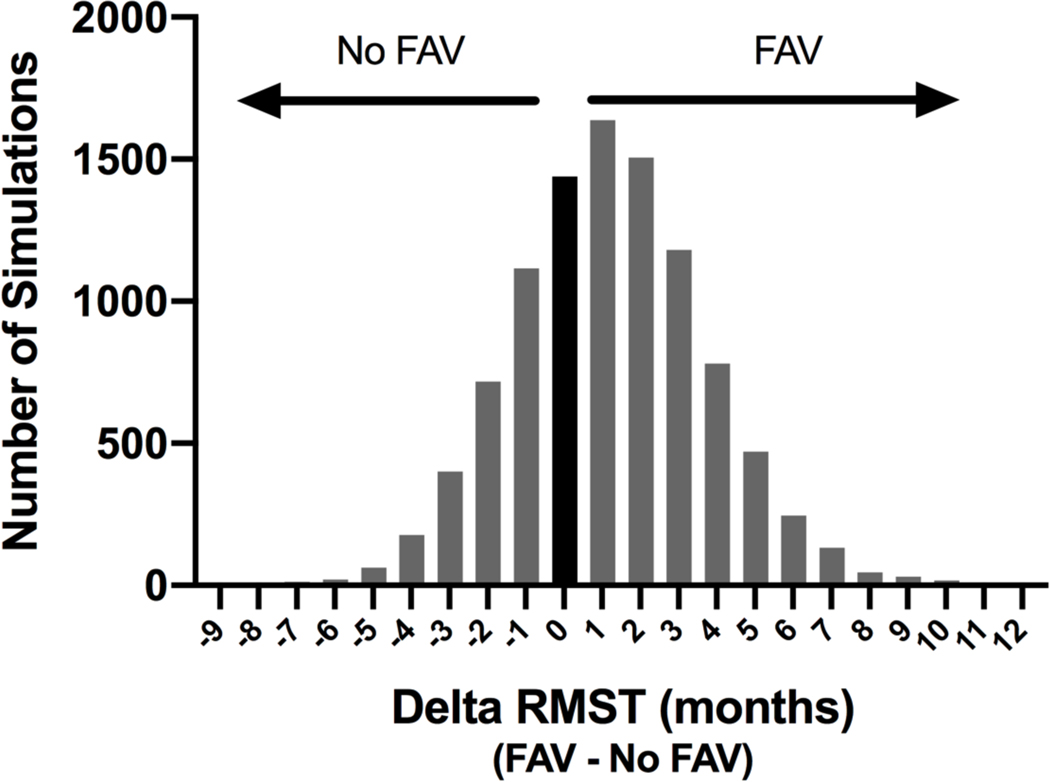
In probabilistic sensitivity analyses, FAV was the preferred strategy in 81% of simulations in the baseline analysis and 99% of simulations in the post-2009 analysis when maximizing the probability of transplant-free survival to age 6 years. The delta RMST value for each of the 10,000 simulations (FAV – no FAV) was plotted as a frequency distribution. A value of 0 indicates the two strategies had equal expected RMST; a positive value indicates FAV has a greater expected RMST (preferred strategy) and a negative value indicates expectant management has a greater expected RMST. FAV was the preferred strategy in 67% of simulations in the baseline analysis when maximizing RMST to age 6 (Figure 4).
Figure 4. Frequency Histogram of Difference in Restricted Mean Transplant-free Survival Time (RMST) for Fetal Aortic Valvuloplasty (FAV) versus Expectant Management.
The delta RMST value (months) to age 6 years for each of the 10,000 simulations (FAV – No FAV) was plotted as a frequency distribution. The Y axis is the number of simulations with that delta RMST value. A value of 0 indicates the two strategies had equal expected RMST and is represented by the black bar. A positive value indicates FAV had a greater expected RMST (preferred strategy) and a negative value indicates expectant management (No FAV) had a greater expected RMST.
