Supplemental Digital Content is available in the text
Keywords: COVID-19, infection, pandemic, SARS-CoV-2, surgery
Objective:
To evaluate the perioperative morbidity and mortality of patients with COVID-19 who undergo urgent and emergent surgery.
Summary Background Data:
Although COVID-19 infection is usually associated with mild disease, it can lead to severe respiratory complications. Little is known about the perioperative outcomes of patients with COVID-19.
Methods:
We examined patients who underwent urgent and emergent surgery at 2 hospitals in New York City from March 17 to April 15, 2020. Elective surgical procedures were cancelled throughout and routine, laboratory based COVID-19 screening was instituted on April 1. Mortality, complications, and admission to the intensive care unit were compared between patients with COVID-19 detected perioperatively and controls.
Results:
Among 468 subjects, 36 (7.7%) had confirmed COVID-19. Among those with COVID-19, 55.6% were detected preoperatively and 44.4% postoperatively. Before the routine preoperative COVID-19 laboratory screening, 7.7% of cases were diagnosed preoperatively compared to 65.2% after institution of screening (P = 0.0008). The perioperative mortality rate was 16.7% in those with COVID-19 compared to 1.4% in COVID-19 negative subjects [aRR = 9.29; 95% confidence interval (CI), 5.68–15.21]. Serious complications were identified in 58.3% of COVID-19 subjects versus 6.0% of controls (aRR = 7.02; 95%CI, 4.96–9.92). Cardiac arrest, sepsis/shock, respiratory failure, pneumonia, acute respiratory distress syndrome, and acute kidney injury were more common in those with COVID-19. The intensive care unit admission rate was 36.1% in those with COVID-19 compared to 16.4% of controls (aRR = 1.34; 95%CI, 0.86–2.09).
Conclusions:
COVID-19 is associated with an increased risk for serious perioperative morbidity and mortality. A substantial number of patients with COVID-19 are not identified until after surgery.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), now known as coronavirus disease 2019 (COVID-19), is a novel coronavirus that was first recognized in the Hubei province of China in late 2019.1–3 COVID-19 typically results in mild respiratory disease but may lead to respiratory failure and critical illness in approximately 5% of patients.2 COVID-19 has spread rapidly across the world and was declared a pandemic by the World Health Organization (WHO) on March 11, 2020.4
The spread of COVID-19 has had a significant impact on surgical services worldwide and posed a number of challenges. First, the emergence of COVID-19 has placed a significant strain on hospital systems due to the largescale increase in hospitalizations for coronavirus-associated disease.2 The need to reallocate resources has limited the availability of operating room facilities and staff. These resource constraints, along with efforts to promote social distancing, have led to recommendations to postpone elective surgical procedures when feasible.5,6
Second, patients with either symptomatic or occult COVID-19 infections who undergo surgery may be at increased risk for adverse outcomes.7 Given that severe COVID-19 related infections result in pneumonia and acute respiratory distress syndrome, surgical patients who typically require mechanical ventilation, are immunosuppressed perioperatively, and who often have underlying comorbidities may be at particular risk for increased morbidity.8 To date, there are limited data describing the perioperative outcomes of patients with COVID-19.7,9
Despite resource constraints and the possible increased risk of perioperative complications, some patients with COVID-19 infections will require urgent or emergent surgical interventions. Further, as many patients with COVID-19 infections are asymptomatic or demonstrate only mild symptomatology, there is a significant risk that patients with occult or unrecognized COVID-19 infections may undergo surgery in areas where community spread of the virus is widespread.10 We performed a cohort study to determine the outcomes of patients with recognized or occult COVID-19 infection who underwent surgery at 2 urban hospital system in New York City during a widespread outbreak of COVID-19 in the region.
METHODS
Patients and Procedures
We performed a retrospective cohort study of patients who underwent surgery at New York Presbyterian Hospital Columbia University Irving Medical Center and Weill Cornell Medical Center during the period of widespread community infection of COVID-19 in the New York City region. Data on all patients who underwent surgery between March 17, 2020 and April 15, 2020 were abstracted from electronic medical records. Patients were classified as COVID-19 positive if they had preoperative documentation of a positive COVID-19 test or documentation of a positive COVID-19 test within 21 days (before or after) their surgical procedure.11 Institutional Review Board approval was obtained from both Columbia University Vagelos College of Physicians and Surgeons and Weill Cornell Medical College.
