Gammaherpesviruses establish lifelong chronic infections in cells of the immune system that can lead to lymphomas and other diseases. To facilitate colonization of a host, gammaherpesviruses encode gene products that manipulate processes involved in cellular proliferation and differentiation. Whether and how these viral gene products function in specific cells of the immune system is poorly defined. We report here the use of a viral genetic system that allows for deletion of specific viral genes in discrete populations of cells. We employ this system in an in vivo model to demonstrate cell-type-specific requirements for a particular viral gene. Our findings reveal that a viral gene product can function in distinct cellular subsets to direct gammaherpesvirus pathogenesis.
KEYWORDS: B cells, M2, MHV68, activation-induced cytidine deaminase, gammaherpesvirus, latency, murid herpesvirus 4, murine gammaherpesvirus 68, reactivation, tumor virus
ABSTRACT
Gammaherpesviruses (GHVs) are DNA tumor viruses that establish lifelong, chronic infections in lymphocytes of humans and other mammals. GHV infections are associated with numerous cancers, especially in immunocompromised hosts. While it is known that GHVs utilize host germinal center (GC) B cell responses during latency establishment, an understanding of how viral gene products function in specific B cell subsets to regulate this process is incomplete. Using murine gammaherpesvirus 68 (MHV68) as a small-animal model to define mechanisms of GHV pathogenesis in vivo, we generated a virus in which the M2 gene was flanked by loxP sites (M2.loxP), enabling the use of Cre-lox technology to define M2 function in specific cell types in infection and disease. The M2 gene encodes a protein that is highly expressed in GC B cells that promotes plasma cell differentiation and viral reactivation. M2 was efficiently deleted in Cre-expressing cells, and the presence of loxP sites flanking M2 did not alter viral replication or latency in mice that do not express Cre. In contrast, M2.loxP MHV68 exhibited a deficit in latency establishment and reactivation that resembled M2-null virus, following intranasal (IN) infection of mice that express Cre in all B cells (CD19-Cre). Nearly identical phenotypes were observed for M2.loxP MHV68 in mice that express Cre in germinal center (GC) B cells (AID-Cre). However, colonization of neither draining lymph nodes after IN infection nor the spleen after intraperitoneal (IP) infection required M2, although the reactivation defect was retained. Together, these data confirm that M2 function is B cell-specific and demonstrate that M2 primarily functions in AID-expressing cells to facilitate MHV68 dissemination to distal latency reservoirs within the host and reactivation from latency. Our study reveals that a viral latency gene functions within a distinct subset of cells to facilitate host colonization.
IMPORTANCE Gammaherpesviruses establish lifelong chronic infections in cells of the immune system that can lead to lymphomas and other diseases. To facilitate colonization of a host, gammaherpesviruses encode gene products that manipulate processes involved in cellular proliferation and differentiation. Whether and how these viral gene products function in specific cells of the immune system is poorly defined. We report here the use of a viral genetic system that allows for deletion of specific viral genes in discrete populations of cells. We employ this system in an in vivo model to demonstrate cell-type-specific requirements for a particular viral gene. Our findings reveal that a viral gene product can function in distinct cellular subsets to direct gammaherpesvirus pathogenesis.
INTRODUCTION
Gammaherpesviruses (GHVs) are DNA tumor viruses that include the human pathogens Epstein-Barr virus (EBV) and Kaposi sarcoma-associated herpesvirus (KSHV). GHVs are lymphotropic viruses with a biphasic infection cycle that is characterized by two distinct stages: productive lytic replication or chronic, lifelong latency (1). Lytic replication is characterized by temporally regulated viral gene expression, replication of viral DNA, and production of infectious viral progeny (1). After resolution of acute infection, latency is established and viral genomes are maintained within germinal center (GC) and memory B cells (2). During latency, viral gene expression is restricted to a few latency gene products and noncoding RNAs (3, 4). These molecules function to manipulate host-cell physiology, maintain viral genomes, and thwart immune surveillance (5–10). In response to specific stimuli, latent viral genomes can reinitiate the lytic cycle, a process called reactivation (11). Reactivation enables infection of new hosts and is thought to help maintain latent reservoirs within a chronically infected host (11, 12).
To define the processes used by GHVs to infect a living organism and cause disease, we and others utilize the natural rodent pathogen murine gammaherpesvirus 68 (MHV68, also known as murid herpesvirus 4 or MuHV-4) (12). The MHV68 genome is colinear with EBV and KSHV, and like other GHVs, MHV68 preferentially infects B lymphocytes and can cause cancer, especially in immunocompromised animals (13, 14). Because MHV68 readily infects and establishes chronic infection in inbred, outbred, and genetically modified mouse strains (11, 15), MHV68 serves as a tractable small animal model for studying the function of viral gene products and the host immune response to GHV infections.
During latency, establishment GHVs are thought to augment and usurp the GC reaction (11, 16, 17). The GC reaction is a B cell maturation process that is characterized by rapid cellular proliferation and the accumulation of mutations in the immunoglobulin locus, which encodes the B cell receptor and antibodies and is mediated by the enzyme activation-induced cytidine deaminase (AID) (18–20). Subversion of the GC response by GHVs is thought to expand the reservoir of latently infected cells and promote the infection of long-lived memory B cells (MBCs) (21). While it is well known that GHVs establish and maintain chronic infection within GC B cells, requirements for specific viral gene products in this process are still being investigated.
The MHV68 M2 gene is highly expressed in GC B cells, and while M2 has no known viral or cellular homologs at the sequence level, it is hypothesized to be a functional homolog of the EBV latent membrane protein 2A (LMP2A) and the KSHV K1 protein (22). Transcription of M2 within B cells is driven by multiple closely linked promoters, which generate two spliced and one unspliced M2 transcript. The spliced transcripts encode the M2 protein and are apparently regulated in a B cell-specific manner, as spliced transcripts are not detected during lytic infection, whether in cell culture or the lung epithelium (4, 23). The M2 protein functions as a scaffold that interacts with membrane-associated signaling molecules to mimic B cell-receptor activation and promote calcium-mediated activation of the nuclear factor of activated T cells (NFAT) pathway (24). NFAT activation driven by M2 induces the plasma cell-associated transcription factor, interferon regulatory factor 4 (IRF4), which enforces a gene expression program involved in plasma cell differentiation and promotes production of anti-inflammatory cytokines, such as interleukin-10 (IL-10) (10, 24). Studies using M2-null MHV68 (M2.Stop) and viruses with specific mutations in putative signaling residues demonstrated that M2 facilitates latency establishment in the spleen after intranasal (IN), but not intraperitoneal (IP), inoculation of mice (25–27). M2 is also generally required for viral reactivation from latency in vivo, a phenotype that correlates with reduced plasma cell infection by M2-null virus (28). Forced retroviral expression of M2 in primary murine B cells in tissue culture and following in vivo transfer drives B cells toward a GC B cell phenotype, which ultimately differentiates into plasma cells (28, 29). While detection of spliced M2 transcripts in B cells, but not other cell types, suggests a B cell-specific function, distinct requirements for M2 in unique B cell subsets during viral colonization of the host are not defined.
Since GC B cells are critical early targets for GHV infection, determining how specific viral gene products function within these cells to permit latency is fundamental to understanding GHV pathogenesis. We previously reported development of a viral genetic platform that allows for dissection of cell-type-specific roles of viral gene products in vivo (30). Building from this technology, we engineered a recombinant MHV68 in which the gene encoding M2 was flanked by loxP sequences (floxed; M2.loxP) to enable the conditional deletion of M2 in cells that express Cre recombinase. We used this system to define the function of M2 in specific cell types in mice, especially GC B cells, during latency establishment by MHV68.
RESULTS
Generation and validation of M2.loxP MHV68.
