Abstract
Background
Coagulation abnormalities in COVID-19 patients have not been addressed in depth.
Objective
To perform a longitudinal evaluation of coagulation profile of patients admitted to the ICU with COVID-19.
Methods
Conventional coagulation tests, rotational thromboelastometry (ROTEM), platelet function, fibrinolysis, antithrombin, protein C and S were measured at days 0, 1, 3, 7 and 14. Based on median total maximum SOFA score, patients were divided in two groups: SOFA ≤ 10 and SOFA > 10.
Results
Thirty patients were studied. Some conventional coagulation tests, as aPTT, PT and INR remained unchanged during the study period, while alterations on others coagulation laboratory tests were detected. Fibrinogen levels were increased in both groups. ROTEM maximum clot firmness increased in both groups from Day 0 to Day 14. Moreover, ROTEM–FIBTEM maximum clot firmness was high in both groups, with a slight decrease from day 0 to day 14 in group SOFA ≤ 10 and a slight increase during the same period in group SOFA > 10. Fibrinolysis was low and decreased over time in all groups, with the most pronounced decrease observed in INTEM maximum lysis in group SOFA > 10. Also, D-dimer plasma levels were higher than normal reference range in both groups and free protein S plasma levels were low in both groups at baseline and increased over time, Finally, patients in group SOFA > 10 had lower plasminogen levels and Protein C than patients with SOFA <10, which may represent less fibrinolysis activity during a state of hypercoagulability.
Conclusion
COVID-19 patients have a pronounced hypercoagulability state, characterized by impaired endogenous anticoagulation and decreased fibrinolysis. The magnitude of coagulation abnormalities seems to correlate with the severity of organ dysfunction. The hypercoagulability state of COVID-19 patients was not only detected by ROTEM but it much more complex, where changes were observed on the fibrinolytic and endogenous anticoagulation system.
Introduction
More than thirteen million people have been diagnosed with coronavirus disease 2019 (COVID-19) worldwide [1] since severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first identified in China in December 2019 [2]. Almost one third of the hospitalized patients with COVID-19 are admitted to the ICU [2–4].
Coagulation abnormalities, mainly thrombotic complications, have been described in COVID-19 patients [5–11]. The incidence of arterial and venous thrombotic complications in COVID-19 patients admitted to the ICU may reach 31% [12]. Indeed, it has been shown that D-dimer and fibrinogen degradation products (FDP) values were higher in patients with most severe SARS-CoV-2 infection than in patients with milder forms of disease [6]. Moreover, Chen et al. have demonstrated that deceased patients with COVID-19 exhibited D-dimer concentrations approximately seven times higher than recovered patients [13]. Additionally, low platelet count was show to be associated with increased risks of more severe forms of disease and increased hospital mortality in COVID-19 patients [10]. Although D-dimer, FDP and platelet count have been used as clinical indicators of SARS-CoV-2 infection severity [14], coagulation abnormalities in COVID-19 patients have not been addressed in depth.
Conventional coagulation tests (CCT) such as prothrombin time (PT), international normalized ratio (INR), thrombin time (TT) and activated partial thromboplastin time (aPTT) are unable to reflect the complexity of hemostatic impairment observed in intensive care unit (ICU) patients [15]. Rotational thromboelastometry (ROTEM) represents a point-of-care test that assess the viscoelastic properties of whole blood, providing a real-time evaluation of clot formation kinetics, i.e., clot formation, stabilization and dissolution, at the bedside [16]. To the best of our knowledge, a comprehensive and longitudinal analysis of the coagulation profile, including CCT, ROTEM, platelet function, fibrinolysis, and endogenous inhibitors of coagulation (antithrombin, protein C and S) have not been described in ICU COVID-19 patients so far.
We hypothesized that ICU patients diagnosed with COVID-19 have a prothrombotic profile that may be explained by an impaired endogenous anticoagulation system. Moreover, the degree of coagulation abnormalities reflects the severity of the disease. Therefore, our objective was to perform a comprehensive longitudinal evaluation of coagulation, fibrinolysis, and endogenous anticoagulation system of patients admitted to the ICU with severe COVID-19.
Materials and methods
Study design and setting
We performed a single center prospective longitudinal study in an ICU of a private tertiary care hospital in São Paulo, Brazil. The study was approved by the Local Ethics Committee at Hospital Israelita Albert Einstein and by Comissão Nacional de Ética em Pesquisa (CONEP) with waiver of informed consent (CAAE: 30175220.3.0000.0071). This study is reported in accordance with the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) statement [17].
Study participants
Thirty patients aged ≥18 years old admitted to the ICU with confirmed diagnosis of COVID-19 were included in this study. Laboratory confirmation of SARS-CoV-2 infection was based on positive reverse-transcriptase-polymerasechain-reaction (RT-PCR) assay [18].
Exclusion criteria included pregnancy, previous known coagulopathy, currently use of systemic anticoagulants or anti-platelet therapy or vitamin K antagonists, moribund patients and patients who presented cardiac arrest.
Participants were recruited between March 29, 2020 through May 13, 2020 and they could represent the majority of severe patients infected by COVID-19, once there were few exclusion criteria and the participants were recruited with waiver of informed consent once it was an observational study without any intervention and consecutive patients admitted in the Intensive Care Unit were recruited following the inclusion and exclusion criteria until we completed 30 patients included in the study.
Laboratory analysis
Laboratory tests were performed at the time of study inclusion (baseline), and at days 1, 3, 7 and 14 after enrollment unless the patient had died or was discharged from the hospital.
Conventional coagulation tests
Conventional coagulation tests included: platelet count (XE 2100, Sysmex, São Paulo, Brazil), plasma fibrinogen concentration [Clauss method (Hemosil QFA thrombin (bovine), IL Instrumentation Laboratory Company, Bedford MA, USA], aPTT (Hemosil Synthasil, IL Instrumentation Laboratory Company, Bedford MA, USA), PT and INR (Hemosil PT-Fibrinogen HS Plus, IL Instrumentation Laboratory Company, Bedford MA, USA) and ionic calcium (ABL 800 FLEX, Radiometer Medical ApS, Brønshøj, Denmark).
Rotational thromboelastometry
Rotational thromboelastometry analyses were performed with EXTEM (extrinsic coagulation pathway assessment), INTEM (intrinsic coagulation pathway assessment) and FIBTEM (extrinsic coagulation pathway assessment with additional platelet inhibition using cytochalasin D) tests according to the manufacturer’s instructions [16]. The following parameters were recorded during ROTEM analysis: clotting time [CT; seconds (sec)], which represents the beginning of the test until clot firmness of 2 mm; clot formation time (CFT; sec), which represents time between detection of a clot firmness of 2 and 20 mm and maximum clot firmness (MCF; mm), which represents the greatest amplitude of thromboelastometric trace and reflects clot “strength” [19, 20]. ROTEM tests were performed by laboratory technicians. Blood samples of approximately 3 ml were collected by venipuncture into a tube with citrate (3.2%; Sarsted1, Wedel, Germany). Blood samples were immediately processed for ROTEM analysis. The analyses were performed by pipetting 340 μl of citrated whole blood and 20 μl of 0.2 M calcium chloride with specific activators into a cup. There was no change in methodology for test performance nor test controls (Rotrol N and Rotrol P) throughout the study period [15, 20].