On March 13, 2020, as the number of cases of COVD-19 in the region began increasing, hospital level guidance was put in place to limit the performance of elective surgical procedures that could be delayed for more than 2 weeks. As the number of confirmed COVID-19 cases continued to rise, revised policy guidance was implemented on March 23, 2020 to further limit procedures to only those cases that were urgent or emergent and needed to be performed within 48 hours. During this period, all surgical procedure requests were reviewed by clinical and administrative staff.
On April 1, 2020, a program to allow preoperative testing of patients for COVID-19 was initiated. The decision to perform COVID-19 testing was at the discretion of the attending surgeon. Testing for COVID-19 was performed by RT-PCR using the cobas SARS-CoV-2 test by Roche Diagnostics until April 10, 2020 after which time testing using the Xpert Xpress SARS-CoV-2 test by Cepheid became available. Patients with COVID-19 were categorized based on the date of the first laboratory confirmation of the disease in relation to the date of surgery. Among the COVID-19 positive patients, those with any of the following symptoms before surgery were classified as symptomatic: fever, myalgia, fatigue, rigor cough, dyspnea, sore throat, diarrhea, chest pain, anosmia, or loss of taste.
Clinical Characteristics and Outcomes
Demographic characteristics included date of the procedure, age, sex, race/ethnicity, body mass index, and tobacco use. Preoperative clinical characteristics including significant medical comorbidities and medications were recorded. The type of surgical procedure (ENT, cardiac, colorectal, general surgical, gynecologic, neurosurgery, orthopedic, skin/breast, thoracic, urologic, vascular, and other) and urgency (urgent vs emergent) of the procedure were noted. Emergent procedures were those operations that in general required performance within 12 hours to minimize the risk of serious morbidity or morality. Intraoperative variables included American Society of Anesthesiology (ASA) class, type of intubation (rapid sequence) and airway, type of anesthesia, vital signs, transfusion requirements, and operative time.
Perioperative complications were assessed using the criteria of the American College of Surgeon's National Surgical Quality Improvement Project (NSQIP) classification schema.12 A composite of serious complications was created and included the occurrence of any of the following: myocardial infarction, cardiac arrest, shock, respiratory failure, stroke, pneumonia, or venous thromboembolism (either pulmonary embolism or deep venous thrombosis). In addition to surgical complications, we examined return to operating room, intensive care unit (ICU) admission, transfusion (both intraoperative and postoperative) and in-hospital mortality. Given that follow-up time was short, we only measured complications during the index hospitalization.
Statistical Analysis
The clinical and demographic characteristics and outcomes were compared between the COVID-19 positive and COVID-19 negative cohorts using χ2 or Fisher exact tests. Marginal multivariable log-linear models with Poisson distributions were used to examine the association between COVID-19 status and ICU admission, serious complications, and death while controlling for other clinical and demographic variables and hospital clustering. Purposeful selection was used for model building. In the multivariable model, patient's age was included a priori in all models. Other potential confounders were investigated for their effect on the association between COVID-19 infection and each outcome. We found that sex, comorbidity, functional status, ASA class, and urgency of the procedure changed the risk estimates (>10%) for ICU admission and serious complications, and were therefore included in the multivariable models. However, for the outcome of death, we only included ASA class to avoid over-fitting the model. Parsimonious models in which only ASA classification and COVID-19 status were included are also reported. Results are reported as risk ratios (RR) with 95% confidence intervals.
Among COVID-19 positive patients, the severity of disease was classified as mild, severe or critical based on the criteria of the Chinese Center for Disease Control and Prevention.2 Mild disease including patients without pneumonia or mild pneumonia, severe disease as those with tachypnea (respiratory frequency ≥30/min), hypoxia (oxygen saturation ≤93%), a partial pressure of arterial oxygen to fraction of inspired oxygen ratio <300, or lung infiltrates of >50% within 24 to 48 hours, and critical disease as those with respiratory failure, septic shock or multiple organ dysfunction.2
We performed a number of sensitivity analyses. First, we performed a nested 1:1 case-control analysis in which each COVID-19 positive patient was matched to one COVID-19 negative control who underwent a similar surgical procedure with similar ASA status and age (within 10 years). McNemar χ2 tests were used to compare the discordant pairs on the outcomes of ICU admission, severe complications, and death. Conditional logistic regression models were developed to estimate the odds ratio (OR) of the outcomes of interest for the COVID-19 positive patients where OR were reported.