In a secondary analysis substituting probabilities for fetal demise, technical success, and biventricular circulation from the previously published IFCIR data, FAV no longer conferred a survival benefit compared to expectant management. Probability of transplant-free survival from fetal diagnosis to age 6 with expectant management was 66% (95% CI 52% - 78%) compared to 64% (95% CI 55% - 74%). Expectant management was the preferred strategy to maximize transplant-free survival in 59% of simulations. If the probability of biventricular circulation after a technically successful procedure exceeded 52%, then FAV was preferred.
Discussion
The choice between continued expectant management versus attempted FAV facing fetal cardiologists and families of fetuses with severe mid-gestational AS and evolving HLHS remains one of the most controversial in our field. To aid in this choice, we used decision analysis to compare the risk of harms and benefits of these two management pathways. We found that FAV, when performed at an experienced center with appropriately selected candidates, modestly increases the probability of medium-term survival to ages 3 and 6 years despite the upfront risks. Prior to our analysis, postnatal survival in biventricular patients after FAV had been shown to be higher than in single ventricle patients, but FAV carries risks of a technically unsuccessful procedure and fetal demise as well as single ventricle circulation despite FAV.13 These factors had not been accounted for when considering only postnatal survival.
Our decision model also identified individual and institutional thresholds above or below which FAV no longer conferred a survival benefit. When we repeated our analyses with data on technical success, fetal demise, and probability of postnatal biventricular circulation from the International Fetal Cardiac Intervention Registry study, which excluded the first 100 cases from our institution, FAV did not improve survival probability.11 Part of the reason for this is the higher probability of fetal demise and lower probability of technical success reported in the IFCIR. In congenital heart procedures, as in many other medical and nonmedical fields, higher volume is associated with better outcomes, particularly for complex and rare procedures.27 FAV is precisely such a procedure, requiring both careful patient selection and an experienced multidisciplinary team (maternal fetal medicine, fetal cardiology, pediatric interventional cardiology, anesthesiology, radiology). We are unable in this analysis to determine the relative contribution of subspecialists to the outcome. Our baseline analysis determined that if the risk of fetal demise exceeds 12%, the expected 6-year survival benefit of FAV is lost and expectant management is favored. This relatively narrow range for fetal demise rate reflects both the importance of patient selection and of technically proficient and experienced centers.
The lower rate of postnatal biventricular circulation after technical success reported in the IFCIR data, as compared to our institutional data, was also a contributing factor to the absence of a survival benefit with FAV. Threshold analysis using IFCIR data in the model revealed that FAV would be preferred if probability of biventricular circulation surpassed 52%. There were no standardized patient selection criteria among the 18 centers included in the IFCIR, which may have contributed to FAV being performed on fetuses with very low probability of left ventricular recovery and postnatal biventricular circulation. In addition, postnatal management strategy (single versus biventricular) is a clinical decision based on best-available data, center experience, and provider and parent preference. As such, there may be variability in postnatal management unrelated to patient-level factors. Furthermore, survival with a biventricular circulation, especially in borderline cases, requires multidisciplinary care and expertise, which may limit the generalizability of our biventricular survival estimates to other institutions.