Normal coagulation profile on the ROTEM was defined according to reference values for CT, CFT and MCF (INTEM CT: 100–240 sec, INTEM CFT: 30–110 sec, INTEM MCF: 50–72 mm; EXTEM CT: 38–79 sec, EXTEM CFT: 34–159 sec, EXTEM MCF: 50–72 mm; FIBTEM MCF: 9–25 mm) [15, 19, 20]. Hypocoagulability in ROTEM was defined as prolongation of CT (INTEM CT >240 sec or EXTEM CT >79 sec) and/or CFT (INTEM CFT >110 sec or EXTEM CFT >159 sec) and/or MCF reduction (MCF INTEM or EXTEM MCF <50 mm or FIBTEM MCF <9 mm) [15, 19]. Hypercoagulability in ROTEM was defined as a reduction in clotting time (INTEM CT <100 sec or EXTEM CT <38 sec), or clot formation time (INTEM CFT <30 sec or EXTEM CFT <34 sec) and/or an increase in MCF (MCF INTEM or EXTEM MCF >72 mm or FIBTEM MCF >25 mm) [15, 19].
Platelet function test
Platelet function test was assessed in whole blood samples using impedance aggregometry (The ROTEM®‐Platelet; TEM Innovations GmbH, Munich Germany) [21]. Platelets were activated with arachidonic acid (ARATEM test) and adenosine diphosphate (ADPTEM) [21].
Fibrinolysis and endogenous anticoagulation system
D-dimer (Hemosil D-dimer HS 500 and hemosil D-dimer HS 500 controls, L Instrumentation Laboratory Company, Bedford MA, USA), serum plasminogen (Plasmin Inhibitor, IL Instrumentation Laboratory Company, Bedford MA, USA), alpha-2 antiplasmin (Plasmin Inhibitor, IL Instrumentation Laboratory Company, Bedford MA, USA), antithrombin (IL Instrumentation Laboratory Company, Bedford MA, USA), protein C (IL Instrumentation Laboratory Company, Bedford MA, USA) and free protein S (IL Instrumentation Laboratory Company, Bedford MA, USA) were measured.
Data collection
All study data were retrieved from Epimed Monitor System (Epimed Solutions, Rio de Janeiro, Brazil), which is an electronic structured case report form where patients data are prospectively entered by trained ICU case managers [22].
Collected clinical variables included demographics, comorbidities, Simplified Acute Physiology score (SAPS 3 score) at ICU admission [23], Sequential Organ Failure Assessment score (SOFA score) [24] at ICU admission, and at days 1, 3, 7 and 14 after enrollment unless the patient had died or was discharged from the ICU, total maximum SOFA score (from the time of study inclusion (baseline) up to 14 after enrollment unless the patient had died or was discharged from the ICU) [25], body-mass index, treatment measures (i.e, hydroxychloroquine, macrolides, corticosteroids, interleukin-6 receptor antagonist, convalescent plasma and lopinavir-ritonavir), supportive therapy [use of vasopressors, mechanical ventilation, noninvasive mechanical ventilation and renal replacement therapy (RRT)] during ICU stay, hospital length of stay (LOS) prior to ICU admission, ICU and hospital LOS, and ICU mortality.
Blood component transfusion [platelet concentrate, fresh frozen plasma (FFP) and cryoprecipitate] and hemostatic agents [fibrinogen concentrate, prothrombin complex concentrate (PCC) and tranexamic acid] were collected. The presence of thrombotic or hemorrhagic events and the use of prophylactic or therapeutic doses of low-molecular-weight heparin (LMWH) or unfractionated heparin (UFH) during ICU stay were recorded.
Statistical analysis
Based on median total maximum SOFA score, patients were divided in two groups: group SOFA ≤ 10 and group SOFA > 10. Categorical variables were presented as n/n total (%). Continuous variables were presented as median with interquartile range (IQR). Categorical variables were compared between groups with Fisher’s exact test, and continuous variables were compared using independent t test or Mann-Whitney U test in case of non-normal distribution, tested by the Kolmogorov-Smirnov test.
To account for longitudinal (repeated measurements) and correlated response continuous variables, between-group differences and within-group differences over time were assessed using generalized estimating equations (GEE), with group (SOFA ≤ 10 and group SOFA > 10) and study time points (time) as predictors. P values for group effect, time effect, and time-group interaction were presented. When a time effect was detected in pooled patients, each time point (Day 1, 3, 7 and 14) was compared against Day 0. When a group effect or a time-group interaction were detected, between group comparisons (group SOFA>10 vs. group SOFA ≤10) were performed at each time point. The Bonferroni method was used to account for multiple comparisons.
Two-tailed tests were used and when p<0.05, the test was considered statistically significant. No adjustment was made for missing data. The SPSS™ (IBM™ Statistical Package for the Social Science version 26.0) was used for statistical analyses, and GraphPad Prism version 8.0.0 (GraphPad Software, San Diego, California, USA) was used for graph plotting.
Results
Baseline characteristics of patients
From March 29, 2020 through May 13, 2020, thirty patients were included in this study. The median (IQR) age of pooled patients was 61 (52–83) years, 50.0% were man and median (IQR) SAPS III of 49 (41–61). Of the 30 patients, 24 (80.0%) had one or more coexisting medical conditions. Obesity [12/29 (41.4%)], systemic hypertension [12/30 (40.0%)] and diabetes mellitus [11/30 (36.7%)] were the most common coexisting conditions. Most patients received invasive mechanical ventilation [27/30 (90.0%)] and/or vasopressors [27/30 (90.0%)] during the ICU stay. Clinical characteristics of patients are shown in Table 1.
Table 1. Characteristics of study participants.
| Characteristics | All patients (n = 30) | SOFA ≤10 (n = 16) | SOFA >10 (n = 14) | P value |
|---|---|---|---|---|
| Age, years (median, IQR) | 61 (52–83) | 53 (45–64) | 78 (60–85) | 0.002a |
| Men, n (%) | 15/30 (50.0) | 7/16 (43.8) | 8/14 (57.1) | 0.715b |
| SAPS III score, points (median, IQR)§ | 49 (41–61) | 50 (43–64) | 47 (41–55) | 0.307a |
| SOFA score D0, points (median, IQR)# | 6 (4–8) | 5 (3–6) | 8 (6–9) | 0.002a |
| Maximum SOFA score, points (median, IQR)# | 10 (7–12) | 7 (6–9) | 13 (11–14) | <0.001a |
| Number of coexisting conditions, (median, IQR) | 2 (1–3) | 1 (0–2) | 3 (2–3) | <0.001a |
| Coexisting conditions, n (%) | ||||
| Obesity | 12/29 (41.4) | 7/16 (43.8) | 5/13 (38.5) | 1.000b |
| Systemic hypertension | 12/30 (40.0) | 5/16 (31.3) | 7/14 (50.0) | 0.457b |
| Diabetes mellitus | 11/30 (36.7) | 2/16 (12.5) | 9/14 (64.3) | 0.007b |
| Malignancy | 4/30 (13.3) | 2/16 (12.5) | 2/14 (14.3) | 1.000b |
| Congestive heart failure | 3/30 (10.0) | 0/16 (0.0) | 3/14 (21.4) | 0.090b |
| COPD / Asthma | 3/30 (10.0) | 1/16 (6.3) | 2/14 (14.3) | 0.586b |
| Chronic kidney disease | 4/30 (13.3) | 1/16 (6.3) | 3/14 (21.4) | 0.315b |
| Coronary artery disease | 2/30 (6.7) | 0/16 (0.0) | 2/14 (14.3) | 0.209b |
| Body-mass index | 29.3 (24.4–32.2) | 29.7 (24.2–32.5) | 27.2 (24.4–31.4) | 0.793a |
| Treatment, n (%) | ||||
| Macrolides | 28/30 (93.3) | 16/16 (100.0) | 12/14 (85.7) | 0.209b |
| Glucocorticoids | 25/30 (83.3) | 12/16 (75.0) | 13/14 (92.9) | 0.336b |
| Hydroxychloroquine | 24/30 (80.0) | 13/16 (81.3) | 11/14 (78.6) | 1.000b |
| Convalescent plasma | 10/30 (33.3) | 6/16 (37.5) | 4/14 (28.6) | 0.709b |
| Interleukin-6 receptor antagonist | 3/30 (10.0) | 2/16 (12.5) | 1/14 (7.1) | 1.000b |
| Lopinavir-ritonavir | 2/30 (6.7) | 1/16 (6.3) | 1/14 (7.1) | 1.000b |
| Support during ICU stay, n (%) | ||||
| Vasopressors | 27/30 (90.0) | 13/16 (81.3) | 14/14 (100.0) | 0.228b |
| Mechanical ventilation | 27/30 (90.0) | 13/16 (81.3) | 14/14 (100.0) | 0.228b |
| Noninvasive ventilation | 6/30 (20.0) | 3/16 (18.8) | 3/14 (21.4) | 1.000b |
| Renal replacement therapy | 10/30 (33.3) | 0/16 (100.0) | 10/14 (71.4) | <0.001b |
| Time from symptom onset to study inclusion, days (median, IQR) | 9 (6–14) | 9 (6–15) | 8 (6–13) | 0.951c |
| Hospital LOS prior ICU admission, days (median, IQR) | 1 (0–3) | 2 (1–3) | 1 (0–2) | 0.400c |
Values represent median (IQR) or n (%). ICU: intensive care unit, SAPS III: simplified acute physiology score III
§: scores on SAPS III range from 0 to 217, with higher scores indicating more severe illness and higher risk of death, SOFA: sequential organ failure assessment score
#: SOFA score ranges from 0 to 24, with higher scores indicating more severe organ dysfunction, COPD: chronic obstructive pulmonary disease, LOS: length of stay. P values were calculated with the use of (a) Independent t-test, (b) Fisher's exact test or (c) Mann-Whitney U test.
