Supplemental Digital Content is available in the text.
Keywords: bloodstream infections, Enterococcus species, frequency, infection control, severe acute respiratory syndrome-coronavirus-2
OBJECTIVES:
We aimed to assess the frequency of ICU-acquired bloodstream infections in coronavirus disease 2019 patients.
DESIGN:
Retrospective observational study.
SETTING:
The emergency expansion of an ICU from eight general beds to 30 coronavirus disease 2019 beds.
PARTICIPANTS:
Patients with coronavirus disease 2019 admitted to the ICU of Luigi Sacco Hospital (Milan, Italy) for greater than or equal to 48 hours between February 21, 2020, and April 30, 2020.
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
The frequency of bloodstream infections per 1,000 days of ICU stay was calculated in 89 coronavirus disease 2019 patients, and the cumulative probability of bloodstream infection was estimated using death and ICU discharge as competing events. Sixty patients (67.4%) experienced at least one of the 93 recorded episodes of bloodstream infection, a frequency of 87 per 1,000 days of ICU stay (95% CI, 67–112).The patients who experienced a bloodstream infection had a higher Sequential Organ Failure Assessment score upon ICU admission (9.5; interquartile range, 8–12 vs 8, interquartile range, 5–10; p = 0.042), a longer median ICU stay (15 d; interquartile range, 11–23 vs 8, interquartile range, 5–12; p < 0.001), and more frequently required invasive mechanical ventilation (98.3% vs 82.8%; p = 0.013) than those who did not. The median time from ICU admission to the first bloodstream infection episode was 10 days. Gram-positive bacteria accounted for 74 episodes (79.6%), with Enterococcus species being the most prevalent (53 episodes, 55.8%). Thirty-two isolates (27.3%) showed multidrug resistance.
CONCLUSIONS:
Coronavirus disease 2019 seemed to increase the frequency of bloodstream infections (particularly Enterococcus-related bloodstream infection) after ICU admission. This may have been due to enteric involvement in patients with severe coronavirus disease 2019 and/or limitations in controlling the patient-to-patient transmission of infectious agents in extremely challenging circumstances.
The coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) has led to the most serious crisis that the healthcare systems of many countries have had to confront for a century. Although most SARS-CoV-2 infections evolve asymptomatically or are associated with mild symptoms, 15–20% of patients require hospitalization, of whom approximately 16% will need intensive care and ventilatory support (1, 2). The first wave of the COVID-19 pandemic has been characterized by a rapid surge in the number of critically ill patients and a need to provide more ICU beds and reassign doctors and nurses. Furthermore, we have been reminded of the need to maintain conventional practices of hospital infection control even in periods of intense pressure by the paradoxical increase in the rate of ICU-acquired methicillin-resistant Staphylococcus aureus infections that occurred during the SARS outbreak of 2003, despite the mandatory use of respiratory and contact precautions (3).
Concerns have been raised that the expansion of critical care capacity required to manage COVID-19 may increase the rate of nosocomial infections (4), but little is known about the bacterial and fungal complications that may arise in critically ill, hospitalized patients with COVID-19. Given the increasing number of bloodstream infections (BSIs) observed among the patients admitted to our dedicated COVID-19 ICU (particularly Enterococcus-related BSIs), the aim of this retrospective observational study was to estimate the frequency of BSIs and BSI-related microbiological patterns in this population.
MATERIALS AND METHODS
Study Design
This retrospective observational study was carried out in the dedicated COVID-19 ICU at Luigi Sacco Hospital, a reference center for infectious disease emergencies in northern Italy.
Setting
The unit was created by converting the hospital’s eight single-cubicle general ICU into a strictly isolated 30-bed ICU located in an infectious disease ward prevalently equipped with two-bedded, negative-pressure rooms (Supplementary Fig. 1, Supplemental Digital Content 1, http://links.lww.com/CCM/F955 [legend: map of the ICU; and Supplementary Fig. 2, Supplemental Digital Content 2, http://links.lww.com/CCM/F956 [legend: map of the 2-bedded rooms]). The physician/patient and nurse/patient ratios changed during the study period from, respectively, 0.5 and 1 per shift to 0.2 and 0.5 at the height of the epidemic (the last week of March). Great emphasis was placed on preventive measures aimed at keeping healthcare workers safe, including the routine use of gloves, N95 masks, face shields or goggles, and Tyvek coveralls for each procedure.
Aseptic techniques and sterile fields were used during the placement of indwelling intravascular and urinary catheters in accordance with local protocols. All of the operators changed previously used personal protective equipment and wore sterile gloves and gowns before performing invasive procedures. However, during the first 7 days of the study, there was a shortage of sterile gowns and some had to be reused. All three available single rooms had a dedicated medical cart containing all of the equipment required for medical and nursing procedures, but the two-bedded rooms were equipped with only one medical cart for both patients.
