Supplemental Digital Content is Available in the Text.
Using magnetic resonance spectroscopy in vivo, we show GABA and glutamate alterations in children aged 7 to 13 years with migraine, which are associated with migraine characteristics.
Keywords: Migraine, MR spectroscopy, Glutamate, Pediatric, Macromolecule-suppressed GABA, GABA-Edited MRS
Abstract
Migraine is one of the top 5 most prevalent childhood diseases; however, effective treatment strategies for pediatric migraine are limited. For example, standard adult pharmaceutical therapies are less effective in children and can carry undesirable side effects. To develop more effective treatments, improved knowledge of the biology underlying pediatric migraine is necessary. One theory is that migraine results from an imbalance in cortical excitability. Magnetic resonance spectroscopy (MRS) studies show changes in GABA and glutamate levels (the primary inhibitory and excitatory neurotransmitters in the brain, respectively) in multiple brain regions in adults with migraine; however, they have yet to be assessed in children with migraine. Using MRS and GABA-edited MRS, we show that children (7-13 years) with migraine and aura had significantly lower glutamate levels in the visual cortex compared to controls, the opposite to results seen in adults. In addition, we found significant correlations between metabolite levels and migraine characteristics; higher GABA levels were associated with higher migraine burden. We also found that higher glutamate in the thalamus and higher GABA/Glx ratios in the sensorimotor cortex were associated with duration since diagnosis, i.e., having migraines longer. Lower GABA levels in the sensorimotor cortex were associated with being closer to their next migraine attack. Together, this indicates that GABA and glutamate disturbances occur early in migraine pathophysiology and emphasizes that evidence from adults with migraine cannot be immediately translated to pediatric sufferers. This highlights the need for further mechanistic studies of migraine in children, to aid in development of more effective treatments.
1. Introduction
Migraine often begins in childhood, and roughly 20% of sufferers experience their first attack before 5 years of age.54 Early intervention can decrease migraine frequency, with those receiving earlier interventions more likely to achieve remission.25 However, treatment strategies for children are limited, in part due to limited knowledge about pediatric migraine biology.46 Migraine is often managed similarly in children as adults, despite evidence that children with migraine present with different symptoms.28 Standard medications to prevent migraine in adults have shown to be no more effective than placebo in children, and may carry side effects.28,30,54 To improve treatments for children, we need to understand the underlying biology of pediatric migraine.
There is compelling evidence that adult migraine results from an imbalance of excitation/inhibition in the brain, which changes cyclically until a migraine occurs (known as the migraine cycle).11,12 During the interictal period, cortical excitability increases proportionally with time until the next attack. During the ictal period, or shortly thereafter, the brain returns to baseline activity and begins the cycle again.11,12 Neurophysiological studies suggest this cortical hyperexcitability results from abnormal thalamic control,9 resulting in altered communication in thalamocortical networks,48 which underlie important processes in multisensory integration; this altered communication is associated with clinical migraine symptoms.9,24 Excitability of the sensory cortices is set by activity in these thalamocortical loops. Between attacks, there is evidence that adults with migraine have reduced function in thalamocortical connectivity,10 resulting in increased excitability in sensory and visual cortices. For example, the sensorimotor cortex of migraineurs shows enhanced responses to sensory stimuli, and the degree of enhancement correlates with headache frequency.50 Similarly, transcranial magnetic stimulation (TMS) studies involving adults with migraine indicate visual cortex hyperexcitability.5
The primary neurochemicals associated with inhibition and excitation in the brain are GABA and glutamate, respectively. These can be measured in vivo noninvasively using magnetic resonance spectroscopy (MRS). A recent review of MRS studies showed that, in adults with migraine, GABA and glutamate levels are increased in multiple brain areas.52 For example, adults with migraine show increased glutamate2,19,45,55 and increased GABA levels1,4 in the visual cortex, and increased glutamate in the thalamus.2
Despite indirect evidence of abnormal cortical excitability in children with migraine,39,44 GABA and glutamate levels remain uninvestigated. The GABA/glutamate ratio may be used to index the inhibitory/excitatory balance and may show a stronger effect than changes in either neurochemical alone. Using advanced MRS methods, this study compares GABA and glutamate in the thalamus, sensorimotor, and visual cortices of children with and without migraine. Although the most predominant symptom of migraine is severe headache, 20% of migraine sufferers experience visual disturbances, known as aura, which is associated with increased responsiveness of the visual cortex.26 Subsequently, GABA and glutamate levels in the visual cortex were compared between children with migraine with and without aura, and children without migraine. Finally, this study explores the association between these neurochemical levels and migraine characteristics to improve our understanding of pediatric migraine.
2. Materials and methods
2.1. Ethics statement
The study protocol was approved by the Conjoint Health Research Ethics Board (CHREB), University of Calgary. All the study participants provided informed assent and their parents provided informed consent at time of enrollment.
2.2. Participants
Thirty-five children with migraine (migraine group) aged 7 to 13 years with a diagnosis of migraine from their family physician were recruited from the Vi Riddell Pain Clinic at the Alberta Children's Hospital and the local community. Participants were included if they had received a physician diagnosis of migraine, which was confirmed using the ICHD-III beta diagnostic criteria,32 with no other accompanying neurological, psychiatric, or systematic disorders (eg, Attention Deficit Hyperactivity Disorder (ADHD), autism), they met the standard Magnetic Resonance Imaging (MRI) safety criteria (eg, no metal implants or devices), and were not taking preventative medications such as triptans.
Thirty-one age- and sex-matched children without migraine (control group) were recruited using the Healthy Infants and Children Clinical Research Program (HICCUP). The same exclusion criteria of no neurological, psychiatric, or systemic disorders and standard MRI safety criteria were applied for the control participants; in addition, control participants were excluded if they had any history of migraine or other headache disorder.
2.3. Migraine diary
The parents of children with migraine were asked to keep a migraine diary for 30 days preceding their appointment and 7 days after, which was sent to their computer or mobile device. In this diary, they were asked to record if their child had had a migraine that day and, if so, the length and pain level of the attack using the Wong–Baker FACES pain rating scale49 from 1 to 10, along with how they treated the migraine. If the child did not have a migraine in the 7 days after the appointment, they were asked to provide the date of the following migraine. Using the migraine diaries, the “position in the migraine cycle” at the time of scanning was calculated as the number of days since the last migraine divided by the number of days between the last migraine and the next. A higher number represents being further along in the cycle and, subsequently, closer to the next migraine, while accounting for interindividual differences in overall length of the migraine cycle. Children with migraine were excluded if they did not experience a migraine in the 30 days preceding their appointment, or if they experienced a migraine on the day of the appointment.
