Abstract
Background: Randomized trials and observation studies have revealed conflicting results regarding the interaction between clopidogrel and proton pump inhibitors (PPIs). The aim of our study was to provide laboratory evidence regarding whether PPIs blunt the antiplatelet reactivity of clopidogrel.
Methods: We included records of Asian patients who received clopidogrel treatment for cardiovascular or cerebrovascular events and the VerifyNow P2Y12 assay for platelet reactivity monitoring. The responsiveness of antiplatelet effect to clopidogrel was analyzed according to 3 criteria:
-
(1)
percentage of platelet inhibition (PI) > 20%,
-
(2)
absolute P2Y12 reaction unit (PRU) < 235, and
-
(3)
PRU < 262.
Results: Patients treated without PPIs did not differ significantly from those concomitantly treated with PPIs in terms of levels of PI (25.7% ± 24.3% vs 23.0 ± 25.3%, P = .4315), PRU (187.3 ± 74.0 vs 197.4 ± 77.3, P = .3373), or responsiveness to antiplatelet (adjusted absolute risk, 3.5%; 95% confidence interval, − 10.7 to 17.7%; P = .6297). Patients treated with lansoprazole, esomeprazole, pantoprazole, and rabeprazole exhibited no significant differences in PRU or PI levels compared with those treated without PPIs. By contrast, patients treated with dexlansoprazole exhibited a significantly decreased level of PI (25.7% ± 24.3% vs 14.0% ± 21.6%, P = .0297) and responsiveness to clopidogrel under the criterion PI > 20% (adjusted absolute risk: 10.5%; 95% confidence interval: 2.6% to 43.6%; P = .0274).
Conclusion: No robust interaction between clopidogrel and PPIs was found, but caution should be exercised in the concomitant use of dexlansoprazole and clopidogrel in Asians.
Keywords: clopidogrel, P2Y12 inhibitor, platelets, proton-pump inhibitor, VerifyNow assay
1. Introduction
Aspirin has been known associated with gastrointestinal mucosa damage[1] and increased risk of peptic ulcer.[2,3] Clopidogrel is an alternative antiplatelet which inhibits platelet aggregation through irreversibly binding to P2Y12 receptor.[4] Besides, clopidogrel is associated with less gastrointestinal discomfort and hemorrhage events when compared to aspirin.[5] Recently, dual antiplatelet therapy (DAPT), defined as use P2Y12 receptor inhibitors (such as clopidogrel, ticagrelor and prasugrel) with aspirin, has been highly recommended for patients with acute coronary syndrome or thrombotic events following percutaneous coronary intervention.[6,7] Besides, DAPT has been recommended for patients with transient ischemic attack or minor acute ischemic stroke and should be continued for 21–90 days.[8,9]
While DAPT carries a higher risk of gastrointestinal bleeding,[10,11] prophylactic prescription of proton pump inhibitors (PPIs) for patients with DAPT increases.[12] Clopidogrel is also a prodrug, which requires 2 sequential oxidative steps through the hepatic cytochrome P450 2C19 (CYP2C19) and CYP3A4/5 to form an active metabolite.[13,14] Concern has remained regarding the interaction between PPIs and clopidogrel since PPIs were reported inhibit hepatic CYP2C19.[15] Previous studies, the Clopidogrel and the Optimization of Gastrointestinal Events Trial (COGENT),[13] Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation 44, [16] Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel Thrombolysis In Myocardial Infarction 38,[16] and Platelet Inhibition and Patient Outcomes[17] have revealed conflicting data regarding the effects of concomitant use of clopidogrel and PPIs on cardiovascular events. Accordingly, we aimed to conduct this study using VerifyNow P2Y12 Assay to investigate whether the use of PPIs may blunt the antiplatelet effects of clopidogrel.
