Abstract
Purpose of Review:
In 2018, the 6th World Symposium on Pulmonary Hypertension (WSPH) proposed lowering the mean pulmonary artery pressure (mPAP) threshold that defines pulmonary hypertension (PH) from ≥25 to >20 mmHg. The historical context and evolution of the PH definition and the data used to rationalize recent changes are reviewed here.
Recent Findings:
There is accumulating data on the clinical significance of mildly elevated mPAPs (21-24 mmHg). Studies have demonstrated lower exercise capacity and an increased risk of progression to overt PH (mPAP ≥25 mmHg) in specific at risk patient populations (e.g. systemic sclerosis and idiopathic pulmonary fibrosis). Further, large registries across diverse PH populations have identified increased mortality in patients with mPAPs 21-24 mmHg. While the clinical sequelae of lowering the mPAP threshold remain unclear, this uncertainty has fueled recent debates within the PH community.
Summary:
The changes to the PH definition proposed by the 6th WSPH are supported by normative hemodynamic data in healthy individuals as well as studies demonstrating an association between mPAPs above this normal range and increased mortality. Whether the higher mortality observed in patients with mildly elevated mPAPs is directly attributable to pulmonary vascular disease that is amenable to therapeutic intervention remains to be determined.
Keywords: Pulmonary hypertension, mean pulmonary artery pressure, pulmonary vascular resistance
Introduction
Proceedings of the 6th World Symposium on Pulmonary Hypertension (WSPH) were published in early 2019, addressing a number of important issues including pathobiology, genomics, clinical diagnosis and classification, risk stratification and clinical management. The World Symposia, which occur every five years, are perhaps best known for establishing the PH clinical classification system and defining the hemodynamic criteria for PH [mean pulmonary artery pressure (mPAP) ≥25 mmHg] and pulmonary arterial hypertension [PAH; mPAP ≥25 mmHg, pulmonary artery wedge pressure (PAWP) ≤15 mmHg, and pulmonary vascular resistance (PVR) >3 Wood units (WU)] [1–5]. Over the past decade, including during past symposia in 2008 and 2013, experts in the field have hotly debated these deeply rooted hemodynamic criteria, but only recently have they taken action. The 6th WSPH Task Force on Hemodynamic Definitions and Clinical Classification proposed a lower mPAP cut-off, reducing the diagnostic threshold from 25 to 20 mmHg for all PH subgroups. The Task Force did not alter the PAWP or PVR cut-offs for PAH [3, 6, 7**] but did add both PAWP ≤15 mmHg and PVR ≥3 WU to the definition of all forms of pre-capillary PH, such as group 3 and group 4 patients. This announcement generated considerable controversy and spirited debate among academic institutions as well as the broader PH community [8, 9]. In the following review, we aim to put these new hemodynamic definitions in historical context. Further, we will explore the medical literature used to justify these changes and lay out the potential clinical benefits and pit-falls should these new definitions be widely adopted.
PH Hemodynamics Throughout History
Stimulated by the increased incidence of PH attributable to widespread anorexigen use, experts in pulmonary vascular disease and right ventricular failure convened the first WSPH in 1973 [5]. During this initial meeting, a mPAP >25 mmHg was chosen as the hemodynamic threshold for diagnosing PH, while a mPAP of 15-25 mmHg was regarded as “borderline” abnormal. Nevertheless, those in attendance conceded that the mPAP cut-off was “empirically and arbitrarily defined.” Notably, more than a decade earlier, a World Health Organization sponsored expert committee on Chronic Cor Pulmonale reported that mPAP is generally ≤15 mmHg in normal adults and never exceeds 20 mmHg [10]. Thus, at its inception, the hemodynamic definition of PH was swathed in uncertainty and acknowledged limitations. In order to address these shortcomings, the inaugural WSPH called for large multi-center registries and accumulation of hemodynamic data to better characterize the normal pulmonary circulation.