2.4. Questionnaires
All participants completed the following questionnaires: Headache Impact Test (HIT-6),27 Pediatric Migraine Disability Assessment (PedMIDAS),23 Revised Children's Anxiety and Depression Scale (RCADS) short version,14 Puberty Status Scale,6 and the Edinburgh Handedness Scale.35 The HIT-6 is a 6-item self-report survey that assesses the negative impact of headaches on normal daily activity. Responses range from 36 to 78, with higher scores representing more negative impact. PedMIDAS is a pediatric version of the self-report Migraine Disability Assessment (MIDAS) questionnaire commonly used in adults, and has been validated in children and adolescents ranging from 6 to 18.23 Responses range from 0 to 90 with higher scores representing more negative impact. The RCADS is a self-report measure developed to assess anxiety and depression symptoms among children and adolescents, and has been validated in children and adolescents ranging from 6 to 18.8 Responses range from 0 to 45 for anxiety and 0 to 30 for depression, with higher scores representing more symptoms. The Pubertal Status Scale is a self-report measure based on the Tanner pubertal staging, and scores are categorised into the following stages: prepubertal, early pubertal, midpubertal, late pubertal, and postpubertal.6
2.5. MR acquisition
Scanning was performed on a 3T GE 750w MR scanner using a 32-channel head coil. A T1-weighted anatomical image was collected for voxel placement (BRAVO; TE/TR = 2.7/7.4 ms, 1 mm3 isotropic voxels). The 3 × 3 × 3 cm3 voxels were placed in the thalamus (midline centred), right sensorimotor cortex (the hand-knob of the motor cortex was used for initial localization and then the voxel was centered between the precentral gyrus and postcentral gyrus; the voxel was then rotated such that the coronal and sagittal planes aligned with the cortical surface53), and the occipital cortex (as close to aligning with the parieto-occipital sulcus as possible, without including cerebellum, midline centred; Fig. 1). GABA-edited spectroscopy data were collected using macromolecule-suppressed MEGA-PRESS (TR/TE = 1800/80 ms, 20 ms editing pulses at 1.9 and 1.5 ppm, 256 averages) from each brain area. Separate PRESS data (TR/TE = 1800/35 ms, 64 averages) were also acquired from each region to quantify glutamate.3
Figure 1.
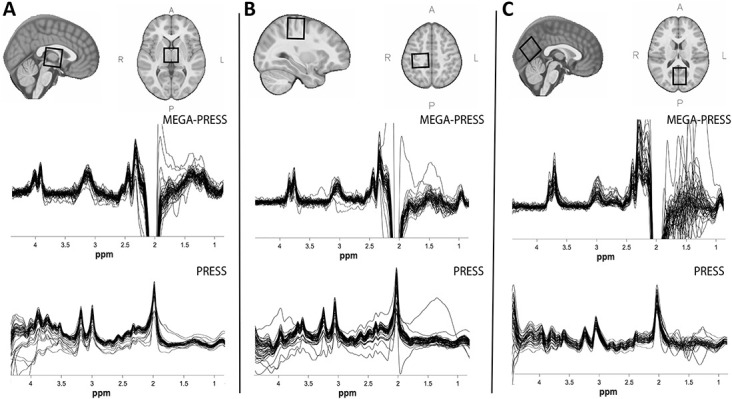
Example voxel placement (top row) and overlay of all (migraine and control) MEGA-PRESS (middle row) and PRESS spectra (bottom row) for (A) thalamus, (B) sensorimotor cortex, and (C) visual cortex.
2.6. Magnetic resonance spectroscopy analysis
MEGA-PRESS data were analysed using Gannet3.1,15 which included the following preprocessing steps: coil combination, frequency and phase correction, apodization, and down-weighting of motion-corrupted averages. Tissue correction, including tissue-specific water visibility, and T1 and T2 relaxations of both water and metabolites, was performed using voxel tissue fractions obtained by generating a subject-specific voxel mask registered to each individual tissue segmented T1 anatomical image.21 GABA was quantified relative to water.
PRESS data were preprocessed with the FID-A42 toolbox using the following preprocessing steps: coil combination, removal of motion-corrupted averages, frequency drift correction, and zero-order phase correction.34 LCModel Version 6.3-1J40 was used to apply eddy current correction and quantification relative to water. Basis sets for quantification (including alanine, aspartate, glycerophosphocholine, phosphocholine, creatine, phosphocreatine, GABA, glutamate, glutamine, lactate, inositol, N-acetyl aspartate, N-acetylaspartylglutamate, scyllo-inositol, glutathione, glucose, and taurine) were simulated using the FID-A toolbox based on exact sequence timings and RF pulse shapes. Metabolite values were corrected for tissue composition, including tissue-specific water visibility, and T1 and T2 relaxations of both water and metabolites17 using the tissue fractions generated from Gannet3.1. Glutamate was quantified both on its own (Glu) and as a combination (Glx) of glutamate and its precursor, glutamine. Glutamate and glutamine overlap on the spectra due to their similar chemical compositions; subsequently, it is difficult to separate the individual signals. Data quality was assessed by visual inspection and metabolite linewidth, and spectra with a linewidth over 0.1 ppm were excluded.
2.7. Statistical analysis
Statistical analysis was conducted using SPSS (IBM. 2017. IBM SPSS Statistics for Macintosh, Version 25.0. Armonk, NY: IBM). Demographic data (Table 1) were compared between children with and without migraine using independent t-tests for age, and χ2 tests for sex and pubertal status. Clinical migraine scores and anxiety and depression scores were compared between groups using analyses of covariance (ANCOVAs) with age included as a covariate. In addition, voxel tissues fractions were compared across groups using ANCOVAs with age included as a covariate (no significant differences were seen in voxel tissue fractions; see Supplemental Table 1, available at http://links.lww.com/PAIN/B134).
Table 1.