Teasing apart the function of GHV gene products in vivo is complicated in part by the biphasic infection cycle, as well as the convoluted dissemination process required to establish splenic latency. Traditional methods of viral mutagenesis result in complete ablation of a gene of interest. This approach discounts the role of multifunctional proteins that are required in different phases of infection or within distinct cell types. M2 encodes a latency-specific gene product that facilitates MHV68 establishment of latency and reactivation and promotes B cell differentiation in infected mice (3, 27–29). To better define roles for the MHV68 latency gene product M2 within distinct populations of B lymphocytes, we generated a recombinant virus to enable cell-type-specific deletion of the M2 gene in infected cells that express Cre recombinase. We previously demonstrated the feasibility and utility of this approach in a study that defined B cell-specific requirements for ORF73, the gene that encodes the MHV68 latency-associated nuclear antigen (mLANA) (30).
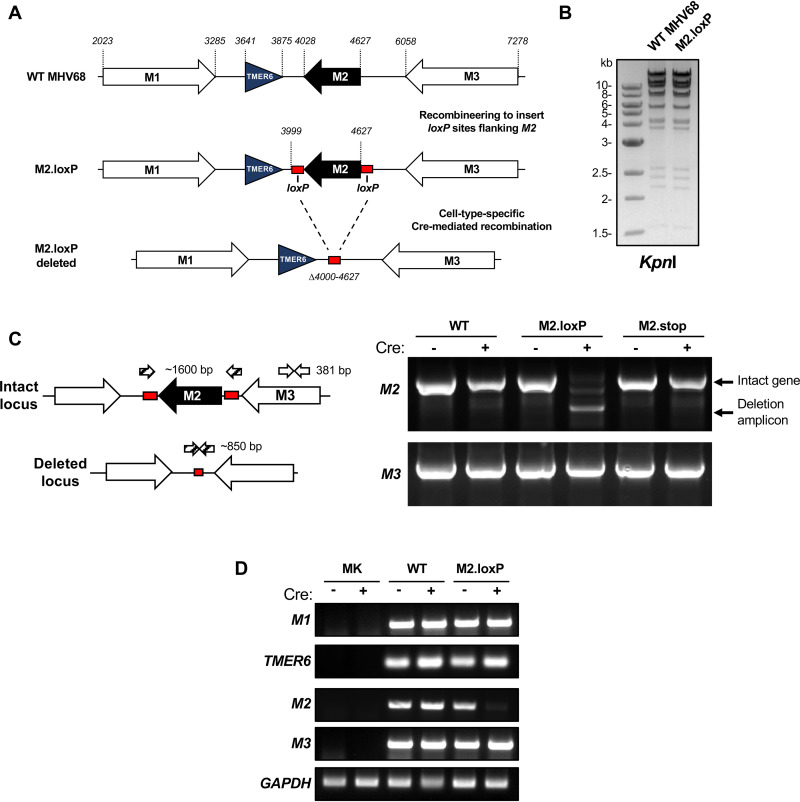
In the new MHV68 recombinant, loxP sites were inserted flanking the M2 gene within the MHV68 bacterial artificial chromosome (BAC), and viral stocks were generated (M2.loxP MHV68; Fig. 1A). Appropriate BAC modification was confirmed by restriction fragment length polymorphism analysis (RFLP; Fig. 1B) and sequencing of the targeted genetic locus. To confirm that floxed M2 was efficiently deleted in the presence of Cre, we infected either Vero or Vero-Cre cells with wild-type (WT) MHV68, M2.Stop, or M2.loxP and then evaluated M2 locus integrity by PCR. Although intact M2 was readily detected in Vero cells infected with M2.loxP, only the M2 deletion amplicon was detected in Vero-Cre cells infected with M2.loxP (Fig. 1C). The full-length M2 locus was detected in both cell types infected by WT MHV68 and M2.Stop. Furthermore, an adjacent viral gene, M3, remained intact when M2 was deleted, suggesting that viral gene deletion is correctly targeted and specific (Fig. 1C). We also performed reverse transcriptase PCR (RT-PCR) to evaluate transcription through the region to confirm that neither loxP insertion nor M2 deletion inhibited adjacent transcripts. Indeed, transcription of M1, TMER6, and M3 was not overtly impacted by the presence of loxP sites or deletion of the M2 locus in cell culture (Fig. 1D). These results demonstrate that Cre-mediated recombination efficiently deletes the M2 gene from the M2.loxP MHV68 genome in cell culture and further suggest that this is accomplished with minimal impact on adjacent viral gene expression.
FIG 1.
Development and validation of M2.loxP MHV68. (A) Schematic depiction of the insertion of loxP sites flanking M2 in the MHV68 genome and its deletion in the presence of Cre recombinase. loxP sites were inserted after nucleotides 3999 and 4627 in the MHV68 genome. Cre-mediated recombination removes the M2 coding region (Δ4000-4627). (B) BAC DNA was digested with the indicated restriction endonuclease, and digestion products were resolved by agarose gel electrophoresis to evaluate the overall genetic integrity of the newly derived M2.loxP BAC. (C) Vero or Vero-Cre cells were infected with the indicated viruses at an MOI of 0.05 PFU/cell. Total DNA was isolated on day 4 postinfection, and PCR was performed as illustrated in the schematic to detect the intact or deleted M2 locus (1,600 bp intact, 850 bp deleted) or the adjacent M3 locus (381 bp) as a control. (D) Vero or Vero-Cre cells were infected with the indicated viruses at an MOI of 0.05 PFU/cell. Total RNA was isolated on day 4 postinfection, and RT-PCR was performed to detect M1, TMER6, M2, M3, and GAPDH transcripts. Primers and amplicon sizes are provided in Table 3.
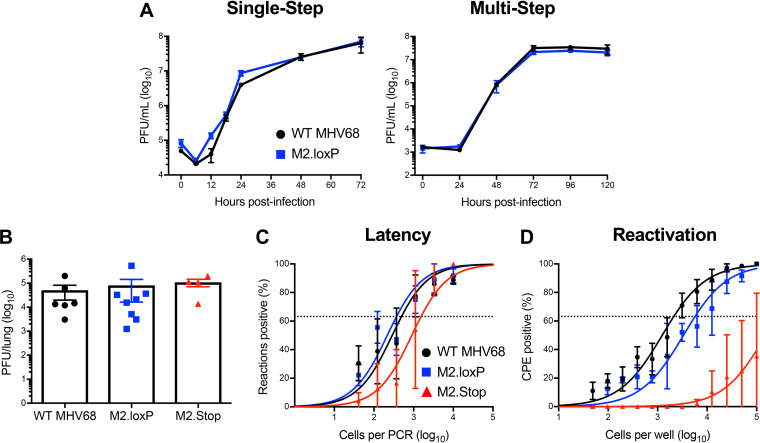
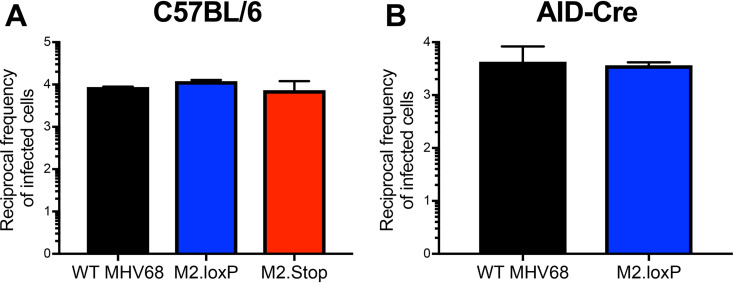
To ensure that the presence of loxP sites flanking M2 did not inadvertently attenuate the virus, we evaluated M2.loxP infection relative to control viruses. In cultured cells, M2.loxP MHV68 replicated with similar kinetics to WT MHV68 in both single- and multistep growth analyses (Fig. 2A). Further, M2.loxP MHV68 titers were equivalent to WT and M2-null MHV68 on day 7 after IN inoculation in lungs of C57BL/6 mice, a time point that approximates the peak of acute viral replication in vivo (Fig. 2B). This finding confirms that the presence of loxP sites did not impact acute viral replication in vivo, and M2.Stop results further demonstrate that M2 is not required for lytic replication. However, while M2-null virus exhibited a modest defect in latency establishment, M2.loxP established latency in the spleen after IN inoculation of C57BL/6 mice at levels similar to WT MHV68 (Fig. 2C and Table 1). M2.loxP also reactivated efficiently from explanted splenocytes, while M2.Stop was again attenuated (Fig. 2D and Table 2). Together, these data confirm previous findings for M2.Stop and demonstrate that the insertion of loxP sites flanking the M2 gene does not impair MHV68 latency establishment in or reactivation from the spleen of WT mice.
