Abstract
Given the numerous chemicals used in society, it is critical to develop tools for accurate and efficient evaluation of potential risks to human and ecological receptors. Fish embryo acute toxicity tests are 1 tool that has been shown to be highly predictive of standard, more resource-intensive, juvenile fish acute toxicity tests. However, there is also evidence that fish embryos are less sensitive than juvenile fish for certain types of chemicals, including neurotoxicants. The utility of fish embryos for pesticide hazard assessment was investigated by comparing published zebrafish embryo toxicity data from pesticides with median lethal concentration 50% (LC50) data for juveniles of 3 commonly tested fish species: rainbow trout, bluegill sunfish, and sheepshead minnow. A poor, albeit significant, relationship (r2 = 0.28; p < 0.05) was found between zebrafish embryo and juvenile fish toxicity when pesticides were considered as a single group, but a much better relationship (r2 = 0.64; p < 0.05) when pesticide mode of action was factored into an analysis of covariance. This discrepancy is partly explained by the large number of neurotoxic pesticides in the dataset, supporting previous findings that commonly used fish embryo toxicity test endpoints are particularly insensitive to neurotoxicants. These results indicate that it is still premature to replace juvenile fish toxicity tests with embryo-based tests such as the Organisation for Economic Co-operation and Development Fish Embryo Acute Toxicity Test for routine pesticide hazard assessment, although embryo testing could be used with other screening tools for testing prioritization.
Keywords: Embryo, Neurotoxicity, Pesticide, Risk assessment, Zebrafish
INTRODUCTION
Given the widespread use of chemicals in society for purposes ranging from agriculture to manufacturing to medicine, it is critical to develop tools to accurately and efficiently assess their hazards and risks to human and ecological receptors. Such tools could be used to screen chemicals rapidly during product development and to prioritize substances for further investigation. Despite efforts to reduce animal testing by the use of in vitro assays and in silico models, whole animal testing remains a critical component of chemical screening and prioritization.
One promising approach that has emerged for evaluating chemical hazards is the exposure of fish embryos, typically from zebrafish (Danio rerio), to a test compound from shortly after fertilization until several hours to days after hatch. The aim of this type of test is to evaluate embryo toxicity as a range finder for conducting tests with juvenile fish, supplement other toxicity tests, or, ultimately, as an alternative to standard testing with juvenile fish 1–3. The Organisation for Economic Co-operation and Development (OECD) recently formalized a version of this test in the form of test guideline 236: Fish Embryo Acute Toxicity Test (FET) 4.
The advantages of the FET, as well as embryo testing in general, versus standard fish toxicity test designs have been identified through numerous studies (reviewed in Braunbeck et al. 5) and by interlaboratory validation efforts 6. One advantage to working with embryos is that they allow massive simultaneous screening of many chemical substances because they can be manipulated and exposed in multiwell plates by hand or by robot 7, thereby reducing the time, cost, and effort associated with hazard screening. Another advantage, particularly with zebrafish and several other model fish species, is that offspring are produced in abundance and develop rapidly, providing an ample supply of test organisms that can be tested over a short timespan. In addition, because the zebrafish chorion is transparent, it is easy to visualize morphological and developmental abnormalities during testing. Finally, a more controversial advantage of tests involving embryos is that nonfeeding life stages are not protected life stages under a European Union directive 8, while the US National Institutes of Health only recognizes zebrafish as live animals at hatching, which is approximately 72 h post fertilization. Such policies are viewed by some as decreasing the extent to which test organisms may suffer during toxicity testing.
In addition to the advantages of conducting tests with fish embryos, several studies have also shown that fish embryo tests are highly predictive of standard juvenile fish tests (e.g., OECD test guideline 203) 9, which are routinely used for hazard and risk assessment in the United States and the European Union. Belanger et al. 10 and Lammer et al. 2 both report high r2 values of approximately 0.9 with slopes and intercepts close to 1 and 0, respectively, when comparing FET median lethal concentration (LC50) data (primarily from zebrafish) with 96-h juvenile fish LC50 data from standard test species (e.g., bluegill sunfish, Lepomis macrochirus). Both of these studies included data from a wide range of compounds including industrial chemicals and pharmaceuticals. Knöbel et al. 11 also showed similar sensitivities of zebrafish embryos and juvenile fathead minnows (Pimephales promelas) exposed to chemicals representing a number of different modes of action. These studies suggest that fish embryo testing can be a reliable indicator of acute toxicity to fish in general and shows promise as a more cost-effective replacement for more resource-intensive juvenile fish tests.
