Chlamydia was detected in 7.4% of >13,500 pregnant women tested at an academic center in Alabama. Age less than 30 years was strongly associated with infection in adjusted models.
Background
There is a paucity of population-based data on chlamydia in pregnancy despite rising rates in US women. Our objectives were to assess chlamydia prevalence by age group and to identify factors associated with infection in pregnant women to inform screening guidelines.
Methods
This cross-sectional study included pregnant women tested for chlamydia who delivered at the University of Alabama at Birmingham between November 1, 2012, and December 31, 2017. The primary outcome was chlamydia prevalence, defined as a positive urogenital chlamydia nucleic acid amplification test result documented in the electronic medical record. Multivariable logistic regression was used to identify factors associated with infection.
Results
Among 17,796 women who delivered during the study period, 13,657 (77%) had chlamydia testing performed at the University of Alabama at Birmingham. Chlamydia prevalence (95% confidence interval) was 7.4% (7.0%–7.9%). Age-stratified prevalence rates were 14.6%, 4.3%, and 1.7% for women younger than 25 years, 25 to 29 years, and 30 years or older, respectively. Chlamydia in pregnancy remained strongly associated with age (adjusted odds ratio [95% confidence interval], 7.2 [5.6–9.2] for age <25 years, and 2.3 [1.7–3.0] for ages 25–29 years, when compared with >30 years) after adjustment for race, urban residence, and insurance status.
Conclusions
Among pregnant women living in the southeastern United States, chlamydia was detected in 1 of 14 women who were tested. Chlamydia positivity was highest among women younger than 30 years. Study findings support broad screening for chlamydia in pregnancy.
Chlamydia is the most common reportable infection in the United States, with more than 1.8 million cases reported to the Centers for Disease Control and Prevention (CDC) in 2018.1 Women aged 15 to 29 years have high pregnancy rates and disproportionately high rates of chlamydia when compared with men of similar age and older women. Untreated chlamydia infection of the cervix in pregnant women can be transmitted vertically at delivery.2 Because most infections are asymptomatic and effective antibiotic therapy is available, routine universal screening for chlamydia in pregnancy at the first prenatal visit is recommended by the American College of Obstetricians and Gynecologists (ACOG) and the American Academy of Pediatrics.3 In contrast, the CDC and the US Preventive Services Task Force limit chlamydia screening to pregnant women younger than 25 years with risk-based screening in older groups.4,5 Chlamydia infection in pregnancy can lead to preventable adverse birth outcomes including a 2-fold increase in preterm delivery, low birth weight, and neonatal pneumonia in up to 30% of exposed infants.3,6,7 Screening for chlamydia infection in pregnancy has been shown to be cost-effective when the prevalence is ≥3%.8,9
Although population-based surveillance data for chlamydia in pregnant women are not available in the United States because of incomplete and inconsistent reporting of pregnancy status for chlamydia cases in women, 3.5% of women tested positive for chlamydia in a US reference laboratory in 2005 to 2008.10 There is regional variability in the prevalence of chlamydia with higher rates in the South (e.g., 777 cases per 100,000 women in Alabama compared with 693 cases per 100,000 women nationwide).1 Risk factors for chlamydia infection in nonpregnant women include younger age, Black race, and history of sexually transmitted infection (STI).11,12 Risk factor–based screening is inadequate if providers and women are not aware of sex partner risk and regional chlamydia prevalence. Our study objectives here were to determine the prevalence of chlamydia in pregnancy according to maternal age, identify additional factors associated with infection, and evaluate the association between age and chlamydia in pregnancy.
MATERIALS AND METHODS
Study Design and Population
The cross-sectional study design included women with chlamydia testing performed in pregnancy who delivered at the University of Alabama at Birmingham’s (UAB’s) Women and Infants Center between November 1, 2012, and December 31, 2017. The UAB provides prenatal care for women with and without underlying medical conditions in Jefferson County (Birmingham) and serves as a referral center for women with complicated pregnancies throughout Alabama. For women with more than one pregnancy during the study period, the initial pregnancy was used for analysis. The standard practice for diagnostic testing in outpatient obstetric clinics was to perform a urogenital chlamydia screening test at the initial prenatal visit with repeat testing based on positivity, symptoms, or exposure. Study data were extracted from the electronic medical record using an algorithm that captured relevant test results and deidentified sociodemographic information for the study population. Unique ID numbers were assigned.