Second, we compared the perioperative outcomes of the COVID-19 positive patients to expected outcomes based on predicted probabilities of adverse events using the ACS NSQIP Surgical Risk Calculator.13 The ACS NSQIP Surgical Risk Calculator uses patient-level clinical, demographic, and procedural characteristics to estimate the predicted probability of an adverse event. The risk calculator has been tested and validated for a variety of procedures.14,15 Finally, our primary analysis allowed a window of 21 days between the date of surgery and the date of COVID-19 testing to maximize capture of asymptomatic or mildly symptomatic COVID-19 patients. We performed a sensitivity analysis in which only patients who tested positive for COVID-19 within 14 days (before or after) were considered in the COVID-19 positive cohort.
We estimated the predicted probability of adverse events among COVID-19 positive patients and compared this estimate to the observed rate of each event. All analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, North Carolina). All tests were 2-sided, and a P-value <0.05 was considered statistically significant.
RESULTS
A total of 468 subjects, including 36 (7.7%) with laboratory confirmed COVID-19 were identified (Table 1, Supplemental Table 1, http://links.lww.com/SLA/C539). The number of daily surgical procedures performed declined from 62 on March 17, 2020 to 15 on April 15, 2020. Among those with COVID-19, the diagnosis was confirmed in 55.6% preoperatively and in 44.4% postoperatively. Before the availability of preoperative COVID-19 laboratory screening, 7.7% (1 of 13) of cases were diagnosed preoperatively. After the availability of laboratory screening, 65.2% (15 of 23) of cases were diagnosed preoperatively (P = 0.0008) (Fig. 1).
TABLE 1.
Clinical and Demographic Characteristics of the Cohort Stratified by COVID-19 Status
| COVID-19 Negative | COVID-19 Positive | ||||
| N | (%) | N | (%) | P-value | |
| 432 | (92.3) | 36 | (7.7) | ||
| COVID-19 diagnosis | – | ||||
| Preoperative | – | – | 20 | (55.6) | |
| Postoperative | – | – | 16 | (44.4) | |
| COVID-19 symptoms | – | ||||
| Symptomatic | – | – | 17 | (47.2) | |
| Asymptomatic | – | – | 19 | (52.8) | |
| Age (yr) | 0.38 | ||||
| <40 | 103 | (24.4) | 24 | (66.7) | |
| 41–50 | 52 | (12.0) | 3 | (8.3) | |
| 51–60 | 73 | (16.9) | 4 | (11.1) | |
| 61–70 | 95 | (22.0) | 10 | (27.8) | |
| >70 | 109 | (25.2) | 8 | (22.2) | |
| Sex | 0.05 | ||||
| Male | 191 | (44.2) | 22 | (61.1) | |
| Female | 241 | (55.8) | 14 | (38.9) | |
| Race/ethnicity | 0.04 | ||||
| White | 175 | (40.5) | 9 | (25.0) | |
| Black | 54 | (12.5) | 5 | (13.9) | |
| Hispanic | 93 | (21.5) | 15 | (41.7) | |
| Other | 110 | (25.5) | 7 | (19.4) | |
| Insurance | 0.003 | ||||
| Private | 198 | (45.8) | 9 | (25.0) | |
| Medicare | 156 | (36.1) | 13 | (36.1) | |
| Medicaid | 68 | (15.7) | 14 | (38.9) | |
| Other | 10 | (2.3) | 0 | – | |
| Surgery | 0.22 | ||||
| ENT | 27 | (6.3) | 2 | (5.6) | |
| Cardiac | 30 | (6.9) | 1 | (2.8) | |
| Colorectal | 35 | (8.1) | 0 | – | |
| General surgery | 68 | (15.7) | 8 | (22.2) | |
| Gynecologic | 58 | (13.4) | 7 | (19.4) | |
| Neurosurgery | 32 | (7.4) | 2 | (5.6) | |
| Orthopedic | 28 | (6.5) | 3 | (8.3) | |
| Thoracic | 36 | (8.3) | 2 | (5.6) | |
| Urologic | 18 | (4.2) | 2 | (5.6) | |
| Vascular | 33 | (7.6) | 7 | (19.4) | |
| Skin and breast | 49 | (11.3) | 2 | (5.6) | |
| Other | 18 | (4.2) | 0 | – | |
| Oncologic surgery | 161 | (37.3) | 5 | (13.9) | 0.005 |
| Urgency of procedure | 0.19 | ||||
| Urgent | 330 | (76.4) | 24 | (66.7) | |
| Emergent | 102 | (23.6) | 12 | (33.3) | |
| ASA class | 0.0002 | ||||
| 1 | 25 | (5.8) | 1 | (2.8) | |
| 2 | 148 | (34.3) | 12 | (33.3) | |
| 3 | 217 | (50.2) | 13 | (36.1) | |
| 4 | 41 | (9.5) | 8 | (22.2) | |
| 5 | 1 | (0.2) | 2 | (5.6) | |
FIGURE 1.