Group demographics and questionnaire results (mean and SD).
| Migraine | Control | Comparison | |
|---|---|---|---|
| N | 29 (12 female) | 27 (14 female) | X2 (1, 57) = 0.414, P = 0.600 |
| Age (y) | 10.19 (1.3) | 9.9 (1.5) | t(55) = −1.009, P = 0.317 |
| HIT-6 | 59.07 (7.2) | 43.5 (4.8) | F(1, 53) = 80.54, P < 0.001 |
| PedMIDAS | 21.5 (19.8) | 2.0 (3.6) | F(1, 54) = 24.87, P < 0.001 |
| RCADS depression | 7.6 (4.5) | 6.1 (4.2) | F(1, 54) = 1.754, P = 0.191 |
| RCADS anxiety | 10.6 (7.5) | 7.5 (5.1) | F(1, 54) = 3.884, P = 0.053 |
| Pubertal status (N) | X2 (3, 57) = 4.488, P = 0.213 | ||
| Pre | 18 | 10 | |
| Early | 7 | 10 | |
| Mid | 4 | 7 | |
| Post | 0 | 0 | |
| No. of migraines in 30 d | 6.3 (6.9) | N/A | N/A |
| Range | 1-30 | N/A | N/A |
| Disease duration (y) | 3.9 (2.5) | N/A | N/A |
HIT, Headache Impact Test; PedMIDAS, Pediatric Migraine Disability Assessment; RCADS, Revised Children's Anxiety and Depression Scale.
GABA, Glx, glutamate, and GABA/Glx from all 3 brain areas were compared between children with migraine and controls using ANCOVAs with age included as a covariate. In addition, data from the visual cortex of children with migraine were also subdivided into 2 groups based on whether the child experienced visual aura symptoms (migraine with aura and migraine without aura) due to known functional and structural differences between the 2 migraine subtypes.26 A 3-group ANCOVA was then used to compare GABA, Glx, glutamate, and GABA/Glx from the visual cortex between migraine with aura, migraine without aura, and controls.
Partial correlation analyses, controlling for age, were used to test the relationship between metabolite levels and migraine characteristics within the migraine group (migraine with aura + migraine without aura). Specifically, the relationship between metabolite levels and (1) how long the child had suffered from migraines (years with migraines), (2) migraine burden as measured by the PedMIDAS, and (3) the position in the migraine cycle.
In a secondary analysis, N-acetyl aspartate, creatine, choline, and inositol were compared between children with and without migraine using ANCOVAs with age as a covariate. No significant differences were detected between groups (see Supplemental Table 2, available at http://links.lww.com/PAIN/B134).
3. Results
3.1. Participants
Participant demographic data and questionnaire results are shown in Table 1; the final sample sizes were 29 children in the Migraine group and 27 children in the Control group. From the 35 children with migraine recruited, 2 children were excluded because they did not experience a migraine during the past 30 days and 3 children were excluded because they experienced a migraine on the day of the scan. Imaging data were not acquired from 1 child with migraine. From the 31 age- and sex-matched controls recruited, 1 child was excluded because migraines developed shortly after participating in the study, 1 child was excluded due to an ADHD diagnosis, and imaging data were not acquired for 2 control children. Some MRS data were not collected due to children requesting to get out of the scanner, and individual voxel data of poor quality was removed on a case-by-case basis. Table 2 shows the final number of spectra for each voxel included for each analysis. There were no significant differences in data quality (measured using linewidth) between the 2 groups.
Table 2.
Number of participants included for each brain region analysis, and group mean linewidth.
| Migraine | Control | |||||
|---|---|---|---|---|---|---|
| Number scanned | Number retained | Mean linewidth (Hz) | Number scanned | Number retained | Mean linewidth (Hz) | |
| Thalamus | ||||||
| GABA | 29 | 25 | 10.41 | 27 | 27 | 9.77 |
| Glu | 29 | 27 | 9.02 | 27 | 27 | 8.85 |
| Sensorimotor cortex | ||||||
| GABA | 29 | 26 | 9.61 | 27 | 26 | 9.14 |
| Glu | 29 | 27 | 8.73 | 27 | 27 | 7.03 |
| Visual cortex | ||||||
| GABA | 26 | 24 | 11.20 | 27 | 27 | 11.09 |
| Glu | 26 | 25 | 10.22 | 26 | 26 | 10.59 |
3.2. Group comparisons
3.2.1. Thalamus
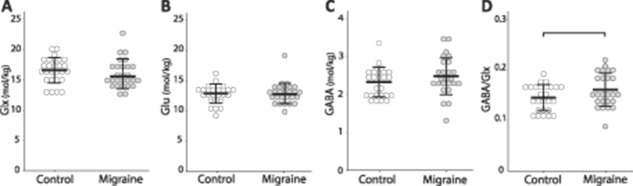
There were no significant differences in Glx (F(1, 51) = 0.241, P = 0.626), Glu (F(1, 51) = 0.001, P = 0.974), or GABA (F(1, 49) = 0.431, P = 0.515) levels in the thalamus between the 2 groups. There was a trend towards higher GABA/Glx ratios in Migraine (F(1, 48) = 2.950, P = 0.092), mainly driven by higher GABA levels (Fig. 2).
Figure 2.
Group comparisons of (A): Glx levels (F(1, 51) = 0.241, P = 0.626); (B): Glu levels (F(1, 51) = 0.001, P = 0.974); (C) GABA levels (F(1, 49) = 0.431, P = 0.515); and (D) GABA/Glx ratios (F(1,48) = 2.950, P = 0.092) in the thalamus. Each circle represents an individual subject.
3.2.2. Sensorimotor cortex
There were no significant differences between groups in any of the metabolites in the sensorimotor cortex (Glx: F(1, 50) = 0.870, P = 0.356; Glu: F(1, 50) = 1.047, P = 0.311; GABA: F(1, 49) = 0.927, P = 0.340; GABA/Glx: F(1, 47) = 1.258, P = 0.268; Fig. 3).
Figure 3.
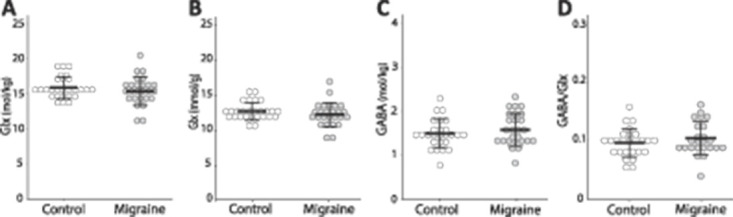
Group comparisons of (A) Glx levels (F(1,50) = 0.870, P = 0.356); (B) Glu levels (F(1, 50) = 1.047, P = 0.311); (C): GABA levels (F(1, 49) = 0.927, P = 0.340); and (D) GABA/Glx ratios (F(1, 47) = 1.258, P = 0.268) in the sensorimotor cortex. Each circle represents an individual subject.