Study Outcomes
The primary study outcome was the prevalence of urogenital chlamydia infection during pregnancy. Prevalence was defined as the number of women with at least one positive chlamydia test result among those screened who delivered at our center during the study period. Chlamydia trachomatis testing was performed on urine samples, vaginal swabs, and cervical swabs using highly sensitive nucleic acid amplification test with Roche Amplicor in 2012 and Cobas 4800 between 2013 and 2017 for clinic patients, and BD Viper in 2012 to 2013 and Aptima Hologic between 2014 and 2017 for women tested in the emergency department (ED) or as inpatients. The performance of these nucleic acid amplification tests performed on samples collected from the female genital tract or urine is similar with high sensitivity >90% and specificity >99%.13 Chlamydia testing performed outside the UAB system or before referral was not available. For pregnancy outcome definitions, preterm delivery occurs before 37 weeks’ gestation, low birth weight is <2500 g, fetal loss before 20 weeks is a spontaneous abortion, and fetal loss after 20 weeks is an intrauterine fetal demise. Neonatal death occurs within 28 days of birth.
Potential Factors of Interest
Variables of interest were maternal age (continuous and categorized into 13–24, 25–29, and 30+ years based on current prenatal screening guidelines), self-reported race (non-Hispanic Black, non-Hispanic White, Asian, multiple and other), ethnicity (Hispanic, non-Hispanic), insurance status (private, public [Medicaid or Medicare], none), urban residence (defined as a resident of Jefferson County, the most populous county in Alabama with 650,000 residents) versus nonurban, and location of chlamydia testing (clinic, ED, inpatient).
Statistical Analysis
To describe the study population, χ2 procedures were used to analyze categorical variables with stratification by chlamydia test results. Bivariate analysis was used to explore associations between variables of interest and chlamydia positivity. Logistic regression was used to calculate unadjusted and adjusted odds ratios (ORs) along with 95% confidence intervals (95% CIs) for the outcome of interest. We included all variables with significance in crude models at a P value <0.05 in the multivariable model. The analysis was performed using SAS version 9.4 (Cary, NC).
Ethics
The study was approved by the UAB Institutional Review Board with waiver of informed consent.
RESULTS
A total of 17,796 pregnant women delivered at UAB hospital during the 5-year study period. Among these, 13,657 women (77%) had chlamydia testing performed at UAB. The prevalence (95% CI) of chlamydia infection in this group was 7.4% (7.0%–7.9%; Fig. 1). Table 1 shows the characteristics of the total sample of pregnant women, and Table 2 shows differences in characteristics stratified by chlamydia test results. The median age for our study sample was 27 years, and 51% were Black women. Chlamydia prevalence varied by age: 14.6% in women younger than 25 years, 4.3% in women aged 25 to 29 years, and 1.7% in women 30 years and older (Table 1). The median age of pregnant women with chlamydia was 22 years compared with 27 years in those without chlamydia (P < 0.001; Table 2). We also observed significant differences in chlamydia positivity by race and ethnicity (P < 0.001; Table 2). Among pregnant women with chlamydia, 93% lived in urban areas compared with 79% of women without chlamydia (P < 0.001). A larger proportion of women with chlamydia had public insurance compared with those without chlamydia (82% [79.1%–84.0%] vs. 64% [63.1%–64.8%], P < 0.001). No difference was noted in testing location (P = 0.13) or birth outcomes (P = 0.81) according to chlamydia positivity (Table 2). Preterm delivery occurred in 15.2% of women with chlamydia and 15.7% of women without chlamydia (P = 0.68).
Figure 1.