Compared with patients in group SOFA ≤ 10 [16/30 (53.3%) patients], patients in group SOFA > 10 [14/30 (46.7%)] were older [median (IQR), 78 (60–85) vs. 53 (45–64) years, p = 0.002], had a higher SOFA score at study inclusion [median (IQR), 8 (6–9) vs. 5 (3–6) points, p = 0.002], a higher number of coexisting conditions [median (IQR), 3 (2–3) vs. 1 (0–2), p<0.001], had diabetes mellitus more frequently [9/14 (64.3) vs. 2/16 (12.5), p = 0.007] and received renal replacement therapy more frequently [10/14 (71.4) vs. 0/16 (0.0), p<0.001] (Table 1).
Anticoagulants, blood transfusion and clinical outcomes
During the study period, 22/30 (73.3%) patients received anticoagulants as deep vein thrombosis (DVT) prophylaxis and 7/30 (23.3%) patients received systemic anticoagulation (Table 2). The proportion of patients receiving anticoagulants as DVT prophylaxis and as systemic anticoagulation did not differ between the groups (p = 0.830) (Table 2). Unfractionated heparin was more commonly used as DVT in group SOFA > 10 compared to group SOFA ≤ 10 [7/10 (70.0%) vs. 2/12 (16.7%), p = 0.027] (Table 2).
Table 2. Administered treatments and clinical outcomes.
| Characteristics | All patients (n = 30) | SOFA ≤10 (n = 16) | SOFA >10 (n = 14) | P value |
|---|---|---|---|---|
| Anticoagulants, n (%) | 0.830a | |||
| DVT prophylaxis | 22/30 (73.3) | 12/16 (75.0) | 10/14 (71.4) | |
| UFH | 9/22 (40.9) | 2/12 (16.7) | 7/10 (70.0) | 0.027a |
| LMWH | 13/22 (59.1) | 10/12 (83.3) | 3/10 (30.0) | |
| Systemic anticoagulation | 7/30 (23.3) | 3/16 (18.8) | 4/14 (28.6) | |
| UFH | 5/7 (71.4) | 1/3 (33.3) | 4/4 (100.0) | 0.143a |
| LMWH | 2/7 (28.6) | 2/3 (66.7) | 0/4 (0.0) | |
| Transfused blood product, n (%) | ||||
| Red blood cells | 4/30 (13.3) | 0 (0.0) | 4/14 (28.6) | 0.037a |
| Platelet concentrate | 0/30 (0.0) | 0 (0.0) | 0 (0.0) | |
| Fresh frozen plasma | 0/30 (0.0) | 0 (0.0) | 0 (0.0) | |
| Cryoprecipitate | 0/30 (0.0) | 0 (0.0) | 0 (0.0) | |
| Thrombotic events, n (%) | 6/30 (20.0) | 3/16 (18.8) | 3/14 (21.4) | 1.000a |
| DVT | 4/6 (66.7) | 2/3 (66.7) | 2/3 (66.7) | |
| Pulmonary embolism | 2/6 (33.3) | 1/3 (33.3) | 1/3 (33.3) | |
| Hemorrhagic events, n (%) | 3/30 (10.0) | 2/16 (12.5) | 1/14 (7.1) | 1.000a |
| Outcomes# | ||||
| Died in ICU | 4/30 (13.3) | 0/16 (0.0) | 4/14 (28.6) | 0.014a |
| Discharged from ICU | 25/30 (83.3) | 16/16 (100.0) | 9/14 (64.3) | |
| Still in ICU as of 06/10/2020 | 1/30 (3.3) | 0/16 (0.0) | 1/14 (7.1) | |
| ICU LOS (days), median (IQR)# | 15 (7–22) | 7 (6–15) | 22 (16–35) | <0.001b |
| Hospital LOS (days), median (IQR)# | 26 (15–38) | 17 (13–30) | 38 (25–51) | 0.012b |
Values represent median (IQR) or n (%). ICU: intensive care unit, DVT: deep vein thrombosis, HFH: unfractionated heparin, LMWH: low-molecular-weight heparin, LOS: length of stay.
#: the number of patients who died, were discharged, and were still admitted in the ICU as of June 10, 2020 were recorded, and ICU and hospital length of stay also were determined. P values were calculated with the use of (a) Fisher's exact test and (b) Mann-Whitney U test.
Four patients (13.3%) received red blood cells transfusion, all of them in group SOFA > 10 (Table 2). No patient received platelet concentrate, FFP, cryoprecipitate, fibrinogen concentrate, PCC or tranexamic acid during the study period. Thrombotic events occurred in 6/30 (20.0%) patients and hemorrhagic events were observed in 3/30 (10.0%) patients. The incidence of thrombotic and hemorrhagic events did not differ between the groups (Table 2).
As of June 10, 2020, one patient [1/30 (3.3%)] was still hospitalized in the ICU while 25/30 (83.3%) of patients were discharged alive from the ICU. Four [4/30 (13.3%)] patients died at the ICU (Table 2). Compared with patients in group SOFA ≤ 10, patients in group SOFA > 10 had a higher ICU mortality [4/14 (28.6%) vs. 0/16 (0.0%), p = 0.014], exhibited a higher ICU [median (IQR), 22 (16–35) vs. 7 (6–15), p<0.001], and hospital [38 (25–51) vs. 17 (13–30), p = 0.012] LOS (Table 2).
Laboratory analysis
Arterial pH increased in both groups from Day 0 to Day 14 (p<0.001 for time effect), while ionized calcium increased from Day 0 to Day 14 in group SOFA ≤ 10, and decreased in group SOFA > 10 (p = 0.038 for time-group interaction) (Table 3). Peripheral temperature remained stable during study period (Table 3).