FIG 2.
M2.loxP exhibits normal lytic replication and latency in C57BL/6 mice. (A) 3T12 fibroblasts were infected with WT MHV68 or M2.loxP at an MOI of 5 PFU/cell (single-step, left panel) or 0.05 PFU/cell (multistep, right panel). Viral titers were determined by plaque assay at the indicated times postinfection. Results are means of triplicate samples. Error bars represent standard deviations. (B to D) C57BL/6 mice were infected IN with 1,000 PFU of the indicated viruses. (B) Mice were sacrificed on day 7 postinfection, and viral titers in lung homogenates were determined by plaque assay. (C and D) Mice were sacrificed on day 16 postinfection. (C) Single-cell suspensions of spleen cells were serially diluted, and the frequencies of cells harboring MHV68 genomes were determined using a limiting-dilution PCR analysis. (D) Reactivation frequencies were determined by ex vivo plating of serially diluted cells on an indicator monolayer. Cytopathic effect was scored 2 to 3 weeks postplating. Groups of 3 to 5 mice were pooled for each infection and analysis. Results are means of 2 to 3 independent infections. Error bars represent the standard error of the means.
TABLE 1.
Frequency of MHV68 latently infected cells
| Mouse strain | Virus | Route of infection | Cell population | Day postinfection | Latency frequency |
|---|---|---|---|---|---|
| C57BL/6 | WT MHV68 | IN | Spleen | 16 | 1/500 |
| M2.Stop | IN | Spleen | 16 | 1/1,500 | |
| M2.loxP | IN | Spleen | 16 | 1/350 | |
| CD19-Cre | WT MHV68 | IN | Spleen | 16 | 1/80 |
| M2.loxP | IN | Spleen | 16 | 1/900 | |
| WT MHV68 | IP | Spleen | 16 | 1/200 | |
| M2.loxP | IP | Spleen | 16 | 1/300 | |
| AID-Cre | WT MHV68 | IN | Spleen | 16 | 1/150 |
| M2.loxP | IN | Spleen | 16 | 1/1,200 | |
| WT MHV68 | IP | Spleen | 16 | 1/250 | |
| M2.loxP | IP | Spleen | 16 | 1/1,000 | |
| WT MHV68 | IP | PEC | 16 | 1/500 | |
| M2.loxP | IP | PEC | 16 | 1/600 |
TABLE 2.
Frequency of reactivation competent cells
| Mouse strain | Virus | Route of infection | Cell population | Day postinfection | Reactivation frequencya |
|---|---|---|---|---|---|
| C57BL/6 | WT MHV68 | IN | Spleen | 16 | 1/2,000 |
| M2.Stop | IN | Spleen | 16 | BLD | |
| M2.loxP | IN | Spleen | 16 | 1/1,400 | |
| CD19-Cre | WT MHV68 | IN | Spleen | 16 | 1/1,900 |
| M2.loxP | IN | Spleen | 16 | 1/94,000 | |
| WT MHV68 | IP | Spleen | 16 | 1/1,000 | |
| M2.loxP | IP | Spleen | 16 | BLD | |
| AID-Cre | WT MHV68 | IN | Spleen | 16 | 1/7,800 |
| M2.loxP | IN | Spleen | 16 | BLD | |
| WT MHV68 | IP | Spleen | 16 | 1/4,400 | |
| M2.loxP | IP | Spleen | 16 | 1/62,000 | |
| WT MHV68 | IP | PEC | 16 | 1/3,100 | |
| M2.loxP | IP | PEC | 16 | 1/1,800 |
BLD, below the limit of detection of 1 in 100,000 cells.
M2 is required in CD19+ B cells for latency establishment and reactivation.
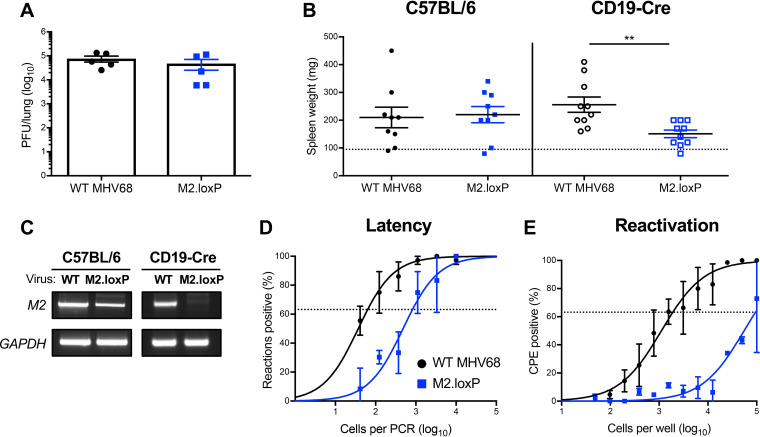
RT-PCR analyses suggest that a properly spliced M2 transcript capable of yielding the functional M2 protein is only present in B cells (4, 23, 31). From this observation, it is hypothesized that M2 primarily functions in B cells to promote MHV68 latency and reactivation. To more conclusively test this hypothesis, we infected mice that express Cre recombinase under the control of the B cell-specific CD19 promoter (CD19-Cre) to facilitate M2 deletion in all B cells. On day 7 after IN inoculation, titers of M2.loxP and WT MHV68 were equivalent in lungs (Fig. 3A). However, splenomegaly, a consequence of the infectious mononucleosis-like syndrome caused by MHV68, did not occur following infection with M2.loxP (Fig. 3B). PCR analysis of the M2 locus demonstrated that floxed M2 was deleted in these mice (Fig. 3C). Latency establishment by M2.loxP also was 10-fold lower than WT MHV68 in spleens of CD19-Cre mice on day 16 postinfection (Fig. 3D and Table 1), and M2.loxP reactivation from explanted splenocytes was ca. 100-fold lower than that of WT MHV68 (Fig. 3E and Table 2), indicating a further 10-fold reduction over the latency defect. The deficits exhibited by M2.loxP following infection of CD19-Cre mice appear to phenocopy M2.Stop infection of WT C57BL/6 mice (Fig. 2), indicating that M2 primarily functions in B lymphocytes to facilitate latency establishment and reactivation following IN infection.
FIG 3.
M2 deletion in CD19+ cells impairs MHV68 latency establishment and reactivation. CD19-Cre mice were infected IN with 1,000 PFU of the indicated viruses. (A) Mice were sacrificed on day 7 postinfection, and viral titers in lung homogenates were determined by plaque assay. (B to E) Mice were sacrificed on day 16 postinfection. (B) Spleens were harvested and weighed as a measure of splenomegaly. The dashed line indicates the average mass of spleens from mock-infected mice. Each dot represents one mouse. Spleen weights from C57BL/6 mice infected in Fig. 2 are shown for comparison. (C) DNA was isolated from infected spleens, and PCR was performed to evaluate the integrity of the M2 locus. Cellular GAPDH serves as an amplification control. (D) Single-cell suspensions of spleen cells were serially diluted, and the frequencies of cells harboring MHV68 genomes were determined using a limiting-dilution PCR analysis. (E) Reactivation frequencies were determined by ex vivo plating of serially diluted cells on an indicator monolayer. Cytopathic effect was scored 2 to 3 weeks postplating. Groups of 3 to 5 mice were pooled for each infection and analysis. Results are means of 2 to 3 independent infections. Error bars represent the standard error of the means. ** denotes P < 0.01 in a two-tailed Student’s t test.
M2 is required in AID-expressing B cells for MHV68 latency and reactivation.
During latency establishment, GHVs are thought to usurp GC reactions to gain access to the pool of long-lived MBCs. At early latency time points, the majority of MHV68-infected cells exhibit a GC phenotype (2). M2 expression alone can drive naive B cells toward a GC B cell phenotype and steer infected B cell differentiation toward a plasma cell phenotype (28, 29). Due to the direct role of M2 in B cell maturation processes and the importance of GC B cells during early latency time points, we hypothesized that M2 expression functions within GC B cells to promote latency establishment.