Although acute toxicity tests with fish embryonic life stages have shown promise for reducing the costs and effort associated with chemical hazard evaluation, there are still several uncertainties associated with their utility for evaluating specific classes of chemicals. Recent studies have reported a lower sensitivity of zebrafish embryos to neurotoxicants, which was hypothesized to be the result of different modes of action in embryos versus juveniles or the lack of biotransformation pathways in the embryonic life stage 11, 12. Therefore, there is still a need to better define the domain of applicability of fish embryo tests 5.
Previous datasets used to evaluate correlations between tests conducted with embryo and juvenile fish life stages have contained a relatively low number of pesticides (15 and 26 pesticides in Lammer et al. 2 and Belanger et al. 10, respectively). Pesticides represent a heterogeneous group of chemicals in terms of their molecular structure and mode of action, but many pesticides, especially those that target insects, act through some type of neurotoxic mode of action. Although many neurotoxicants represent older insecticide chemistries (e.g., organophosphates, carbamates) that have already been widely evaluated in both hazard and risk assessment, several of the newer insecticide chemistries, including pyrethroids, neonicotinioids, and more recent neonicotinoid-like nicotinic acetylcholine receptor antagonists (e.g., butenolides, sulfoximines), also disrupt some aspect of nervous systems. For this reason, the domain of applicability of fish embryo toxicity tests across pesticides is uncertain and should be evaluated to understand its limitations for future routine hazard assessment. In the present study we expand on previous studies by comparing zebrafish embryo developmental screening data from Padilla et al. 13 with standard juvenile fish toxicity data exclusively for pesticides.
MATERIALS AND METHODS
Dataset development
Zebrafish embryo toxicity data for 161 pesticides were obtained from a previously published dataset as part of the US Environmental Protection Agency (USEPA) ToxCast™ Phase I Chemical Library 13. Briefly, at 6 h to 8 h after fertilization, zebrafish embryos (typically 2 embryos/concentration) were exposed to 11 different chemical concentrations ranging from 0.001 μM to 80 μM for 120 h. Mortality, hatch, and malformations were assessed. The measurement endpoint for the zebrafish embryo test is the half-maximal activity concentration (AC50), which is a combined toxicity score that includes lethality and malformations (see Padilla et al. 13 for full description of the AC50 calculation). In the present study, the raw mortality data used to generate the zebrafish embryo AC50 values in the Padilla et al. 13 dataset were also used to derive the median lethal concentration (LC50) values to determine whether integration of malformation data (i.e., the AC50) yields different results than for mortality data alone (i.e., the LC50). Briefly, LC50 values were calculated as the geometric mean of the highest concentration with 0% mortality and the lowest concentration with 100% mortality based on the raw mortality data reported by Padilla et al. 13. This methodology was used previously by Scholz et al. 14. Because the zebrafish embryo toxicity test design reported in Padilla et al. 13 differs somewhat from other commonly used test designs 4, the zebrafish toxicity dataset used in the present study was also compared with other available zebrafish embryo toxicity data as compiled in Scholz et al. 14 using linear regression to ensure that differences in test design did not affect the overall results and conclusions.
The zebrafish embryo toxicity dataset for pesticides was compared with juvenile toxicity data for 3 of the most commonly tested fish species used in US pesticide risk assessments: the freshwater rainbow trout (Oncorhynchus mykiss; n = 133) and bluegill sunfish (L. macrochirus; n = 121), and the estuarine/marine sheepshead minnow (Cyprinodon variegatus, n = 77); juvenile fish toxicity data were compiled from a database of pesticide registrant-submitted data and federal laboratory data that is maintained by the USEPA Office of Pesticide Programs (Supplemental Data, Table S1). Juvenile toxicity data for these species were retained in the dataset if they met the following criteria: 1) the endpoint was mortality (i.e., LC50 reported); 2) the study duration was 96 h; 3) the test was performed on technical-grade active ingredient, not pesticide formulations; and 4) the percentage of active ingredient was equal to or greater than 90% (70–90% was also accepted if it could be demonstrated from the original study that LC50 values were derived from studies conducted with technical-grade active ingredients and were corrected for the percentage of active ingredient). If multiple LC50 values were available for a chemical–species pair, the geometric mean was used.