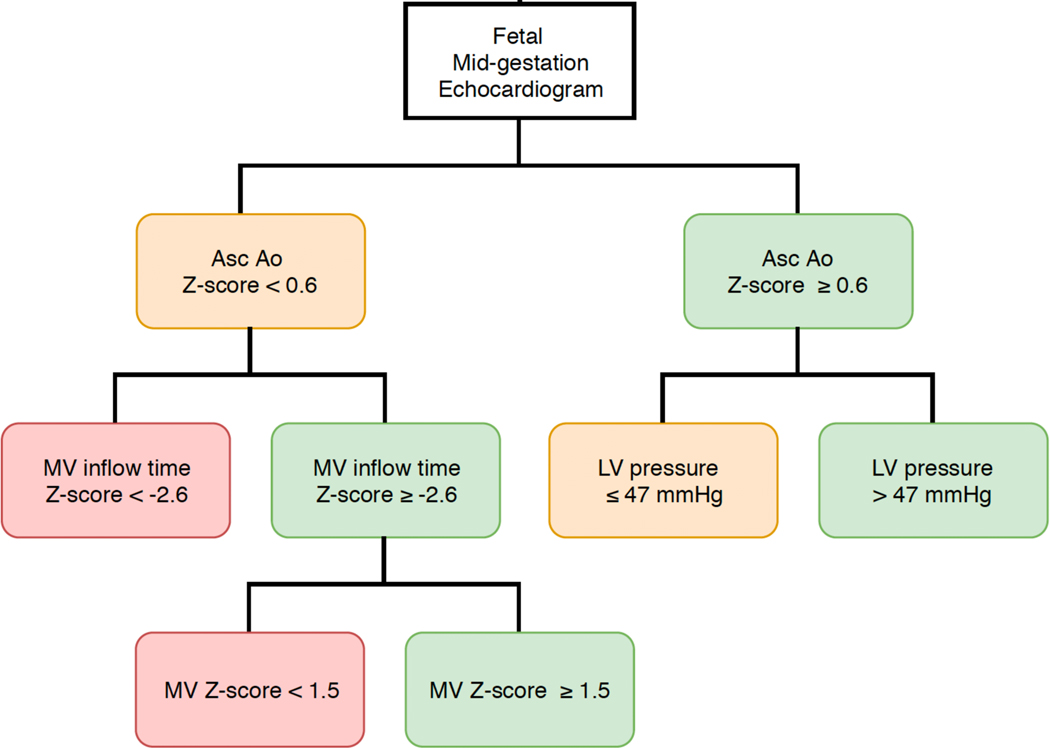
Incorporating previously published (or locally developed) prediction models for the likelihood of biventricular circulation into our decision model would enable personalized decision making.16 Friedman et al. previously published a classification and regression tree analysis that identified probability of biventricular circulation based on fetal echocardiographic parameters. We adapted this figure to demonstrate the application of our model-identified threshold for probability of biventricular circulation with technical success to existing prediction models (Figure 5). For example, if fetal echocardiographic parameters, such as left ventricular pressure and ascending aortic Z-score, predict a probability of biventricular circulation below 26%, our decision model supports expectant management. However, if technical success and risk of fetal demise improve with refinement of technique, this threshold falls to 7%. Since 2009, the risk of procedure-related fetal demise after FAV at our institution has decreased to 4%. Notably, our estimates were derived from relatively small numbers, with fortunately few demises, so caution should be taken in their interpretation.
Figure 5. Classification and Regression Tree Analysis (CART) for Mid-gestation Echocardiographic Parameters.
Our model identified a threshold probability of biventricular circulation above which fetal aortic valvuloplasty provides a 6-year transplant-free survival benefit. The threshold value (26%) is applied to the previously published CART diagram, which identified probabilities of postnatal biventricular circulation based on fetal echocardiographic parameters.16 Green boxes exceed the threshold, i.e., perform FAV; orange is within +/− 10% of the threshold; red boxes fall below the threshold, i.e., No FAV. Adapted with permission from Friedman et al, Improved technical success, postnatal outcome and refined predictors of outcome for fetal aortic valvuloplasty. Ultrasound Obstet Gynecol. 2018;52:212-220.16
Although 6-year transplant-free survival with FAV does not necessarily confer a longer-term benefit, we anticipate that the survival benefit will persist and possibly increase over decades of follow-up. Biventricular patients avoid risks for Fontan-related complications or dependence on a systemic right ventricle and tricuspid valve. Although the risks of single ventricle physiology are well known, the long-term prognosis for biventricular patients after FAV remains uncertain, particularly the risk of developing left ventricular diastolic dysfunction resulting in left atrial and pulmonary hypertension.28,29 Therefore, as longer term follow-up data emerge from our FAV cohort and others as well as from the SVR trial and other single ventricle cohorts, analyses such as ours will need to be repeated.