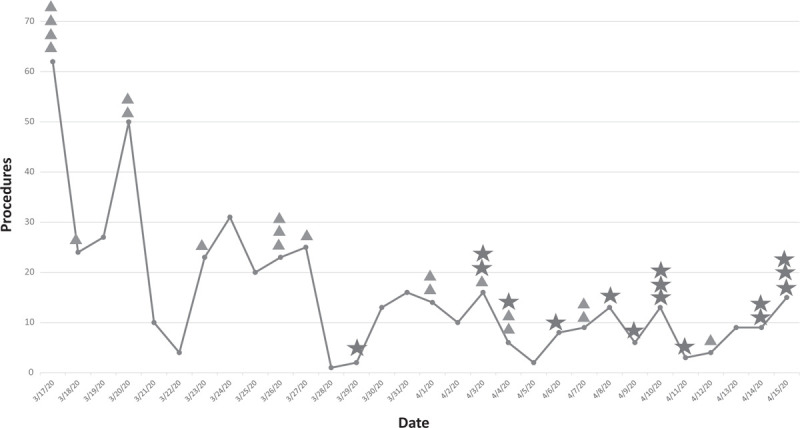
Total emergent and urgent surgical procedures from March 17 until April 15, 2020. Triangles represent COVID-19 patients diagnosed postoperatively, stars represent COVID-19 patients diagnosed preoperatively.
Patients diagnosed with COVID-19 were more often non-White (P = 0.04) and Medicaid recipients (P = 0.003) compared to those without COVID-19. Overall, 22.2% of those with COVID-19 were over 70 years of age compared to 25.2% of subjects without COVID-19 (P = 0.68). Compared to the controls, subjects ultimately diagnosed with COVID-19 more commonly had underlying COPD (8.3% vs 3.9%; P = 0.13) and coronary artery disease (30.6% vs 12.0% P = 0.003). The majority of procedures were classified as urgent (vs emergent) in both cohorts (P = 0.19) General surgical procedures followed by gynecologic procedures were the most common procedural types for both cohorts. Patients with COVID-19 were more commonly classified as ASA class 4 (22.2% vs 9.5%) or 5 (5.6% vs 0.23%) compared to those without COVID-19.
Serious perioperative complications were noted in 58.3% of COVID-19 positive patients compared to 5.6% of COVID-19 negative patients (P < 0.0001) (Table 2). Individually, cardiac arrest (16.7% vs 1.2%; P < 0.0001), shock (13.9% vs 0.9%; P = 0.0002), respiratory failure (33.3% vs 2.6%; P < 0.0001), pneumonia (50.0% vs 2.8%; P < 0.0001), acute kidney injury (22.2% vs 3.5%; P < 0.0001) and acute respiratory distress syndrome (ARDS) (8.3% vs 0%; P = 0.0004) were all more common among subjects with COVID-19. Similarly, the rates of intraoperative transfusion (16.7% vs 6.3%; P = 0.02), postoperative transfusion (22.2% vs 7.9%; P = 0.007), and ICU admission (36.1% vs 16.4%; P = 0.004) were more frequent in those with COVID-19. The rate of reoperation (2.8% vs 3.9%; P = 0.36) did not differ between the cohorts. Patients diagnosed with COVID-19 required oxygen postoperatively more than COVID-19 negative patients (50.0% vs 15.1%; P < 0.0001). The perioperative mortality rate was 16.7% among those with COVID-19 compared to 1.4% in the controls (P < 0.0001).
TABLE 2.