Table 3. Arterial pH, ionized calcium, peripheral temperature, conventional coagulation tests and hemoglobin.
| Parameters | Reference range | Day 0 | Day 1 | Day 3 | Day 7 | Day 14 | P value |
|---|---|---|---|---|---|---|---|
| Arterial pH | 7.35–7.45 | ||||||
| All patients | 7.40 (7.35–7.41) | 7.38 (7.31–7.40) | 7.40 (7.34–7.43) | 7.42 (7.38–7.45) | 7.46 (7.43–7.50)* | <0.001a | |
| SOFA≤10 | 7.40 (7.36–7.41) | 7.39 (7.35–7.42) | 7.43 (7.40–7.45) | 7.43 (7.42–7.47) | 7.45 (7.42–7.48) | <0.001b | |
| SOFA >10 | 7.39 (7.31–7.40) | 7.32 (7.27–7.39)# | 7.34 (7.30–7.39)# | 7.41 (7.32–7.45)# | 7.47 (7.44–7.50) | 0.001c | |
| Ionized calcium (mmol/L) | 1.14–1.31 | ||||||
| All patients | 1.12 (1.09–1.15) | 1.15 (1.12–1.20) | 1.13 (1.10–1.16) | 1.17 (1.15–1.21) | 1.15 (1.06–1.24) | 0.030a | |
| SOFA≤10 | 1.14 (1.13–1.21) | 1.15 (1.12–1.19) | 1.13 (1.10–1.14) | 1.16 (1.15–1.19) | 1.24 (1.17–1.25) | 0.151b | |
| SOFA >10 | 1.12 (1.09–1.12)# | 1.14 (1.10–1.20) | 1.13 (1.09–1.21) | 1.19 (1.16–1.26) | 1.09 (1.06–1.17) | 0.038c | |
| Temperature (°C) | |||||||
| All patients | 36.4 (36.0–37.1) | 36.6 (36.2–37.0) | 36.5 (36.2–37.1) | 36.2 (36.0–36.7) | 36.1 (36.0–36.6) | 0.149a | |
| SOFA≤10 | 36.2 (36.0–36.8) | 36.6 (36.4–37.2) | 36.7 (36.2–37.2) | 36.3 (36.2–36.7) | 36.1 (36.0–36.6) | 0.351b | |
| SOFA >10 | 36.5 (35.9–37.5) | 36.5 (36.0–37.0) | 36.3 (35.3–36.7) | 36.1 (35.9–36.8) | 36.1 (35.7–36.6) | 0.324c | |
| Platelets (x109/L) | 150–450 | ||||||
| All patients | 226 (176–261) | 236 (182–268) | 272 (230–314)* | 349 (242–444)* | 306 (229–480)* | <0.001a | |
| SOFA≤10 | 243 (185–274) | 236 (210–307) | 271 (252–310) | 419 (307–462) | 469 (232–679) | 0.009b | |
| SOFA >10 | 197 (141–237)# | 220 (160–264) | 277 (197–314) | 282 (175–349)# | 286 (212–383) | 0.213c | |
| Fibrinogen (g/dL) | 200–400 | ||||||
| All patients | 600 (480–680) | 642 (470–722) | 625 (513–782) | 532 (348–592)* | 397 (303–537)* | <0.001 | |
| SOFA≤10 | 633 (503–690) | 642 (524–722) | 592 (513–680) | 482 (348–592) | 372 (298–439) | 0.365b | |
| SOFA >10 | 552 (480–680) | 610 (470–851) | 700 (513–822) | 564 (473–646) | 470 (303–550) | 0.048c | |
| Prothrombin time (sec) | 70–100 | ||||||
| All patients | 82 (76–89) | 81 (69–89) | 78 (70–88) | 85 (73–88) | 80 (67–89) | 0.323a | |
| SOFA≤10 | 82 (78–93) | 78 (70–86) | 78 (70–88) | 85 (78–87) | 83 (67–97) | 0.226b | |
| SOFA >10 | 83 (67–86) | 86 (69–89) | 75 (67–86) | 76 (70–89) | 77 (63–87) | 0.463c | |
| INR | 0.96–1.30 | ||||||
| All patients | 1.13 (1.07–1.18) | 1.14 (1.07–1.25) | 1.17 (1.10–1.25) | 1.11 (1.07–1.21) | 1.17 (1.07–1.34) | 0.503a | |
| SOFA≤10 | 1.13 (1.05–1.17) | 1.16 (1.09–1.25) | 1.16 (1.07–1.24) | 1.10 (1.09–1.16) | 1.12 (1.02–1.33) | 0.303b | |
| SOFA >10 | 1.13 (1.09–1.28) | 1.10 (1.07–1.26) | 1.19 (1.10–1.29) | 1.18 (1.07–1.27) | 1.21 (1.10–1.39) | 0.143c | |
| aPTT (sec) | 25.6–35.5 | ||||||
| All patients | 28.8 (27.2–32.6) | 27.8 (25.6–32.8) | 28.6 (25.9–33.2) | 27.9 (26.4–31.9) | 28.1 (25.6–35.7) | 0.079a | |
| SOFA≤10 | 28.0 (27.0–31.1) | 27.2 (25.6–31.2) | 26.7 (25.6–31.0) | 27.4 (27.0–30.8) | 28.1 (23.7–31.9) | 0.497b | |
| SOFA >10 | 30.0 (28.3–33.0) | 28.7 (25.6–33.4) | 30.8 (27.0–38.0) | 28.4 (26.0–33.3) | 28.3 (26.6–36.7) | 0.512c | |
| Hemoglobin (g/dL) | 13.5–17.5 | ||||||
| All patients | 12.1 (11.2–12.9) | 11.4 (10.2–12.2)* | 10.5 (9.6–11.8)* | 10.2 (9.3–11.1)* | 9.2 (8.7–10.6)* | <0.001a | |
| SOFA≤10 | 12.2 (11.3–12.9) | 11.4 (10.7–12.1) | 10.6 (9.7–11.8) | 10.7 (10.1–11.1) | 9.2 (9.1–11.7) | 0.483b | |
| SOFA >10 | 11.7 (11.2–13.4) | 11.3 (10.1–13.2) | 10.5 (9.0–11.8) | 9.8 (8.3–10.3) | 9.3 (8.1–10.2) | 0.618c |
Values represent median (IQR). INR: international normalized ratio, aPTT: activated partial thromboplastin time. P values were calculated with the use of generalized estimating equations (GEE): (a): time effect, (b): group effect and (c): time-group interaction. Pairwise comparisons significant at the 0.05 level:
(*): time effect—pooled patients: each time point vs. Day 0.
(#): between group comparisons (group SOFA>10 vs. group SOFA ≤10) at each time point.
Conventional coagulation tests
Platelet count increased from baseline to Day 14 in both groups (p<0.001 for time effect), although lower platelet count was observed over the time in group SOFA > 10 compared to group SOFA ≤ 10 (p = 0.009 for group effect) (Table 3). Fibrinogen levels were increased in both groups at baseline, with the highest values observed at day 1 in group SOFA ≤ 10 and at day 3 in group SOFA > 10 (p<0.001 for time effect). Patients in group SOFA ≤ 10 exhibited a more pronounced decrease in plasma fibrinogen levels at study end than patients in group SOFA > 10 (p = 0.048 for time-group interaction) (Table 3). Prothrombin time, INR, aPTT, and hemoglobin remained unchanged during the study period (Table 3).