2. Methods
2.1. Design and study patients
The VerifyNow P2Y12 assay (Accumetrics, San Diego, CA, USA), a new device of point-of-care test, is effective in evaluating platelet aggregation inhibition induced by clopidogrel or other P2Y12 receptor inhibitors.[18,19] This study was a retrospective electronic medical record review. From January 1, 2016, to May 31, 2019, the list of patients who received the test of VerifyNow P2Y12 assay was obtained from the laboratory information system of Far Eastern Memorial Hospital, New Taipei, Taiwan. The electronic medical records for patients tested with VerifyNow P2Y12 assay were reviewed. The patients who were treated with clopidogrel were included. The exclusion criteria were
-
(1)
patients who were aged < 20-year-old, and
-
(2)
use of other P2Y12 inhibitor, such as prasugrel and ticagrelor.
Subsequently, we categorized patients under clopidogrel treatment into 2 groups: group 1, patients treated without PPI, and group 2, patients concomitantly treated with PPIs. Concomitant treatment with PPIs was defined as any treatment involving the combination of clopidogrel and PPIs in the 7 days prior to phlebotomy for the VerifyNow P2Y12 assay. This study was approved by the Institutional Review Board of Far Eastern Memorial Hospital, Taiwan (reference number: 108099E).
2.2. Data collection
Data on age; sex; medical history of hypertension, diabetes mellitus, dyslipidemia, ischemic stroke, myocardial infarction, and chronic kidney disease; concurrent medications; and the clinical indication of antiplatelet use for the period from January 1, 2016 to May 31, 2019, were obtained from the electronic medical records and laboratory information system of Far Eastern Memorial Hospital. A cardiovascular event was defined as the development of acute coronary syndrome or coronary artery thrombosis managed through percutaneous coronary intervention. A cerebrovascular event was defined as the occurrence of transient ischemic attack or acute ischemic stroke. The medical record review was performed by authors SF Lin and PC Lin.
2.3. VerifyNow P2Y12 assay
The VerifyNow P2Y12 assay was completed within 4 hours after phlebotomy, and the assay results are expressed as either absolute P2Y12 reaction units (PRU) or as percentage of platelet inhibition (PI).[19,20] The PI value was adjusted according to the platelet base function value using the following formula: PI = (platelet base function − PRU)/PRU × 100%. To determine favorable antiplatelet effects engendered by clopidogrel (low on-treatment platelet reactivity, LTPR) in patients, we adopted 3 universal standards:
Study of Prasugrel compared with clopidogrel For Japanese patients with acute coronary syndrome undergoing percutaneous coronary intervention study showed that PRU level of < 262 was more optimal cutoff value for Asians.[26]
2.4. Statistical analysis
Patient characteristics are presented as mean and standard deviation for continuous variables and number and frequencies for categorical variables. To compare the characteristics of patients in groups 1 and 2, we used Student t test for continuous variables and Pearson chi-squared test or Fisher exact test for categorical variables. Moreover, to thoroughly investigate the interaction of clopidogrel and each PPI, we used both additive and multiplicative models (as a sensitivity analysis). Regarding the additive model, we used a generalized linear model (with an identity function being the link function and the data distribution being binomial); in this model, responsiveness to clopidogrel (as determined using the 3 standards for LTPR) served as the dependent variable and treatment with or without PPIs served as the independent variable. Regarding the multiplicative model, we used a logistic regression model, with responsiveness to clopidogrel (LTPR) being the dependent variable and treatment with or without PPIs serving as the independent variable. A covariate of DAPT was used for adjustment in the multiple regression analyses for both models. All analyses were performed using SAS 9.4 software (SAS Inc., Cary, NC).
3. Results
3.1. Patients
At first, a total of 237 patients were initially enrolled, 25 of whom were excluded because of ticagrelor use. Of the remaining 212 clopidogrel users, 122 (57.5%) and 90 (42.5%) were categorized into groups 1 and 2, respectively. Table 1 presents the characteristics of the patients in the 2 groups. Compared with group 1, group 2 had a higher proportion of patients who received DAPT and higher proportion of patients with cerebrovascular events. No significant differences were observed between the 2 groups in terms of age; sex; comorbidities of hypertension, diabetes mellitus, dyslipidemia, ischemic stroke, myocardial infarction, and chronic kidney disease; or concurrent medications with a potential to increase and reduce the antiplatelet effects of clopidogrel (Supplemental Table I).