3.2.3. Visual cortex
There were no significant differences in Glx (F(1, 48) = 1.989, P = 0.165), Glu (F(1, 48) = 2.812, P = 0.100), GABA (F(1, 48) = 0.582, P = 0.449), or GABA/Glx ratios (F(1, 47) = 1.276, P = 0.264; Fig. 4) in the visual cortex between the 2 groups.
Figure 4.
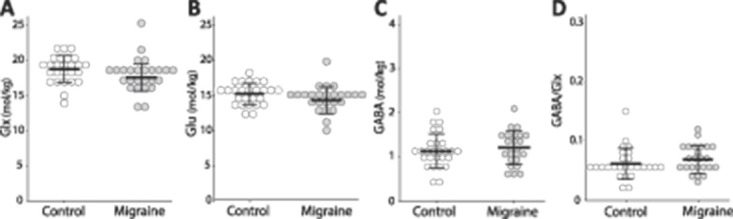
Group comparisons in (A): Glx levels (F(1, 48) = 1.989, P = 0.165); (B): Glu levels (F(1, 48) = 2.812, P = 0.100); (C): GABA levels (F(1, 48) = 0.582, P = 0.449); and (D) GABA/Glx ratios (F(1, 47) = 1.276, P = 0.264) in the visual cortex. Each circle represents an individual subject.
3.2.3.1. Aura
Of the 25 children with migraine included in the visual cortex analyses, 9 reported aura either before or during their migraine, 15 reported no aura, and 1 participant did not specify (who was removed from the following analysis). There was a significant effect of group on Glu levels (F(2, 46) = 3.317, P = 0.045) when comparing children with migraine and aura, children with migraine without aura, and controls. Levels of Glu were significantly lower in migraine with aura (P = 0.022) compared to Controls (Fig. 5). There were no significant differences in GABA (F(2, 46) = 0.458, P = 0.635), Glx (F(2, 46) = 2.798, P = 0.071), or GABA/Glx ratios (F(2, 45) = 1.024, P = 0.367) in the visual cortex between the 3 groups.
Figure 5.
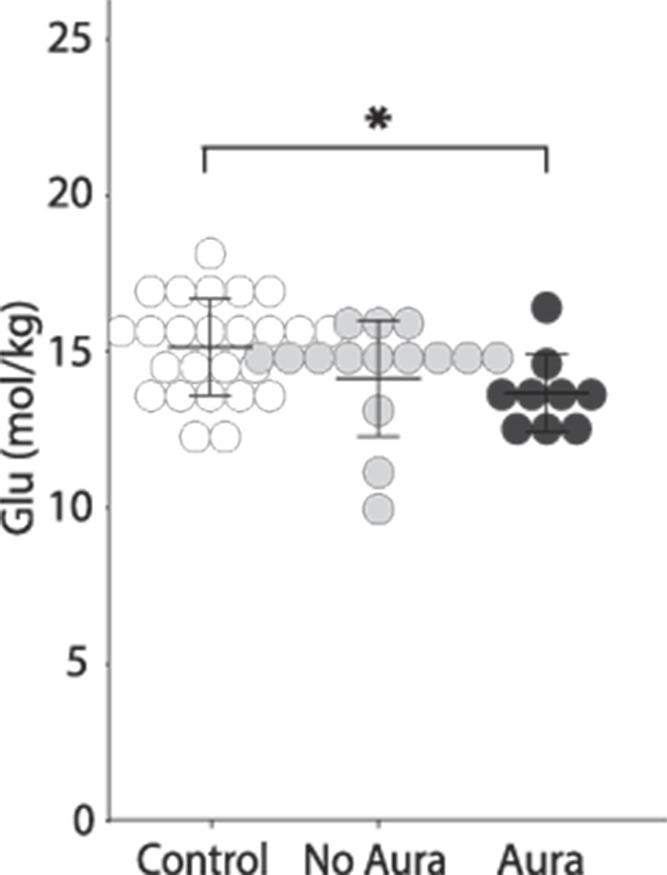
Glu levels in Migraine with and without aura, and Controls. Migraine with aura had significantly lower glutamate levels in the visual cortex compared to Control (P = 0.019). There was no significant difference between Migraine without aura and control (P = 0.135) or between the Migraine with and without aura groups (P = 0.324).
3.3. Migraine characteristics
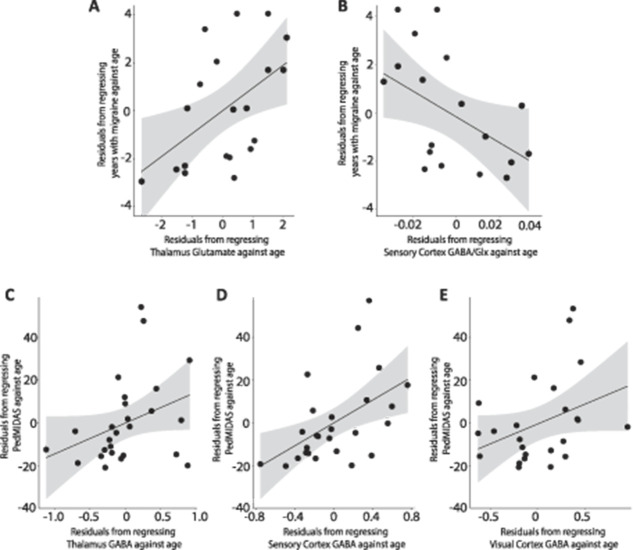
In Migraine, higher glutamate levels in the thalamus (r(17) = 0.514, P = 0.025, Fig. 6A) and higher GABA/Glx ratios in the sensorimotor cortex (r(15) = −0.562, P = 0.019, Fig. 6B) were associated with a greater number of years with migraine.
Figure 6.