Only one of the two infectious disease physicians experienced in nosocomial infection control and antibiotic stewardship could do a daily ICU round during the study period. Additionally, routine surveillance for multiresistant agents (nasal, cutaneous, and rectal swabs upon admission and during hospitalization) and the isolation of positive patients had to be suspended during the first weeks of the pandemic.
Microbiology test ordering practices based on clinical criteria remains instead unchanged from the pre-COVID19 period.
Participants
The study involved all of the COVID-19 patients admitted to the ICU for greater than or equal to 48 hours between February 21, 2020, and April 30, 2020.
Outcomes
The primary outcome was the cumulative frequency of microbiologically proven BSIs.
The secondary outcomes were the characterization of the causative agents and the differences in the frequency of BSIs and the prevalence of specific causative organisms between the study period (February 21, 2020, and April 30, 2020) and the same period in 2018 and 2019.
Definitions
BSIs were defined using the Center for Disease and Control criteria (5). The isolation of a common skin organism usually associated with contamination had to be confirmed in two sets of blood cultures in order to be considered a BSI (6). An ICU-acquired BSI was defined as a BSI diagnosed greater than or equal to 48 hours after ICU admission. A polymicrobial BSI was defined as a BSI with more than one organism isolated from a single set of blood cultures or different sets of blood cultures during a 48-hour period. In order to be considered a new episode, a BSI had to fulfill the criteria for an ICU-acquired BSI due to a different organism 48 hours after the initial infection; the isolation of the same microorganism in repeated sets of blood cultures was considered a recurrent BSI.
The patients who stayed in the ICU during overlapping periods or for a maximum of 4 weeks were defined as being epidemiologically linked (7), and the presence of nosocomial transmission was considered ascertained if genotypically related strains were detected in two or more epidemiologically linked patients.
Laboratory Procedures
The species causing BSIs were identified by means of Vitek MS matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (bioMérieux, Marcy l’Etoile, France). Antimicrobial susceptibility and resistance detection of the clinical isolates were determined using the automated Vitek 2 system (bioMérieux). The interpretation of susceptibility patterns was performed according to the European Committee on Antimicrobial Susceptibility Testing (8).
In accordance with local multidrug resistance (MDR) surveillance policy, all of the MDR bacteria isolated from blood cultures are routinely collected and stored at –80°C. The genetic relationships of the vancomycin-resistant Enteroccoccus species strains isolated from COVID-19 patients were explored by means of automated repetitive extragenic palindromic polymerase chain reaction (DiversiLab Enterococcus kit, bioMérieux) (9, 10). The amplified fragments were separated by means of capillary electrophoresis and their band patterns were compared using an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA). The isolates whose band patterns were greater than 95% similar were considered to be genetically related (9).
Statistical Analysis
The demographic and clinical data are recorded as absolute numbers, proportions, percentages, or median values and interquartile ranges (IQRs). The characteristics of the patients who developed one or more BSI were compared with those of the patients without a BSI using the χ2 test (or Fisher exact test where necessary) for categorical variables and Wilcoxon rank-sum test for continuous variables. The crude occurrence rate was estimated as the number of first BSI episodes per patient-days at risk with the 95% CI being computed using Poisson distribution. The cumulative frequency of the first BSI episodes over time was estimated using death and ICU discharge as competing events. The occurrence rate BSIs in 2018, 2019, and 2020 were compared using the Poisson regression model, and the proportion of Enterococcus species among the first BSI episodes in 2018, 2019, and 2020 were compared using the χ2 test.
Ethics Statement
The study was approved by the Comitato Etico Interaziendale Area 1. Informed consent was waived in the case of patients undergoing mechanical ventilation.
RESULTS
Eighty-nine critically ill COVID-19 patients (77.5% male; median age, 61.5 yr; IQR, 53.1–68.7) were admitted to our ICU for at least 48 hours during the study period. Their characteristics are shown in Table 1. At the time of admission, their median Sequential Organ Failure Assessment (SOFA) score was 9 (IQR, 7–12). The median length of ICU stay was 12 days (IQR, 8–18 d); 93.3% of the patients required invasive mechanical ventilation and 44 (49.4%) died.
TABLE 1.