Rotational thromboelastometry
The majority of patients in both groups exhibited a hypercoagulability state based on ROTEM (Fig 1). ROTEM (INTEM and EXTEM) maximum clot firmness slightly increased in both study groups from Day 0 to Day 14 (p<0.001 for time effect) (Table 4). ROTEM–FIBTEM maximum clot firmness was high in both groups during the study period, with a slight decrease from Day 0 to Day 14 in group SOFA ≤ 10 and a slight increase during the same period in group SOFA > 10 (p = 0.050 for time-group interaction) (Table 4).
Fig 1. Coagulation profile accordingly to rotational thromboelastometry (ROTEM).
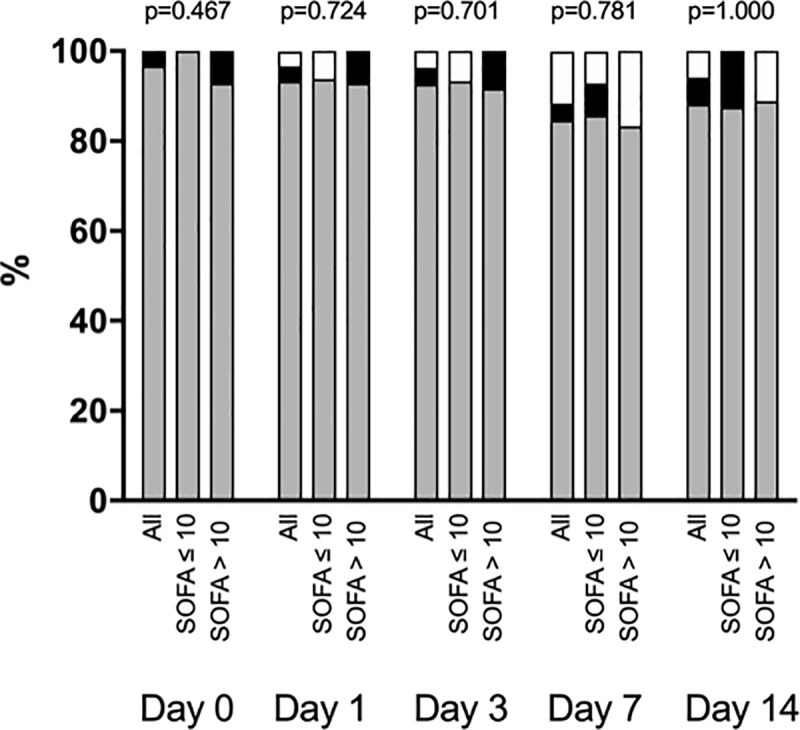
White filled bars represent normal coagulation profile, grey filled bars represent a hypercoagulability state and black filled bars represent a hypocoagulability state. P values comparing group SOFA>10 vs. group SOFA ≤10 at each time point were calculated with the use of Fisher's exact test.
Table 4. Rotational thromboelastometry and platelet function test.
| Parameters | Reference range | Day 0 | Day 1 | Day 3 | Day 7 | Day 14 | P value |
|---|---|---|---|---|---|---|---|
| ROTEM—INTEM | |||||||
| Clotting time (sec) | 100–240 | ||||||
| All patients | 164 (155–184) | 160 (156–191) | 173 (159–196) | 171 (157–193) | 163 (153–190) | 0.300a | |
| SOFA≤10 | 165 (141–180) | 157 (153–168) | 161 (142–180) | 172 (162–193) | 171 (148–188) | 0.035b | |
| SOFA >10 | 159 (157–184) | 176 (156–194) | 184 (172–214)# | 169 (152–185) | 157 (153–194) | 0.378c | |
| Clot formation time (sec) | 30–110 | ||||||
| All patients | 48 (46–59) | 47 (42–57) | 44 (39–55) | 42 (39–48) | 41 (34–47) | 0.096a | |
| SOFA≤10 | 48 (45–55) | 46 (41–52) | 40 (35–47) | 42 (37–47) | 34 (29–49) | 0.026b | |
| SOFA >10 | 53 (47–62) | 51 (43–63)# | 52 (45–62)# | 43 (40–61) | 43 (39–46) | 0.567c | |
| Maximum clot firmness (mm) | 50–72 | ||||||
| All patients | 70 (67–72) | 71 (69–73)* | 73 (70–76) | 75 (70–78)* | 76 (69–79)* | <0.001a | |
| SOFA≤10 | 70 (68–72) | 72 (70–74) | 74 (72–76) | 75 (70–79) | 78 (70–80) | 0.312b | |
| SOFA >10 | 70 (66–73) | 71 (67–73) | 72 (70–76) | 75 (71–77) | 75 (69–78) | 0.914c | |
| Maximum lysis (%) | £15 | ||||||
| All patients | 10 (6–12) | 8 (5–10)* | 6 (3–9)* | 3 (2–6)* | 4 (2–6)* | <0.001a | |
| SOFA≤10 | 11 (6–13) | 8 (4–10) | 7 (6–9) | 5 (2–6) | 5 (2–6) | 0.223b | |
| SOFA >10 | 9 (6–12) | 8 (5–11) | 3 (2–7) | 2 (1–3)# | 3 (2–7) | 0.004c | |
| ROTEM—EXTEM | |||||||
| Clotting time (sec) | 38–79 | ||||||
| All patients | 72 (66–79) | 73 (66–88) | 78 (70–84)* | 73 (62–88) | 72 (61–80) | 0.013a | |
| SOFA≤10 | 76 (71–81) | 75 (67–94) | 76 (68–78) | 74 (68–81) | 78 (61–83) | 0.486b | |
| SOFA >10 | 68 (66–71) | 72 (64–75) | 85 (77–94)# | 68 (61–89) | 67 (65–80) | 0.001c | |
| Clot formation time (sec) | 34–159 | ||||||
| All patients | 54 (44–61) | 56 (45–64) | 48 (41–63) | 49 (41–57) | 47 (41–56) | 0.123a | |
| SOFA≤10 | 53 (44–61) | 52 (43–62) | 45 (40–64) | 46 (37–56) | 38 (31–59) | 0.124b | |
| SOFA >10 | 54 (50–64) | 60 (45–73) | 56 (47–63) | 53 (45–62) | 51 (44–55) | 0.839c | |
| Maximum clot firmness (mm) | 50–72 | ||||||
| All patients | 73 (69–74) | 73 (70–75) | 75 (71–77) | 74 (70–79)* | 74 (69–79) | <0.001a | |
| SOFA≤10 | 73 (70–75) | 74 (71–76) | 76 (71–77) | 75 (70–79) | 75 (69–80) | 0.344b | |
| SOFA >10 | 71 (68–73) | 72 (68–73) | 73 (71–80) | 74 (72–78) | 74 (69–78) | 0.415c | |
| Maximum lysis (%) | £15 | ||||||
| All patients | 10 (7–12) | 9 (6–13) | 8 (5–10)* | 8 (3–11) | 7 (5–11) | 0.006a | |
| SOFA≤10 | 10 (8–12) | 9 (6–11) | 8 (6–9) | 8 (4–12) | 7 (4–11) | 0.863b | |
| SOFA >10 | 9 (6–15) | 10 (7–13) | 8 (4–11) | 4 (3–10) | 8 (5–11) | 0.617c | |
| ROTEM—FIBTEM | |||||||
| Maximum clot firmness (mm) | 9–25 | ||||||
| All patients | 36 (32–38) | 37 (30–40) | 41(30–44) | 37 (31–45) | 35 (28–47) | 0.080a | |
| SOFA≤10 | 37 (30–41) | 37 (30–41) | 40 (28–43) | 42 (31–46) | 32 (27–49) | 0.836b | |
| SOFA >10 | 36 (33–38) | 36 (31–40) | 42 (36–46) | 37 (32–42) | 38 (31–43) | 0.050c | |
| PLATELET function test | |||||||
| ARATEM test (sec) | 70–153 | ||||||
| All patients | 79 (54–110) | 87 (58–113) | 81 (62–112) | 114 (89–124)* | 110 (83–154)* | 0.014a | |
| SOFA≤10 | 79 (53–108) | 88 (65–112) | 85 (71–110) | 121 (115–154) | 140 (85–182) | 0.068b | |
| SOFA >10 | 79 (54–122) | 76 (36–113) | 62 (43–112) | 95 (65–110)# | 109 (83–116) | 0.161c | |
| ADPTEM test (sec) | 56–139 | ||||||
| All patients | 96 (65–111) | 105 (72–128) | 92 (76–117) | 127 (86–138) | 112 (67–134) | 0.004a | |
| SOFA≤10 | 105 (83–126) | 105 (89–127) | 92 (78–116) | 135 (115–148) | 126 (73–159) | 0.024b | |
| SOFA >10 | 85 (56–107) | 106 (51–135) | 91 (61–118) | 82 (63–129)# | 96 (67–116) | 0.121c |
Values represent median (IQR). SOFA: sequential organ failure assessment score. P values were calculated with the use of generalized estimating equations (GEE): (a): time effect, (b): group effect and (c): time-group interaction. Pairwise comparisons significant at the 0.05 level:
(*): time effect—pooled patients: each time point vs. Day 0.