While transplant-free survival is an important and relevant outcome, it does not capture all the costs and quality of life decrements associated with both single and biventricular postnatal management. Cost and quality of life were not considered in our model due to the absence of data for these procedures and population. Although much progress has been made in quantifying disease-specific and general health-related quality of life in children with CHD, these measures have not yet been used as a surrogate for utilities in cost-effectiveness analyses. Given the modest benefit of FAV with regards to medium-term transplant-free survival, further research on both cost and quality of life of all possible strategies would be necessary to inform incremental cost-effectiveness analyses and help guide decision making from both individual and societal perspectives.
Besides the aforementioned assumptions, additional limitations merit consideration. First, the model values fetal demise as equivalent to a postnatal death. It is beyond the scope of this study to consider the relative impact of the timing of a death, but it may factor into the decision making of some parents.30 Second, we assume survival of a single ventricle patient who had a technically successful procedure is equivalent to someone who did not undergo FAV. It is possible that additional antegrade aortic flow, even if insufficient to support an entire cardiac output, confers a survival benefit. However, this has not been evaluated to date, so the model conservatively assumed no benefit of FAV in the single ventricle patient. Third, we included a non-cardiac death (e.g., car accident) in our survival estimate. This may underestimate the benefit of FAV on survival. All of the above limitations and assumptions biased our results against FAV as the preferred strategy, so the model’s estimated comparative benefit from FAV could be considered conservative.
Conclusion
Fetal aortic valvuloplasty for the management of mid-gestation AS with evolving HLHS modestly increases the probability of transplant-free survival and life expectancy to ages 3 and 6 years. When restricting the analysis to post-2009 to reflect our current practices and outcomes, the survival benefits of FAV increase further. Expectant management is favored if risk of fetal demise exceeds 12% or probability of biventricular circulation falls below 26%, suggesting that an experienced team and careful application of selection criteria are imperative. Additional follow-up of patients receiving FAV into the second decade of life and beyond are needed.
What is Known
Fetal aortic valvuloplasty (FAV) for mid-gestation aortic stenosis increases the probability of postnatal biventricular circulation but carries risks, including fetal demise, a technically unsuccessful procedure, and single ventricle postnatal circulation despite technical success.
What the Study Adds
Despite these upfront risk, our model suggests that FAV confers a modest medium-term transplant-free survival benefit compared to expectant management.
The survival benefit of FAV was lost if risk of fetal demise exceeded 12% or probability of biventricular circulation fell below 26%.
Acknowledgments
The authors thank Russell Gongwer and Felicia Trachtenerg of the New England Research Institute for their expert assistance with data analysis from the Pediatric Heart Network Single Ventricle Reconstruction trial.
Financial Support
Financial support was provided by the Benderson and Nomellini Family Funds, the Tupper Research Fund (JBW), and NIH/NHLBI T32 HL007572-32 (SSP). These funders had no involvement in the design of the study; the collection, analysis, and interpretation of the data; and the decision to approve publication of the finished manuscript.