The first multicenter Primary PH registry was sponsored by the NIH and initiated in 1981 [11, 12]. In keeping with the first WSPH definition, patients were included based on mPAP>25 mmHg at rest or >30 mmHg with exertion and a PAWP ≤12 mmHg. Of note, PVR was not a part of the definition at that time, though it was understood that this was tantamount to differentiating pre- and post-capillary disease. Patients included in this seminal study had a mean mPAP of 60±18 mmHg and a mean indexed PVR of 26±14 WU m2, reflecting the severity of disease in this cohort. Importantly, the inclusion criteria established by the Primary PH registry served as the criteria for enrollment in the first randomized, controlled trial in PAH patients investigating epoprostenol [13].
As time progressed and physicians’ arsenal of PAH medications began to grow [13], the World Symposia on PH were re-established. During the second and third World Symposia [1, 4], the clinical classification system, composed of five PH subgroups, was introduced, the term primary pulmonary hypertension was eliminated in favor of PAH, and the PVR >3 WU cut-off was added to the definition of PAH. Both data and rationale for the selection of PVR >3 WU were conspicuously absent [1], and in 2008, the 4th WSPH decided to again remove it from the PAH definition [6]. Aiding in the decision to remove the PVR criteria were the results of a large systematic review that was performed by the 4th World Symposium’s PAH Diagnosis working group [14**]. Analysis of 1,187 subjects from 47 studies identified a mean (SD) supine mPAP of 14 (3.3) mmHg, thus delineating an upper limit of normal of 20.6 mmHg (2SD above the mean). Similarly, a population distribution of PVR was constructed, demonstrating a mean (SD) of 0.925 (0.375) WU. Though the authors warned that this PVR distribution was not Gaussian, it served as the primary reason that PVR >3 was deemed arbitrary and removed from the PAH definition. Seemingly in contradiction, the authors only slightly modified the mPAP PH criteria (from >25 to ≥25 mmHg), though they admitted that mPAPs ranging from 21-24 mmHg were abnormal and warranted additional population-based studies.
In 2013, the 5th WSPH re-addressed these same questions. In a systematic fashion, the attendees discussed the need to decrease the mPAP PH criteria from 25 to 20 mmHg and whether or not to re-introduce PVR into the PAH definition [3]. Though they cited the same systematic review performed in 2008 [14**] as well as increasing evidence that borderline mPAPs (21-24 mmHg) were associated with worse exercise capacity and increased risk of developing “bona fide” PH [15–17], the decision was made to retain the 25 mmHg threshold. This decision was largely based upon the exclusion of patients with borderline PH (mPAP 21-24 mmHg) from clinical trials and the fact that the data informing their natural history was limited to specific patient populations such as those with systemic sclerosis (SSc) or idiopathic pulmonary fibrosis. The attendees again added PVR >3 WU back to the PAH definition. They argued that this component of the definition was important to ensure a thorough hemodynamic assessment via right heart catheterization (RHC) with measurement of PAWP and cardiac output (CO), as well as to decrease the likelihood that high-output and left heart disease patients would be inaccurately classified. Interestingly, an antithetical argument was made for maintaining the PAWP ≤15 mmHg cut-off instead of dropping it to 12 mmHg. Despite evidence that PAWP <15 does not rule out left heart disease [18], the authors chose to retain the more sensitive criteria because it was “widely memorized among physicians” and a staple of therapeutic trial inclusion criteria [3].
In summary, we can contextualize the most recent changes to the PH definition by reviewing the choices of previous symposia (Figure 1). Though dogmatically accepted and taught to generations of clinicians across the world, it is clear that the former PH and PAH hemodynamic criteria were rather arbitrarily selected, often debated, and intermittently altered. The decision to maintain the criteria of mPAP ≥25 mm Hg and include PVR was, in part, motivated by a desire to avoid over-diagnosis and inappropriate treatment. Further, the available evidence on whether borderline mPAPs were clinically relevant in broader patient populations and across PH subgroups remained inconclusive.
Figure 1. Changes over time to the hemodynamic definition of pulmonary hypertension.