Statistical analysis
All statistical analyses were performed in R 3.1.2 15. Linear regression of zebrafish and juvenile toxicity data were conducted on log10-transformed values. After visual inspection of the data and regression analysis (see Results section), juvenile toxicity data among the 3 fish species were considered similar enough based on high r2 values (see Results section) to warrant combining them into a single, simplified dataset to compare with zebrafish embryo toxicity data. This consolidation of the dataset was performed by calculating the geometric mean of LC50 values for all species for which toxicity data were available for a given chemical and provided a larger dataset for comparison of embryo versus juvenile test sensitivity relationships. Statistical analyses were conducted by combining all pesticide toxicity data and by subdividing chemicals based on the acute toxicity mode of action in fish. The fish toxicity mode of action classifications of pesticides were based on the MOAtox database 16 and included the broad mode of action categories of narcosis, acetylcholinesterase (AChE) neurotoxicity, non-AChE neurotoxicity (i.e., all neurotoxicants not acting through inhibition of AChE), reactivity, and electron transport chain inhibition (Supplemental Data, Table S1). The target pest classifications were derived from the primary pesticidal activity of the chemical and included plants, insects, fungi, acarines, and bacteria (Supplemental Data, Table S1, and C. Russom, United States Environmental Protection Agency, Office of Research and Development, Duluth, MN, unpublished data). An analysis of covariance (ANCOVA) was performed for the mode of action-based analysis by including mode of action as a factor. For the ANCOVA, model simplification was performed by initially allowing each mode of action to have different slopes and intercepts and then using analysis of variance (ANOVA) to determine whether simpler models (e.g., different intercepts but a single slope) accounted for significantly less variation than the more complex model. Statistical significance for all analyses was based on an α level of 0.05.
To assess variation in zebrafish embryo toxicity as a result of differences in testing methodologies, the AC50 values used in the present study from the USEPA ToxCast dataset 13 were compared with the LC50 values collected under the OECD 4 study design (compiled by Schulz et al. 14) for a subset of overlapping chemicals. Linear regression was performed to compare the 2 datasets.
RESULTS
Juvenile fish sensitivity to pesticides
Juvenile fish toxicity values for the 3 species of fish were similar for each of the chemicals, resulting in r2 values ranging from 0.74 to 0.77 across pairwise species comparisons (Table 1). Based on the visualization of the y-intercept and data trend, rainbow trout and bluegill sunfish exhibited comparable levels of sensitivity, whereas the sheepshead minnow was slightly less sensitive than the other 2 species for the chemicals examined.
Table 1.
Regression results for comparison of pesticide toxicity datasets for 3 species of juvenile fish and zebrafish embryo based on median lethal (LC50) and adverse effect (AC50) concentrations
| Dataset comparisona | Regression results | |||
|---|---|---|---|---|
| x-axis | y-axis | No. | r2 | y-intercept |
| Rainbow trout LC50 | Bluegill sunfish LC50 | 115 | 0.78* | 0.02 |
| Rainbow trout LC50 | Sheepshead minnow LC50 | 74 | 0.77* | 0.18 |
| Bluegill sunfish LC50 | Sheepshead minnow LC50 | 69 | 0.75* | 0.12 |
| Zebrafish embryo AC50 | Rainbow trout LC50 | 134 | 0.26* | −0.54 |
| Bluegill sunfish LC50 | 119 | 0.31* | −0.51 | |
| Sheepshead minnow LC50 | 77 | 0.23* | −0.40 | |
| All juvenile fish LC50 | 140 | 0.28* | −0.43 | |
| Zebrafish embryo LC50b | Rainbow trout LC50 | 101 | 0.15* | −0.50 |
| Bluegill sunfish LC50 | 94 | 0.17* | −0.52 | |
| Sheepshead minnow LC50 | 56 | 0.07 | −0.33 | |
| All juvenile fish LC50 | 106 | 0.14* | −0.41 | |
LC50/AC50 data are log10-transformed.
LC50 converted from AC50 based on visualization of mortality data pattern (see Materials and Methods section and Padilla et al. 13 for further details).
Linear regression p < 0.05.