(#): between group comparisons (group SOFA>10 vs. group SOFA ≤10) at each time point.
Platelet function test
Median (IQR) values of ARATEM and ADPTEM tests remained within the normal range during the study period, although both ARATEM (p = 0.014 for time effect) and ADPTEM (p = 0.004 for time effect) slightly increased over time in both groups (Table 4).
Fibrinolysis and endogenous inhibitors of coagulation
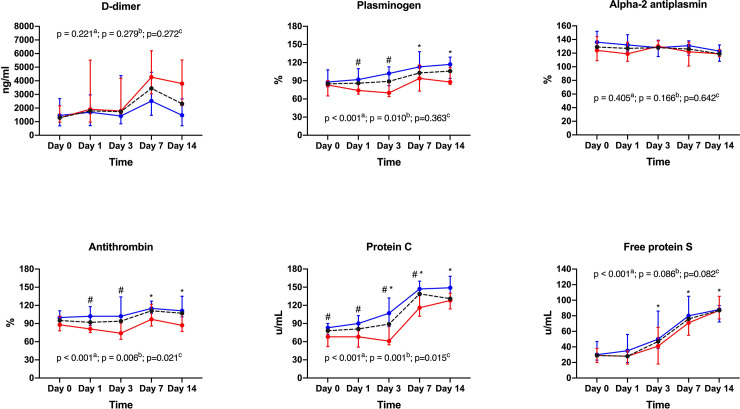
Fibrinolysis (INTEM and EXTEM maximum lysis) was low and decreased over time in all groups, with the most pronounced decrease observed in INTEM maximum lysis in group SOFA > 10 (p = 0.004 for time-group interaction) (Table 4). D-dimer plasma levels were 2 to 3-fold higher than normal reference range in both groups (Fig 2; S1 Table). Plasminogen median values remained within the normal range during the study period, although with a slight increase over time in both groups (p<0.001 for time effect). Alpha-2 antiplasmin remained unchanged during the study period (Fig 2; S1 Table).
Fig 2. Fibrinolysis and endogenous inhibitors of coagulation.
Antithrombin slightly increased over time in group SOFA ≤ 10 while it remained stable in group SOFA > 10 (p = 0.021 for time-group interaction) (Fig 2; S1 Table). Protein C plasma levels increased over time in both groups, although patients in group SOFA > 10 exhibited lower values in comparison to patients in group SOFA ≤ 10 (p = 0.015 for time-group interaction) (Fig 2; S1 Table). Free Protein S plasma levels were low in both groups at baseline and increased over time with no between-group differences (p<0.001 for time effect) (Fig 2; S1 Table).
Discussion
In this prospective longitudinal single center study, we demonstrated that patients admitted to the ICU with severe SARS-CoV-2 infection exhibited a pronounced hypercoagulability state, characterized by increased plasma fibrinogen levels, decreased free protein S plasma levels, and decreased fibrinolysis. The severity of coagulation derangements seems to correlate with the intensity of organ dysfunction according to the SOFA score. Finally, the hypercoagulability state of severe COVID-19 patients was detected by ROTEM and modifications on some coagulation tests related to the fibrinolytic and endogenous anticoagulation system, while the more common conventional coagulation tests, as aPTT (sec) INR and platelets, remained unchanged.
Approximately 5% of patients infected with SARS-CoV-2 become critically ill, developing organ dysfunction and failure [26]. Coagulopathy is frequently observed in COVID-19 patients [5–11] and has been associated with worse outcomes [3, 11, 27, 28]. For instance, Tang and cols. showed that abnormal conventional coagulation tests, especially markedly elevated D-dimer and FDP levels during hospitalization, were associated with poor prognosis in COVID-19 patients [11]. Moreover, disseminated intravascular coagulation (DIC) was more frequently observed in non-survivals (71.4% vs. 0.6%, respectively) compared to survivors [11].
The hemostatic disorders observed in critically ill COVID-19 patients are complex and multifactorial. The combination of varying degrees of hypoxemia, immune-mediated endothelial damage/dysfunction, and systemic inflammation have been implicated in the physiopathology of hypercoagulability state observed in COVID-19 patients [29]. Therefore, several authors have demonstrated that COVID-19 infection is associated with a high rate of venous and arterial thrombotic manifestations, such as DVT, pulmonary embolism, catheter-related thrombosis and ischemic stroke [5, 12, 30, 31]. Therefore, caution for thromboembolic events should be advised especially in the most severe patients, and the strategy for DVT prophylaxis / anticoagulation individualized.
Panigada and cols. recently demonstrated that patients admitted to the ICU infected with COVID-19 exhibited hypercoagulability by thromboelastography [8]. Similarly, we found that most patients admitted to the ICU with severe SARS-CoV-2 infection exhibited a pronounced hypercoagulability state, characterized by increased ROTEM INTEM, EXTEM and FIBTEM maximum clot firmness. Nevertheless, we did not observe consumption coagulopathy and laboratory findings suggesting DIC as observed by Tang and cols [11]. Our results are in line with other studies that did not show a DIC state in severe critical ill COVID-19 patients [5, 8, 30].
Hypercoagulability may be related to augmented levels of pro-coagulant factors, reduced levels of the naturally occurring anti-coagulant factors or both. Fibrinogen levels, which were initially high in both groups at ICU admission, decreased during the ICU stay. The decrease was more pronounced in patients with lower maximum SOFA score (group SOFA ≤ 10) and after day 7. Also, the naturally occurring anti-coagulant factors, such as protein C and antithrombin were lower in the sicker patients (SOFA >10). The differences observed over time between the groups in our study may reflect a higher pro-coagulant tendency in the higher SOFA group and may explain the worst clinical outcomes observed in the group of patients with SOFA > 10.
Protein C has anti-coagulant, profibrinolytic, and anti-inflammatory effects [33]. It has been shown that septic patients with low plasma protein C concentrations have a higher incidence of organ dysfunction and worse outcomes [33–35]. Furthermore, the occurrence of fibrinolysis suppression in patients with septic shock was associated with disease severity and lower survival [36]. Additionally, it has been shown that patients with acute respiratory distress syndrome (ARDS) have higher levels of plasminogen activator inhibitor-1 (PAI-1), which contribute to fibrin deposition in lung parenchyma [37]. This abnormality has been shown also during the SARS-CoV-1 pandemic [38].