Characteristics of the Study Population
| Characteristics | Overall (n = 89) | BSI Not Acquired (n = 29) | BSI Acquired (n = 60) | p |
|---|---|---|---|---|
| Females, n (%) | 20 (22.5) | 5 (17.2) | 15 (25.0) | 0.589 |
| Median age (IQR), yr | 61.5 (53.1–68.7) | 59.2 (50.7–69.6) | 61.5 (53.7–68.5) | 0.875 |
| Median Sequential Organ Failure Assessment score upon admission (IQR) | 9.00 (7.0–12.0) | 8.00 (5.0–10.0) | 9.50 (8.0–12.0) | 0.042 |
| Median Charlson Comorbidity Index (IQR) | 2.00 (1.0–3.0) | 3.00 (1.0–3.0) | 2.00 (1.0–3.0) | 0.069 |
| Median days from symptom onset to ICU (IQR) | 11.0 (8.0–15.0) | 12.0 (8.0–16.0) | 10.5 (7.7–15.0) | 0.364 |
| Median time from hospitalization to ICU (IQR) | 1.0 (0.0–4.0) | 1.0 (0.0–3.0) | 2.0 (0.0–5.0) | 0.246 |
| Median length of ICU stay, d, (IQR) | 12.0 (8.0–18.0) | 8.0 (5.0–12.0) | 15.0 (11.0–23.0) | < 0.001 |
| Mechanical ventilation, n (%) | 83 (93.3) | 24 (82.8) | 59 (98.3) | 0.013 |
BSI = bloodstream infection, IQR = interquartile range.
Italicized values indicate p < 0.05.
During their ICU stay, 60 patients (67.4%) experienced one or more of the 93 recorded BSI episodes. The median time from ICU admission to the first BSI episode was 10 days (Fig. 1). The patients who developed one or more BSIs had a higher median SOFA score upon admission (9.5; IQR, 8–12 vs 8; IQR, 5–10; p = 0.042), a longer median ICU stay (15 d; IQR, 11–23 d vs 8 d; IQR, 5–12; p < 0.001), and more frequently required invasive mechanical ventilation (98.3% vs 82.8%; p = 0.013) than those who did not.
Figure 1.
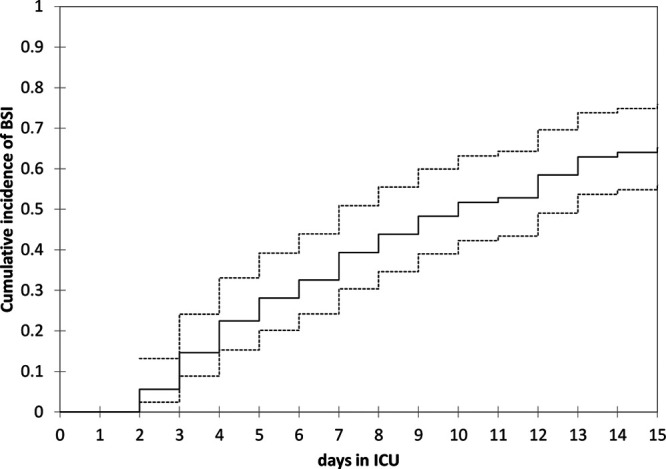
Cumulative frequency of bloodstream infections (BSIs) in critically ill coronavirus disease 2019 patients admitted to the ICU. The continuous line represents the estimated cumulative frequency and the dashed lines the upper and lower 95% CI.
Characteristics of the BSIs and the Microorganisms Involved
Most of the BSIs were monomicrobial (71/93, 76.3%) and 20.4% were recurrent BSIs (Table 2). The source of bacteremia was a central line-associated BSI in 28 cases (30.1%), ventilator-associated pneumonia in 13 cases (14%), and unknown in 52 cases (55.9%). None of the BSIs was attributable to urinary tract infections.
TABLE 2.