Perioperative Outcomes Stratified by COVID-19 Status
| COVID-19 Negative | COVID-19 Positive | ||||
| N | (%) | N | (%) | P-value | |
| Operative time (min) | 0.34 | ||||
| Median (IQR) | 83 (44–153) | 69 (46–149) | |||
| ≤60 min | 158 | (36.6) | 16 | (44.4) | |
| 61–120 min | 118 | (27.3) | 10 | (27.8) | |
| 121–180 min | 73 | (16.9) | 2 | (5.6) | |
| >180 min | 83 | (19.2) | 8 | (22.2) | |
| Estimated blood loss | 0.84 | ||||
| Median (IQR) | 20 (5–100) | 15 (5–100) | |||
| <500 cc | 402 | (93.1) | 33 | (91.7) | |
| 500–1000 cc | 23 | (5.3) | 2 | (5.6) | |
| >1000 cc | 5 | (1.2) | 1 | (2.8) | |
| Unknown | 2 | (0.5) | 0 | – | |
| Transfusion | |||||
| Intraoperative | 27 | (6.3) | 6 | (16.7) | 0.02 |
| Postoperative | 34 | (7.9) | 8 | (22.2) | 0.007 |
| Complications | |||||
| Surgical site infection | 4 | (0.9) | 2 | (5.6) | 0.06 |
| Abscess | 3 | (0.7) | 0 | – | 0.79 |
| Myocardial infarction | 0 | – | 1 | (2.8) | 0.08 |
| Cardiac arrest | 5 | (1.2) | 6 | (16.7) | <0.0001 |
| Sepsis/shock | 4 | (0.9) | 5 | (13.9) | 0.0002 |
| Respiratory failure | 11 | (2.6) | 12 | (33.3) | <0.0001 |
| Pneumonia | 12 | (2.8) | 18 | (50.0) | <0.0001 |
| Acute respiratory distress syndrome | 0 | – | 3 | (8.3) | 0.0004 |
| Deep vein thrombosis/pulmonary embolism | 4 | (0.9) | 0 | – | 0.73 |
| Stroke | 1 | (0.2) | 1 | (2.8) | 0.14 |
| Acute kidney injury | 15 | (3.5) | 8 | (22.2) | <0.0001 |
| Serious complication∗ | 26 | (6.0) | 21 | (58.3) | <0.0001 |
| Reoperation | 17 | (3.9) | 1 | (2.8) | 0.36 |
| Oxygen therapy | 65 | (15.1) | 18 | (50.0) | <0.0001 |
| ICU admission | 71 | (16.4) | 13 | (36.1) | 0.004 |
| In hospital mortality | 6 | (1.4) | 6 | (16.7) | <0.0001 |
IQR, Interquartile Range.
Serious complications defined as myocardial infarction, cardiac arrest, sepsis/shock, respiratory failure, stroke, pneumonia, or deep vein thrombosis/pulmonary embolism.
In an unadjusted model the risk ratio for ICU admission among patients with COVID-19 was 2.20 [95% confidence interval (CI), 1.27–3.79] but was not statistically significant in a multivariable model (aRR = 1.34; 95% CI, 0.86–2.09) (Table 3). In adjusted models, COVID-19 infection was associated with an increased risk of serious complications (aRR = 7.02; 95% CI, 4.96–9.92) and death (aRR = 9.29; 95% CI, 5.68–15.21). In addition to COVID-19 status, ASA classification was significantly associated with ICU admission and serious complications.
TABLE 3.
Association Between Clinical Characteristics and Adverse Outcomes
| ICU admission | Serious complication | In hospital mortality | ||||
| Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted‡ | |
| COVID-19 diagnosis | ||||||
| Negative | Referent | Referent | Referent | Referent | Referent | Referent |
| Positive | 2.20 (1.27–3.79)∗ | 1.34 (0.86–2.09) | 9.69 (9.10–10.32)† | 7.02 (4.96–9.92)† | 12.00 (9.28–15.51)† | 9.29 (5.68–15.21)† |
| Age (yr) | – | 0.99 (0.99–0.99)† | – | 1.00 (1.00–1.01) | – | – |
| Sex | ||||||
| Male | – | Referent | – | Referent | – | – |
| Female | – | 0.74 (0.66–0.84)† | – | 0.82 (0.80–0.85)† | – | – |
| Comorbidity§ | ||||||
| No | – | Referent | – | Referent | – | – |
| Yes | – | 0.87 (0.78–0.98)∗ | – | 1.98 (1.90–2.07)† | – | – |
| Functional status | ||||||
| Independent | – | Referent | – | Referent | – | – |
| Partially/totally dependent | – | 1.19 (0.82–1.75) | – | 1.64 (1.06–2.53)∗ | – | – |
| ASA class | ||||||
| 1–2 | – | Referent | – | Referent | – | Referent |
| 3 | – | 5.37 (4.41–6.54)† | – | 2.60 (1.64–4.13)† | – | 5.22 (0.51–52.91) |
| 4–5 | – | 16.23 (7.23–36.45)† | – | 4.97 (4.12–5.99)† | – | 10.89 (0.87–135.66) |
| Urgency of procedure | ||||||
| Urgent | – | Referent | – | Referent | – | – |
| Emergent | – | 1.44 (1.41–1.48)† | – | 1.62 (1.45–1.81)† | – | – |
P-value <0.05.
P-value <0.0001. Values represent risk ratios (95% confidence intervals). Age was included as a continuous variable.
Model failed to converge with additional covariates.