There are some pathophysiological changes secondary to COVID-19 infection that may contribute to a greater chance of patients infected with COVID-19 to develop thrombotic complications as an increased angiotensin II expression secondary to angiotensin-converting enzyme 2 receptor binding and consequently augmented plasminogen activator inhibitor C-1 expression with a reduced fibrinolysis in the anticoagulation system [39].
Further, angiotensin II–mediated pulmonary vasoconstriction can predispose to stasis and hypercoagulability, as can COVID-19 induction of antiphospholipid antibodies and complement during cytokine storms, causing vasculitis and microthromboses [40].
Patients in group SOFA > 10 had lower plasminogen levels than patients with SOFA <10, which may represent less fibrinolysis activity during a state of hypercoagulability and, consequently, a greater probability of microthrombi formation in the microcirculation of different organs. This might explain the higher rate of organ dysfunction in group SOFA > 10. Nevertheless, our data do not allow us to confirm or refute this hypothesis. We did not evidence differences in the fibrinolytic behavior in both groups accordingly to ROTEM. One possible explanation for this fact is the high values of fibrinogen present in both groups, which compromise detection of increased thrombotic activity by ROTEM maximum lysis. The hypothesis that the fibrinolytic system is overwhelmed during COVID-19 infection has been commented in a recent paper discussing the association of COVD-19 and the fibrinolytic pathway [7].
We believe that despite several studies addressing the alterations in the coagulation system of COVID-19 patients, [41–46] our study continues to bring important news because it was one of the few that evaluated the behavior of the coagulation system, of critically critical patients infected with COVID-19, during two weeks of hospitalization. Also, our study was one of the few where we tried to assess the impact of the degree of change in the coagulation system and its impact on the evolution of organ dysfunctions presented by patients during the two weeks of study.
Several mechanics can explain the relationship between viral infection and our findings, as endothelial cell disruption, tissue factor expression, and activation of the coagulation cascade by cytokines released during viral infections are other possible mechanisms of thrombosis. This pro-inflammatory state can promote microthrombosis in the vascular lung system and consequently promoting more hypoxia with local impact creating a deleterious positive thrombo-inflammatory feedback loop [39, 47, 48].
Our study has some limitations. First, it is a single-center study with a relatively small sample size. However, to the best of our knowledge, it was the first study to perform a comprehensive and longitudinal assessment of coagulation profile in critically ill COVID-19 patients during the first two weeks of ICU stay. Secondly, during the study period, all the ICU beds available in our department were destinated to COVID-19 patients. Thus, inclusion of a control group without severe SARS-CoV-2 infection was not possible. Nevertheless, the coagulation profile of patients admitted to the ICU in our center has been recently addressed [15]. Also, a recent study has already demonstrated a higher incidence of thrombotic complications were diagnosed in COVID-19 ARDS patients than in patients with non-COVID-19 ARDS [5]. Third, all patients were already receiving anticoagulants as DVT prophylaxis or systemic anticoagulation and these could change the ROTEM results. Fourth, we did not evaluate all factors involved in fibrinolysis, such as PAI-1 and plasmin, which preclude us to fully understand the role of fibrinolytic system on COVID-19 induced coagulopathy and also other laboratory tests as prothrombin fragment 1+2, thrombin-anti-thrombin complexes and endogenous thrombin potential assays were not done to better understand the hypercoagulability state of such patients. Finally, we not measured any marker for endothelial dysfunction which probably contribute to the modifications on the coagulation system of the severe patients infected with SARS-CoV-2. Nevertheless, our study was the first to demonstrate some aspects of coagulation disorders that can occur in critical patients infected by COVID-19, especially the deficiency of naturally anti-coagulant factors.
Conclusion
Patients admitted to the ICU with COVID-19 have a pronounced hypercoagulability state, characterized by impaired endogenous anticoagulation system and decreased fibrinolysis. Moreover, the magnitude of coagulation abnormalities seems to correlate with the severity of organ dysfunction. The hypercoagulability state of patients infected with SARS-CoV-2 was detected by ROTEM and other coagulation tests but not with usual coagulation tests. Our findings highlight the role of rotational thromboelastometry when monitoring the coagulation system in ICU patients with COVID-19 and also demonstrated that the mechanisms to explain the hypercoagulability state of patients infected with SARS-CoV-2 is very complex and need more studies.
Supporting information
(DOCX)
Acknowledgments
We thank Helena Spalic for proofreading this manuscript.
List of abbreviations
- ARDS
acute respiratory distress syndrome
- aPTT
activated partial thromboplastin time
- CFT
clot formation time
- CCT
conventional coagulation tests
- COPD
chronic obstructive pulmonary disease
- COVID-19
coronavirus disease 2019
- CT
clotting time
- DVT
deep vein thrombosis
- HFH
unfractionated heparin
- ICU
intensive care unit
- INR
international normalized ratio
- LMWH
low-molecular-weight heparin
- LOS
length of stay
- MCF
maximum clot firmness
- PAI-1
plasminogen activator inhibitor-1
- PCC
prothrombin complex concentrate
- PT
prothrombin time
- RRT
renal replacement therapy
- ROTEM
rotational thromboelastometry
- RT-PCR
reverse-transcriptase-polymerasechain-reaction
- SAPS III
simplified acute physiology score III
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- SOFA
sequential organ failure assessment score
- TT
thrombin time
- UFH
unfractionated heparin
Data Availability
All relevant data are available from Dryad (doi:10.5061/dryad.pg4f4qrn3).