Characteristics of the Isolates and Types of Bloodstream Infection
| Microorganisms | Isolates (n = 117) | Bloodstream Infection Episodes (n = 93) | Monomicrobial, n = 71 (76.3%) | Polymicrobial, n = 22 (23.7%) | Recurrent, n = 19 (20.4%) |
|---|---|---|---|---|---|
| Gram-positive, n (%) | 85 (72.6) | 74 (79.6) | 52 (73.2) | 22 (100) | 14 (73.7) |
| Enterococcus speciesb | 53 (45.3) | 53 (55.8) | 32 (45.1) | 22 (100) | 11 (57.9) |
| Vancomycin-resistant Enterococcus faecium | 5 (4.3) | 5 (5.4) | 3 (4.2) | 2 (9.1) | 1 (5.3) |
| Staphylococcus aureus | 7 (6) | 7 (7.5) | 3 (4.2) | 4 (18.2) | 2 (10.5) |
| Methicillin-resistant S. aureus | 5 (4.3) | 5 (5.4) | 2 (2.8) | 3 (13.6) | 1 (5.3) |
| Coagulase-negative Staphylococci | 24 (20.5) | 24 (25.8) | 16 (22.5) | 8 (36.4) | 5 (26.3) |
| Gemella sanguinis | 1 (0.8) | 1 (0.8) | 1 (0.8) | 0 (0.0) | 0 (0.0) |
| Gram-negative, n (%) | 29 (24.8) | 27 (29.0) | 16 (22.5) | 12 (54.5) | 10 (52.6) |
| Enterobacteralesa | 19 (16.2) | 19 (20.4) | 10 (14.1) | 9 (40.9) | 5 (26.3) |
| Extended spectrum beta lactamase-positive Enterobacterales | 6 (5.1) | 6 (6.5) | 3 (4.2) | 3 (13.6) | 2 (10.5) |
| Carbapenemase-producing Enterobacterales | 10 (8.5) | 10 (10.8) | 6 (8.5) | 4 (18.2) | 2 (10.5) |
| Enterobacter species | 6 (5.1) | 6 (6.5) | 4 (5.6) | 2 (9.1) | 3 (15.8) |
| Cephalosporin-resistant Enterobacter | 4 (3.4) | 4 (4.3) | 3 (4.2) | 1 (4.5) | 1 (3.6) |
| Pseudomonas aeruginosa | 2 (1.7) | 2 (2.2) | 1 (1.4) | 1 (4.5) | 1 (5.3) |
| MDR P. aeruginosa | 1 (0.8) | 1 (1.1) | 1 (1.4) | 0 (0.0) | 1 (5.3) |
| Stenotrophomonas maltophilia | 1 (0.8) | 1 (1.1) | 1 (1.4) | 0 (0.0) | 1 (5.3) |
| MDR S. maltophilia | 1 (0.8) | 1 (1.1) | 1 (1.4) | 0 (0.0) | 1 (5.3) |
| Acinetobacter baumannii | 1 (0.8) | 1 (1.1) | 0 (0.0) | 1 (4.5) | 0 (0.0) |
| Yeasts, n (%) | 3 (2.6) | 3 (3.2) | 3 (4.2) | 0 (0.0) | 0 (0.0) |
| Candida albicans | 3 (2.6) | 3 (3.2) | 4 (4.2) | 0 (0.0) | 0 (0.0) |
MDR = multidrug resistant.
aEnterobacterales: Klebsiella pneumoniae (16 [85%]), Klebsiella oxytoca (1 [5%]), Serratia marcescens (1 [5%]), and Escherichia coli (1 [5%]).
bEnterococcus species: Enterococcus faecium (26 [49.1%]), Enterococcus faecalis (26 [49.1%]), and Enterococcus hirae (1 [1.8%]).
One hundred and seventeen isolates were identified (Table 2). The majority (85, 72.6%) were Gram-positive bacteria, prevalently Enterococcus species (53, 45.3%), including 26 (49.1%) Enterococcus faecalis, 26 (49.1%), Enterococcus faecium, and one (1.8%) Enterococcus hirae, followed by coagulase-negative staphylococci (24, 20.5%) and S. aureus (9, 7.6%). Gram-negative bacteria, mainly Enterobacterales (19/29), accounted for 24.8% of the isolates, and Candida species for 2.6%. Overall, 10 of the 85 Gram-positive isolates (11.7%; including five vancomycin-resistant E. faecium isolates) and 22 of the 29 Gram-negative bacteria (75.8%) were MDR.
Comparison of the Frequencies of BSIs During the COVID-19 and Pre-COVID-19 Periods
As shown in Figure 2A, the frequency of BSIs between February 21, 2020, and April 30, 2020 (87/1,000 d of ICU stay; 95% CI, 67–112) was significantly higher than that during the same period in 2018 (24/1,000 d of ICU stay; 95% CI, 11–54) or 2019 (19/1,000 d of ICU stay; 95% CI, 8–45) (p < 0.001). When the first BSI episode in each patient was considered, the proportion of BSIs caused by Enterococcus species was also significantly higher in 2020 (71.7% vs 33.3% and 20%; p = 0.016) (Fig. 2B).
Figure 2.