Comorbidity includes presence of asthma, COPD, seizure disorder, CVA/TIA, hypertension, coronary artery disease, congestive heart failure, arrhythmia, peripheral vascular disease, cancer, diabetes mellitus, chronic kidney disease, end stage renal disease, organ transplant.
Using the Chinese centers for disease control classification of disease severity, among the COVID-19 positive patients, the severity of disease was classified as mild in 16 (44.4%), severe in 6 (16.7%) and critical in 14 (38.9%). Within the COVID-19 positive cohort, symptomatic patients were more likely than asymptomatic patients to experience a severe complication (94.1% vs 26.3%; P < 0.0001) and in-hospital mortality (23.5% vs 10.5%; P = 0.03). When stratified by ASA classification, COVID-19 positive patients were at increased risk of severe complications regardless of preoperative ASA category (Supplemental Tables 2 and 3, http://links.lww.com/SLA/C539). The risk ratio for death for COVID-19 positive compared to COVID-19 negative patients was 16.69 (95% CI, 4.08–68.26) for ASA 3 patients and 6.30 (95% CI, 0.70–56.35) for ASA class 4–5 patients (Supplemental Table 3, http://links.lww.com/SLA/C539). Similarly, when stratified by urgency of the procedure, COVID-19 patients who underwent both urgent and emergent procedures were at increased risk for serious complication. The risk ratio for death for COVID-19 positive compared to COVID-19 negative patients was 55.00 (95% CI, 17.22–175.66) for those who underwent urgent surgery and 3.40 (95% CI, 0.68–17.03) among those who underwent emergent operations. There were no statistically significant differences in the rates of serious complications or mortality between patients diagnosed with COVID-19 preoperatively compared to those diagnosed postoperatively.
These findings were robust in a series of sensitivity analyses. In a matched analysis, matches were identified for 97.2% of the COVID-19 positive patients (Table 4). The matched cohort was well balanced for ASA classification, procedure type, urgency of the procedure, sex, and age. The proportion of serious complications was 57.1% in COVID-19 positive patients compared to 14.3% in the COVID-19 negative cohort (P = 0.0006; OR = 8.50; 95% CI, 2.02–75.85) whereas the mortality rate was 17.1% versus 5.7%, respectively (P = 0.025; OR = 6.73; 95% CI 1.22-infinity). The 1 COVID-19 patient that was not matched experienced a serious complication but was not admitted to the ICU and was discharged from the hospital alive. Based on the ACS NSQIP Surgical Risk Calculator the predicted probability of serious complications in the COVID-19 patients was 12.4% whereas the observed risk in the cohort was 58.3%. Similarly, the predicted probability of death was 4.9% whereas the observed mortality rate was 16.7%. These findings were largely unchanged in another sensitivity analysis in which the window for COVID-19 positivity was limited to detection within 14 days of surgery (Supplemental Table 4, http://links.lww.com/SLA/C539).
TABLE 4.
Matched Analysis of COVID-19 Positive and Negative Surgical Patients
| COVID-19 Positive | COVID-19 Negative | ||
| (N = 35) | (N = 35) | P-value | |
| Age median (IQR) | 61 (38–70) | 60 (44–72) | 0.96 |
| Male | 21 (60.0) | 17 (48.6) | 0.34 |
| ASA | 0.46 | ||
| 1–2 | 12 (34.3) | 12 (34.3) | |
| 3 | 13 (36.1) | 17 (48.2) | |
| 4–5 | 10 (27.8) | 6 (17.1) | |
| Any comorbidity | 26 (74.3) | 24 (68.6) | 0.60 |
| Functional status | 0.72 | ||
| Independent | 30 (85.7) | 31 (88.6) | |
| Dependent | 5 (14.3) | 4 (11.4) | |
| Urgency of procedure | 1.00 | ||
| Emergent | 12 (34.3) | 12 (34.3) | |
| Urgent | 23 (65.7) | 23 (65.7) |
| McNemar's Chi-Square Test | Exact conditional Logistic Regression OR (95% CI) | |||
| ICU admission | 13 (37.1) | 7 (20.0) | 0.0833 | 3.00 (0.75–17.23) |
| Serious complications | 20 (57.1) | 5 (14.3) | 0.0006 | 8.50 (2.02–75.85)∗ |
| Death | 6 (17.1) | 1 (2.9) | 0.0253 | 6.73 (1.22-infinity) |
A nested 1:1 case-control study was conducted as the sensitivity analysis where each COVID-19 positive patient was matched to 1 negative control patient with a similar surgical procedure and ASA status as well as the difference of age within 10 years. McNemar Chi-Square test was used to compare the discordant pairs on the outcomes of ICU admission, severe complications, and death. Conditional logistic regression models were employed to estimate the odds ratio of outcomes of interest for the COVID-19 positive patients compared to negative controls. IQR, Interquartile Range.