Zebrafish embryo and juvenile fish comparison
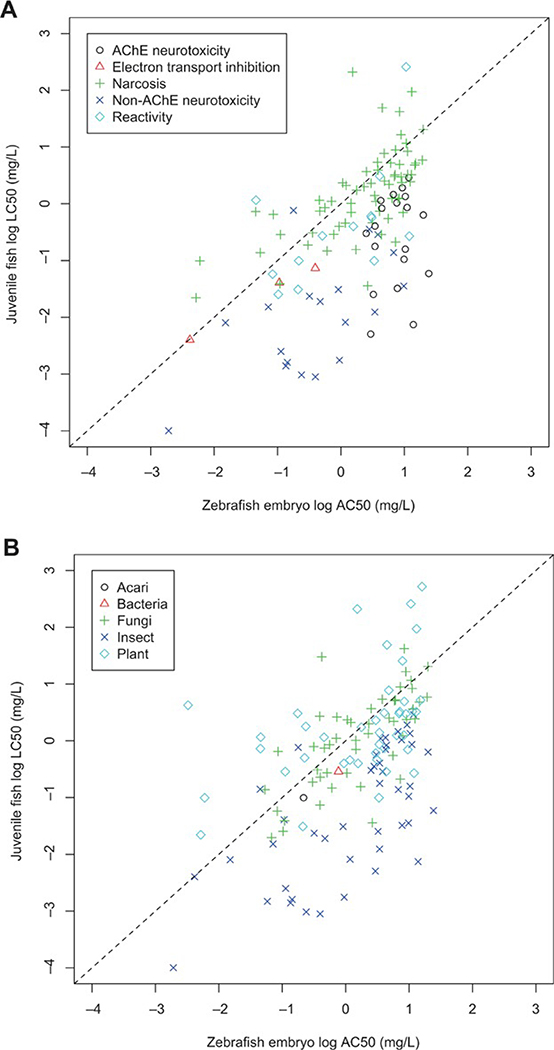
The relationship between zebrafish embryo AC50 and juvenile fish toxicity values was weak when all pesticides were analyzed together, with r2 values ranging from 0.23 to 0.31 across pairwise comparisons between zebrafish embryos (predictor) and the 3 different species of juvenile fish examined (Table 1). When pesticide toxicity data were further divided by the chemical mode of action (Figure 1A) and by target pest (Figure 1B), a pattern emerged indicating different relative sensitivities between zebrafish embryos and juvenile fish for different pesticide groups. In particular, toxicity values for insecticides, which are mostly comprised of neurotoxic compounds, fell below the 1:1 toxicity line, whereas herbicides and fungicides, which primarily act through narcosis in fish 16, were closer to the 1:1 toxicity line (Figure 1A and B). The 1:1 toxicity line represents equal chemical sensitivity between the 2 different life stages in terms of AC50/LC50 with a slope of 1 and a y-intercept of 0. This pattern, suggesting lower sensitivity of zebrafish embryos to insecticides compared with juvenile fish, was further supported by the results of the ANCOVA (Table 2). The ANCOVA analysis indicates that the relationship between both zebrafish embryo toxicity and mode of action on juvenile fish toxicity was significant (p < 0.05), but the difference in slopes among mode of action groups was not significant (p = 0.997). However, when different intercepts (but not slopes) were fitted for different mode of action groups, the explanatory power of mode of action (i.e., the amount of variability accounted for by mode of action) on juvenile toxicity was much higher (r2 = 0.64), with significantly different intercepts (p < 0.05) for all mode of action groups except for electron transport inhibitors, which were only represented by 3 chemicals.
Figure 1.
Scatterplots of zebrafish embryo log10 half-maximal activity concentration (AC50; x-axis) and juvenile fish log10 96-h median lethal concentration (LC50; y-axis) values by (A) chemical mode-of-action and (B) pesticide target group. Dotted line represents a 1:1 toxicity relationship between fish embryo (x-axis) and juvenile fish (y-axes) toxicity values. AChE = acetyl cholinesterase.
Table 2.
Analysis of covariance results for comparison of juvenile and zebrafish embryo toxicity pesticide datasets based on chemical mode of action
| Mode of action | y intercept | r2 | Slope |
|---|---|---|---|
| AChE neurotoxicity | −1.1* | 0.64 | 0.63a |
| Electron transport inhibition | −0.85 | ||
| Narcosis | 0.26* | ||
| Non-AChE neurotoxicity | −0.30* | ||
| Reactivity | 0.46* |
Significant difference in y-intercept among mode of action groups: p < 0.05.
There is a single slope for the entire dataset because the model with 1 slope was not significantly different (i.e., showed lower explanatory power) than the model with multiple slopes for different mode of action categories.
AChE = acetylcholinesterase.