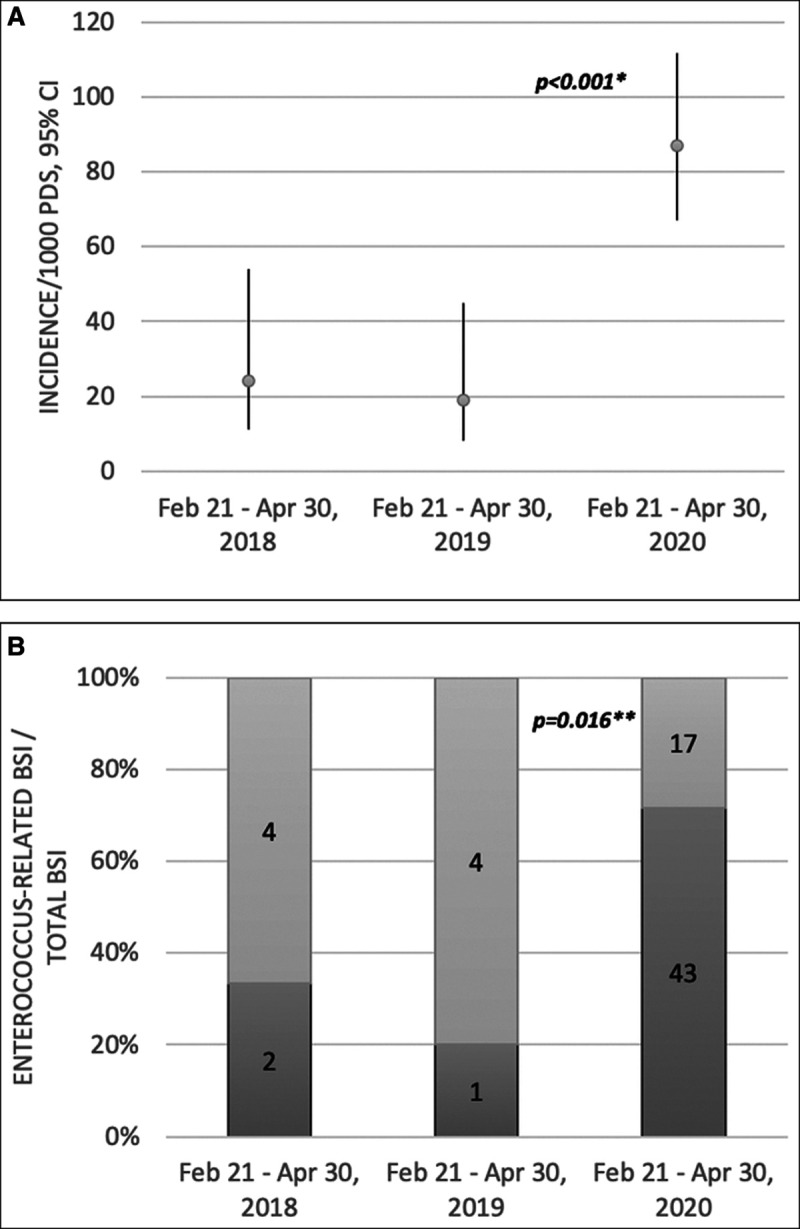
A, Comparison of the frequency of bloodstream infections (BSIs) between February 21 and April 30 in 2018, 2019, and 2020. B, Comparison of the proportion of first BSI episodes attributable to Enterococcus species in each patient between February 21 and April 30 in 2018, 2019, and 2020. 1000 PDS = 1,000 patient-days of ICU stay, Feb = February, Apr = April. *Frequencies were compared using Poisson regression model. **The proportions of Enterococcus species were compared using the χ2 test.
Characteristics of the Enterococcus-Related BSIs Associated With COVID-19
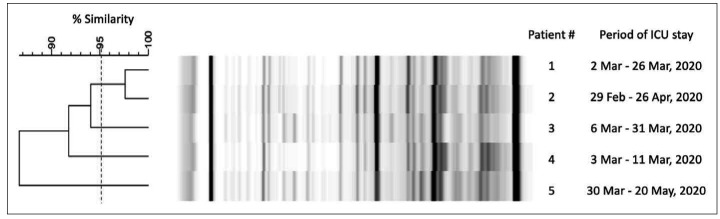
All of the Enterococcus BSIs recorded during the study period were defined as hospital-acquired BSIs, and all involved epidemiologically linked patients. Thirty-six out of 43 patients (83.7%) who developed Enterococcus bacteremia shared the same room or stayed in the same room within 4 weeks. These thirty-six accounted for 42 BSIs (21 E. faecalis bacteremia and 21 E. faecium bacteremia including five vancomycin-resistance E. faecium). Two out of five vancomycin-resistant E. faecium strains seemed to be genetically related; the remaining three were unrelated singletons (Fig. 3).
Figure 3.
Molecular characterization of the five vancomycin-resistant Enterococcus faecium strains collected from blood cultures in 2020. The analysis showed a strict genotypic relation between two strains.
DISCUSSION
The findings of this study reveal a worryingly high frequency of BSIs in the critically ill COVID-19 patients admitted to our ICU during the first weeks of the epidemic in Milan, Italy. The main characteristics of the patients who experienced at least one BSI episode were a more compromised clinical status at the time of ICU admission, a need for invasive ventilation during their stay in the ICU, and a longer ICU stay, all of which are in line with previous descriptions of other ICU populations (11).