Footnotes
Disclosures
None
References
- 1.Danford DA, Cronican P. Hypoplastic left heart syndrome: progression of left ventricular dilation and dysfunction to left ventricular hypoplasia in utero. Am Heart J. 1992;123:1712–1713. http://www.ncbi.nlm.nih.gov/pubmed/1595559. [DOI] [PubMed] [Google Scholar]
- 2.Simpson JM, Sharland GK. Natural history and outcome of aortic stenosis diagnosed prenatally. Heart. 1997;77:205–210. doi: 10.1136/hrt.77.3.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gardiner HM, Kovacevic A, Tulzer G, Sarkola T, Herberg U, Dangel J, Öhman A, Bartrons J, Carvalho JS, Jicinska H, Fesslova V, Averiss I, Mellander M, Fetal Working Group of the AEPC. Natural history of 107 cases of fetal aortic stenosis from a European multicenter retrospective study. Ultrasound Obstet Gynecol. 2016;48:373–381. doi: 10.1002/uog.15876 [DOI] [PubMed] [Google Scholar]
- 4.Mäkikallio K, McElhinney DB, Levine JC, Marx GR, Colan SD, Marshall AC, Lock JE, Marcus EN, Tworetzky W. Fetal aortic valve stenosis and the evolution of hypoplastic left heart syndrome: patient selection for fetal intervention. Circulation. 2006;113:1401–1405. doi: 10.1161/CIRCULATIONAHA.105.588194 [DOI] [PubMed] [Google Scholar]
- 5.Simpson JM. Hypoplastic left heart syndrome. Ultrasound Obstet Gynecol. 2000;15:271–278. doi: 10.1046/j.1469-0705.2000.00086.x [DOI] [PubMed] [Google Scholar]
- 6.Schidlow DN, Tworetzky W, Wilkins-Haug LE. Percutaneous fetal cardiac interventions for structural heart disease. Am J Perinatol. 2014;31:629–636. doi: 10.1055/s-0034-1383884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allan LD, Maxwell DJ, Carminati M, Tynan MJ. Survival after fetal aortic balloon valvoplasty. Ultrasound Obstet Gynecol. 1995;5:90–91. doi: 10.1046/j.1469-0705.1995.05020090.x [DOI] [PubMed] [Google Scholar]
- 8.Maxwell D, Allan L, Tynan MJ. Balloon dilatation of the aortic valve in the fetus: a report of two cases. Br Heart J. 1991;65:256–258. doi: 10.1136/hrt.65.5.256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohl T, Sharland G, Allan LD, Gembruch U, Chaoui R, Lopes LM, Zielinsky P, Huhta J, Silverman NH. World experience of percutaneous ultrasound-guided balloon valvuloplasty in human fetuses with severe aortic valve obstruction. Am J Cardiol. 2000;85:1230–1233. doi: 10.1016/S0002-9149(00)00733-5 [DOI] [PubMed] [Google Scholar]
- 10.McElhinney DB, Tworetzky W, Lock JE. Current Status of Fetal Cardiac Intervention. Circulation. 2010;121:1256–1263. doi: 10.1161/CIRCULATIONAHA.109.870246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moon-Grady AJ, Morris SA, Belfort M, Chmait R, Dangel J, Devlieger R, Emery S, Frommelt M, Galindo A, Gelehrter S, Gembruch U, Grinenco S, Habli M, Herberg U, Jaeggi E, Kilby M, Kontopoulos E, Marantz P, Miller O, Otaño L, Pedra C, Pedra S, Pruetz J, Quintero R, Ryan G, Sharland G, Simpson J, Vlastos E, Tworetzky W, Wilkins-Haug L, Oepkes D, International Fetal Cardiac Intervention Registry. International Fetal Cardiac Intervention Registry. J Am Coll Cardiol. 2015;66:388–399. doi: 10.1016/j.jacc.2015.05.037 [DOI] [PubMed] [Google Scholar]