USEPA ToxCast versus OECD FET designs
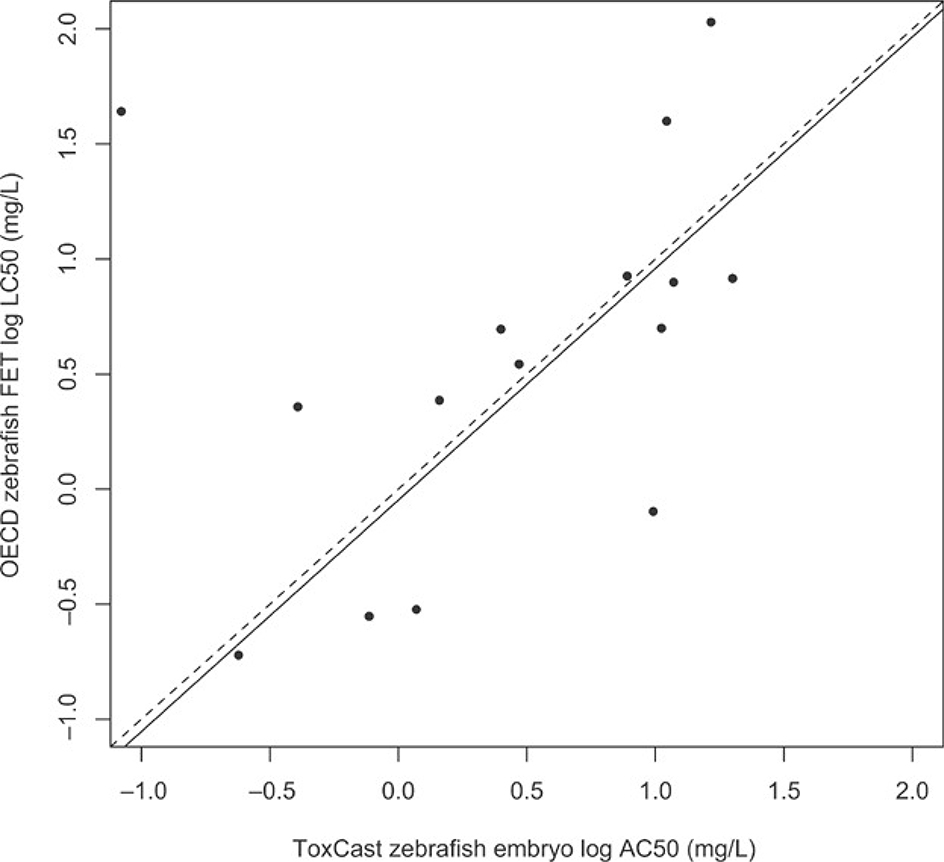
A comparison of zebrafish toxicity data generated under USEPA 13 and OECD FET 4 study designs revealed a strong relationship between toxicity endpoints generated under the 2 methodologies (Figure 2; r2 = 0.65; slope = 1; p < 0.05). A single outlier, chlorothalonil, was removed from the linear regression analysis (but not from Figure 2) because it had highly divergent endpoint values for the 2 test designs that did not follow the overall trend for the other chemicals. When that single outlier was removed, the slope and intercept for the relationship between the 2 datasets were nearly 1 and 0, respectively.
Figure 2.
Scatterplot of zebrafish embryo data generated by the US Environmental Protection Agency’s ToxCast Program (x-axis) and under the Organisation for Economic Co-operation and Development (OECD) Fish Embryo Toxicity Test (FET) Protocol (y-axis). Dotted line represents a 1: toxicity relationship between the x- and y-axes. Solid line represents the best-fit relationship between the 2 datasets based on linear regression. Note: outlier (chlorothalonil) was removed before linear regression was performed. AC50 = half-maximal activity concentration; LC50 = median lethal concentration.
DISCUSSION
Several previous studies have shown significant relationships between embryo and juvenile fish acute toxicity values across a wide range of chemical classes 2, 10. This has led to development of the FET as an OECD test guideline 4 along with recommendations that the FET replace standard juvenile fish toxicity testing 5. However, Klüver et al. 12 noted that there is a potential discrepancy in the relationship between FET and juvenile fish data for neurotoxic chemicals; in particular, embryos are often less sensitive than juvenile stage fish, which has been attributed, in part, to differences in metabolic capacities or cardiovascular physiologies between the 2 life stages.
The present study confirms this pattern for neurotoxicants within a large sample of pesticides, indicating that the use of malformation and survival endpoints within fish embryo tests may not be adequately sensitive for hazard screening of pesticides, which include many neurotoxicants. Conversely, as demonstrated in previous studies, the relationship between embryo and juvenile fish acute toxicity values was much stronger for pesticides known to act more generically via narcosis in aquatic animals, such as many fungicides and herbicides. These results suggest that although the fish embryo tests can be valuable for evaluating chemical hazards to fish for some types of pesticides, they should be used with caution in the evaluation of chemicals targeting insects or animals in general. The toxicity pattern of AChE inhibitors is particularly different from the other mode of action groups, because it forms a nearly vertical column of data points below the 1:1 toxicity line for zebrafish embryo (x-axis) and juvenile fish (y-axis) toxicity data. Such a pattern suggests that the measurement endpoints evaluated in zebrafish embryo assays are not only less sensitive to AChE inhibitors than juvenile fish, but also show little resolution for distinguishing among this group of chemicals (i.e., narrow spread of AC50/LC50 values across different AChE inhibitors).