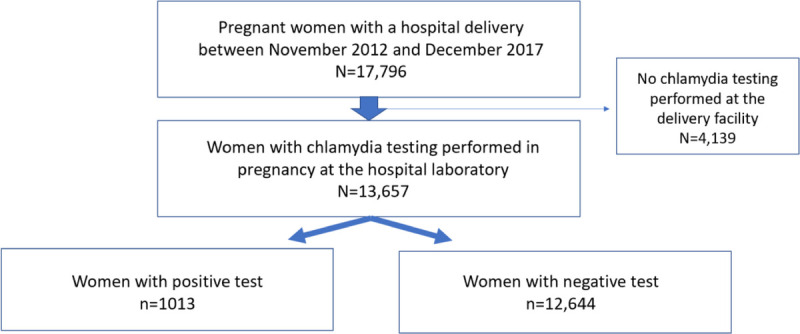
Study flow diagram.
TABLE 1.
Characteristics of Pregnant Women Tested for Chlamydia
| Total Sample* (n = 13,657) | Chlamydia Positive* (n = 1013) |
Chlamydia Negative* (n = 12,644) |
|
|---|---|---|---|
| Age at delivery, median (IQR), y | 26.8 (9.2) | 21.6 (5.4) | 27.3 (8.9) |
| Age category, n (%) | |||
| <25 y | 5303 | 774 (14.6) | 4529 (85.4) |
| 25–29 y | 3828 | 164 (4.3) | 3664 (95.7) |
| 30+ y | 4526 | 75 (1.7) | 4451 (98.3) |
| Race/Ethnicity, n (%) | |||
| Black | 7016 | 804 (11.5) | 6219 (88.5) |
| White | 3561 | 82 (2.3) | 3479 (97.7) |
| Hispanic | 2501 | 95 (3.8) | 2404 (96.2) |
| Asian | 244 | 8 (3.3) | 236 (96.7) |
| Multiple | 129 | 15 (11.6) | 115 (88.4) |
| Other | 206 | 9 (4.4) | 196 (95.6) |
| Urban residence, n (%) | 10,867 | 944 (8.7) | 9923 (91.3) |
| Insurance status, n (%) | |||
| Public | 8918 | 827 (9.3) | 8091 (90.7) |
| Private | 3425 | 100 (2.9) | 3325 (97.1) |
| Uninsured | 1314 | 86 (6.5) | 1228 (93.5) |
| Location of testing, n (%) | |||
| Clinic | 12,169 | 899 (7.4) | 11,270 (92.6) |
| ED | 1474 | 111 (7.5) | 1363 (92.5) |
| Inpatient | 14 | 3 (21.4) | 11 (78.6) |
| Preterm delivery, n (%) | 2139 | 154 (7.2) | 1985 (92.8) |
| Birth weight, n (%) | |||
| <2500 g | 1794 | 153 (8.5) | 1641 (91.5) |
| ≥2500 g | 10,930 | 797 (7.3) | 10,133 (92.7) |
| Missing | 933 | 63 (6.8) | 870 (93.2) |
| Birth outcomes, n (%) | |||
| Live birth | 13,409 | 998 (7.4) | 12,411 (92.6) |
| SAB/Stillbirth/IUFD | 228 | 17 (7.5) | 211 (92.5) |
| Neonatal death | 23 | 1 (4.3) | 22 (95.7) |
*Row percentages.
IUFD indicates intrauterine fetal demise; SAB, spontaneous abortion.
TABLE 2.