Associations between migraine characteristics and neurochemical levels. (A) Positive correlation between Glu in the thalamus and the number of years with migraine, controlling for age (r(18) = 0.532, P = 0.016). (B) Negative correlation between the GABA/Glx ratio in the thalamus and the number of years with migraine, controlling for age (r(17) = −0.456, P = 0.05). (C) Positive association between GABA in the thalamus and the PedMIDAS score, controlling for age (r(23) = 0.370, P = 0.068). (D) Positive correlation between GABA in the sensorimotor and the PedMIDAS score, controlling for age (r(24) = 0.514, P = 0.007). (E) Positive association between GABA in the visual cortex and the PedMIDAS score, controlling for age (r(22) = 0.361, P = 0.084). Gray shading indicates the 95% confidence intervals on the partial correlations.
In Migraine, higher levels of GABA in the sensorimotor cortex were associated with higher PedMIDAS scores (r(23) = 0.516, P = 0.008, Fig. 6D), indicating children with higher GABA levels were more impacted by their migraines. This association was also seen in the thalamus and visual cortex but did not reach statistical significance. (thalamus: r(22) = 0.365, P = 0.080, visual cortex: r(21) = 0.352, P = 0.099, Figs. 6C and E).
3.3.1. Migraine cycle
In Migraine, lower GABA (and, consequently, GABA/Glx) levels in the thalamus were associated with being further along in the migraine cycle and subsequently, closer to the next migraine (GABA: r(15) = −0.507, P = 0.038, Fig. 7; GABA/Glx: r(15) = −0.559, P = 0.020).
Figure 7.
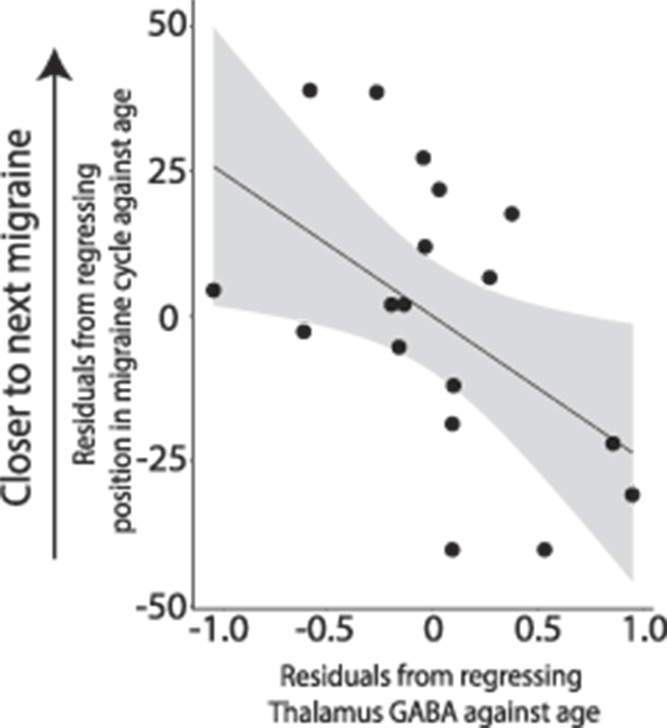
Negative correlation between levels of GABA in the thalamus and the position of the child in their migraine cycle, controlling for age (r(16) = −0.499, P = 0.035).
4. Discussion
To the best of our knowledge, this is the first study to measure GABA and glutamate in pediatric migraine and one of only a few studies on migraine to consider multiple voxel locations. We found (1) migraine with aura had significantly lower Glu levels in the visual cortex compared to controls; (2) metabolite levels in the migraine group correlate with migraine characteristics; and (3) the migraine group had a higher GABA:Glx ratio in the thalamus as compared to the control group (although this did not reach statistical significance), which was primarily driven by an increase in GABA.
It has been suggested that increased glutamate is a driving force behind migraine, which is supported by the literature showing increased Glu19,55 or Glx2,7 in migraine in adults, including the analysis of populations of migraine with or without aura. In the one other study directly comparing glutamate levels in the visual cortex between migraine with and without aura,55 the migraine without aura group had significantly higher Glu levels than the control group. Although the migraine with aura group also had higher levels than controls, this did not reach statistical significance. By contrast, we found a decrease in glutamate levels in the visual cortex of migraine with aura, and a trend towards decreased glutamate in migraine without aura compared to controls.
Aura in children is difficult to assess but is generally thought to have a similar presentation in adults and children.28 Aura is thought to be caused by hypoperfusion in the occipital lobe,18 and those who suffer from migraine with aura show higher cortical responsiveness and higher resting-level functional connectivity in the visual cortex compared to those who suffer from migraine without aura and healthy controls.41 Therefore, our finding that children with migraine and aura show a greater difference to controls than children without aura is in line with the evidence from other imaging modalities. However, the finding of decreased levels of glutamate is unexpected, and may be a contributing factor as to why medications that are effective in adult migraineurs are not as effective in children.38 This opposite finding emphases the need to study migraine biology in young, pediatric samples.
A change from lower glutamate levels in pediatric migraine to higher glutamate levels in adult migraine may be a result of development. Cortical excitability is known to decrease over time through pruning of glutamatergic synapses. Contingent negative variation, a measure of cortical excitability, has been shown to decrease from childhood to adulthood. Interestingly, contingent negative variation in migraine patients does not decrease as much over time as in healthy controls or in migraine patients who went into remission.43 This implies migraine progression is related to alterations in cortical development. Synaptic pruning is triggered by GABA receptors, which increase in number at pubertal onset36. Subsequently, GABA alterations early in development may produce future glutamatergic alterations. Therefore, increasing our understanding of early migraine biology in pediatrics has important implications for developing targeted, early interventions, a crucial step into reducing migraine impact throughout the lifespan.
Many suggest hormonal changes are a pivotal factor in migraine due to dramatic changes in prevalence between males and females. Before puberty, there is slightly higher prevalence of migraine in males, whereas after puberty, prevalence increases dramatically in females, affecting roughly 2 females:1 male,31 but the mechanism behind this change is not clear. We suggest the aforementioned GABA and glutamate changes are important factors. However, due to the narrow age range used in this study, the majority of participants were classed as in the pre/early stages using the pubertal status questionnaire. Therefore, although it is likely that puberty has a modulatory effect on brain development and migraine, these effects are minimized here.