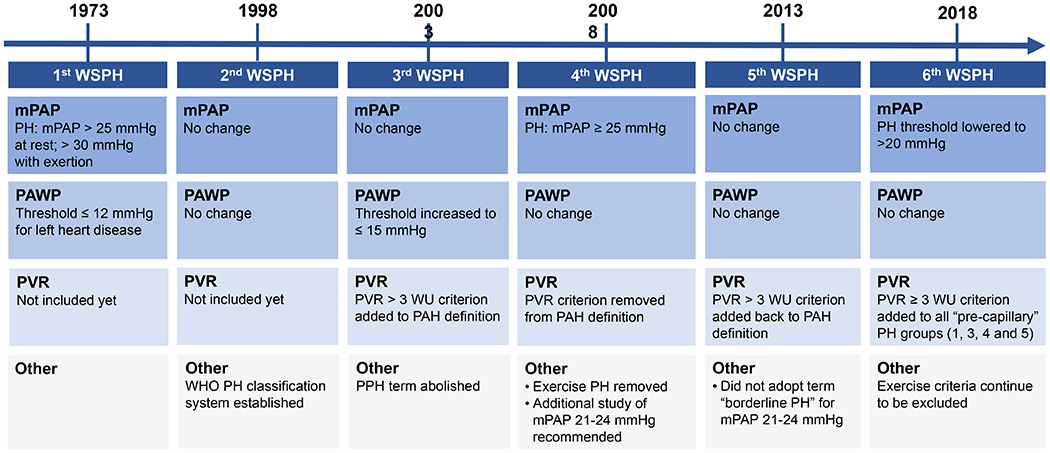
Since the 1st World Symposium on Pulmonary Hypertension (WSPH) in 1973, there have been a number of changes to the hemodynamic criteria for diagnosing PH and pre-capillary PH.
Rationalizing the New PH Definition
The 6th WSPH once again assigned a task force to examine the appropriateness of the PH and PAH hemodynamic definitions, and, similar to prior symposia, the authors acknowledged that the upper limit of normal for mPAP is 20 mmHg. However, unlike in years past, the authors made the bold decision to drop the mPAP threshold to >20 mmHg for all PH groups. Rationalizing their decision, the authors focused on the increasingly evident association of borderline mPAPs with poor clinical outcomes in multiple, large patient populations [7**]. Additionally, the task force decided to maintain a conservative PVR cut-off of ≥3 WU, as it had already demonstrated prognostic power in the decision to correct congenital shunts as well as in predicting outcomes in heart transplant, advanced lung disease, and chronic thromboembolism.
There has been interest in the natural history of patients with “borderline” mPAPs for a number of years and data continue to accumulate. Expectedly, the majority of evidence is from patients with known PH risk factors, such as SSc, advanced lung disease, and left heart disease, as they frequently undergo RHC. Some of the earliest data come from patients with SSc, in which mPAPs ranging from 21-24 mm Hg have been associated with worse functional class, lower exercise capacity, and future development of PAH [15, 16, 19*, 20]. For instance, Valerio et al. and Coghlan et al. found that 27% (5 year follow-up) and 33% (3 year follow-up) of SSc patients with a baseline mPAP 21-24 mmHg, respectively, surpass the more stringent threshold of ≥25 mmHg on repeat RHC [19*, 20]. The average (SD) mPAP in these patients who eventually were diagnosed with PAH on follow up RHC was 31.3 (4.2) mmHg [19*], illustrating that the increases in mPAP were significant. Notably, among patients with a baseline mPAP of 21-24 mmHg, there was a high proportion with PVR <3 WU in both studies, with a mean (SD) of 2.9 (0.6) and 2.3 (0.8) WU, respectively. Although patients with SSc-PAH are known to have worse survival compared to idiopathic PAH patients [21], studies have not detected a survival difference between SSc patients with a mPAP ≤20 mmHg versus those with a mPAP of 21-24 mmHg [16, 19*, 20]. Importantly, these studies were not limited to patients who developed SSc-PAH, and a substantial proportion developed PH due to lung disease and left heart disease. Patients who did develop PAH on repeat RHC had outcomes akin to other SSc-PAH patients.