Table 1.
Characteristics of patients treated with and without PPIs.
| Characteristics | Group 1 Patient Treated without PPI (N = 122) | Group 2 Patient Treated with PPI (N = 90) | P value |
| Age (yr) | 65.9 ± 11.5 | 66.9 ± 12.0 | .5113 |
| Sex, N (%) | .0965 | ||
| Female | 38/122 (31.2%) | 39 (42.2%) | |
| Male | 84/122 (68.9%) | 54 (57.8%) | |
| Comorbidity, N (%) | |||
| Hypertension | 51/122 (41.8%) | 30/90 (33.3%) | .2097 |
| Diabetes mellitus | 40/122 (32.8%) | 23/90 (25.6%) | .2548 |
| Dyslipidemia | 50/122 (41.0%) | 30/90 (33.3%) | .2560 |
| Ischemic stroke | 29/122 (20.5%) | 31/90 (34.4%) | .0881 |
| Myocardial infarction | 9/122 (7.4%) | 10/90 (11.1%) | .3468 |
| Chronic kidney disease | 5/122 (4.1%) | 3/90 (3.3%) | .7726 |
| Clinical Events, N (%) | .0004∗ | ||
| Cardiovascular events | 69/122 (56.6%) | 29/90 (32.2%) | |
| Cerebrovascular events | 53/122 (43.4%) | 61/90 (67.8%) | |
| Dual antiplatelet, N (%) | 79/122 (64.8%) | 81/90 (90.0%) | <.0001∗ |
| PPI medications, N (%) | |||
| Lansoprazole | – | 44/90 (48.9%) | |
| Dexlansoprazole | – | 24/90 (26.7%) | |
| Pantoprazole | – | 16/90 (17.8%) | |
| Esomeprazole | – | 15/90 (16.7%) | |
| Rabeprazole | – | 8/90 (8.9%) | |
N = number, PPI = proton pump inhibitor.
3.2. VerifyNow P2Y12 assay
Table 2 shows the VerifyNow P2Y12 assay results for groups 1 and 2. Comparing patients treated without PPIs and those treated with any PPIs revealed no significant differences in the levels of PRU (187.3 ± 74.0 vs 197.4 ± 77.3, P = .3373) or PI (25.7% ± 24.3% vs 23.0% ± 25.3%, P = .4315). Regarding individual PPIs, patients treated with lansoprazole, pantoprazole, esomeprazole, and rabeprazole showed no significant differences in PRU or PI levels when compared to the group without PPIs. However, dexlansoprazole showed a reduced PI level compared with those treated without PPIs (14.0% ± 21.6% vs 25.7% ± 24.3%, P = .0297).
Table 2.
Comparison of VerifyNow P2Y12 assay results for patients treated without and with PPIs.
| N | PRU | P value | Base | P value | PI | P value | |
| Group 1:Without PPI (reference group) | 122 | 187.3 ± 74.0 | – | 244.3 ± 56.3 | – | 25.7 ± 24.3% | – |
| Group 2: Any PPIs | 90 | 197.4 ± 77.3 | .3373 | 246.5 ± 55.5 | .7809 | 23.0 ± 25.3% | .4315 |
| Individual PPI | |||||||
| Lansoprazole | 44 | 192.4 ± 82.1 | .7023 | 252.0 ± 60.6 | .4472 | 26.3 ± 26.9% | .9004 |
| Dexlansoprazole | 24 | 207.8 ± 73.1 | .2156 | 225.5 ± 52.7 | .1323 | 14.0 ± 21.6% | .0297∗ |
| Pantoprazole | 16 | 202.1 ± 82.7 | .4578 | 248.3 ± 40.1 | .7856 | 22.7 ± 27.1% | .6440 |
| Esomeprazole | 15 | 195.4 ± 105.5 | .7760 | 237.3 ± 82.5 | .7532 | 26.4 ± 31.8% | .9217 |
| Rabeprazole | 8 | 172.4 ± 57.4 | .5778 | 251.9 ± 78.7 | .7215 | 34.9 ± 21.2% | .3011 |
N = number, PI = percentage of platelet inhibition, PPI = proton pump inhibitor, PRU = absolute P2Y12 reaction unit.