In addition to higher glutamate, a recent systematic review demonstrated that adults with migraine showed higher levels of GABA in various cortical and subcortical regions.37 In the thalamus, we found GABA/Glx to be higher in Migraine, primarily driven by higher GABA, although group comparisons did not reach statistical significance. Higher GABA levels are thought to reflect an increased inhibitory tone. Indeed, there is evidence of reduced thalamic activity in adults with migraine in between attacks,51 which may be due to an increase in inhibition. Within the Migraine group, we found an association between higher GABA levels and higher migraine burden (measured by the PedMIDAS) in all 3 areas (although only the sensorimotor cortex reached statistical significance), indicating that children with higher GABA levels are more affected by their migraines. These higher GABA levels may reflect a compensatory mechanism in response to multiple migraines or hyperexcitability associated with migraine. We also show that higher glutamate in the thalamus and higher GABA/Glx ratios in the sensorimotor cortex are associated with duration since diagnosis, i.e., having migraines longer, even when controlling for current age. This suggests that these imbalances may develop over time. Indeed, there is evidence that alterations in GABA receptors influence the age of onset of migraine,16 and adults who have suffered from migraine longer have an increased inability to habituate to stimuli.29 Taken together, we speculate migraine is a progressive disorder leading to more irregularities in cortical excitability with development. This highlights the potential impact of early targeted interventions on migraine progression.
To control for variations in the length of each individual's migraine cycle, we created a novel metric to show, proportionally, how far a person was through their migraine cycle. This means the position in the cycle, along with changes in the brain associated with this, can be compared across people more accurately than simply looking at the number of days since their last migraine because the duration between migraines varies between people. We found that children in the migraine group with lower GABA levels in the thalamus were further in their migraine cycle, or closer in time to the next migraine. This suggests that, as the migraine cycle progresses, there is a reduction in GABAergic inhibition in the thalamus, which we speculate has a mechanistic role in the development of a migraine. Evidence in adults shows an increase in cortical excitability as the migraine cycle progresses. Cortese et al. (2017) showed a negative correlation between the resting motor threshold and the time elapsed since the last attack; as the days since the last attack increased, the resting motor threshold decreased, indicating an increase in excitability in the motor cortex. Coppola et al. (2016) showed that a reduction in lateral inhibition in the somatosensory cortex was associated with a higher number of days elapsed since the last attack. These changes in cortical excitability may be driven by changes in thalamic activity or excitability over time, evidenced in the alterations in GABA levels seen here.
A limitation of this study is that our sample generally scored less than 30 on the PedMIDAS scale, indicating migraine had a mild impact on their life. Although this may represent an abundance of migraine sufferers and may reflect the typical impact of migraine in this younger sample, it is unknown whether the findings here generalize to more severe migraines that require intensive clinical management. As we see relationships between GABA levels and PedMIDAS scores, it is possible that group differences in GABA may have been detected if our migraine sample had a higher migraine burden. In addition, the cross-sectional design of this study limits the conclusions that can be drawn; for example, although we show a relationship between GABA and migraine burden, it is unknown if GABA will increase in those whose migraine burden increases. It should also be noted that the relationship between neurotransmitter levels measured at rest using MRS and excitability of the cortex is poorly understood, with mixed findings regarding the relationship between MRS and TMS measures of excitability.13,47 Subsequently, the conclusions regarding migraine physiology that can be drawn from this study are limited. Future studies would benefit from comparing both MRS and TMS measures in children with and without migraine.
A strength of this study is the use of a macromolecule-suppressed GABA acquisition, providing increased specificity of GABA, without the contamination of macromolecules, which can account for roughly half the GABA signal in a typical MEGA-PRESS acquisition.20,22 However, a large voxel is needed to offset the inherent low signal-to-noise ratio for GABA,33 resulting in partial volume effects. Tissue correction has been applied to control for metabolite relaxation effects34; however, the voxel will contain tissue from areas surrounding the area of interest, for example, the sensorimotor voxel contains signal from both sensory and motor regions.
In conclusion, we show alterations in excitatory and inhibitory neurotransmitter levels in children with migraine, and that these measures are associated with migraine characteristics. We show that higher GABA levels are associated with higher migraine burden, in line with the adult literature. We also show a reduction in glutamate levels in the visual cortex, the opposite of findings in adults. This highlights the need for further mechanistic studies of migraine in children, to aid in the development of more effective treatments.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/B134.
Acknowledgements
This study was funded by a SickKids CIHR IHDCYH New Investigator Grant. T. Bell was supported by a Harley N. Hotchkiss- Samuel Weiss Postdoctoral Fellowship, University of Calgary. Additional support provided by the Hotchkiss Brain Institute and the Alberta Children's Hospital Research Institute, University of Calgary, and a CFI-JELF award. The funders had no involvement in study design, in the collection, analysis, and interpretation of data, in writing the report, or in the decision to submit the article for publication.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
References
- [1].Aguila MER, Lagopoulos J, Leaver AM, Rebbeck T, Hübscher M, Brennan PC, Refshauge KM. Elevated levels of GABA+ in migraine detected using 1H-MRS. NMR Biomed 2015;28:890–7. [DOI] [PubMed] [Google Scholar]