Evaluating Differences in Characteristics by Chlamydia Test Positivity
| Chlamydia Positive* (n = 1013) | Chlamydia Negative* (n = 12,644) | P† | |
|---|---|---|---|
| Age at delivery, median (IQR), y | 21.6 (5.4) | 27.3 (8.9) | <0.001 |
| Age category, n (%) | |||
| <25 y | 774 (76.4) | 4529 (35.8) | <0.001 |
| 25–29 y | 164 (16.2) | 3664 (29.0) | |
| 30+ y | 75 (7.4) | 4451 (35.2) | |
| Race/Ethnicity, n (%) | |||
| Black | 804 (79.4) | 6219 (49.1) | <0.001 |
| White | 82 (8.1) | 3479 (27.5) | |
| Hispanic | 95 (9.4) | 2404 (19.0) | |
| Asian | 8 (0.8) | 236 (1.9) | |
| Multiple | 15 (1.5) | 115 (0.9) | |
| Other | 9 (0.9) | 196 (1.6) | |
| Urban residence, n (%) | 944 (93.2) | 9923 (78.5) | <0.001 |
| Insurance status, n (%) | |||
| Public | 827 (81.6) | 8091 (64.0) | <0.001 |
| Private | 100 (9.9) | 3325 (26.3) | |
| Uninsured | 86 (8.5) | 1228 (9.7) | |
| Location of testing, n (%) | 0.13 | ||
| Clinic | 899 (88.8) | 11,270 (89.1) | |
| ED | 111 (11.0) | 1363 (10.8) | |
| Inpatient | 3 (0.3) | 11 (0.1) | |
| Preterm delivery, n (%) | 154 (15.2) | 1985 (15.7) | 0.68 |
| Birth weight, n (%) | |||
| <2500 g | 153 (15.1) | 1641 (13.0) | 0.13 |
| ≥2500 g | 797 (78.7) | 10,133 (80.1) | |
| Missing | 63 (6.2) | 870 (6.9) | |
| Birth outcomes, n (%) | |||
| Live birth | 998 (98.5) | 12,411 (98.2) | 0.81 |
| SAB/Stillbirth/IUFD | 17 (1.7) | 211 (1.6) | |
| Neonatal death | 1 (0.1) | 22 (0.2) |
*Column percentages.
†Significance determined using χ2 tests for proportions and t test for continuous variables.
Factors associated with chlamydia infection are shown in Table 3. In crude models, younger age (odds ratios [ORs], 10.1 [95% CI, 8.0–12.9] for age <25 years and 2.7 [95% CI, 2.0–3.5] for ages 25–29 years, both compared with age >30 years); self-reported Black (OR, 5.5 [95% CI 4.4–6.9]), Hispanic (OR, 1.7 [95% CI, 1.2–2.3]), or other race (OR, 2.5 [95% CI, 1.6–3.8]) compared with White race; urban residence (OR, 3.8 [95%, CI 2.9–4.8]); having public insurance (OR, 3.4 [95% CI, 2.8–4.2]); and being uninsured (OR, 2.3 [95% CI 1.7–3.1]) compared with private insurance were significantly associated with chlamydia.
TABLE 3.
Factors Associated With Chlamydia in Pregnancy
| Crude Odds Ratio (95% CI) | |
|---|---|
| Age, y | |
| <25 | 10.1 (8.0–12.9) |
| 25–29 | 2.7 (2.0–3.5) |
| 30+ | Referent |
| Race/Ethnicity | |
| White | Referent |
| Black | 5.5 (4.4–6.9) |
| Hispanic | 1.7 (1.2–2.3) |
| Other | 2.5 (1.6–3.8) |
| Urban residence | 3.8 (2.9–4.8) |
| Insurance status | |
| Private | Referent |
| Uninsured | 2.3 (1.7–3.1) |
| Public | 3.4 (2.8–4.2) |
Table 4 shows 3 adjusted models for the association between age and chlamydia in pregnancy. All models show similar results. In model 3, women younger than 25 years had 7-fold higher odds of chlamydia compared with women older than 30 years (adjusted OR [95% CI], 7.2 [5.6–9.2]). Women aged 25 to 29 years had 2-fold higher odds of having chlamydia compared with women older than 30 years (2.3 [1.7–3.0]). This model was adjusted for race/ethnicity, urban residence, and insurance status.
TABLE 4.
Association Between Age and Chlamydia in Pregnancy
| Crude Odds Ratio (95% CI) |
Model 1, Adjusted Odds Ratio* (95% CI) |
Model 2, Adjusted Odds Ratio† (95% CI) |
Model 3, Adjusted Odds Ratio‡ (95% CI) |
|
|---|---|---|---|---|
| Age, y | ||||
| <25 | 10.1 (8.0–12.9) | 7.9 (6.2–10.1) | 7.8 (6.1–9.9) | 7.2 (5.6–9.2) |
| 25–29 | 2.7 (2.0–3.5) | 2.4 (1.8–3.1) | 2.3 (1.8–3.1) | 2.3 (1.7–3.0) |
| 30+ | Referent | Referent | Referent | Referent |
*Model 1 adjusted for race.
†Model 2 adjusted for race and urban residence.
‡Model 3 adjusted for race, urban residence, and insurance status.