It is possible that current embryo toxicity test endpoints are simply not designed to detect certain potentially sensitive neurotoxic effects in zebrafish. One of the obvious differences between an embryo/larva and a juvenile fish is that the former does not have to engage in feeding behavior because it is living off of the yolk, whereas the juvenile needs to feed to grow and survive. Feeding requires an exquisite coordination among the sensory and motor aspects of the nervous system: if an animal’s nervous system is not functioning correctly, it is highly likely it will be unable to feed efficiently and may not survive. This is not true for an embryo/larva, because even if its nervous system is malfunctioning because of neurotoxicant exposure, it will not starve. Neurotoxicant effects are more likely to be manifested in a juvenile fish as opposed to an embryo given the gross endpoints in each assay. If, however, more subtle endpoints, like behavioral alterations, were included in the embryo assay, effects of neurotoxic compounds may be revealed. For example, assessments of the photomotor response at 28 h to 30 h post fertilization 17 or the locomotor activity at 6 d post fertilization have both been shown to be sensitive to neuroactive chemicals 18–20.
Although some of the discrepancy between zebrafish embryo and juvenile fish toxicity data is linked to pesticide mode of action and the properties of absorption, distribution, metabolism, and excretion of different chemical groups, there are several other potential reasons for the overall poor relationship found in the present analysis. First, potential toxicity outliers were not removed in the present study, because we could not be certain that they did not represent actual differences in toxicity among pesticides. In addition, differences in observed toxicity could be because of a species mismatch, since all embryo data were collected from zebrafish whereas juvenile data were compiled from 3 different species that are commonly tested with pesticides: rainbow trout, bluegill sunfish, and sheepshead minnow. Unfortunately, standardized pesticide toxicity records for juvenile zebrafish were not available to compare with these other fish species 21; however, given the similarity in sensitivity among the 3 juvenile fish species examined in the present study for each of the pesticides evaluated (Table 1), it seems unlikely that zebrafish would be substantially different. This is supported by Belanger et al.’s 10 finding that the regression relationship between zebrafish embryo and juvenile acute toxicity data was not significantly different across the 5 different species examined (fathead minnow, bluegill, rainbow trout, zebrafish, and Japanese medaka), which includes 2 of the 3 species in our juvenile toxicity dataset. Zebrafish testing has focused on earlier life stages, with only a limited number of studies comparing the sensitivity of juvenile zebrafish with standard test species. Two studies with rainbow trout show that zebrafish are generally less sensitive, consistent with the trend of greater sensitivity in salmonids compared with other fish taxa 22, 23. As discussed in Klüver et al. 12, it is also possible that the use of juvenile fish toxicity studies in which chemical concentrations were not analytically confirmed may have increased the variability or error associated with our dataset; unfortunately, there are insufficient data to conduct a robust analysis if all LC50 values from studies with unmeasured (i.e., nominal) concentrations are removed. Loss of compounds during toxicity exposures through degradation, uptake, volatilization, and absorption may also not be comparable between standard fish toxicity test chambers and the multiwell plates used in zebrafish testing. The pooling of freshwater and saltwater species in the juvenile toxicity dataset was not considered to be a significant source of uncertainty or confounding factor in the present study. Previous research has shown that although salinity can alter chemical specific toxicity (particularly for ionic compounds), freshwater and saltwater species generally show similar sensitivity 24.
The poor relationship between zebrafish embryo and juvenile toxicity data may also be a result of the differences in test design employed by Padilla et al. 13 versus the commonly used OECD FET protocol 4, 14. Most notably, Padilla et al. 13 used more test concentrations but fewer individuals (n = 2) per concentration, which may have impacted the precision of AC50/LC50 estimates, but it seems unlikely that the toxicity measure would vary greatly on a log scale. Another difference between the standard OECD FET and the zebrafish data used in the present study is the computation of a 6-d AC50 that includes malformations that may not be lethal. To address these particular issues, the embryo data used in the present study were compared with data generated under the OECD protocol (Figure 2), and indicate a fairly strong concordance for a subset of chemicals tested under both methodologies (r2 = 0.65; slope =1; p < 0.05).
Zebrafish embryo testing has clear utility in toxicity testing, especially for decreasing the resources used in hazard screening and reducing the number of juvenile fish tested. However, the importance of adopting a given toxicity test in chemical regulation requires a firm understanding of the test limitations for risk assessment and decision-making. In the present study, zebrafish embryos were less sensitive to many pesticides, especially those that target animals through specific mode of actions rather than more generic cellular narcosis. Although part of this discrepancy could be the result of issues of experimental methodology, we suggest that it would be prudent to further evaluate the patterns of sensitivity of zebrafish embryos to pesticides and consider other lines of evidence that could reduce uncertainty before using such a test to replace more traditional juvenile fish toxicity tests. As mentioned above, perhaps including more neurological endpoints in the embryo assay design could increase the sensitivity of fish embryo tests for neurotoxic pesticides.