- [2].Bathel A, Schweizer L, Stude P, Glaubitz B, Wulms N, Delice S, Schmidt-Wilcke T. Increased thalamic glutamate/glutamine levels in migraineurs. J Headache Pain 2018;19:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bell T, Boudes ES, Loo RS, Barker GJ, Lythgoe DJ, Edden RAE, Lebel RM, Wilson M, Harris AD. In vivo Glx and Glu measurements from GABA-edited MRS at 3 T. NMR Biomed 2020: e4245 doi: 10.1002/nbm.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bigal ME, Hetherington H, Pan J, Tsang A, Grosberg B, Avdievich N, Friedman B, Lipton RB. Occipital levels of GABA are related to severe headaches in migraine. Neurology 2008;70:2078–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Brigo F, Storti M, Tezzon F, Manganotti P, Nardone R. Primary visual cortex excitability in migraine: a systematic review with meta-analysis. Neurol Sci 2013;34:819–30. [DOI] [PubMed] [Google Scholar]
- [6].Carskadon MA, Acebo C. A self-administered rating scale for pubertal development. J Adolesc Heal 1993;14:190–5. [DOI] [PubMed] [Google Scholar]
- [7].Chan YM, Pitchaimuthu K, Wu QZ, Carter OL, Egan GF, Badcock DR, McKendrick AM. Relating excitatory and inhibitory neurochemicals to visual perception: a magnetic resonance study of occipital cortex between migraine events. PLoS One 2019;14:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chorpita BF, Yim L, Moffitt C, Umemoto LA, Francis SE. Assessment of symptoms of DSM-IV anxiety and depression in children: a revised child anxiety and depression scale. Behav Res Ther 2000;38:835–55. [DOI] [PubMed] [Google Scholar]
- [9].Coppola G, Bracaglia M, Di Lenola D, Iacovelli E, Di Lorenzo C, Serrao M, Evangelista M, Parisi V, Schoenen J, Pierelli F. Lateral inhibition in the somatosensory cortex during and between migraine without aura attacks: correlations with thalamocortical activity and clinical features. Cephalalgia 2016;36:568–78. [DOI] [PubMed] [Google Scholar]
- [10].Coppola G, Vandenheede M, Di Clemente L, Ambrosini A, Fumal A, De Pasqua V, Schoenen J. Somatosensory evoked high-frequency oscillations reflecting thalamo-cortical activity are decreased in migraine patients between attacks. Brain 2005;128:98–103. [DOI] [PubMed] [Google Scholar]
- [11].Cortese F, Coppola G, Di Lenola D, Serrao M, Di Lorenzo C, Parisi V, Pierelli F. Excitability of the motor cortex in patients with migraine changes with the time elapsed from the last attack. J Headache Pain 2017;18:16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cosentino G, Fierro B, Vigneri S, Talamanca S, Paladino P, Baschi R, Indovino S, Maccora S, Valentino F, Fileccia E, Giglia G, Brighina F. Cyclical changes of cortical excitability and metaplasticity in migraine: evidence from a repetitive transcranial magnetic stimulation study. PAIN 2014;155:1070–8. [DOI] [PubMed] [Google Scholar]
- [13].Dyke K, Pépés SE, Chen C, Kim S, Sigurdsson HP, Draper A, Husain M, Nachev P, Gowland PA, Morris PG, Jackson SR. Comparing GABA-dependent physiological measures of inhibition with proton magnetic resonance spectroscopy measurement of GABA using ultra-high-field MRI. Neuroimage 2017;152:360–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ebesutani C, Reise SP, Chorpita BF, Ale C, Regan J, Young J, Higa-McMillan CWJ. The Revised Child Anxiety and Depression Scale-Short Version: scale reduction via exploratory bifactor modeling of the broad anxiety factor. Psychol Assess 2012;24:833–45. [DOI] [PubMed] [Google Scholar]
- [15].Edden RAE, Puts NAJ, Harris AD, Barker PB, Evans CJ. Gannet: a batch-processing tool for the quantitative analysis of gamma-aminobutyric acid–edited MR spectroscopy spectra. J Magn Reson Imaging 2014;40:1445–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].García-Martín E, Martínez C, Serrador M, Alonso-Navarro H, Navacerrada F, Esguevillas G, García-Albea E, Agúndez JAG, Jiménez-Jiménez FJ. Gamma-aminobutyric acid (gaba) receptors rho (gabrr) gene polymorphisms and risk for migraine. Headache 2017;57:1118–35. [DOI] [PubMed] [Google Scholar]
- [17].Gasparovic C, Song T, Devier D, Bockholt HJ, Caprihan A, Mullins PG, Posse S, Jung RE, Morrison La. Use of tissue water as a concentration reference for proton spectroscopic imaging. Magn Reson Med 2006;55:1219–26. [DOI] [PubMed] [Google Scholar]
- [18].Goadsby PJ, Holland PR, Martins-Oliveira M, Hoffmann J, Schankin C, Akerman S. Pathophysiology of migraine: a disorder of sensory processing. Physiol Rev 2017;97:553–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].González De La Aleja J, Ramos A, Mato-Abad V, Martínez-Salio A, Hernández-Tamames JA, Molina JA, Hernández-Gallego J, Álvarez-Linera J. Higher glutamate to glutamine ratios in occipital regions in women with migraine during the interictal state. Headache 2013;53:365–75. [DOI] [PubMed] [Google Scholar]
- [20].Harris AD, Puts NAJ, Barker PB, Edden RAE. Spectral-editing measurements of GABA in the human brain with and without macromolecule suppression. Magn Reson Med 2015;74:1523–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Harris AD, Puts NAJ, Edden RAE. Tissue correction for GABA-edited MRS: considerations of voxel composition, tissue segmentation, and tissue relaxations. J Magn Reson Imaging 2015;42:1431–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Harris AD, Saleh MG, Edden RAE. Edited 1 H magnetic resonance spectroscopy in vivo: methods and metabolites. Magn Reson Med 2017;77:1377–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hershey AD, Powers SW, Vockell ALB, LeCates S, Kabbouche MA, Maynard MK. PedMIDAS: development of a questionnaire to assess patients' satisfaction. Neurology 2001;11:2034–9. [DOI] [PubMed] [Google Scholar]
- [24].Hodkinson DJ, Wilcox SL, Veggeberg R, Noseda R, Burstein R, Borsook D, Becerra L. Increased amplitude of thalamocortical low-frequency oscillations in patients with migraine. J Neurosci 2016;36:8026–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kienbacher C, Wöber C, Zesch HE, Hafferl-Gattermayer A, Posch M, Karwautz A, Zormann A, Berger G, Zebenholzer K, Konrad A, Wöber-Bingöl Ç. Clinical features, classification and prognosis of migraine and tension-type headache in children and adolescents: a long-term follow-up study. Cephalalgia 2006;26:820–30. [DOI] [PubMed] [Google Scholar]