3.3. Proportions of responsiveness to clopidogrel treatment
The 2 groups’ responsiveness to clopidogrel (LTPR), which was defined by criteria of
-
(1)
PI > 20%,
-
(2)
PRU < 235, and
-
(3)
PRU < 262 were shown in Table 3.
Table 3.
Responsiveness (LTPR) to clopidogrel in patients treated without and with PPIs.
| Addictive Models | |||||||
| Criteria in Defining LTPR | Group 1 Responders for Treated without PPI | Group 2 Responders for Treated with PPI | P value | Crude AR Difference (95% CI) | P value | Adjusted AR Difference (95% CI)† | P value |
| (1) By inhibition >20% | |||||||
| Any PPIs | 63/122 (51.6%) | 41/90 (45.6%) | .3811 | 6.1% (−7.5–19.7%) | 0.3800 | 3.5% (−10.7–17.7%) | .6297 |
| Individual PPI | |||||||
| Lansoprazole | 63/122 (51.6%) | 23/44 (52.3%) | .9425 | −0.6% (−17.9– 16.6%) | 0.9425 | −3.0% (−20.6–14.7%) | .7406 |
| Dexlansoprazole | 63/122 (51.6%) | 6/24 (25.0%) | .0169∗ | 26.6% (7.2–46.1%) | 0.0073∗ | 10.5% (2.6–43.6%) | .0274∗ |
| Pantoprazole | 63/122 (51.6%) | 7/16 (43.8%) | .5529 | 7.9% (−18.0–33.8%) | 0.5501 | 5.6% (−20.5–31.8%) | .6722 |
| Esomeprazole | 63/122 (51.6%) | 8/17 (47.1%) | .7134 | 11.6% (−14.7–38.0%) | 0.3863 | 9.4% (−17.2–36.0%) | .4871 |
| Rabeprazole | 63/122 (51.6%) | 6/8 (75.0%) | .2004 | −23.4% (−54.7–7.9%) | 0.1434 | −26.9% (−58.9–5.1%) | .0991 |
| (2) By PRU <235 | |||||||
| Any PPIs | 82/122 (67.2%) | 53/90 (58.9%) | .2129 | 8.3% (−4.8– 21.5%) | 0.2145 | 4.1% (−9.8–17.9%) | .5657 |
| Individual PPI | |||||||
| Lansoprazole | 82/122 (67.2%) | 27/44 (61.4%) | .4836 | 5.9% (−10.8–22.5%) | 0.4904 | 1.9% (−15.2– 19.0%) | .8274 |
| Dexlansoprazole | 82/122 (67.2%) | 12/24 (50.0%) | .1075 | 17.2% (−4.5–38.9%) | 0.1195 | 12.0% (−10.7–34.7%) | .2989 |
| Pantoprazole | 82/122 (67.2%) | 8/16 (50.0%) | .1741 | 17.2% (−8.7–43.1%) | 0.1923 | 14.2% (−11.7– 40.0%) | .2830 |
| Esomeprazole | 82/122 (67.2%) | 8/15 (53.3%) | .2853 | 13.9% (−12.7– 40.5%) | 0.3062 | 9.4% (−18.5– 37.3%) | .5091 |
| Rabeprazole | 82/122 (67.2%) | 7/8 (87.5%) | .2316 | −20.3% (−44.7–4.1%) | 0.1030 | −25.5% (−50.8–0.2%) | .0484∗ |
| (3) By PRU <262 | |||||||
| Any PPIs | 89/122 (73.0%) | 59/90 (65.6%) | .2463 | 7.4% (−5.2–20.0%) | 0.2496 | 3.1% (−9.6–15.9%) | .6296 |
| Individual PPI | |||||||
| Lansoprazole | 89/122 (73.0%) | 31/44 (66.7%) | .7511 | 2.5% (−13.1–18.1%) | 0.7541 | −1.1% (−16.3–14.0%) | .8843 |
| Dexlansoprazole | 89/122 (73.0%) | 12/24 (50.0%) | .0260∗ | 23.0% (1.5–44.5%) | 0.0364∗ | 15.8% (−6.8–38.4%) | .1695 |