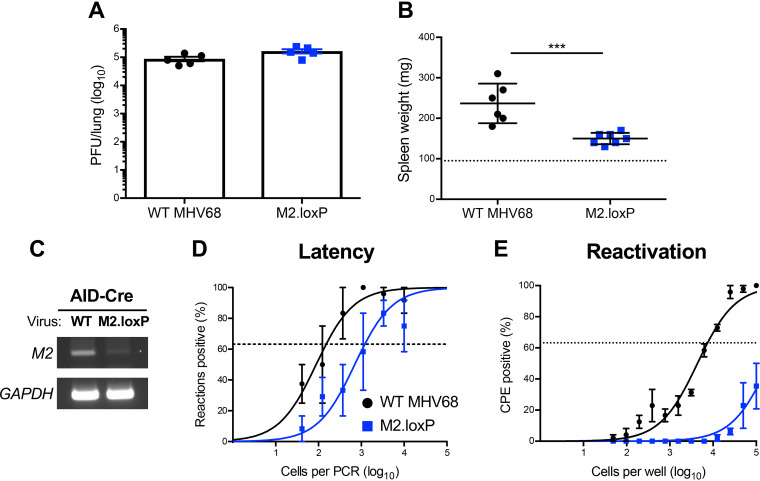
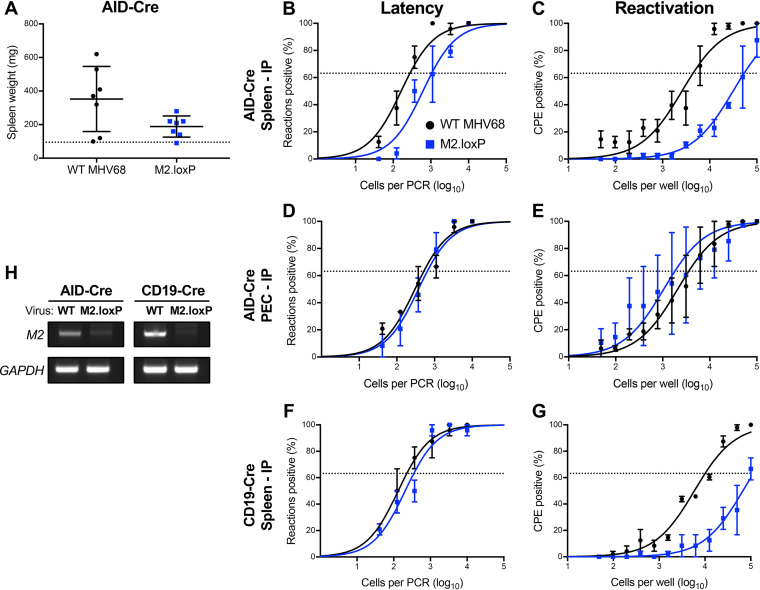
To determine how M2 functions within the GC B cell compartment, we evaluated M2.loxP infection in mice that express Cre recombinase under the control of the promoter for activation-induced cytidine deaminase (AID), the enzyme responsible for somatic hyper-mutation (SHM) and class-switch recombination (CSR) in GC B cells (19). Following IN infection of AID-Cre mice, M2.loxP viral titers in the lung were equivalent to those of WT MHV68 on day 7 postinfection (Fig. 4A). A PCR analysis of the M2 locus demonstrated that M2 was deleted in these mice (Fig. 4C). Similar to results in CD19-Cre mice, M2.loxP caused reduced splenomegaly relative to WT virus on day 16 postinfection (Fig. 4B), and M2.loxP latency in the spleen was 10-fold lower than that of WT MHV68 at this time point (Fig. 4D and Table 1). Additionally, M2.loxP reactivation was below the limit of detection of 1 in 100,000 cells (Fig. 4E and Table 2), which indicates that M2 expression in AID-expressing B cells potentiates MHV68 reactivation. These data strongly suggest that M2 functions within the GC compartment to promote efficient latency establishment in and reactivation from the spleen following intranasal infection.
FIG 4.
M2 deletion in AID-expressing cells impairs MHV68 latency and reactivation. AID-Cre mice were infected IN with 1,000 PFU of the indicated viruses. (A) Mice were sacrificed on day 7 postinfection, and viral titers in lung homogenates were determined by plaque assay. (B to E) Mice were sacrificed on day 16 postinfection. (B) Spleens were harvested and weighed as a measure of splenomegaly. The dashed line indicates the average mass of spleens from mock-infected mice. Each dot represents one mouse. (C) DNA was isolated from infected spleens, and PCR was performed to evaluate the integrity of the M2 locus. Cellular GAPDH serves as an amplification control. (D) Single-cell suspensions of spleen cells were serially diluted, and the frequencies of cells harboring MHV68 genomes were determined using a limiting-dilution PCR analysis. (E) Reactivation frequencies were determined by ex vivo plating of serially diluted cells on an indicator monolayer. Cytopathic effect was scored 2 to 3 weeks postplating. Groups of 3 to 5 mice were pooled for each infection and analysis. Results are means of 2 to 3 independent infections. Error bars represent the standard error of the means. *** denotes P < 0.001 in a two-tailed Student’s t test.
M2 is not required within GC B cells for colonization of lymphoid tissue.
Following IN inoculation, MHV68 drains from the lungs to the mediastinal lymph nodes (MLNs) (32). From MLNs, the virus disseminates via the blood to distal latency reservoirs, such as the spleen (33). We previously demonstrated that ORF73, the viral gene that encodes the MHV68 LANA homolog, is not necessary in CD19+ B cells for initial viral deposition in MLNs, despite its critical importance in hematogenous dissemination (30). Whether M2 is required for MLN infection and its requirement in specific B cell subsets have not been tested. We therefore evaluated MLN infection for M2.loxP, M2.Stop, and WT MHV68 in either C57BL/6 or AID-Cre mice. In C57BL/6 mice, all three viruses were detected at equivalent levels in MLNs on day 16 after IN infection. In agreement with these findings, M2.loxP also established latency at levels similar to those of WT MHV68 in the MLNs of AID-Cre mice (Fig. 5A and B). These data support the conclusion that M2 is not necessary for latency in draining lymphoid tissue. Given that latency in the spleen is reduced in the absence of M2 following IN inoculation, we hypothesize that MHV68 facilitates viral dissemination to the spleen after initial seeding of the draining MLN.
FIG 5.
M2 expression is not required for MHV68 colonization of draining lymph nodes. (A) C57BL/6 or (B) AID-Cre mice were infected IN with 1,000 PFU of the indicated virus. Single-cell suspensions of MLNs were serially diluted, and the frequencies of cells harboring MHV68 genomes were determined using a limiting-dilution PCR analysis. Reciprocal frequencies are shown. Groups of 3 to 5 mice were pooled for each infection and analysis. Results are means of 2 to 3 independent infections. Error bars represent the standard error of the means.
Intraperitoneal (IP) inoculation with MHV68 provides a more permissive route to the spleen, bypassing viral gene requirements for trafficking that may impact latency establishment following IN inoculation (27, 34). Similar to observations for MLNs, M2-null MHV68 achieves normal levels of latency in the spleen after IP inoculation but still exhibits a reactivation defect (27, 28, 35). To determine how deletion of M2 from AID-expressing B cells influenced chronic MHV68 infection after IP inoculation, we compared infections of M2.loxP and WT MHV68 in AID-Cre mice. Analogous to observations following IN inoculations, M2.loxP exhibited reduced splenomegaly compared to WT virus following IP infection of AID-Cre mice (Fig. 6A). While the frequency of cells harboring latent M2.loxP genomes in spleens of AID-Cre mice was only slightly reduced compared to that of WT MHV68, M2.loxP was severely impaired in its capacity to reactivate from splenic latency (Fig. 6B and C). In peritoneal exudate cells (PECs), M2.loxP and WT MHV68 latency and reactivation were equivalent, which is consistent with the notion that MHV68 does not use AID-expressing cells to access or reactivate from cells present in the peritoneum (Fig. 6D and E). We also infected CD19-Cre mice as a control to confirm that phenotypes were similar when M2 was deleted in B cells in general. Again, M2.loxP established latency in the spleen at levels similar to WT MHV68 but still exhibited a reduction in reactivation (Fig. 6F and G). Reduced detection of the M2 locus in spleens from M2.loxP-infected AID-Cre and CD19-Cre mice indicates that M2 was efficiently deleted from both mouse strains after IP infection (Fig. 6H). Together, these data suggest that M2 is not required for viral latency establishment within primary lymphatic tissues but functions after deposition in draining lymph nodes to promote viral dissemination to the spleen.