The unprecedentedly high frequency of BSIs (87/1,000 d of ICU stay) is much higher than the rates observed in our ICU during the same period in 2018 (24/1,000 d of ICU stay) or 2019 (19/1,000 d of ICU stay), which were in the range of those reported in other mixed medical/surgical ICUs in the pre-COVID-19 period (5.2; 19.8/1,000 d of ICU stay) (11–13).
Since clinical criteria adopted for microbiological tests ordering did not differ between the analyzed periods, it is unlikely that the unexpected number of bacteremia episodes we observed was attributable to an increased sampling during the COVID-19 period.
A recent Italian study by Giacobbe et al (14) also found an unexpectedly high frequency of BSIs in critically ill COVID-19 patients (47/1,000 patient-days), although this is lower than that in our ICU. One of the possible reasons for this difference may be the greater severity of the disease among the patients admitted to our ICU (median SOFA score 9 vs 4), which is also supported by the higher case-fatality rate in our population (49.4% vs 25%) (15). The proportion of patients who developed a BSI is also much higher than that reported in some other studies conducted in China and the United States (1–11.9%) (15–17), although the observation periods of these studies were shorter than ours and it is known that the occurrence of BSIs is greatly influenced by the duration of observation (18).
Another factor may explain differences in the frequency of BSIs in different ICU settings, which is the proportion of intubated patients, as it is known that mechanical ventilation is associated with an increased risk of BSI (11). More than 83% of our patients underwent mechanical ventilation, as did 87.3% of the patients in a study carried out by Grasselli et al (19) and most of the critically ill patients admitted to different ICUs in northern Italy during the first weeks of the epidemic, whereas the proportion of ICU-treated COVID-19 patients requiring mechanical ventilation in studies conducted in China and the United States varies from 37.6% to 67.4% (15–18, 20–22).
The very high frequency of Enterococcus species (55.8%) among the isolates of both mono- and polymicrobial BSIs was unexpected. It was significantly higher than that observed in our hospital in the previous 2 years (20–33%), which was already higher than the 12.4% recorded in the 2018 ECDC epidemiological report on ICU-acquired BSIs (23), although in line with the 28% observed in other European settings (24). The intrinsic and acquired antimicrobial resistance of Enterococcus species represents an increasing nosocomial problem worldwide, especially in severely ill and immunocompromised patients. These bacteria are intrinsically tolerant of unfavorable conditions, which allows them to survive for long periods in a hospital environment and makes them difficult to control (25, 26). It has also been shown that Enterococcus species frequently colonize the respiratory tract of intubated patients and are frequently transmitted from one to another, thus increasing the risk of nosocomial infections (27).
The abrupt surge in the number of critically ill COVID-19 patients admitted to our ICU during the study period required rapid operational changes that can significantly challenge the ability of healthcare staff to respect fully the usual infection control practices aimed at reducing the patient-to-patient transmission of pathogens that frequently contaminate a hospital environment. For example, during the SARS epidemic in Hong Kong, Yap et al (3) observed an increase in methicillin-resistant S. aureus acquisition in the ICU and suggested that the excessive use of gloves (especially when worn together with long-sleeved gowns) may have contributed to reducing compliance to hand hygiene and the advised practice of changing gloves and cleansing hands before and after each contact with a patient, and between caring for dirty and clean body-sites in the same patient.
A careful reassessment of the infection control procedures adopted in our ICU during the emergency phase of the pandemic revealed two major critical issues that may have increased the risk of cross contamination: there was a shortage of sterile gowns during the first 7 days of the epidemic and the two-bedded rooms were equipped with only one medical cart. Medical charts are prone to surface contamination, since they are handled by physicians, nurses, and other medical staff several times a day. In specific, medical carts are considered high-touch surfaces accounting for a median of three touches per operator’s interaction (28).
It is also worth noting that our usual practice of screening patients for MDR microorganisms upon admission and putting possibly colonized/infected patients in preventive isolation could not be fully implemented during the study period, because the ICU was rapidly overwhelmed and there were only three single rooms that could be used for isolation purposes. At the same time, the antimicrobial stewardship service provided by infectious diseases specialists had to be reduced. It is, therefore, possible that the combination of all of these factors played a role in favoring episodes of cross-contamination that eventually increased the number of bacteramia episodes in our extremely fragile population.
In particular, our finding that a high percentage (83.7%) of patients who developed Enterococcus bacteremia had stayed in the same room for overlapping periods or within few weeks suggests that the degree of nosocomial transmission might have been relevant.