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Organization WH. Coronavirus disease 2019 (COVID-19) Situation Report– 178. Data as received by WHO from national authorities by 10:00 CEST, 16 July 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200716-covid-19-sitrep-178.pdf?sfvrsn=28ee165b_22020
- 2.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. Jama. 2020. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petrilli CM, Jones SA, Yang J, Rajagopalan H, O'Donnell L, Chernyak Y, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966 10.1136/bmj.m1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020. 10.1007/s00134-020-06062-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han H, Yang L, Liu R, Liu F, Wu KL, Li J, et al. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020. 10.1515/cclm-2020-0188 [DOI] [PubMed] [Google Scholar]
- 7.Mortus JR, Manek SE, Brubaker LS, Loor M, Cruz MA, Trautner BW, et al. Thromboelastographic Results and Hypercoagulability Syndrome in Patients With Coronavirus Disease 2019 Who Are Critically Ill. JAMA Netw Open. 2020;3(6):e2011192 10.1001/jamanetworkopen.2020.11192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V, et al. Hypercoagulability of COVID-19 patients in intensive care unit: A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020. 10.1111/jth.14850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ranucci M, Ballotta A, Di Dedda U, Bayshnikova E, Dei Poli M, Resta M, et al. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost. 2020. 10.1111/jth.14854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A meta-analysis. Clin Chim Acta. 2020;506:145–8. 10.1016/j.cca.2020.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–7. 10.1111/jth.14768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klok FA, Kruip M, van der Meer NJM, Arbous MS, Gommers D, Kant KM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. Bmj. 2020;368:m1091 10.1136/bmj.m1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barrett CD, Moore HB, Yaffe MB, Moore EE. ISTH interim guidance on recognition and management of coagulopathy in COVID-19: A Comment. Journal of Thrombosis and Haemostasis.n/a(n/a). 10.1111/jth.14860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crochemore T, Correa TD, Lance MD, Solomon C, Neto AS, Guerra JCC, et al. Thromboelastometry profile in critically ill patients: A single-center, retrospective, observational study. PLoS One. 2018;13(2):e0192965 10.1371/journal.pone.0192965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whiting D, DiNardo JA. TEG and ROTEM: technology and clinical applications. Am J Hematol. 2014;89(2):228–32. 10.1002/ajh.23599 [DOI] [PubMed] [Google Scholar]
- 17.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–7. 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 18.Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lier H, Vorweg M, Hanke A, Gorlinger K. Thromboelastometry guided therapy of severe bleeding. Essener Runde algorithm. Hamostaseologie. 2013;33(1):51–61. 10.5482/HAMO-12-05-0011 [DOI] [PubMed] [Google Scholar]
- 20.Crochemore T, Piza FMT, Rodrigues RDR, Guerra JCC, Ferraz LJR, Correa TD. A new era of thromboelastometry. Einstein (Sao Paulo). 2017;15(3):380–5. 10.1590/S1679-45082017MD3130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petricevic M, Konosic S, Biocina B, Dirkmann D, White A, Mihaljevic MZ, et al. Bleeding risk assessment in patients undergoing elective cardiac surgery using ROTEM((R)) platelet and Multiplate((R)) impedance aggregometry. Anaesthesia. 2016;71(6):636–47. 10.1111/anae.13303 [DOI] [PubMed] [Google Scholar]
- 22.Zampieri FG, Soares M, Borges LP, Salluh JIF, Ranzani OT. The Epimed Monitor ICU Database(R): a cloud-based national registry for adult intensive care unit patients in Brazil. Rev Bras Ter Intensiva. 2017;29(4):418–26. 10.5935/0103-507X.20170062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moreno RP, Metnitz PG, Almeida E, Jordan B, Bauer P, Campos RA, et al. SAPS 3—From evaluation of the patient to evaluation of the intensive care unit. Part 2: Development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med. 2005;31(10):1345–55. 10.1007/s00134-005-2763-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vincent JL, Moreno R, Takala J, Willatts S, De MA, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–10. 10.1007/BF01709751 [DOI] [PubMed] [Google Scholar]
- 25.Moreno R, Vincent JL, Matos R, Mendonca A, Cantraine F, Thijs L, et al. The use of maximum SOFA score to quantify organ dysfunction/failure in intensive care. Results of a prospective, multicentre study. Working Group on Sepsis related Problems of the ESICM. Intensive Care Med. 1999;25(7):686–96. 10.1007/s001340050931 [DOI] [PubMed] [Google Scholar]
- 26.Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. N Engl J Med. 2020. [DOI] [PubMed] [Google Scholar]
- 27.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–62. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020. 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joly BS, Siguret V, Veyradier A. Understanding pathophysiology of hemostasis disorders in critically ill patients with COVID-19. Intensive Care Med. 2020. 10.1007/s00134-020-06088-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thrombosis research. 2020;191:9–14. 10.1016/j.thromres.2020.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. Journal of the American College of Cardiology. 2020;75(23):2950–73. 10.1016/j.jacc.2020.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarangi PP, Lee HW, Kim M. Activated protein C action in inflammation. Br J Haematol. 2010;148(6):817–33. 10.1111/j.1365-2141.2009.08020.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brunkhorst F, Sakr Y, Hagel S, Reinhart K. Protein C concentrations correlate with organ dysfunction and predict outcome independent of the presence of sepsis. Anesthesiology. 2007;107(1):15–23. 10.1097/01.anes.0000267531.39410.d3 [DOI] [PubMed] [Google Scholar]
- 34.Mesters RM, Helterbrand J, Utterback BG, Yan B, Chao YB, Fernandez JA, et al. Prognostic value of protein C concentrations in neutropenic patients at high risk of severe septic complications. Crit Care Med. 2000;28(7):2209–16. 10.1097/00003246-200007000-00005 [DOI] [PubMed] [Google Scholar]
- 35.Yan SB, Helterbrand JD, Hartman DL, Wright TJ, Bernard GR. Low levels of protein C are associated with poor outcome in severe sepsis. Chest. 2001;120(3):915–22. 10.1378/chest.120.3.915 [DOI] [PubMed] [Google Scholar]
- 36.Semeraro F, Colucci M, Caironi P, Masson S, Ammollo CT, Teli R, et al. Platelet Drop and Fibrinolytic Shutdown in Patients With Sepsis. Critical care medicine. 2018;46(3):e221–e8. 10.1097/CCM.0000000000002919 [DOI] [PubMed] [Google Scholar]
- 37.Bertozzi P, Astedt B, Zenzius L, Lynch K, LeMaire F, Zapol W, et al. Depressed bronchoalveolar urokinase activity in patients with adult respiratory distress syndrome. N Engl J Med. 1990;322(13):890–7. 10.1056/NEJM199003293221304 [DOI] [PubMed] [Google Scholar]
- 38.Gralinski LE, Bankhead A 3rd, Jeng S, Menachery VD, Proll S, Belisle SE, et al. Mechanisms of severe acute respiratory syndrome coronavirus-induced acute lung injury. mBio. 2013;4(4). 10.1128/mBio.00271-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dolhnikoff M, Duarte-Neto AN, de Almeida Monteiro RA, et al. Pathological evidence of pulmonary thrombotic phenomena in severe COVID-19. J Thromb Haemost. Published online April 15, 2020) 10.1111/jth.14844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Medcalf RL, Keragala CB, Myles PS. Fibrinolysis and COVID-19: a plasmin paradox. J Thromb Haemost. 2020. 10.1111/jth.14960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hardy M, Douxfils J, Bareille M, Lessire S, Gouin-Thibault I, Fontana P, et al. Studies on hemostasis in COVID-19 deserve careful reporting of the laboratory methods, their significance and their limitations. J Thromb Haemost. 2020. August 13: 10.1111/jth.15061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Collett LW, Gluck S, Strickland RM, Reddi BJ. Evaluation of coagulation tatus using viscoelastic testing in intensive care patients with coronavirus disease 2019 (COVID-19): An observational point prevalence cohort study. Aust Crit Care. 2020. July 21:S1036-7314(20)30254-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Creel-Bulos C, Auld SC, Caridi-Scheible M, Barker N, Friend S, Gaddh M, Kempton CL, Maier C, Nahab F, Sniecinski R. Fibrinolysis Shutdown and Thrombosis in A COVID-19 ICU. Shock. 2020. August 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ibañez C, Perdomo J, Calvo A, Ferrando C, Reverter JC, Tassies D, Blasi A. High D dimers and low global fibrinolysis coexist in COVID19 patients: what is going on in there? J Thromb Thrombolysis. 2020. July 15:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nougier C, Benoit R, Simon M, Desmurs-Clavel H, Marcotte G, Argaud L, et al. Hypofibrinolytic state and high thrombin generation may play a major role in SARS-COV2 associated thrombosis. J Thromb Haemost. 2020. July 15:10.1111/jth.15016. 10.1111/jth.15016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pavoni V, Gianesello L, Pazzi M, Stera C, Meconi T, Frigieri FC. Evaluation of coagulation function by rotation thromboelastometry in critically ill patients with severe COVID-19 pneumonia. J Thromb Thrombolysis. 2020. 10.1007/s11239-020-02130-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wool GD, Miller JL. The Impact of COVID-19 Disease on Platelets and Coagulation Pathobiology. 2020. October 13:1–13. 10.1159/000512007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Escher R, Breakey N, Lämmle B. Severe COVID-19 infection associated with endothelial activation. Thromb Res. 2020. June;190:62) 10.1016/j.thromres.2020.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]