P-value <0.05.
DISCUSSION
These findings suggest that COVID-19 poses a substantial risk for patients undergoing urgent and emergent surgical procedures. COVID-19 is associated with significantly increased risk for serious perioperative morbidity and mortality. Nearly half of the cases of COVID-19 in patients who undergo surgery were not identified until after the surgical intervention.
To date, there are limited data describing outcomes of patients with COVID-19 or other coronavirus-related infections who undergo surgery.7,9,16,17 A series of 34 patients from China with occult COVID-19 infections at the time of surgery reported dismal outcomes including development of pneumonia in all patients, ARDS in 32%, shock in 29% and a perioperative mortality rate of 21%.7 Prior cases and case series of patients with H1N1 influenza who underwent surgery during the pandemic of 2009 suggest that these patients also seemed to be at increased risk of developing ARDS.16,17 We noted that patients with COVID-19 were at a substantially higher risk for perioperative morbidity and mortality. Within our cohort of COVID-19 positive patients, 58% experienced serious complications and the perioperative mortality rate was 17%.
Surgery seems to exacerbate the disease course of COVID-19. Severe or critical COVID-19-associated disease was identified in 56% of patients in our series, a rate over 2 and half times more frequent than population-based estimates from China.2 The vast majority of prior reports have found that most COVID-19 infections result in only mild disease. A number of factors including the physiologic stress of surgery, the need for mechanical ventilation, and the increased risk of other infections could all theoretically exacerbate the course of COVD-19 in patients undergoing operative interventions. Our cohort included only patients who underwent urgent and emergent procedures, a group that is at significant risk for adverse events. However, even compared to controls undergoing similar operations during the same time period and after adjusting for other perioperative risk factors, those with COVID-19 had higher morbidity and mortality.
Epidemiologic data suggests that a significant number of patients with COVID-19 infections are asymptomatic.10 A cross sectional sample of consecutive women admitted to a labor and delivery unit in New York found that 15% of patients who were positive for COVID-19 and that 88% of these women were asymptomatic.10 Among patients who develop symptoms, the median incubation period from exposure to the onset of symptoms is just over 5 days.11 There is thus a significant risk that surgical patients without overt symptoms may harbor COVID-19 in areas where broad community spread of the virus has occurred.
Not surprisingly, nearly half of the subjects in our series had unrecognized COVID-19 at the time of surgery. The poor outcomes for COVID-19 patients we noted highlight the importance of identifying carriers before performing a surgical intervention. Accurate identification of COVID-19 carriers allows delaying surgery if feasible or implementation of protocols for known COVID-19 patients when surgery cannot be delayed.18–20 Given the significant risk of transmission to operating room personnel, knowledge of COVID-19 status may also help to reduce exposures for healthcare workers.21 Given these considerations, preoperative COVID-19 testing may be prudent in patients scheduled for urgent procedures when feasible.10,19
We acknowledge a number of important limitations. First, COVID-19 testing was not performed in all patients in the cohort. Our experience spans the initial phase of rapid spread of COVID-19 in the community and before the ability to perform preoperative testing in all subjects. Although there may have been some unrecognized COVID-19 patients in the control group, the evolution of the disease will likely be similar in other regions. Second, at the time of data lock 7.3% of the controls and 36.1% of COVID-19 patients remained hospitalized. We felt that timely reporting of our data was paramount. Further, any bias would likely result in underreporting of adverse outcomes in the COVID-19 positive cohort given that a much higher percentage of these patients remained hospitalized. Third, patients in the COVID-19 cohort had more significant underlying comorbidity. However, our findings of increased morbidity and mortality in the COVID-19 subjects were of a large magnitude and noted in a variety of sensitivity analyses including after multivariable adjustment, matching, and comparison to estimates from the NSQIP surgical calculator. Fourth, as with any observational study, decision making was at the discretion of individual surgeons and not standardized. Forth, although cases were reviewed after March 23, surgical urgency and whether to proceed with an operation was at the discretion of the attending surgeon. Lastly, although an extensive review of all medical records was performed, we cannot exclude the possibility that some clinical parameters including symptoms and comorbidities were underreported.