Borderline mPAPs have also been associated with the development of “bona fide” PH and worse clinical outcomes in patients with interstitial lung disease [17, 22*] and large, diverse cohorts referred for RHC [23**–25*]. Maron et al. and Assad et al. investigated RHCs performed primarily in patients with known or suspected left heart disease from the large Veterans Affairs Clinical Assessment, Reporting, and Tracking (VA CART) program and Vanderbilt University, respectively. In the VA CART study, >21,000 patients were observed, with a mortality hazard rate (HR) increase of 1.183 (95% CI 1.004-1.393) for every 1 mmHg increase in mPAP starting at 19 mmHg [23**]. Importantly, the association of borderline mPAPs with increased mortality remained after adjustment for comorbidities and in subgroups with PVR <3 WU or PAWP ≤15 mmHg. In the Vanderbilt University database, 18% of the more than 4,000 patients analyzed had a mPAP 19-24 mmHg. Similar to the VA CART study, survival was worse in these patients compared to those with a normal mPAP (≤18 mmHg) with a HR increase of 1.31 (95% CI 1.04-1.65) and an unadjusted 5-year survival of 75% vs 83%, respectively [24]. Only 70 patients with an initial mPAP 19-24 mmHg underwent repeat RHC, but 61% of them had a mPAP ≥25 mmHg on follow up. Lastly, Douschan et al. found a similar association between increasing mPAP and mortality in a patient cohort with a more even distribution of PH group assignments (26% group I, 20% group II, 27% group III, 18% group IV, and 9% group V) [25*]. In an unbiased classification and regression tree analysis, the authors identified mPAP prognostic discrimination groups of < 17, 17-26, and >26 mmHg associated with increasing mortality. They proceeded to analyze their cohort according to the normal distribution of mPAPs in healthy adults and found a progressive increase in mortality in those with “high-normal” mPAPs (18-20 mmHg) and “borderline” mPAPs (21-24 mmHg) compared to “low-normal” mPAPs (<18 mmHg). Again, despite a mPAP of 1 SD (high-normal) or 2 SDs (borderline) above the mean, patients in these two groups largely had a PVR <3 WU.
Though the above registry and retrospective cohort data convincingly argues that mildly elevated mPAPs are associated with significant clinical outcomes, there are much fewer data demonstrating distinct pulmonary vascular remodeling in these patients. In other words, does a mPAP of 21-24 mmHg represent an early timepoint on the spectrum of pulmonary vascular remodeling and right ventricular dysfunction, or is it simply a marker of co-existing medical comorbidities? Limited evidence comes from a few reports in patients with SSc and borderline elevated mPAPs [26–28]. van der Bruggen and colleagues reported the case of a patient with SSc, dyspnea, reduced diffusion capacity for carbon monoxide, and normal chest computed tomography. Lung biopsy was performed and demonstrated extensive pulmonary arterial abnormalities, including medial hypertrophy, intimal fibrosis, and mild macrophage infiltration [26]. There was no evidence of pulmonary venous remodeling or interstitial lung disease. Concurrent RHC revealed a mPAP of 22 mmHg and PVR of 2.5 WU. Despite treatment with sildenafil, the patient’s symptoms worsened and she was diagnosed with PAH four years later (mPAP 44 mmHg, PVR 8.8 WU). Though obviously limited as a single case report, this is an illustrative example of the potential histopathological changes associated with mild mPAP elevations. Nagel et al and Lamia et al have also demonstrated subclinical right ventricular (RV) dysfunction in patients with a mPAP of 21-24 mmHg. Nagel and colleagues analyzed exercise RHCs in 112 SSC patients with normal, borderline, and overtly elevated mPAPs [27]. They found that patients with borderline mPAPs had a less robust increase in CI and consistently lower pulmonary arterial compliance, potentially representing decreased RV contractile reserve. Lamia and colleagues investigated RV regional contraction by speckle tracking echocardiography in PAH patients, patients with a mPAP 21-24 mmHg, and healthy controls. They identified significant RV contractile dysynchrony in both the PAH and borderline mPAP groups compared to controls [28]. Notably, in 11 of the 13 patients with mPAP 21-24 mmHg and abnormal RV synchrony, RV ejection fraction was normal, suggesting a state of silent RV dysfunction mirroring the “borderline” mPAP.