FIG 6.
M2 expression in B cells is necessary for reactivation, but not latency establishment, following IP inoculation. AID-Cre (A to E, H) or CD19-Cre (F to H) mice were infected IP with 1,000 PFU of the indicated virus and sacrificed on day 16 postinfection. (A) Spleens were harvested and weighed as a measure of splenomegaly. The dashed line indicates the average mass of spleens from mock-infected mice. Each dot represents one mouse. Single-cell suspensions of spleen cells (B, F) or PECs (D) were serially diluted, and the frequencies of cells harboring MHV68 genomes were determined using a limiting-dilution PCR analysis. Reactivation frequencies of splenocytes (C, G) or PECs (E) were determined by ex vivo plating of serially diluted cells on an indicator monolayer. Cytopathic effect was scored 2 to 3 weeks postplating. (H) DNA was isolated from infected spleens of the indicated mouse strain, and PCR was performed to evaluate the integrity of the M2 locus. Cellular GAPDH serves as an amplification control. Groups of 3 to 5 mice were pooled for each infection and analysis. Results are means of 2 to 3 independent infections. Error bars represent the standard error of the means. ns denotes nonsignificant, and ** denotes P < 0.01 in a two-tailed Student’s t test.
M2 deletion from AID-expressing cells only moderately reduces plasmablast infection.
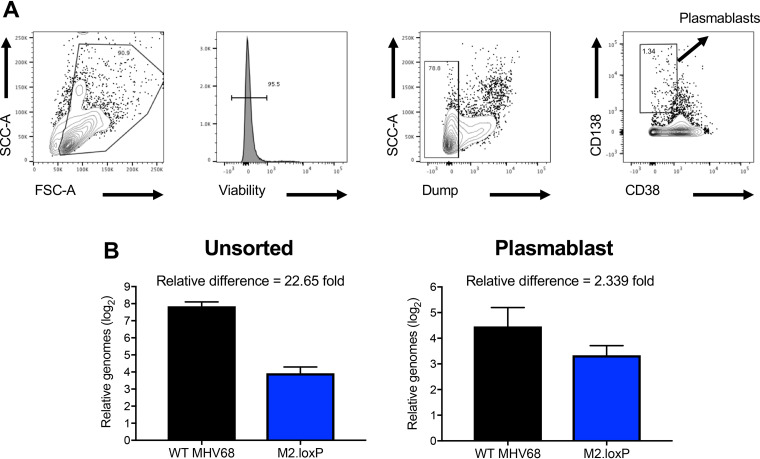
M2-driven plasma cell differentiation accounts for ∼90% of MHV68 ex vivo reactivation (28). M2 expression in BCL-1 lymphoma cell lines promotes features of plasmacyte morphology, including increased size, granularity, and secretion of high levels of IgM. The notion that M2 drives plasmablast differentiation is consistent with reports that M2.Stop virus persists in naive B lymphocytes that have not undergone class switching (IgD+IgG2a–) (28, 36). To determine whether M2 deletion from AID-expressing cells influences infection of plasmablasts in the spleen, we sorted CD138+/CD38lo cells from spleens of infected AID-Cre mice (37) (Fig. 7A) and performed quantitative PCR (qPCR) to quantify viral genomes. Interestingly, by this method of quantification, we observed a 4.5-cycle difference, indicative of a ca. 20-fold deficit, in M2.loxP genome levels compared to those of WT virus in unsorted cells (Fig. 7B). However, M2.loxP DNA in the plasmablast population was essentially equivalent to WT MHV68 (Fig. 7C). We suspect that the larger impact on the general level of viral genomes detected in this experiment reflects that qPCR provides an absolute quantification of viral genomes in the population, rather than the evaluation of cells with at least one genome that is provided by the standard LD-PCR approach. This suggests that, while similar numbers of cells harbor MHV68 genomes (Fig. 6), there are fewer viral genomes per cell upon deletion of M2 from AID-expressing cells. In contrast, the absence of a severe phenotype for plasmablast infection suggests that infection of plasmablasts is either less dependent on M2 or does not require prior infection of an AID-expressing cell.
FIG 7.
Loss of M2 in AID-expressing cells does not prevent infection of plasmablasts. AID-Cre mice were infected IP with 1,000 PFU of the indicated virus and were sacrificed on day 14 postinfection. Spleens were harvested and stained as indicated for FACS-based sorting. Representative plots in panel A demonstrate the gating strategy used for sorting of CD138+CD38lo plasmablasts. (B) DNA was isolated from unsorted splenocytes or sorted plasmablasts, and qPCR was performed on 50 ng of DNA to detect MHV68 genomes or cellular GAPDH as a control.
DISCUSSION
Gammaherpesviruses have evolved to potently manipulate and usurp immune responses generated toward viral antigens. For instance, studies in SAP- and IL-21 receptor-deficient mice demonstrate that MHV68 utilizes the general GC response to its own infection as a means to expand the latent cellular reservoir and generate long-lived reservoirs like MBCs (21, 38, 39). Understanding how specific viral genes engage these immune pathways is important for understanding the basic biology of GHV infection. Through the generation of null and point mutant viruses, overexpression studies, and utilization of fluorescent tagging, the MHV68 M2 protein is already linked to manipulation of B cell activation and differentiation (22, 27–29, 35), but when and where M2 exerts its functions to facilitate chronic viral infection and reactivation from latency are not known. To better define M2 functions in MHV68 pathogenesis, we describe here the generation and in vivo characterization of M2.loxP, a recombinant virus with the capacity to conditionally delete the M2 locus in a Cre-dependent manner. Using this system, we demonstrate that loss of M2 from AID-expressing B cells essentially recapitulates M2-null virus phenotypes in latency establishment and reactivation. We therefore conclude that M2 primarily functions within the GC B cell compartment to facilitate chronic MHV68 infection.
While our results indicate that the presence of loxP sites flanking M2 in the MHV68 genome does not overtly influence lytic replication and latency, there are potential caveats to consider. Although our data indicate in cell culture that loxP insertion and M2 deletion do not alter transcription within this region of the viral genome, it remains possible that cell-type-specific nuances of viral-gene transcription or genome chromatinization are altered for M2.loxP. To better characterize the potential off-target effects that could occur following M2 deletion, further analysis such as single-cell RNA or DNA sequencing is required. Additionally, if paired with mechanisms for marking infected cells, such as expression of a fluorescent or enzymatic reporter (2, 40), the impact of M2 deletion on downstream infection of specific cell populations could be evaluated. Moreover, a marking approach could further enhance evaluations of M2 functions in signal transduction (24, 41–43) and impacts on cellular transcription (24), especially manipulation of the germinal center response.
As mentioned earlier, M2-null MHV68 accumulates in naive B cells during latency establishment and does not infect plasma cells (28, 36). Our qPCR data following sorting of plasmablast populations suggests that either MHV68 passage through AID-expressing cells only minimally influences plasmablast infection or that these cells are directly infected by the virus. Use of marking approaches as described above to facilitate isolation of the infected cells could enhance attempts to further phenotype the infected plasmablast population. For instance, detection of undeleted M2 in plasmablasts following infection of CD19-Cre mice with M2.loxP would support the notion that these cells are directly infected in the spleen. Infection of mice that express Cre in plasma cells could also be performed in future experiments to further understand this unexpected result.