In sum, these data suggest that patients with COVID-19 who undergo urgent and emergent surgery are at increased risk for perioperative morbidity and mortality. These findings have a number of important clinical implications. First, as a significant number of patients with COVID-19 are asymptomatic and only identified postoperatively, symptom-based screening lacks appropriate sensitivity to accurately detect these patients. As such, institutional protocols that include universal, preoperative laboratory screening may increase the recognition of COVID-19 carriers. Second, given the extremely poor perioperative outcomes of subjects with COVID-19, every effort should be made to utilize nonoperative therapies or to delay surgery whenever feasible. Given the increasing burden of COVID-19, protocols for the detection and management of those at risk for the disease should be implemented to optimize outcomes.
Acknowledgments
Dr. Wright has served as a consultant for Clovis Oncology and received research funding from Merck.
Footnotes
A.K., Z.N.Z., and J.W. contributed equally to work.
The authors declare no conflict of interests.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.annalsofsurgery.com).
REFERENCES
- 1.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA 2020; 323:1574–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in china: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA 2020; 323:1239–1242. [DOI] [PubMed] [Google Scholar]
- 3.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. WHO Director-General's opening remarks at the media briefing on COVID-19-11 March 2020. Available at: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020. Accessed April 10, 2020. [Google Scholar]
- 5. American College of Surgeons. COVID-19: Guidance for Triage of Non-Emergent Surgical Procedures. Available at: https://www.facs.org/covid-19/clinical-guidance/triage. Accessed April 10, 2020. [Google Scholar]
- 6. American College of Surgeons. COVID-19: Recommendations for Management of Elective Surgical Procedures. Available at: https://www.facs.org/covid-19/clinical-guidance/elective-surgery. Accessed April 10, 2020. [Google Scholar]
- 7.Lei S, Jiang F, Su W, et al. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. EClinicalMedicine 2020; 21:100331.doi: 10.1016/j.eclinm.2020.100331. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hogan BV, Peter MB, Shenoy HG, et al. Surgery induced immunosuppression. Surgeon 2011; 9:38–43. [DOI] [PubMed] [Google Scholar]
- 9.Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet 2020; 395:809–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sutton D, Fuchs K, D’Alton M, et al. Universal screening for SARS-CoV-2 in women admitted for delivery. N Engl J Med 2020; 382:2163–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lauer SA, Grantz KH, Bi Q, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med 2020; 172:577–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. ACS NSQIP. User guide for the 2017 ACS NSQIP participant use data file (PUF).Available at: https://www.facs.org/-/media/files/quality-programs/nsqip/nsqip_puf_userguide_2017.ashx. Accessed April 14, 2020. [Google Scholar]
- 13. ACS NSQIP Surgical Risk Calculator. Available at: https://riskcalculator.facs.org/RiskCalculator/. Accessed April 17, 2020. [Google Scholar]
- 14.Vaziri S, Wilson J, Abbatematteo J, et al. Predictive performance of the American College of Surgeons universal risk calculator in neurosurgical patients. J Neurosurg 2018; 128:942–947. [DOI] [PubMed] [Google Scholar]
- 15.Eisenstein S, Stringfield S, Holubar SD. Using the National Surgical Quality Improvement Project (NSQIP) to perform clinical research in colon and rectal surgery. Clin Colon Rectal Surg 2019; 32:41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galbraith JG, Butler JS, Pead M, et al. H1N1 infection in emergency surgery: a cautionary tale. Int J Surg Case Rep 2010; 1:4–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Person B, Bahouth H, Brauner E, et al. Surgical emergencies confounded by H1N1 influenza infection - a plea for concern. World J Emerg Surg 2010; 5:6.doi:10.1186/1749-7922-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurihara H, Bisagni P, Faccincani R, et al. Covid-19 outbreak in Northern Italy: viewpoint of the Milan Area Surgical Community. J Trauma Acute Care Surg 2020; 88:719–724. [DOI] [PubMed] [Google Scholar]
- 19.Zheng MH, Boni L, Fingerhut A. Minimally invasive surgery and the novel coronavirus outbreak: lessons learned in China and Italy. Ann Surg 2020; 272:e5–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. American College of Surgeons. COVID-19: considerations for optimum surgeon protection before, during, and after operation. Available at: https://www.facs.org/covid-19/clinical-guidance/surgeon-protection. Accessed April 15, 2020. [Google Scholar]
- 21.Heinzerling A, Struckey MJ, Scheuer T, et al. Transmission of COVID-19 to health care personnel during exposures to a hospitalized patient-Solano county, California, February 2020. MMWR Morb Mortal Wkly Rep 2020; 69:472–476. [DOI] [PMC free article] [PubMed] [Google Scholar]