The Pro-Con Debate
The changes to the PH and PAH hemodynamic definitions described above, regardless of available evidence, were certain to stir up significant dissent (Table 1). A number of PH experts have argued that, outcome data aside, lowering the threshold for the diagnosis of PH to a mPAP >20 mmHg will come with practical difficulties and unintended consequences [8, 29]. First, given the paucity of data linking mild elevations in mPAP with histological changes or RV maladaptation, opponents of the new definition are skeptical that PH is a significant driver of the observed increased mortality. For example, the largest investigation of patients with borderline mPAPs to date primarily included patients with left heart disease, and in that study the increased mortality associated with increasing mPAP persisted even in a subgroup with PVR <3 WU. It seems unlikely that pulmonary vascular disease is driving mortality in these patients, when RV afterload is not substantially altered [29]. Second, little data exist on how diagnostic algorithms perform at this mPAP threshold. Will clinicians be able to reliably differentiate between WHO PH groups [29]? Additionally, retaining other “arbitrary” cut-offs, such as a PVR ≥3 WU and a PAWP ≤15 mmHg appears to be at odds with the rationale for lowering the mPAP threshold. If future changes to other hemodynamic thresholds are forthcoming, the rolling tide of small changes to the pre-capillary PH definition could serve to confuse non-specialists and alter referral patterns [8]. Furthermore, with the mPAP threshold decreased, the diagnosis of pre-capillary PH will now weigh more heavily on accurate determinations of CO and PAWP, both of which are prone to error even at expert centers. Third, a diagnosis of PAH based on the proposed new definition could result in undue pressure on clinicians to consider treatment, which is neither FDA approved nor evidence-based in patients with a mPAP of 21-24 mmHg. Alternatively, a diagnosis of PH due to left heart disease in a patient with a mPAP of 21-24 mmHg changes little, as no effective therapies are currently available. Complicating matters further, if therapy is initiated it may be falsely interpreted as effective, even though disease progression may have never occurred in the first place. Thus, while admitting that this patient group requires more study, opponents of the proposed new definition cite concrete clinical misgivings and believe that further research, including therapeutic trials in some select PH subgroups, should be done before the field is disrupted by definitional changes.
Table 1.
Lowering mPAP threshold from 25 mmHg → 20 mmHg
| Pros | Cons |
|---|---|
| • Borderline mPAP associated with increased mortality and possibility of progression to mPAP ≥ 25 mmHg • Requires lower PAWP and/or lower CO for diagnosis (selects for more severe disease) • Will spur inclusion of patients with mPAP 21-24 mmHg in registries and randomized, controlled trials |
• Elevated mPAP not necessarily driver of increased mortality • More emphasis on CO and PAWP, both error-prone measurements • Unclear diagnostic algorithms and treatment options for patients with mPAP < 25 mmHg |
Proponents of the definition change are obviously more optimistic regarding the potential research and trial-based benefits of lowering the mPAP threshold, while discounting concerns of rampant over-diagnosis and therapeutic dilemmas as inflated [9, 30, 31]. First and foremost, based on unpublished analyses of large clinical PAH registries, the authors of the new hemodynamic criteria report that less than 10% of patients would be re-classified as having pre-capillary PH [9]. Similarly, a University of Michigan analysis of 131 patients revealed that the new definition resulted in reclassification of only 4 patients, 3% of the cohort [30, 32*]. This was largely due to keeping the PVR threshold of ≥3 WU, which advocates of the new definition point to as a stop-gap against over-diagnosis. Indeed by retaining a PVR ≥3 WU, the definition proposed by the 6th WSPH dictates either a lower PAWP or a more depressed cardiac output, thus identifying patients more likely to have clinically significant pulmonary vascular disease (Figure 2). Proponents also argue that the new definition will be most influential in patient populations with an increased risk of pre-capillary PH, where early screening programs are already in place, such as SSc and heritable PAH. Likewise, the definition change should encourage the inclusion of these patients in prospective clinical registries and aide in their recruitment for randomized controlled therapeutic trials, few of which have been performed or are currently ongoing [31, 33*]. Additionally, though less likely to fall within the new hemodynamic definition of pre-capillary PH, patients with PH due to left heart disease and advanced lung disease also warrant more pathophysiologic and clinical investigation. Perhaps enrolling patients with a mPAP >20 mmHg from these PH subgroups will enable new insights into pathobiology and heart-lung interactions.
Figure 2. Effect of different mean pulmonary artery pressure thresholds on the proportion of cardiac output and pulmonary artery wedge pressure combinations leading to an elevated pulmonary vascular resistance.