| Pantoprazole | 89/122 (73.0%) | 10/16 (62.5%) | .3827 | 10.5% (−14.6–35.5%) | 0.4125 | 7.8% (−16.0– 31.6%) | .5201 |
| Esomeprazole | 89/122 (73.0%) | 9/15 (60.0%) | .2942 | 13.0% (−13.1–39.0%) | 0.3292 | 6.7% (−20.9–34.1%) | .6384 |
| Rabeprazole | 89/122 (73.0%) | 7/8 (87.5%) | .3643 | −14.6% (−38.8–9.7%) | 0.2393 | −21.7% (−46.9–3.5%) | .0917 |
AR = absolute risk, LTPR = low on-treatment platelet reactivity, PPI = proton pump inhibitor.
Statistical significance (P <.05).
Model was adjusted with covariate of dual antiplatelet use.
Generally, group 1 and 2 showed no significant difference for the responsiveness to clopidogrel. However, regarding individual PPIs, group 1 had a significantly higher percentage of patients with favorable responses to treatment than did group 2 only for dexlansoprazole under the criteria PI > 20% (51.6% vs 25.0%, P = .0169) and PRU < 262 (73.0% vs 50.0%, P = .0260).
3.4. Regression analyses for responsiveness to clopidogrel
Figure 1 presents the regression analysis results regarding the interaction between clopidogrel and PPIs. Concerning responsiveness to clopidogrel, no significant difference was observed between the 2 groups in the crude and adjusted models (Table 3). Regarding individual PPIs, patients treated with dexlansoprazole and rabeprazole exhibited significant differences in responsiveness to treatment compared with those treated without PPIs.
Figure 1.
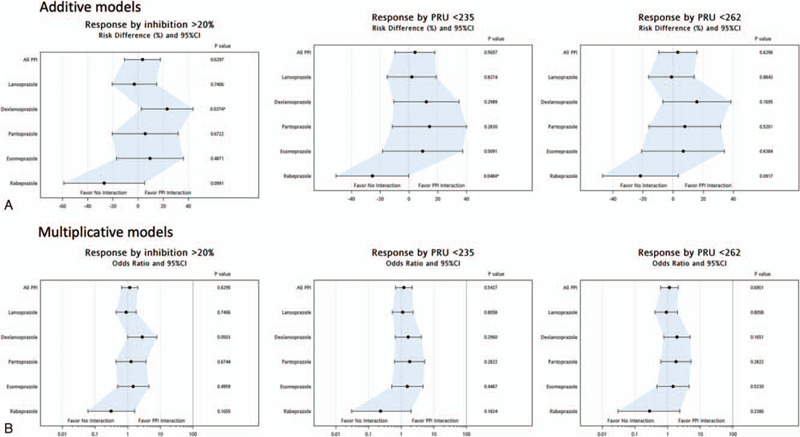
Interaction between clopidogrel and proton pump inhibitors in (A) additive, and (B) multiplicative models.
On the criterion PI > 20%, dexlansoprazole was determined to blunt the antiplatelet effects of clopidogrel in the crude (absolute risk [AR], 26.6%; 95% confidence interval [CI], 7.2–46.1%; P = .0073) and adjusted (AR, 10.5%; 95% CI, 2.6–43.6%; P = .0274) models. On the criterion PRU < 262, patients treated with dexlansoprazole showed significant differences from those treated without PPIs in the crude model (AR, 23.0%; 95% CI, 1.5–44.5%; P = .0364) but not in the adjusted model (AR, 7.8%; 95% CI, − 16.0 to 31.6%; P = .5201). On the criterion PRU < 235, rabeprazole showed no significant interaction with clopidogrel in the adjusted model (AR, − 25.5%; 95% CI, −50.8 to 0.2%; P = .0484). In a sensitivity analysis, the multiplicative model showed the same findings as did the additive model (Table 4).