MHV68 colonization of the host is thought to require viral dissemination from primary sites of infection into draining lymph nodes, initial expansion in the lymph node, and subsequent relay via the blood to secondary lymphoid organs, such as the spleen (11, 30, 32). Our data with both M2.Stop and M2.loxP demonstrate that M2 is not required for MHV68 to reach the draining lymph nodes. Similarly, using IP infection to bypass trafficking requirements for colonization of the spleen, both M2-null (27, 35, 36) and M2.loxP MHV68 efficiently establish latency in the spleen, though viral reactivation is severely impaired. These findings suggest that M2 is not directly required for MHV68 to establish latency in lymphoid tissue but is required for downstream colonization events. Since M2.loxP accumulates to levels that are equivalent to those of WT virus in the MLN after IN infection of AID-Cre mice but does not efficiently infect the spleen, it is reasonable to conclude that MHV68 traffics through a GC B cell to facilitate the seeding of distal latency reservoirs. Clearly, this is not absolute, as viral genomes are still detected in the spleen following IN infection of Cre-expressing mice. It is notable that infection with 100 PFU of an M2.Stop virus resulted in a greater reduction in splenic latency after IN infection than observed here. We expect that utilization of a lower inoculating dose would lead to an even more potent reduction in latency establishment than we observed using 1,000 PFU of virus.
Since AID expression correlates with B cell maturation, we consider it unlikely that the M2.loxP defect corresponds to the accumulation of M2.Stop virus in naive B cells, since M2 would presumably still be present in naive B cells, due to a lack of AID expression (44). M2-driven reactivation facilitating seeding of the spleen is possible; however, another virus in which we conditionally delete ORF50, the gene that encodes the replication and transcription activator (RTA), suggests that reactivation/lytic replication in B cells is not required for splenic latency after IN inoculation (A. Gupta, E. Salinas, S. M. Owens, D. G. Oldenburg, D. W. White, and J. C. Forrest, manuscript in preparation). We speculate that M2’s function in augmenting signaling within GC B cells is critical to enhancing MHV68 trafficking to secondary sites of latency in the mouse. We anticipate pairing cell-type-specific ablation of M2 function with single-cell transcriptomic and proteomic approaches in future experiments to evaluate these possibilities.
MATERIALS AND METHODS
Ethics statement.
Mouse experiments were carried out in accordance with National Institutes of Health, United States Department of Agriculture, and University of Arkansas for Medical Sciences (UAMS) Division of Laboratory Animal Medicine and Institutional Animal Care and Use Committee (IACUC) guidelines. The protocol supporting this study was approved by the UAMS IACUC (animal use protocol 3817). Mice were anesthetized prior to inoculations and sacrificed humanely at the end of experiments.
Cells and viruses.
NIH 3T12 (ATCC CCL-164), 3T12 Flp+ (30), BHK21 (ATCC CCL-10), Vero (ATCC CCL-81), and Vero-Cre (45), were cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM l-glutamine (cDMEM). Cells were maintained at 37°C in 5% CO2 and ∼99% humidity. Murine embryonic fibroblasts (MEFs) were harvested from C57BL/6 mouse embryos as previously described (34). Previously described viruses used in this study include FRT BAC MHV68 (WT MHV68) (30) and M2.null MHV68 (M2.STOP) (27).
To generate the M2.loxP BAC, loxP sites were inserted adjacent to the 5′ and 3′ ends of M2 in an FRT BAC template by two successive rounds of en passant mutagenesis as previously described (30) utilizing the following primers:
M2loxpUP_for 5′-GTCTGTCACGCTTCTCCTTCCAGGCGTGTTTAAAGAAAAAATAACTTCGTATAGCATACATTATACGAAGTTATGTTATGTTCTGCGTTAGCACTAGGGATAACAGGGTAATCGATTT-3′, M2loxpUP_rev 5′-GGTTAATTGGTTGTAACACTGGCCAGGCGTGTTTAAAGAAAAAATAACTTCGTATAGCATACATTATACGAAGTTATGTTATGTTCTGCGTTAGCACCTTCACTGTTACTCCTCGCC-3′, M2loxpDWN_for 5′-GTGGGGGTGTTGGGGCCATTACCTGAAAACGAAACCTCATATAACTTCGTATAGCATA, and 5′-M2loxpDWN_rev 5′-CCTTTTACTGGAGGGGGTTTCAACAGGCACTAGTCTGATGATAACTTCGTATAATGTATGCTATACGAAGTTATATGAGGTTTCGTTTTCAGGTGCCAGTGTTACAACCAATTAACC-3′.
Viruses were passaged in Flp-expressing 3T12 fibroblasts to remove the BAC cassette, and titers were quantified as previously described (30).
Nucleic acid isolation and PCR.
Vero or Vero-Cre cells were mock-infected or infected with MHV68 at a multiplicity of infection (MOI) of 0.05 PFU/cell. Nucleic acid was isolated from cells at 4 days postinfection. Total DNA was isolated using a GenCatch blood and tissue genomic mini-prep kit (Epoch Life Science). PCR for the detection of the full-length M2 gene was performed using primers in Table 2 using GoTaq polymerase (Promega) and reaction conditions as previously described (30).
Total RNA was isolated using a GenCatch total RNA extraction kit (Epoch Life Science) and converted to cDNA using SuperScript II reverse transcriptase (Thermo Fisher) as previously described (46). Reverse transcription-PCR (RT-PCR) for M1, TMER6, M2, M3, and GAPDH transcripts was performed using GoTaq polymerase (Promega) using primers described in (3) (Table 2). Cycling conditions were 20 cycles of 94°C for 1 min, 62°C for 1 min, and 72°C for 1 min.
Mouse infections and tissue harvests.
Male and female, C57BL/6, heterozygous CD19-Cre [B6.129P2(C)-Cd19tm1(cre)Cgn/J], and heterozygous AID-Cre [B6.129P2-Aicdatm1(cre)Mnz/J] mice were purchased from Jackson Laboratories. Note: all Cre-encoding mice used were heterozygous for Cre expression. Mice were bred and maintained according to all local, state, and federal guidelines under the supervision of the UAMS Division of Laboratory Animal Medicine. Eight-to-ten-week-old mice were anesthetized using isoflurane and inoculated with 1,000 PFU of virus diluted in incomplete DMEM (20 μl) for IN inoculations or injected with 1,000 PFU of virus diluted in incomplete DMEM (200 μl) for IP inoculations. Splenocytes and peritoneal exudate cells (PECs) were harvested as previously described (47). Cells from draining lymph nodes were isolated as previously described (30).
Splenocyte isolation and limiting-dilution analyses.
Spleens were homogenized using a tenBroek tissue disrupter. Red blood cells were lysed by incubating tissue homogenate in 8.3 g/liter ammonium chloride for 10 minutes at room temperature with shaking. Cells were filtered through 40-μM mesh to reduce clumping. Frequencies of cells harboring MHV68 genomes were determined using limiting-dilution (LD) PCR analysis as previously described (48). Frequencies of latently infected cells capable of reactivating were determined using a limiting-dilution analysis for cytopathic effect induced on an indicator BHK21 monolayer as previously described (34).
Plaque assays.
Plaque assays were performed as previously described (49) using BHK21 cells (2 × 105 cells/well). Briefly, infected cells were overlaid with 1.5% methylcellulose in DMEM supplemented with 2.5% calf serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM l-glutamine and incubated at 37°C for 4 to 6 days. Cell monolayers were stained with a solution of crystal violet in formalin for identification and quantification of plaques.
Cell sorting.
Splenocytes were isolated and sorted by flow cytometry. Blocking and detection antibodies were diluted in phosphate-buffered saline (PBS) with 0.2% bovine serum albumin (BSA) and 1 mM EDTA. Splenocytes were blocked with anti-CD16/32 (BD Biosciences) for 15 minutes before surface staining for 30 minutes in the dark at 4°C. Dead cells were labeled using fixable viability dye eFlour 780 (eBioscience) per the manufacturer’s instructions. Surface stains include dump gate (CD3, Ter119, CD11b, CD11c-PerCp5.5), B220-redFluor 710 (Tonbo), CD19-BV650, CD38-PE-Cy7, and CD138-PE (BioLegend). Cells were sorted on a FACS Aria using FACS Diva software. Data were analyzed using FlowJo software (v10.6.2).
Nucleic acid isolation and qPCR.