- 12.McElhinney DB, Marshall AC, Wilkins-Haug LE, Brown DW, Benson CB, Silva V, Marx GR, Mizrahi-Arnaud A, Lock JE, Tworetzky W. Predictors of technical success and postnatal biventricular outcome after in utero aortic valvuloplasty for aortic stenosis with evolving hypoplastic left heart syndrome. Circulation. 2009;120:1482–1490. doi: 10.1161/CIRCULATIONAHA.109.848994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freud LR, McElhinney DB, Marshall AC, Marx GR, Friedman KG, del Nido PJ, Emani SM, Lafranchi T, Silva V, Wilkins-Haug LE, Benson CB, Lock JE, Tworetzky W, Tweddell J, Hoffman G, Mussatto K, Fedderly R, Berger S, Jaquiss R, Ghanayem N, Frisbee S, Litwin S, Mitchell M, Ittenbach R, Gaynor J, Wernovsky G, Nicolson S, Spray T, Walter ED, Hübler M, Alexi-Meskishvili V, Miera O, Weng Y, Loforte A, Berger F, Hetzer R, Ghanayem N, Allen K, Tabbutt S, Atz A, Clabby M, Cooper D, Eghtesady P, Frommelt P, Gruber P, Hill K, Kaltman J, Laussen P, Lewis A, Lurito K, Minich L, Ohye R, Schonbeck J, Schwartz S, Singh R, Goldberg C, Feinstein J, Benson D, Dubin A, Cohen M, Maxey D, Mahle W, Pahl E, Villafañe J, Bhatt A, Peng L, Johnson B, Marsden A, Daniels C, Rudd N, Caldarone C, Mussatto K, Morales D, Ivy D, Gaynor J, Tweddell J, Deal B, Furck A, Rosenthal G, Ohye R, Ghanayem N, Cheatham J, Tworetzky W, Martin G, Anderson P, Sleeper L, Mahony L, Colan S, Atz A, Breitbart R, Gersony W, Gallagher D, Geva T, Margossian R, McCrindle B, Paridon S, Schwartz M, Stylianou M, et al. Fetal aortic valvuloplasty for evolving hypoplastic left heart syndrome: postnatal outcomes of the first 100 patients. Circulation. 2014;130:638–645. doi: 10.1161/CIRCULATIONAHA.114.009032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown DW, Dipilato AE, Chong EC, Lock JE, McElhinney DB. Aortic valve reinterventions after balloon aortic valvuloplasty for congenital aortic stenosis: Intermediate and late follow-up. J Am Coll Cardiol. 2010;56:1740–1749. doi: 10.1016/j.jacc.2010.06.040 [DOI] [PubMed] [Google Scholar]
- 15.Tworetzky W, Wilkins-Haug L, Jennings RW, van der Velde ME, Marshall AC, Marx GR, Colan SD, Benson CB, Lock JE, Perry SB. Balloon Dilation of Severe Aortic Stenosis in the Fetus. Circulation. 2004;110:2125–2131. doi: 10.1161/01.CIR.0000144357.29279.54 [DOI] [PubMed] [Google Scholar]
- 16.Friedman KG, Sleeper LA, Freud LR, Marshall AC, Godfrey ME, Drogosz M, Lafranchi T, Benson CB, Wilkins-Haug LE, Tworetzky W. Improved technical success, postnatal outcome and refined predictors of outcome for fetal aortic valvuloplasty. Ultrasound Obstet Gynecol. 2018;52:212–220. doi: 10.1002/uog.17530 [DOI] [PubMed] [Google Scholar]
- 17.Ohye RG, Sleeper L a, Mahony L, Newburger JW, Pearson GD, Lu M, Goldberg CS, Tabbutt S, Frommelt PC, Ghanayem NS, Laussen PC, Rhodes JF, Lewis AB, Mital S, Ravishankar C, Williams I a, Dunbar-Masterson C, Atz AM, Colan S, Minich LL, Pizarro C, Kanter KR, Jaggers J, Jacobs JP, Krawczeski CD, Pike N, McCrindle BW, Virzi L, Gaynor JW. Comparison of shunt types in the Norwood procedure for single-ventricle lesions. N Engl J Med. 2010;362:1980–1992. doi: 10.1056/NEJMoa0912461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newburger JW, Sleeper LA, Frommelt PC, Pearson GD, Mahle WT, Chen S, Dunbar-Masterson C, Mital S, Williams IA, Ghanayem NS, Goldberg CS, Jacobs JP, Krawczeski CD, Lewis AB, Pasquali SK, Pizarro C, Gruber PJ, Atz AM, Khaikin S, Gaynor JW, Ohye RG, Pediatric Heart Network Investigators. Transplantation-Free Survival and Interventions at 3 Years in the Single Ventricle Reconstruction Trial. Circulation. 