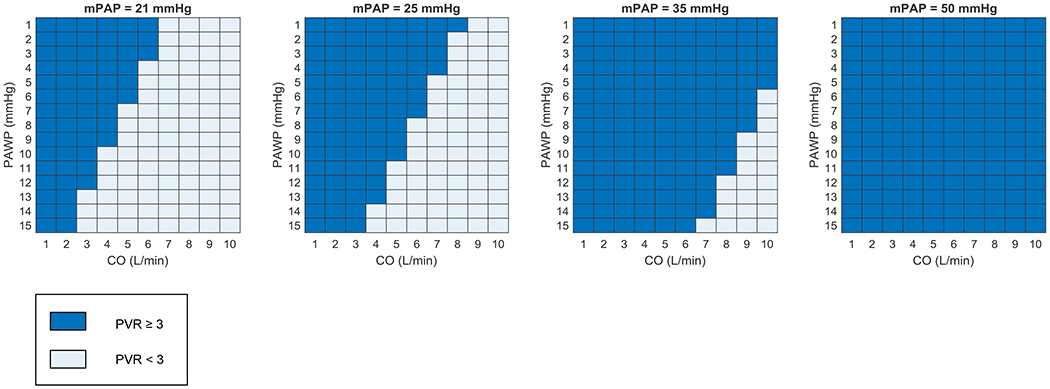
Combinations of cardiac output (CO) and pulmonary artery wedge pressure (PAWP) resulting in a pulmonary vascular resistance (PVR) ≥3 Woods units (WU) are displayed in dark blue and those resulting in a PVR <3 WU are displayed in light blue.
Conclusion
From its inception, the WSPH has continuously debated and frequently altered the hemodynamic definitions of PH in general and pre-capillary PH specifically. The evidence clearly supports that mPAPs ranging from 21-24 mmHg are abnormal and associated with poor outcomes across a diverse range of PH etiologies. Including this group of patients will hopefully stimulate additional research into the relevance of these early pulmonary pressure elevations in terms of both pathophysiology and therapy. Retaining the PVR ≥3 WU threshold for pre-capillary PH appears to safeguard against over-diagnosis and inappropriate treatment of non-group I PH, while simultaneously limiting the patient population that warrants additional study. Indeed, patients with mPAP >20 mmHg and PVR 2-3 WU are still abnormal and deserve closer investigation and therapeutic trials of their own. It is perhaps this group that deserves the terminology “borderline PH.” While opinions regarding the PH hemodynamic changes may vary, clinicians should all be prepared for new patient interactions and unclear treatment algorithms, as the clinical trajectory and appropriate management of these patients is further clarified.
Key Points.
The 6th WSPH proposed lowering the mPAP threshold for the diagnosis of pulmonary hypertension from ≥25 to >20 mmHg while maintaining the PVR ≥3 WU and PAWP ≤15 mmHg cut-offs for pre-capillary disease.
This proposed change was based on evidence demonstrating that the upper limit of normal for mPAP in healthy adults is 20.6 mmHg as well as a strong association of mildly elevated mPAPs (21–24 mmHg) with increased mortality across diverse PH populations.
Whether mPAPs of 21–24 mmHg are representative of pulmonary vascular remodeling that warrants therapeutic intervention is not clear, stimulating debate throughout the PH community as to how to integrate these definition changes into practice.
Acknowledgements
This research was made possible through the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and contributions to the Foundation for the NIH from the Doris Duke Charitable Foundation, Genentech, the American Association for Dental Research, the Colgate-Palmolive Company, and other private donors.
Financial support and sponsorship
This work was supported by the National Institutes of Health Clinical Center intramural funding.
Conflicts of interest
The NIH Clinical Center PAH Program receives support from Aadi Bioscience through a Cooperative Research and Development Agreement. The authors have no personal financial relationship with Aadi Bioscience or any other entity.