DNA from sorted cells was isolated by phenol-chloroform extraction and ethanol precipitation. Then, 50 ng of DNA was diluted in PowerUp SYBR green master mix (Thermo Fisher) and analyzed by quantitative real-time PCR in an Applied Biosystems QuantStudio thermocycler. Cycling conditions were 10 min at 95°C followed by 40 cycles of 15 s at 95°C and 1 min at 60°C, using primers specific to the viral ORF72 gene or cellular GAPDH gene (3, 46). Biological triplicate samples were analyzed in technical duplicate using the ΔΔCT comparative threshold cycle method as previously described (46). The fold change in ORF72 levels for M2.loxP was calculated relative to WT MHV68. Primers used and amplicon sizes are provided in Table 3.
TABLE 3.
Primers used for PCR analysis
| Genomic region | Primer A | Primer B | Size of product (bp) |
|---|---|---|---|
| M1a | ATG CAG CTG GCC ACC TTA TG | CCA TGG TGG GTA GTG GGA GTC | 328 |
| M2a | TCG AGC CAG AGT CCA ACA TCA T | CAA AAT CCA GTG CTA TGG GGT G | 379 |
| M3a | TGG CAC TCA AAC TTG GTT GTG G | TAA CAG GCA GAT TGC CAT TCC C | 381 |
| TMER6 | GCC AGC GTA GCT CAA TTG TTA G | CAA CAG ACT CTT CCC AGC CAG | 88 |
| ORF72a | TTA GCA CTG GGC GTT TCA TG | CAC GTG GAT GTT TTG TGT GTG C | 425 |
| GAPDH | CCT GCA CCA CCA ACT GCT TAG | GTG GAT GCA GGG ATG ATG TTC | 181 |
| M2 deletion | GTG GAT GCT GTC ACC TAC TGA | CCG AGA TCC ACA GAC TAG AC | 1,529 |
The primers pairs previously published by Virgin et al. (3) were used here.
ACKNOWLEDGMENTS
We thank the laboratory of Jason Stumhofer for assistance in designing flow cytometry antibody panels and Andrea Harris in the UAMS Flow Cytometry Core. We thank Scott Tibbetts and Linda van Dyk for help developing the TMER6 RT-PCR analysis.
This work was supported in part by grant R01CA167065 from the NIH National Cancer Institute and start-up funds from the UAMS College of Medicine and Arkansas Biosciences Institute to J.C.F. The Flow Cytometry Core and the work described here were also supported in part by Center for Microbial Pathogenesis and Host Inflammatory Responses award P20GM103625 from the NIH National Institute of General Medical Sciences Centers of Biomedical Research Excellence. S.M.O. was supported by Translational Research Institute (TRI) grant TL1 TR003109 through the NIH National Center for Advancing Translational Sciences. D.W.W. and D.G.O. were supported by the Gundersen Medical Foundation. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Boehmer PE, Nimonkar AV. 2003. Herpes virus replication. IUBMB Life 55:13–22. doi: 10.1080/1521654031000070645. [DOI] [PubMed] [Google Scholar]
- 2.Collins CM, Speck SH. 2012. Tracking murine gammaherpesvirus 68 infection of germinal center B cells in vivo. PLoS One 7:e33230. doi: 10.1371/journal.pone.0033230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Virgin HW IV, Presti RM, Li XY, Liu C, Speck SH. 1999. Three distinct regions of the murine gammaherpesvirus 68 genome are transcriptionally active in latently infected mice. J Virol 73:2321–2332. doi: 10.1128/JVI.73.3.2321-2332.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinez-Guzman D, Rickabaugh T, Wu TT, Brown H, Cole S, Song MJ, Tong L, Sun R. 2003. Transcription program of murine gammaherpesvirus 68. J Virol 77:10488–10503. doi: 10.1128/jvi.77.19.10488-10503.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fowler P, Marques S, Simas JP, Efstathiou S. 2003. ORF73 of murine herpesvirus-68 is critical for the establishment and maintenance of latency. J Gen Virol 84:3405–3416. doi: 10.1099/vir.0.19594-0. [DOI] [PubMed] [Google Scholar]
- 6.Habison AC, Beauchemin C, Simas JP, Usherwood EJ, Kaye KM. 2012. Murine gammaherpesvirus 68 LANA acts on terminal repeat DNA to mediate episome persistence. J Virol 86:11863–11876. doi: 10.1128/JVI.01656-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowden RJ, Simas JP, Davis AJ, Efstathiou S. 1997. Murine gammaherpesvirus 68 encodes tRNA-like sequences which are expressed during latency. J Gen Virol 78:1675–1687. doi: 10.1099/0022-1317-78-7-1675. [DOI] [PubMed] [Google Scholar]
- 8.Gangappa S, van Dyk LF, Jewett TJ, Speck SH, Virgin HW IV. 2002. Identification of the in vivo role of a viral bcl-2. J Exp Med 195:931–940. doi: 10.1084/jem.20011825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Upton JW, van Dyk LF, Speck SH. 2005. Characterization of murine gammaherpesvirus 68 v-cyclin interactions with cellular cdks. Virology 341:271–283. doi: 10.1016/j.virol.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 10.Siegel AM, Herskowitz JH, Speck SH. 2008. The MHV68 M2 protein drives IL-10 dependent B cell proliferation and differentiation. PLoS Pathog 4:e1000039. doi: 10.1371/journal.ppat.1000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barton E, Mandal P, Speck SH. 2011. Pathogenesis and host control of gammaherpesviruses: lessons from the mouse. Annu Rev Immunol 29:351–397. doi: 10.1146/annurev-immunol-072710-081639. [DOI] [PubMed] [Google Scholar]
- 12.Speck SH, Virgin HW IV. 1999. Host and viral genetics of chronic infection: a mouse model of gamma-herpesvirus pathogenesis. Curr Opin Microbiol 2:403–409. doi: 10.1016/s1369-5274(99)80071-x. [DOI] [PubMed] [Google Scholar]
- 13.Olivadoti M, Toth LA, Weinberg J, Opp MR. 2007. Murine gammaherpesvirus 68: a model for the study of Epstein-Barr virus infections and related diseases. Comp Med 57:44–50. [PubMed] [Google Scholar]
- 14.Fujiwara S, Nakamura H. 2020. Animal models for gammaherpesvirus infections: recent development in the analysis of virus-induced pathogenesis. Pathogens 9:116. doi: 10.3390/pathogens9020116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong S, Forrest JC, Liang X. 2017. Murine gammaherpesvirus 68: a small animal model for gammaherpesvirus-associated diseases. Adv Exp Med Biol 1018:225–236. doi: 10.1007/978-981-10-5765-6_14. [DOI] [PubMed] [Google Scholar]
- 16.Flano E, Husain SM, Sample JT, Woodland DL, Blackman MA. 2000. Latent murine gamma-herpesvirus infection is established in activated B cells, dendritic cells, and macrophages. J Immunol 165:1074–1081. doi: 10.4049/jimmunol.165.2.1074. [DOI] [PubMed] [Google Scholar]
- 17.Willer DO, Speck SH. 2005. Establishment and maintenance of long-term murine gammaherpesvirus 68 latency in B cells in the absence of CD40. J Virol 79:2891–2899. doi: 10.1128/JVI.79.5.2891-2899.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mesin L, Ersching J, Victora GD. 2016. Germinal center B cell dynamics. Immunity 45:471–482. doi: 10.1016/j.immuni.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. 2018. Pillars article: class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000. 102: 553–563. J Immunol 201:2530–2540. [PubMed] [Google Scholar]
- 20.Bransteitter R, Pham P, Scharff MD, Goodman MF. 2003. Activation-induced cytidine deaminase deaminates deoxycytidine on single-stranded DNA but requires the action of RNase. Proc Natl Acad Sci U S A 100:4102–4107. doi: 10.1073/pnas.0730835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim IJ, Flano E, Woodland DL, Lund FE, Randall TD, Blackman MA. 2003. Maintenance of long term gamma-herpesvirus B cell latency is dependent on CD40-mediated development of memory B cells. J Immunol 171:886–892. doi: 10.4049/jimmunol.171.2.886. [DOI] [PubMed] [Google Scholar]