Unfortunately, we could not extend our genotyping analysis to all of the Enterococcus species isolates in order to verify the hypothesis of a point source outbreak, because local policy requires only the routine collection and storage of MDR organisms for the purposes of microbiological surveillance. However, the results of our genotyping analyses of the vancomycin-resistant E. faecium strains collected from five patients whose intubation periods overlapped support the view that cross-transmission may have accounted for only some of the observed vancomycin-resistant Enterococcus-related BSIs.
Finally, another possible explanation for the unexpectedly high frequency of BSIs due to Enterococcus species may be the disruption of the gut barrier caused by SARS-CoV-2. As it has been shown that SARS-CoV-2 productively infects human gut enterocytes (29), it is possible that SARS-CoV-2 infection increases intestinal permeability and bacterial translocation, which is a known risk factor for the development of BSIs. Pathologic studies are needed to shed light on the possible enteric barrier damage induced by SARS-CoV-2 such as that previously found to be associated with SARS-CoV-1 (30).
Our study has a number of limitations. First, its single-centre design and the evolving nature of the setting limit the generalizability of the findings; however, we believe that our findings can be generalized to other contexts and settings in which an unexpectedly high flow of critically ill COVID-19 patients exceeds the capacity of healthcare facilities (31). Second, the lack of comparative data (historical or from other hospital settings) makes it difficult to ascertain how much weight should be given to SARS-CoV-2 infection itself or the organizational changes in explaining the observed increase in the frequency of BSIs. In particular, the available evidence concerning BSI episodes in critically ill patients is representative of quite different settings that were not overwhelmed by the surge of the pandemic (i.e., low-threshold ICU admissions involving a smaller percentage of mechanically ventilated patients). Third, the conduction of a prospective study with the collection of microbiological samples was not feasible due to the contingency of the pandemic. Thus, the retrospective design of the study made it impossible to genotype all of the Enterococcus species strains and only the MDR strains collected for surveillance purposes were available to test the hypothesis of between-patient cross-contamination. Finally, the high short-term mortality rate and small sample size prevented us from assessing the impact of the BSIs on in-ICU mortality (18).
CONCLUSIONS
An abrupt surge in the number of critically ill COVID-19 patients seems to have a detrimental effect on the frequency of ICU-acquired BSIs (particularly Enterococcus-related BSIs), but it remains to be seen whether this is due to SARS-CoV-2 infection itself, the emergency disruption of virtuous norms of infection control aimed at avoiding cross-transmission, or both. Our observations indicate the need to strengthen the procedures for the prevention and surveillance of nosocomial infections in the ICUs dedicated to COVID-19 patient.
ACKNOWLEDGMENTS
We thank all of the patients and medical staff (paramedics, nurses, and physicians) who began this fight on one side of the wall and eventually fell ill during the battle. We also thank Dario Garegnani for his excellent technical support and Anna Gigantiello, Cristina Pagani, and Sara Giordana Rimoldi, who were the microbiologists who performed and helped us to interpret the new microbiological analyses. Finally, we thank all the people who have given us unconditional financial support by donating to our institution.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
Drs. Bonazzetti, Morena, and Giacomelli contributed equally.
Drs. Bonazzetti, Morena, Giacomelli, Ridolfo, and Antinori designed the study. Dr. Giacomelli and Ms. Oreni were responsible for the statistical analysis. All of the authors contributed to patient enrolment, and data collection and interpretation. Dr. Giacomelli drew up a preliminary draft of the manuscript, which was critically reviewed by Drs. Colombo, Ridolfo, and Antinori. All of the authors approved the final version of the manuscript.