References
- [1].Barst RJ, McGoon M, Torbicki A et al. Diagnosis and differential assessment of pulmonary arterial hypertension. J Am Coll Cardiol 2004; 43:40S–47S. [DOI] [PubMed] [Google Scholar]
- [2].Simonneau G, Galie N, Rubin LJ et al. Clinical classification of pulmonary hypertension. J Am Coll Cardiol 2004; 43:5S–12S. [DOI] [PubMed] [Google Scholar]
- [3].Hoeper MM, Bogaard HJ, Condliffe R et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol 2013; 62:D42–50. [DOI] [PubMed] [Google Scholar]
- [4].Rich S, editor. Primary Pulmonary Hypertension: Executive Summary from the World Symposium – Primary Pulmonary Hypertesion 1998. Available from the World Health Organization via the Internet; (http://www.who.int/ncd/cvd/pph.htm;). [Google Scholar]
- [5].Hatano S, Strasser T, World Health O. Primary pulmonary hypertension: report on a WHO meeting, Geneva, 15-17 October 1973 / edited by Shuichi Hatano and Toma Strasser. In: Geneva: World Health Organization; 1975. [Google Scholar]
- [6].Badesch DB, Champion HC, Sanchez MA et al. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol 2009; 54:S55–66. [DOI] [PubMed] [Google Scholar]
- [7]**.Simonneau G, Montani D, Celermajer DS et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53. [DOI] [PMC free article] [PubMed] [Google Scholar]; This report of the 6th World Symposium on Pulmonary Hypertension outlines the proposed new hemodynamic criteria as well as the task force’s rationale.
- [8].Gibbs JSR, Torbicki A. Proposed new pulmonary hypertension definition: is 4 mm(Hg) worth re-writing medical textbooks? Eur Respir J 2019; 53. [DOI] [PubMed] [Google Scholar]
- [9].Hoeper MM, Humbert M. The new haemodynamic definition of pulmonary hypertension: evidence prevails, finally! Eur Respir J 2019; 53. [DOI] [PubMed] [Google Scholar]
- [10].Chronic cor pulmonale. Report of an expert committee. World Health Organ Tech Rep Ser 1961; 213:35. [PubMed] [Google Scholar]
- [11].RICH S, DANTZKER DR, AYRES SM et al. Primary Pulmonary Hypertension: A National Prospective Study. Annals of Internal Medicine 1987; 107:216–223. [DOI] [PubMed] [Google Scholar]
- [12].D’Alonzo GE, Barst RJ, Ayres SM et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 1991; 115:343–349. [DOI] [PubMed] [Google Scholar]
- [13].Barst RJ, Rubin LJ, Long WA et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med 1996; 334:296–301. [DOI] [PubMed] [Google Scholar]
- [14]**.Kovacs G, Berghold A, Scheidl S, Olschewski H. Pulmonary arterial pressure during rest and exercise in healthy subjects: a systematic review. Eur Respir J 2009; 34:888–894. [DOI] [PubMed] [Google Scholar]; This study represents the seminal analysis of normal resting and exercise pulmonary vascular hemodynamics. Since its publication, no substantial new investigations have been performed.
- [15].Kovacs G, Maier R, Aberer E et al. Borderline pulmonary arterial pressure is associated with decreased exercise capacity in scleroderma. Am J Respir Crit Care Med 2009; 180:881–886. [DOI] [PubMed] [Google Scholar]
- [16].Bae S, Saggar R, Bolster MB et al. Baseline characteristics and follow-up in patients with normal haemodynamics versus borderline mean pulmonary arterial pressure in systemic sclerosis: results from the PHAROS registry. Ann Rheum Dis 2012; 71:1335–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hamada K, Nagai S, Tanaka S et al. Significance of pulmonary arterial pressure and diffusion capacity of the lung as prognosticator in patients with idiopathic pulmonary fibrosis. Chest 2007; 131:650–656. [DOI] [PubMed] [Google Scholar]
- [18].Prasad A, Hastings JL, Shibata S et al. Characterization of static and dynamic left ventricular diastolic function in patients with heart failure with a preserved ejection fraction. Circ Heart Fail 2010; 3:617–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Valerio CJ, Schreiber BE, Handler CE et al. Borderline mean pulmonary artery pressure in patients with systemic sclerosis: transpulmonary gradient predicts risk of developing pulmonary hypertension. Arthritis Rheum 2013; 65:1074–1084. [DOI] [PubMed] [Google Scholar]
- [20]*.Coghlan JG, Wolf M, Distler O et al. Incidence of pulmonary hypertension and determining factors in patients with systemic sclerosis. Eur Respir J 2018; 51. [DOI] [PubMed] [Google Scholar]; This study highlights the natural history of disease in a small cohort of patients with systemic sclerosis and borderline elevated mean pulmonary artery pressures.