DISCUSSION
Among more than 13,500 women who delivered at our university hospital facility in urban Alabama, the prevalence of chlamydia infection during pregnancy was 7.4%. Women younger than 30 years had a significantly higher prevalence of infection compared with older women. Nearly 1 in 4 infections would have been missed if chlamydia testing had been limited to women younger than 25 years. We also found evidence of disparity in infection rates according to race, urban residence, and socioeconomic status: women who were uninsured or had public insurance (mostly Medicaid) had higher odds of chlamydia infection compared with women with private insurance.
Few contemporary studies estimate the prevalence of chlamydia infection in pregnancy in the United States. An analysis from a large laboratory database (Quest Diagnostics) 10 years ago showed that 59% of women had been tested for chlamydia in pregnancy, and the positivity rate was 3.5%.10 In Atlanta, chlamydia prevalence in pregnant women tested was similar to our study at 9%.14 A CDC-funded system called the Pregnancy Risk Assessment Monitoring System surveyed nearly 13,000 women about STI in pregnancy in 5 states from 2009 to 2011: 2.4% reported chlamydia infection, but this highlights the limitations of self-report and likely underestimates prevalence.12 In a retrospective matched cohort study of 358 pregnant women with and without HIV who delivered at our UAB facility between 2000 and 2014, chlamydia prevalence rates were 17% in women with HIV and 12% in women without HIV (P = 0.2).15
In the current study, 77% of women who delivered had laboratory testing for chlamydia at our center. This likely underestimates the true prenatal screening rate because women referred to the UAB for pregnancy complications may have been tested for chlamydia before the transfer of care. External laboratory records were not incorporated in the current analysis. In a recent CDC analysis from the National Survey of Family Growth (n = 1155), 48% of women with pregnancy in the past year reported that they were tested for chlamydia during prenatal care.16 National surveillance systems capture valuable information about chlamydia infection in women because chlamydia is a reportable condition, but pregnancy status is not consistently included in case reports to the CDC.17 As a result, stratified analysis of population-based national data to look at screening rates and positivity rates according to pregnancy status is not possible.
Current CDC and US Preventive Services Task Force recommendations for universal chlamydia screening in pregnancy is limited to younger woman (<25 years). This guidance is based on prevalence and cost-effectiveness analyses from the United States and other countries.9,18,19 In our study setting where universal screening per ACOG guidelines was standard practice, 1 in 4 cases of chlamydia in pregnancy would have been missed if testing had been restricted to women younger than 25 years. Risk-based STI screening in pregnancy can be limited: for example, in a recent CDC study of pregnant women with primary and secondary syphilis, 49% had no reported risk factor for infection.20 Although chlamydia rates are consistently highest in women younger than 25 years, Hu et al.8 showed that annual chlamydia screening in the general population of women aged 15 to 29 years can be cost-effective. Another factor in support of ACOG’s universal screening guidelines for chlamydia in pregnancy relates to demographic changes in the US population: the mean maternal age at the time of first pregnancy has increased to 26.3 years.21 Because fewer pregnant women are younger than 25 years, age-restricted screening guidelines may lead to reduced screening rates.
Younger age in women has long been associated with higher rates of chlamydia acquisition, and age is the strongest predictor of chlamydia in pregnant women in our study.1 Whether this is due to anatomy (cervical ectropion that resolves with age), immunology (acquired immunity to chlamydia with age), or behavioral patterns (more sex partners or higher risk sex partners in younger women) remains unclear.22 In HIV-discordant couples, pregnancy is associated with 2-fold increased risk of HIV acquisition compared with nonpregnant women (7.4 vs. 3.0 incident infections per 100 person-years).23 Other independent predictors of chlamydia infection in pregnancy in our study included Black race. Persistent disparities in STI rates have been consistently documented in national surveillance reports where chlamydia infection rates are 4 to 5 times higher in Black women compared with White women.1,24,25 Sexual networks have been shown to explain much of the elevated risk of STI acquisition among Black adolescents and women compared with other racial/ethnic groups.26 Lack of health insurance or having public insurance instead of private insurance is another independent predictor of chlamydia in pregnant women in our study. This may be a proxy for access to health care, but it is most likely a proxy for socioeconomic status, which has been associated with STI risk.11 Many adverse outcomes have been documented in adults without medical insurance, including pregnancy outcomes.27 Improving access to high-quality prenatal care is critical to improving health outcomes that depend on the detection and treatment of chlamydia in asymptomatic and symptomatic women.28,29
Our study has important limitations. It is a retrospective study limited to women who delivered at a single center in the Southeastern United States, which may not be representative of other regions or women who reside in predominantly rural regions. Our study was not able to distinguish women who presented for testing because of symptoms, known exposure, or routine screening. Complete information about potential risk factors for chlamydia acquisition (such as sex partner number and characteristics, STI history, drug use, alcohol use, education level, and income) was not routinely available for this analysis. This may have led to bias due to residual confounding that we were unable to adjust for. Our estimates of screening and positivity rates may be underestimates if women were screened and/or treated for chlamydia at other facilities before transfer to our center. There may be a testing bias if women who were not screened had different characteristics from women who were screened. This should have been minimized by the protocol for universal screening. Study strengths include the sample size, the use of highly sensitive diagnostic testing, and the data set quality.