- 22.Rangaswamy US, O'Flaherty BM, Speck SH. 2014. Tyrosine 129 of the murine gammaherpesvirus M2 protein is critical for M2 function in vivo. PLoS One 9:e105197. doi: 10.1371/journal.pone.0105197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeZalia M, Speck SH. 2008. Identification of closely spaced but distinct transcription initiation sites for the murine gammaherpesvirus 68 latency-associated M2 gene. J Virol 82:7411–7421. doi: 10.1128/JVI.00651-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rangaswamy US, Speck SH. 2014. Murine gammaherpesvirus M2 protein induction of IRF4 via the NFAT pathway leads to IL-10 expression in B cells. PLoS Pathog 10:e1003858. doi: 10.1371/journal.ppat.1003858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macrae AI, Usherwood EJ, Husain SM, Flano E, Kim IJ, Woodland DL, Nash AA, Blackman MA, Sample JT, Stewart JP. 2003. Murid herpesvirus 4 strain 68 M2 protein is a B-cell-associated antigen important for latency but not lymphocytosis. J Virol 77:9700–9709. doi: 10.1128/jvi.77.17.9700-9709.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simas JP, Marques S, Bridgeman A, Efstathiou S, Adler H. 2004. The M2 gene product of murine gammaherpesvirus 68 is required for efficient colonization of splenic follicles but is not necessary for expansion of latently infected germinal centre B cells. J Gen Virol 85:2789–2797. doi: 10.1099/vir.0.80138-0. [DOI] [PubMed] [Google Scholar]
- 27.Jacoby MA, Virgin HW IV, Speck SH. 2002. Disruption of the M2 gene of murine gammaherpesvirus 68 alters splenic latency following intranasal, but not intraperitoneal, inoculation. J Virol 76:1790–1801. doi: 10.1128/jvi.76.4.1790-1801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang X, Collins CM, Mendel JB, Iwakoshi NN, Speck SH. 2009. Gammaherpesvirus-driven plasma cell differentiation regulates virus reactivation from latently infected B lymphocytes. PLoS Pathog 5:e1000677. doi: 10.1371/journal.ppat.1000677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terrell S, Speck SH. 2017. Murine gammaherpesvirus M2 antigen modulates splenic B cell activation and terminal differentiation in vivo. PLoS Pathog 13:e1006543. doi: 10.1371/journal.ppat.1006543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salinas E, Gupta A, Sifford JM, Oldenburg DG, White DW, Forrest JC. 2018. Conditional mutagenesis in vivo reveals cell type- and infection stage-specific requirements for LANA in chronic MHV68 infection. PLoS Pathog 14:e1006865. doi: 10.1371/journal.ppat.1006865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ebrahimi B, Dutia BM, Roberts KL, Garcia-Ramirez JJ, Dickinson P, Stewart JP, Ghazal P, Roy DJ, Nash AA. 2003. Transcriptome profile of murine gammaherpesvirus-68 lytic infection. J Gen Virol 84:99–109. doi: 10.1099/vir.0.18639-0. [DOI] [PubMed] [Google Scholar]
- 32.Frederico B, Milho R, May JS, Gillet L, Stevenson PG. 2012. Myeloid infection links epithelial and B cell tropisms of murid herpesvirus-4. PLoS Pathog 8:e1002935. doi: 10.1371/journal.ppat.1002935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feldman ER, Kara M, Oko LM, Grau KR, Krueger BJ, Zhang J, Feng P, van Dyk LF, Renne R, Tibbetts SA. 2016. A gammaherpesvirus noncoding RNA is essential for hematogenous dissemination and establishment of peripheral latency. mSphere 1:e00105-15. doi: 10.1128/mSphere.00105-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weck KE, Barkon ML, Yoo LI, Speck SH, Virgin HI. 1996. Mature B cells are required for acute splenic infection, but not for establishment of latency, by murine gammaherpesvirus 68. J Virol 70:6775–6780. doi: 10.1128/JVI.70.10.6775-6780.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herskowitz JH, Siegel AM, Jacoby MA, Speck SH. 2008. Systematic mutagenesis of the murine gammaherpesvirus 68 M2 protein identifies domains important for chronic infection. J Virol 82:3295–3310. doi: 10.1128/JVI.02234-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herskowitz J, Jacoby MA, Speck SH. 2005. The murine gammaherpesvirus 68 M2 gene is required for efficient reactivation from latently infected B cells. J Virol 79:2261–2273. doi: 10.1128/JVI.79.4.2261-2273.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tellier J, Nutt SL. 2017. Standing out from the crowd: how to identify plasma cells. Eur J Immunol 47:1276–1279. doi: 10.1002/eji.201747168. [DOI] [PubMed] [Google Scholar]
- 38.Collins CM, Speck SH. 2015. Interleukin 21 signaling in B cells is required for efficient establishment of murine gammaherpesvirus latency. PLoS Pathog 11:e1004831. doi: 10.1371/journal.ppat.1004831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Collins CM, Speck SH. 2014. Expansion of murine gammaherpesvirus latently infected B cells requires T follicular help. PLoS Pathog 10:e1004106. doi: 10.1371/journal.ppat.1004106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nealy MS, Coleman CB, Li H, Tibbetts SA. 2010. Use of a virus-encoded enzymatic marker reveals that a stable fraction of memory B cells expresses latency-associated nuclear antigen throughout chronic gammaherpesvirus infection. J Virol 84:7523–7534. doi: 10.1128/JVI.02572-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pires de Miranda M, Alenquer M, Marques S, Rodrigues L, Lopes F, Bustelo XR, Simas JP. 2008. The Gammaherpesvirus m2 protein manipulates the Fyn/Vav pathway through a multidocking mechanism of assembly. PLoS One 3:e1654. doi: 10.1371/journal.pone.0001654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodrigues L, Pires de Miranda M, Caloca MJ, Bustelo XR, Simas JP. 2006. Activation of Vav by the gammaherpesvirus M2 protein contributes to the establishment of viral latency in B lymphocytes. J Virol 80:6123–6135. doi: 10.1128/JVI.02700-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mandal P, Krueger BE, Oldenburg D, Andry KA, Beard RS, White DW, Barton ES. 2011. A gammaherpesvirus cooperates with interferon-alpha/beta-induced IRF2 to halt viral replication, control reactivation, and minimize host lethality. PLoS Pathog 7:e1002371. doi: 10.1371/journal.ppat.1002371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shen HM, Bozek G, Pinkert CA, McBride K, Wang L, Kenter A, Storb U. 2008. Expression of AID transgene is regulated in activated B cells but not in resting B cells and kidney. Mol Immunol 45:1883–1892. doi: 10.1016/j.molimm.2007.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gierasch WW, Zimmerman DL, Ward SL, Vanheyningen TK, Romine JD, Leib DA. 2006. Construction and characterization of bacterial artificial chromosomes containing HSV-1 strains 17 and KOS. J Virol Methods 135:197–206. doi: 10.1016/j.jviromet.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 46.Forrest JC, Paden CR, Allen RD III, Collins J, Speck SH. 2007. ORF73-null murine gammaherpesvirus 68 reveals roles for mLANA and p53 in virus replication. J Virol 81:11957–11971. doi: 10.1128/JVI.00111-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paden CR, Forrest JC, Moorman NJ, Speck SH. 2010. Murine gammaherpesvirus 68 LANA is essential for virus reactivation from splenocytes but not long-term carriage of viral genome. J Virol 84:7214–7224. doi: 10.1128/JVI.00133-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weck KE, Kim SS, Virgin HI, Speck SH. 1999. Macrophages are the major reservoir of latent murine gammaherpesvirus 68 in peritoneal cells. J Virol 73:3273–3283. doi: 10.1128/JVI.73.4.3273-3283.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stahl JA, Paden CR, Chavan SS, MacLeod V, Edmondson RD, Speck SH, Forrest JC. 2012. Amplification of JNK signaling is necessary to complete the murine gammaherpesvirus 68 lytic replication cycle. J Virol 86:13253–13262. doi: 10.1128/JVI.01432-12. [DOI] [PMC free article] [PubMed] [Google Scholar]