Table 4.
Assessment of P2Y12 responsiveness in patients treated without and with PPIs (multiplicative model).
| Multiplicative Model | ||||
| Criteria in Defining LTPR | Crude OR (95% CI) | P value | Adjusted OR (95% CI)† | P value |
| (1) Criteria by inhibition >20% | ||||
| All PPIs | 1.28 (0.74–2.20) | .3814 | 1.15 (0.65–2.04) | .6295 |
| Individual PPI | ||||
| Lansoprazole | 0.98 (0.49–1.94) | .9426 | 0.89 (0.44–1.81) | .7406 |
| Dexlansoprazole | 3.20 (1.19–8.62) | .0212∗ | 2.78 (0.99–7.74) | .0503 |
| Pantoprazole | 1.37 (0.48–3.92) | .5540 | 1.26 (0.43–3.64) | .6744 |
| Esomeprazole | 1.60 (0.54–4.78) | .3980 | 1.47 (0.49–4.44) | .4959 |
| Rabeprazole | 0.36 (0.07–1.83) | .2168 | 0.31 (0.06–1.63) | .1655 |
| (2) Criteria by PRU < 235 | ||||
| All PPI | 1.43 (0.81–2.52) | .2137 | 1.20 (0.67–2.17) | .5427 |
| Individual PPI | ||||
| Lansoprazole | 1.29 (0.63–2.64) | .4841 | 1.10 (0.53–2.229) | .8058 |
| Dexlansoprazole | 2.05 (0.85–4.97) | .1119 | 1.63 (0.65–4.10) | .2960 |
| Pantoprazole | 2.05 (0.72–5.86) | .1804 | 1.80 (0.62–5.22) | .2822 |
| Esomeprazole | 1.79 (0.61–5.30) | .2901 | 1.53 (0.51–4.61) | .4467 |
| Rabeprazole | 0.29 (0.04–2.46) | .2583 | 0.23 (0.03–1.99) | .1834 |
| (2) Criteria by PRU < 262 | ||||
| All PPIs | 1.42 (0.79–2.56) | .2472 | 1.13 (0.61–2.10) | .6901 |
| Individual PPI | ||||
| Lansoprazole | 1.13 (0.53–2.42) | .7512 | 0.91 (0.41–1.99) | .8058 |
| Dexlansoprazole | 2.70 (1.10–6.60) | .0297∗ | 1.93 (0.76–4.86) | .1651 |
| Pantoprazole | 1.62 (0.55–4.80) | .3858 | 1.80 (0.62–5.22) | .2822 |
| Esomeprazole | 1.80 (0.59–5.44) | .2990 | 1.45 (0.47–4.50) | .5230 |
| Rabeprazole | 0.39 (0.05–3.25) | .3808 | 0.28 (0.03–2.35) | .2386 |
LTPR = low on-treatment platelet reactivity, OR = odds ratio, PPI = proton pump inhibitor. Model adjusted using the covariate of dual antiplatelet use.
Statistical significance (P <.05).
Model was adjusted using covariate of dual antiplatelet use.
4. Discussion
The interaction between clopidogrel and PPIs has been proposed to be drug-specific because each PPI inhibits CYP2C19 to varying degrees.[15] Theoretically, the active form of clopidogrel should be decreased by concomitant treatment with CYP2C19 inhibitors.[14] Through the VerifyNow P2Y12 assay, this study offers laboratory evidence regarding the interaction between clopidogrel and PPIs for real-world patients with cardiovascular or cerebrovascular events. Our results are consistent with those revealed by most large clinical trials,[13,16] which have suggested no strong interaction between clopidogrel and PPIs in general.