One in 14 pregnant women who were tested in our academic center during the past 5 years had chlamydia infection. Factors associated with infection included younger age (<30 years), Black race, and lower socioeconomic status. In the midst of rising chlamydia rates in the United States, study findings support current ACOG guidelines for universal chlamydia screening in pregnancy.
Footnotes
Conflict of Interest and Sources of Funding: N.C.W. has received research funding from Amgen and was an expert witness for Pfizer. This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (K23HD090993 to J.D.-O.) and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (1K01AR068400 to N.C.W.) of the National Institutes of Health.
Contributor Information
Jessica S. McKenzie, Email: jessmck@uab.edu.
Nicole C. Wright, Email: ncwright@uab.edu.
Shainela A. Sheikh, Email: shaine29@uab.edu.
Akila Subramaniam, Email: asubramaniam@uabmc.edu.
Alan T. N. Tita, Email: atita@uabmc.edu;atita@uab.edu.
Jodie Dionne-Odom, Email: jdionne@uab.edu.
REFERENCES
- 1.US Centers for Disease Control and Prevention Sexually Transmitted Disease Surveillance Report 2018 2019. Available at: https://www.cdc.gov/nchhstp/newsroom/2019/2018-STD-surveillance-report.html. Accessed March 9, 2020.
- 2.Martin DH Koutsky L Eschenbach DA, et al. . Prematurity and perinatal mortality in pregnancies complicated by maternal Chlamydia trachomatis infections. JAMA 1982; 247:1585–1588. [PubMed] [Google Scholar]
- 3.Wiesenfeld HC. Screening for Chlamydia trachomatis infections in women. N Engl J Med 2017; 376:765–773. [DOI] [PubMed] [Google Scholar]
- 4.LeFevre ML. Screening for chlamydia and gonorrhea: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014; 161:902–910. [DOI] [PubMed] [Google Scholar]
- 5.Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64(RR-03):1–137. [PMC free article] [PubMed] [Google Scholar]
- 6.Berggren EK, Patchen L. Prevalence of Chlamydia trachomatis and Neisseria gonorrhoeae and repeat infection among pregnant urban adolescents. Sex Transm Dis 2011; 38:172–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reekie J Roberts C Preen D, et al. . Chlamydia trachomatis and the risk of spontaneous preterm birth, babies who are born small for gestational age, and stillbirth: A population-based cohort study. Lancet Infect Dis 2018; 18:452–460. [DOI] [PubMed] [Google Scholar]
- 8.Hu D, Hook EW, 3rd, Goldie SJ. Screening for Chlamydia trachomatis in women 15 to 29 years of age: A cost-effectiveness analysis. Ann Intern Med 2004; 141:501–513. [DOI] [PubMed] [Google Scholar]
- 9.Ong JJ Chen M Hocking J, et al. . Chlamydia screening for pregnant women aged 16–25 years attending an antenatal service: A cost-effectiveness study. BJOG 2016; 123:1194–1202. [DOI] [PubMed] [Google Scholar]
- 10.Blatt AJ Lieberman JM Hoover DR, et al. . Chlamydial and gonococcal testing during pregnancy in the United States. Am J Obstet Gynecol 2012; 207:55.e51–55.e558. [DOI] [PubMed] [Google Scholar]
- 11.Noah AJ, Yang TC, Wang WL. The Black-White disparity in sexually transmitted diseases during pregnancy: How do racial segregation and income inequality matter? Sex Transm Dis 2018; 45:301–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams CL Harrison LL Llata E, et al. . Sexually transmitted diseases among pregnant women: 5 states, United States, 2009–2011. Maternal Child Health J 2018; 22:538–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae—2014. MMWR Recomm Rep 2014; 63(Rr-02):1–19. [PMC free article] [PubMed] [Google Scholar]