2014;129:2013–2020. doi: 10.1161/CIRCULATIONAHA.113.006191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newburger JW, Sleeper LA, Gaynor JW, Hollenbeck-Pringle D, Frommelt PC, Li JS, Mahle WT, Williams IA, Atz AM, Burns KM, Chen S, Cnota J, Dunbar-Masterson C, Ghanayem NS, Goldberg CS, Jacobs JP, Lewis AB, Mital S, Pizarro C, Eckhauser A, Stark P, Ohye RG, Pediatric Heart Network Investigators. Transplant-Free Survival and Interventions at 6 Years in the SVR Trial. Circulation. 2018;137:2246–2253. doi: 10.1161/CIRCULATIONAHA.117.029375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uno H, Wittes J, Fu H, Solomon SD, Claggett B, Tian L, Cai T, Pfeffer MA, Evans SR, Wei L-J. Alternatives to Hazard Ratios for Comparing the Efficacy or Safety of Therapies in Noninferiority Studies. Ann Intern Med. 2015;163:127–134. doi: 10.7326/M14-1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marshall AC, Tworetzky W, Bergersen L, McElhinney DB, Benson CB, Jennings RW, Wilkins-Haug LE, Marx GR, Lock JE. Aortic valvuloplasty in the fetus: Technical characteristics of successful balloon dilation. J Pediatr. 2005;147:535–539. doi: 10.1016/j.jpeds.2005.04.055 [DOI] [PubMed] [Google Scholar]
- 22.Wilkins-Haug LE, Tworetzky W, Benson CB, Marshall AC, Jennings RW, Lock JE. Factors affecting technical success of fetal aortic valve dilation. Ultrasound Obstet Gynecol. 2006;28:47–52. doi: 10.1002/uog.2732 [DOI] [PubMed] [Google Scholar]
- 23.Beroukhim RS, Gauvreau K, Benavidez OJ, Baird CW, LaFranchi T, Tworetzky W. Perinatal outcome after prenatal diagnosis of single-ventricle cardiac defects. Ultrasound Obstet Gynecol. 2015;45:657–663. doi: 10.1002/uog.14634 [DOI] [PubMed] [Google Scholar]
- 24.Kim DH, Uno H, Wei L-J. Restricted Mean Survival Time as a Measure to Interpret Clinical Trial Results. JAMA Cardiol. 2017;2:1179–1180. doi: 10.1001/jamacardio.2017.2922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Royston P, Parmar MK. Restricted mean survival time: an alternative to the hazard ratio for the design and analysis of randomized trials with a time-to-event outcome. BMC Med Res Methodol. 2013;13:152. doi: 10.1186/1471-2288-13-152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao L, Claggett B, Tian L, Uno H, Pfeffer MA, Solomon SD, Trippa L, Wei LJ. On the restricted mean survival time curve in survival analysis. Biometrics. 2016;72:215–221. doi: 10.1111/biom.12384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pasquali SK, Li JS, Burstein DS, Sheng S, O’Brien SM, Jacobs ML, Jaquiss RDB, Peterson ED, Gaynor JW, Jacobs JP. Association of center volume with mortality and complications in pediatric heart surgery. Pediatrics. 2012;129:e370–6. doi: 10.1542/peds.2011-1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedman KG, Margossian R, Graham DA, Harrild DM, Emani SM, Wilkins-Haug LE, McElhinney DB, Tworetzky W. Postnatal Left Ventricular Diastolic Function After Fetal Aortic Valvuloplasty. Am J Cardiol. 2011;108:556–560. doi: 10.1016/j.amjcard.2011.03.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friedman KG, Freud L, Escobar-Diaz M, Banka P, Emani S, Tworetzky W. Left Ventricular Remodeling and Function in Children with Biventricular Circulation After Fetal Aortic Valvuloplasty. Pediatr Cardiol. 2015;36:1502–1509. doi: 10.1007/s00246-015-1193-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phillips J, Millum J. Valuing Stillbirths. Bioethics. 2015;29:413–423. doi: 10.1111/bioe.12120 [DOI] [PMC free article] [PubMed] [Google Scholar]