In our study, chemicals were divided into AChE and non-AChE neurotoxicants. However, there may be variation in sensitivity among the different types of non-AChE neurotoxicants for zebrafish embryos and juvenile fish. Because the majority of chemicals in the non-AChE category of our dataset are sodium channel modulators (12 of 20 chemicals)—mostly pyrethroid insecticides—it was not possible to break the analysis down into more specific mode of action categories. Future studies should expand the number of chemicals examined across the different types of neurotoxic mode of actions, including newer chemistries such as the neonicotinoids. These data should become available either through continued testing under OECD guideline 236 or through USEPA ToxCast Phase II. With these data, it may be possible to derive specific regression relationships among the different neurotoxic mode of actions to predict juvenile fish toxicity from embryo data.
Supplementary Material
Acknowledgment
We thank E. Velasquez for her help in compiling the juvenile fish toxicity data used in the analysis. We are grateful to S. Belanger for providing a compilation of zebrafish embryo toxicity data collected under Organisation for Economic Co-operation and Development 4 test guidelines and to S. Scholz for providing valuable advice on project design as well as LC50 calculations for the Padilla et al. 13 dataset. We also thank T. Steeger, S. Raimondo, and Y. Chiari for providing helpful comments on the manuscript, and C. Russom for development of the target pest database.
Footnotes
Disclaimer
The present study has been subjected to review by the USEPA National Health and Environmental Effects Research Laboratory and approved for publication. Approval does not signify that the contents reflect the views of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
Supplemental Data
The Supplemental Data are available on the Wiley Online Library at DOI: 10.1002/etc.3641.
Data Availability
Data are supplied in the Supplemental Data, Table S1.
References
- 1.Embry MR, Belanger SE, Braunbeck TA, Galay-Burgos M, Halder M, Hinton DE, Leonard MA, Lillicrap A, Norberg-King T, Whale G. 2010. The fish embryo toxicity test as an animal alternative method in hazard and risk assessment and scientific research. Aquat Toxicol 2: 79–87. [DOI] [PubMed] [Google Scholar]
- 2.Lammer E, Carr GJ, Wendler K, Rawlings JM, Belanger SE, Braunbeck T. 2009. Is the fish embryo toxicity test (FET) with the zebrafish (Danio rerio) a potential alternative for the fish acute toxicity test? Comp Biochem Physiol C Toxicol Pharmacol 149: 196–209. [DOI] [PubMed] [Google Scholar]
- 3.Nagel R 2002. DarT: The embryo test with the zebrafish Danio rerio—A general model in ecotoxicology and toxicology. ALTEX 19: 38–48. [PubMed] [Google Scholar]
- 4.Organisation for Economic Co-operation and Development. 2013. Test no. 236: Fish embryo acute toxicity (FET) test. OECD guidelines for the testing of chemicals; Paris, France. [Google Scholar]
- 5.Braunbeck T, Kais B, Lammer E, Otte J, Schneider K, Stengel D, Strecker R. 2014. The fish embryo test (FET): Origin, applications, and future. Environ Sci Pollut R 22: 16247–16261. [DOI] [PubMed] [Google Scholar]
- 6.Busquet F, Strecker R, Rawlings JM, Belanger SE, Braunbeck T, Carr GJ, Cenijn P, Fochtman P, Gourmelon A, Hübler N, Kleensang A, Knöbel M, Kussatz C, Legler J, Lillicrap A, Martínez-Jerónimo F, Polleichtner C, Rzodeczko H, Salinas E, Schneider KE, Scholz S, van den Brandhof E-J, van der Ven LTM, Walter-Rohde S, Weigt S, Witters H, Halder M. 2014. OECD validation study to assess intra- and interlaboratory reproducibility of the zebrafish embryo toxicity test for acute aquatic toxicity testing. Regul Toxicol Pharmacol 69: 496–511. [DOI] [PubMed] [Google Scholar]
- 7.Truong L, Reif DM, St Mary L, Geier MC, Truong HD, Tanguay RL. 2014. Multidimensional in vivo hazard assessment using zebrafish. Toxicol Sci 137: 212–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strähle U, Scholz S, Geisler R, Greiner P, Hollert H, Rastegar S, Schumacher A, Selderslaghs I, Weiss C, Witters H, Braunbeck T. 2012. Zebrafish embryos as an alternative to animal experiments—A commentary on the definition of the onset of protected life stages in animal welfare regulations. Reprod Toxicol 33: 128–132. [DOI] [PubMed] [Google Scholar]