- [21].Ramjug S, Hussain N, Hurdman J et al. Idiopathic and Systemic Sclerosis-Associated Pulmonary Arterial Hypertension: A Comparison of Demographic, Hemodynamic, and MRI Characteristics and Outcomes. Chest 2017; 152:92–102. [DOI] [PubMed] [Google Scholar]
- [22]*.Nemoto K, Oh-Ishi S, Akiyama T et al. Borderline pulmonary hypertension is associated with exercise intolerance and increased risk for acute exacerbation in patients with interstitial lung disease. BMC Pulm Med 2019; 19:167. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study highlights the natural history of disease in a cohort of patients with interstitial lung disease and borderline elevated mean pulmonary artery pressures.
- [23]**.Maron BA, Hess E, Maddox TM et al. Association of Borderline Pulmonary Hypertension With Mortality and Hospitalization in a Large Patient Cohort: Insights From the Veterans Affairs Clinical Assessment, Reporting, and Tracking Program. Circulation 2016; 133:1240–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]; This retrospective analysis of a large Veterans Affairs cohort served as the turning point for incorporating patients with mildly elevated pulmonary artery pressures into the pulmonary hypertension definition.
- [24].Assad TR, Maron BA, Robbins IM et al. Prognostic Effect and Longitudinal Hemodynamic Assessment of Borderline Pulmonary Hypertension. JAMA Cardiol 2017; 2:1361–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]*.Douschan P, Kovacs G, Avian A et al. Mild Elevation of Pulmonary Arterial Pressure as a Predictor of Mortality. Am J Respir Crit Care Med 2018; 197:509–516. [DOI] [PubMed] [Google Scholar]; This study represents the most diverse investigation into the natural history of patients with borderline elevated mean pulmonary artery pressures, across pulmonary hypertension subgroups.
- [26].van der Bruggen CE, Nossent EJ, Grunberg K et al. The Real Face of Borderline Pulmonary Hypertension in Connective Tissue Disease. Ann Am Thorac Soc 2016; 13:1428–1430. [DOI] [PubMed] [Google Scholar]
- [27].Nagel C, Marra AM, Benjamin N et al. Reduced Right Ventricular Output Reserve in Patients With Systemic Sclerosis and Mildly Elevated Pulmonary Artery Pressure. Arthritis Rheumatol 2019; 71:805–816. [DOI] [PubMed] [Google Scholar]
- [28].Lamia B, Muir JF, Molano LC et al. Altered synchrony of right ventricular contraction in borderline pulmonary hypertension. Int J Cardiovasc Imaging 2017; 33:1331–1339. [DOI] [PubMed] [Google Scholar]
- [29].Torbicki A Definition of pulmonary hypertension challenged? Nature Reviews Cardiology 2016; 13:250–251. [DOI] [PubMed] [Google Scholar]
- [30].Kovacs G, Olschewski H. Debating the new haemodynamic definition of pulmonary hypertension: much ado about nothing? Eur Respir J 2019; 54. [DOI] [PubMed] [Google Scholar]
- [31].Simonneau G, Hoeper MM. The revised definition of pulmonary hypertension: exploring the impact on patient management. Eur Heart J Suppl 2019; 21:K4–K8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]*.Jaafar S, Visovatti S, Young A et al. Impact of the revised haemodynamic definition on the diagnosis of pulmonary hypertension in patients with systemic sclerosis. Eur Respir J 2019; 54. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates that dropping the mean pulmonary artery pressure to > 20 mmHg, will likely have only a small effect on the prevalence of pre-capillary pulmonary hypertension.
- [33]*.Pan Z, Marra AM, Benjamin N et al. Early treatment with ambrisentan of mildly elevated mean pulmonary arterial pressure associated with systemic sclerosis: a randomized, controlled, double-blind, parallel group study (EDITA study). Arthritis Res Ther 2019; 21:217. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study represents the only randomized controlled trial to date utilizing PAH-specific therapy in patients with mildly elevated mean pulmonary artery pressures (21-24 mmHg) at rest and/or > 30 mmHg with low-intensity exercise.