- 14.Goggins ER Chamberlain AT Kim TG, et al. . Patterns of screening, infection, and treatment of Chlamydia trachomatis and Neisseria gonorrhea in pregnancy. Obstet Gynecol 2020; 135:799–807. [DOI] [PubMed] [Google Scholar]
- 15.Dionne-Odom J Khan MJ Jauk VC, et al. . HIV status and other risk factors for prevalent and incident sexually transmitted infection during pregnancy (2000–2014). Infect Dis Obstet Gynecol 2019; 2019:6584101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leichliter JS Haderxhanaj LT Gift TL, et al. . Sexually transmissible infection testing among pregnant women in the US, 2011–15. Sex Health 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.US Centers for Disease Control and Prevention STD Surveillance Report 2017 2018. Accessed March 16, 2020.
- 18.Ditkowsky J Shah KH Hammerschlag MR, et al. . Cost-benefit analysis of Chlamydia trachomatis screening in pregnant women in a high burden setting in the United States. BMC Infect Dis 2017; 17:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee KC Ngo-Metzger Q Wolff T, et al. . Sexually transmitted infections: Recommendations from the U.S. Preventive Services Task Force. Am Fam Phys 2016; 94:907–915. [PubMed] [Google Scholar]
- 20.Trivedi S Williams C Torrone E, et al. . National trends and reported risk factors among pregnant women with syphilis in the United States, 2012–2016. Obstet Gynecol 2019; 133:27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mathews TJ, Hamilton BE. CDC National Center for Health Statistics. Mean age of mothers is on the rise: United States, 2000–2014. 2016. Hyattsville, MD. Available at: https://www.cdc.gov/nchs/data/databriefs/db232.pdf. [PubMed]
- 22.Darville T Albritton HL Zhong W, et al. . Anti-chlamydia IgG and IgA are insufficient to prevent endometrial chlamydia infection in women, and increased anti-chlamydia IgG is associated with enhanced risk for incident infection. Am J Reprod Immunol 2019; 81:e13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mugo NR Heffron R Donnell D, et al. . Increased risk of HIV-1 transmission in pregnancy: A prospective study among African HIV-1-serodiscordant couples. AIDS 2011; 25:1887–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.US Centers for Disease Control and Prevention Sexually transmitted diseases in women and infants. STD Surveillance Report 2013. 2014. Accessed March 16, 2020.
- 25.US Centers for Disease Control and Prevention STD Surveillance Report, 2015 2016. Accessed March 16, 2020.
- 26.Pelligrino N Zaitzow BH Sothern M, et al. . Incarcerated Black women in the Southern USA: A narrative review of STI and HIV risk and implications for future public health Research, practice, and policy. J Racial Ethn Health Disparities 2017; 4:9–18. [DOI] [PubMed] [Google Scholar]
- 27.Taylor YJ, Liu TL, Howell EA. Insurance differences in preventive care use and adverse birth outcomes among pregnant women in a Medicaid nonexpansion state: A retrospective cohort study. J Womens Health 2019; 29:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Handler A, Johnson K. A call to revisit the prenatal period as a focus for action within the reproductive and perinatal care continuum. Matern Child Health J 2016; 20:2217–2227. [DOI] [PubMed] [Google Scholar]
- 29.Partridge S Balayla J Holcroft CA, et al. . Inadequate prenatal care utilization and risks of infant mortality and poor birth outcome: A retrospective analysis of 28,729,765 U.S. deliveries over 8 years. Am J Perinatol 2012; 29:787–793. [DOI] [PubMed] [Google Scholar]