Dr. Giacomelli received funding from consultancy fees from Mylan and educational support from Gilead. Dr. Antinori has received support for research activities from Pfizer and Merck Sharp & Dome. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020; 323:1239–1242 [DOI] [PubMed] [Google Scholar]
- 2.Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: Early experience and forecast during an emergency response. JAMA. 2020; 323:1545–1546 [DOI] [PubMed] [Google Scholar]
- 3.Yap FH, Gomersall CD, Fung KS, et al. Increase in methicillin-resistant Staphylococcus aureus acquisition rate and change in pathogen pattern associated with an outbreak of severe acute respiratory syndrome. Clin Infect Dis. 2004; 39:511–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rawson TM, Moore LSP, Zhu N, et al. Bacterial and fungal co-infection in individuals with coronavirus: A rapid review to support COVID-19 antimicrobial prescribing [published online ahead of print May 2, 2020]. Clin Infect Dis. doi: 10.1093/cid/ciaa530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008; 36:309–332 [DOI] [PubMed] [Google Scholar]
- 6.Laupland KB, Kirkpatrick AW, Church DL, et al. Intensive-care-unit-acquired bloodstream infections in a regional critically ill population. J Hosp Infect. 2004; 58:137–145 [DOI] [PubMed] [Google Scholar]
- 7.Willemsen I, Mooij M, van der Wiel M, et al. Highly resistant microorganisms in a teaching hospital: The role of horizontal spread in a setting of endemicity. Infect Control Hosp Epidemiol. 2008; 29:1110–1117 [DOI] [PubMed] [Google Scholar]
- 8.The European Committee on Antimicrobial Susceptibility Testing (EUCAST): Clinical Breakpoints and Dosing of Antibiotics. Available at: https://www.eucast.org/clinical_breakpoints/. Accessed August 2, 2020
- 9.Healy M, Huong J, Bittner T, et al. Microbial DNA typing by automated repetitive-sequence based PCR. J Clin Microbiol. 2005; 43:99–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sherer CR, Sprague BM, Campos JM, et al. Characterizing vancomycin-resistant enterococci in neonatal intensive care. Emerg Infect Dis. 2005; 11:1470–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prowle JR, Echeverri JE, Ligabo EV, et al. Acquired bloodstream infection in the intensive care unit: Incidence and attributable mortality. Crit Care. 2011; 15:R100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hugonnet S, Sax H, Eggimann P, et al. Nosocomial bloodstream infection and clinical sepsis. Emerg Infect Dis. 2004; 10:76–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laupland KB, Zygun DA, Davies HD, et al. Population-based assessment of intensive care unit-acquired bloodstream infections in adults: Incidence, risk factors, and associated mortality rate. Crit Care Med. 2002; 30:2462–2467 [DOI] [PubMed] [Google Scholar]
- 14.Giacobbe DR, Battaglini D, Ball L, et al. Bloodstream infections in critically ill patients with COVID-19. Eur J Clin Invest. 2020; 50:e13319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of covid-19 in New York City. N Engl J Med. 2020; 382:2372–2374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020; 395:507–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020; 395:497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pouwels KB, Vansteelandt S, Batra R, et al. Intensive care unit (ICU)-acquired bacteraemia and ICU mortality and discharge: Addressing time-varying confounding using appropriate methodology. J Hosp Infect. 2018; 99:42–47 [DOI] [PubMed] [Google Scholar]
- 19.Grasselli G, Greco M, Zanella A, et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020; 180:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu Y, Xu D, Fu S, et al. Patients with COVID-19 in 19 ICUs in Wuhan, China: A cross-sectional study. Crit Care. 2020; 24:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim L, Garg S, O’Halloran A, et al. Risk factors for intensive care unit admission and in-hospital mortality among hospitalized adults identified through the U.S. coronavirus disease 2019 (COVID-19)-associated hospitalization surveillance network (COVID-NET). Clin Infect Dis. 2020 Jul 16; ciaa1012 [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta S, Hayek SS, Wang W, et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020 Jul 15; e203596 [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.European Centre for Disease Prevention and Control: Healthcare-associated infections acquired in intensive care units. In: ECDC. Annual Epidemiological Report for 2017. 2019, Stockholm: ECDC [Google Scholar]
- 24.Ong DS, Bonten MJ, Safdari K, et al. MARS Consortium: Epidemiology, management, and risk-adjusted mortality of ICU-acquired enterococcal bacteremia. Clin Infect Dis. 2015; 61:1413–1420 [DOI] [PubMed] [Google Scholar]
- 25.Wendt C, Wiesenthal B, Dietz E, et al. Survival of vancomycin-resistant and vancomycin-susceptible enterococci on dry surfaces. J Clin Microbiol. 1998; 36:3734–3736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arias CA, Murray BE. The rise of the enterococcus: Beyond vancomycin resistance. Nat Rev Microbiol. 2012; 10:266–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lund B, Agvald-Ohman C, Hultberg A, et al. Frequent transmission of enterococcal strains between mechanically ventilated patients treated at an intensive care unit. J Clin Microbiol. 2002; 40:2084–2088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huslage K, Rutala WA, Sickbert-Bennett E, et al. A quantitative approach to defining “high-touch” surfaces in hospitals. Infect Control Hosp Epidemiol. 2010; 31:850–853 [DOI] [PubMed] [Google Scholar]
- 29.Lamers MM, Beumer J, van der Vaart J, et al. SARS-CoV-2 productively infects human gut enterocytes. Science. 2020; 369:50–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding Y, He L, Zhang Q, et al. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: Implications for pathogenesis and virus transmission pathways. J Pathol. 2004; 203:622–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paterlini M. On the front lines of coronavirus: The Italian response to covid-19. BMJ. 2020; 368:m1065. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.