- 9.Organisation for Economic Co-operation and Development. 1992. Test guideline 203: Fish, acute toxicity test. OECD guideline for the testing of chemicals; Paris, France. [Google Scholar]
- 10.Belanger SE, Rawlings JM, Carr GJ. 2013. Use of fish embryo toxicity tests for the prediction of acute fish toxicity to chemicals. Environ Toxicol Chem 32: 1768–1783. [DOI] [PubMed] [Google Scholar]
- 11.Knöbel M, Busser FJM, Rico-Rico Á, Kramer NI, Hermens JLM, Hafner C, Tanneberger K, Schirmer K, Scholz S. 2012. Predicting adult fish acute lethality with the zebrafish embryo: Relevance of test duration, endpoints, compound properties, and exposure concentration analysis. Environ Sci Technol 46: 9690–9700. [DOI] [PubMed] [Google Scholar]
- 12.Klüver N, Konig M, Ortmann J, Massei R, Paschke A, Kuhne R, Scholz S. 2015. Fish embryo toxicity test: Identification of compounds with weak toxicity and analysis of behavioral effects to improve prediction of acute toxicity for neurotoxic compounds. Environ Sci Technol 49: 7002–7011. [DOI] [PubMed] [Google Scholar]
- 13.Padilla S, Corum D, Padnos B, Hunter DL, Beam A, Houck KA, Sipes N, Kleinstreuer N, Knudsen T, Dix DJ, Reif DM. 2012. Zebrafish developmental screening of the ToxCast™ Phase I chemical library. Reprod Toxicol 33: 174–187. [DOI] [PubMed] [Google Scholar]
- 14.Scholz S, Ortmann J, Klüver N, Leonard M. 2014. Extensive review of fish embryo acute toxicities for the prediction of GHS acute systemic toxicity categories. Regul Toxicol Pharmacol 69: 572–579. [DOI] [PubMed] [Google Scholar]
- 15.R Core Team. 2014. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria: [cited 2015 August 15]. Available from: http://www.R-project.org [Google Scholar]
- 16.Barron MG, Lilavois CR, Martin TM. 2015. A comprehensive mode of action and acute aquatic toxicity database for predictive model development. Aquat Toxicol 16: 102–107. [DOI] [PubMed] [Google Scholar]
- 17.Kokel D, Bryan J, Laggner C, White R, Cheung CYJ, Mateus R, Healey D, Kim S, Werdich AA, Haggarty SJ, MacRae CA, Shoichet B, Peterson RT. 2010. Rapid behavior-based identification of neuroactive small molecules in the zebrafish. Nat Chem Biol 6: 231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Irons TD, MacPhail RC, Hunter DL, Padilla S. 2010. Acute neuroactive drug exposures alter locomotor activity in larval zebrafish. Neurotoxicol Teratol 32: 91–98. [DOI] [PubMed] [Google Scholar]
- 19.Jarema KA, Hunter DL, Shaffer RM, Behl M, Padilla S. Acute and developmental behavioral effects of flame retardants and related chemicals in zebrafish. Neurotoxicol Teratol 52: 194–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Selderslaghs IW, Hooyberghs J, Blust R, Witters HE. 2013. Assessment of the developmental neurotoxicity of compounds by measuring locomotor activity in zebrafish embryos and larvae. Neurotoxical Teratol 37: 44–56. [DOI] [PubMed] [Google Scholar]
- 21.Raimondo S, Jackson CR, Barron MG. 2010. Influence of taxonomic relatedness and chemical mode of action in acute interspecies estimation models for aquatic species. Environ Sci Technol 44: 7711–7716. [DOI] [PubMed] [Google Scholar]
- 22.Fogels J, Sprague JB. 1977. Comparative short-term tolerance of zebrafish, flagfish, and rainbow trout to five poisons including potential reference toxicants. Water Res 11: 811–817. [Google Scholar]
- 23.Dave G, Andersson K, Berglind R, Hasselrot B. 1981. Toxicity of eight solvent extraction chemicals and of cadmium to water fleas, Daphnia magna, rainbow trout, Salmo gairdneri, and zebrafish, Brachydanio rerio. Comp Biochem Physiol C 69: 83–98. [DOI] [PubMed] [Google Scholar]
- 24.de Zwart D 2002. Observed regularities in species sensitivity distributions for aquatic species In Posthuma L, Suter GW, Traas TP, eds, Species Sensitivity Distributions in Ecotoxicity. Lewis, Boca Raton, FL, USA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are supplied in the Supplemental Data, Table S1.