- [26].Kincses ZT, Veréb D, Faragó P, Tóth E, Kocsis K, Kincses B, Király A, Bozsik B, Párdutz Á, Szok D, Tajti J, Vécsei L, Tuka B, Szabó N. Are migraine with and without aura really different entities? Front Neurol 2019;10:6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kosinski M, Bayliss M, Bjorner J, Ware JJ, Garber W, Batenhorst A, Cady R, Dahlöf C, Dowson A, Tepper S. A six-item short-form survey for measuring headache impact: the HIT-6. Qual Life Res 2003;12:963–74. [DOI] [PubMed] [Google Scholar]
- [28].Kroon Van Diest AM, Ernst MM, Slater S, Powers SW. Similarities and differences between migraine in children and adults: presentation, disability, and response to treatment. Curr Pain Headache Rep 2017;21:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kropp P, Wallasch TM, Müller B, Meyer B, Darabaneanu S, Bosse C, Keller A, Meyer W, Gerber WD. Disease duration of episodic migraine correlates with modified amplitudes and habituation of contingent negative variation. J Neural Transm 2015;122:877–85. [DOI] [PubMed] [Google Scholar]
- [30].Locher C, Kossowsky J, Koechlin H, Lam TL, Barthel J, Berde CB, Gaab J, Schwarzer G, Linde K, Meissner K. Efficacy, safety, and acceptability of pharmacologic treatments for pediatric migraine prophylaxis: a systematic review and network meta-analysis. JAMA Pediatr 2020;02115:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Maleki N, Bernstein C, Napadow V, Field A. Migraine and puberty: potential susceptible brain sites. Semin Pediatr Neurol 2016;23:53–9. [DOI] [PubMed] [Google Scholar]
- [32].McAbee GN, Morse AM, Assadi M. Pediatric aspects of headache classification in the international classification of headache disorders—3 (ICHD-3 beta version). Curr Pain Headache Rep 2016;20:1–6. [DOI] [PubMed] [Google Scholar]
- [33].Mullins PG, McGonigle DJ, O'Gorman RL, Puts NAJ, Vidyasagar R, Evans CJ, Edden RAE. Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. Neuroimage 2014;86:43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Near J, Harris AD, Juchem C, Öz G, Slotboom J, Kreis R. Preprocessing, analysis and quantification in single-voxel magnetic resonance spectroscopy : experts consensus recommendations. NMR Biomed 2020: e4257. doi: 10.1002/nbm.4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 1971;9:97–113. [DOI] [PubMed] [Google Scholar]
- [36].Parato J, Shen H, Smith SS. α4βδ GABA A receptors trigger synaptic pruning and reduce dendritic length of female mouse CA3 hippocampal pyramidal cells at puberty. Neuroscience 2019;398:23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Peek AL, Rebbeck T, Puts NA, Watson J, Aguila ME, Leaver AM. Brain GABA and glutamate levels across pain conditions: a systematic literature review and meta-analysis of 1H-MRS studies using the MRS-Q quality assessment tool. Neuroimage 2020;210:116532. [DOI] [PubMed] [Google Scholar]
- [38].Powers SW, Coffey CS, Chamberlin LA, Ecklund DJ, Klingner EA, Yankey JW, Korbee LL, Porter LL, Hershey AD. Trial of amitriptyline, topiramate, and placebo for pediatric migraine. N Engl J Med 2017;376:115–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Pro S, Tarantino S, Capuano A, Vigevano F, Valeriani M. Primary headache pathophysiology in children: the contribution of clinical neurophysiology. Clin Neurophysiol 2014;125:6–12. [DOI] [PubMed] [Google Scholar]
- [40].Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed 2001;14:260–4. [DOI] [PubMed] [Google Scholar]
- [41].Russo A, Silvestro M, Tessitore A, Tedeschi G. Recent insights in migraine with aura: a narrative review of advanced neuroimaging. Headache 2019;59:637–49. [DOI] [PubMed] [Google Scholar]
- [42].Simpson R, Devenyi GA, Jezzard P, Hennessy TJ, Near J. Advanced processing and simulation of MRS data using the FID appliance (FID-A)—an open source, MATLAB-based toolkit. Magn Reson Med 2017;77:23–33. [DOI] [PubMed] [Google Scholar]
- [43].Siniatchkin M, Jonas A, Baki H, Van Baalen A, Gerber WD, Stephani U. Developmental changes of the contingent negative variation in migraine and healthy children. J Headache Pain 2010;11:105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Siniatchkin M, Reich AL, Shepherd AJ, van Baalen A, Siebner HR, Stephani U. Peri-ictal changes of cortical excitability in children suffering from migraine without aura. PAIN 2009;147:132–40. [DOI] [PubMed] [Google Scholar]
- [45].Siniatchkin M, Sendacki M, Moeller F, Wolff S, Jansen O, Siebner H, Stephani U. Abnormal changes of synaptic excitability in migraine with aura. Cereb Cortex 2012;22:2207–16. [DOI] [PubMed] [Google Scholar]
- [46].Slater SK, Powers SW, O'Brien HL. Migraine in children: presentation, disability and response to treatment. Curr Opin Pediatr 2018;30:775–9. [DOI] [PubMed] [Google Scholar]
- [47].Stagg CJ, Bestmann S, Constantinescu AO, Moreno Moreno L, Allman C, Mekle R, Woolrich M, Near J, Johansen-Berg H, Rothwell JC. Relationship between physiological measures of excitability and levels of glutamate and GABA in the human motor cortex. J Physiol 2011;589:5845–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Tu Y, Fu Z, Zeng F, Maleki N, Lan L, Li Z, Park J, Wilson G, Gao Y, Liu M, Calhoun V, Liang F, Kong J. Abnormal thalamocortical network dynamics in migraine. Neurology 2019;92:e2706–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wong-Baker FACES Foundation. Wong-Baker FACES® Pain Rating Scale, 2018. Retrieved 2017 with permission from http://www.WongBakerFACES.org.
- [50].Xiang J, Leiken K, Degrauw X, Kay B, Fujiwara H, Rose DF, Allen JR, Kacperski JE, O'Brien HL, Kabbouche MA, Powers SW, Hershey AD. Spatial heterogeneity of cortical excitability in migraine revealed by multi-frequency neuromagnetic signals. 2016;17:694–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Younis S, Hougaard A, Noseda R, Ashina M. Current understanding of thalamic structure and function in migraine. Cephalalgia 2019;39:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Younis S, Hougaard A, Vestergaard MB, Larsson HBW, Ashina M. Migraine and magnetic resonance spectroscopy: a systematic review. Curr Opin Neurol 2017;30:246–62. [DOI] [PubMed] [Google Scholar]
- [53].Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, Winkler P. Localization of the motor hand area to a knob on the precentral gyrus A new landmark. Brain 1997:141–57. [DOI] [PubMed] [Google Scholar]
- [54].Youssef PE, Mack KJ. Episodic and chronic migraine in children. Dev Med Child Neurol 2020;62:34‐41. [DOI] [PubMed] [Google Scholar]
- [55].Zielman R, Wijnen JP, Webb A, Onderwater GLJ, Ronen I, Ferrari MD, Kan HE, Terwindt GM, Kruit MC. Cortical glutamate in migraine. Brain 2017;140:1859–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/B134.