COGENT, the first randomized trial in this topic, did not rule out clinically significant cardiovascular interactions between clopidogrel and omeprazole.[13] Omeprazole was reported to have strong inhibitory effects on CYP2C19 metabolism.[15] Our results are similar to the findings of COGENT; that is, no significant interaction existed between clopidogrel and esomeprazole, a strong CYP2C19 inhibitor.[15,27] Rabeprazole was associated with the highest proportion of responsiveness to clopidogrel compared with the other PPIs, although the corresponding samples were very small. This is consistent with previous reports that the clearance of rabeprazole is nonenzymatic and that rabeprazole is not metabolized by CYP2C19.[8,15,28]
We observed an interaction between clopidogrel and the weak CYP2C19 inhibitor dexlansoprazole[15] under the criterion PI > 20%. This finding is explained as follows. Despite being a weak CYP2C19 inhibitor, dexlansoprazole had pharmaceutical formulation of dual delayed form for 24-hour symptom control and an extremely short time to peak level.[29] This could be attributed to the confounding effects of our patients’ CYP2C19 genotype polymorphisms. Asians were reported to exhibit a higher frequency of poor metabolizer genotypes (homozygous loss of function allele) for CYP2C19 (13%–23%) compared with other races (2%–5%).[30] Additionally, pharmacokinetic data obtained for Japanese patients revealed that the plasma dexlansoprazole concentration in patients with poor metabolizer phenotypes was higher than that in those with normal metabolizer phenotypes by 12-fold.[29] The platelet inhibition and patient outcomes trial[17] also revealed increased outcomes of cardiovascular events among groups concomitantly treated with any P2Y12 inhibitor (either clopidogrel or ticagrelor) and PPIs. PPI use is thus likely to be an indicator of higher rates of complication.
Compared to the previous studies, the distinction pints in this study are outlined as follows. First, we provide laboratory evidence regarding the interaction between clopidogrel and different types PPIs in real-world patients. Second, a newer PPI of dexlansoprazole was included in our final analyses. In Taiwan, dexlansoprazole was firstly introduced in 2014, and its medical costs were covered by the National Health Insurance program. Finally, different criteria for responsive to clopidogrel were used. Our analyses showed no robust interaction between clopidogrel and PPIs. The sensitivity analysis results using the multiplicative models were consistent with those obtained using the additive models. These should increase the validity of our findings.
This study has some limitations. Since this was a retrospective observational study, some unmeasurable difference between the group 1 and 2 may exist. First, the CYP2C19 polymorphisms was not examined in our patients. Though dexlansoprazole was found negatively associated with platelet aggregation, the confounding effect by poor metabolizer of CYP2C19 cannot be ruled out. Second, using of VerifyNow P2Y12 assay was dependent on the discretion of each physician. The cost of VerifyNow P2Y12 assay is approximately US$150, which is a relative high price for patients in Taiwan. Physicians may prescribe this test for more complicated cases. However, the proportions of patients receiving such PPIs are comparable to those in previous studies. For the examples, the Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation 44 Study and trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel thrombolysis in myocardial infarction 38 trials revealed that 53 (26.4%) and 4529 (33.3%) individuals concomitantly received P2Y12 inhibitors and PPIs, respectively.[16] Therefore, our 2 groups were comparable and not confounded by the factor of prescription of VerifyNow P2Y12 assay. Third, the studies were limited to ethnic Asian. The poor metabolizers of CYP2C19 may be more common in Asians.[30] Fourth, the sample size was relatively small. This restricted another analytic approach by propensity score matching.[31,32] In this study, VerifyNow P2Y12 assay offered the evidence of clinical phenotype for the interaction between clopidogrel and PPIs. Studies investigating of interaction between clopidogrel and PPIs with CYP2C19 polymorphism and large sample size are needed in the future.
In conclusion, this study of real-world patients provides laboratory evidence with VerifyNow P2Y 12 assay regarding the interaction between clopidogrel and PPIs. No robust association between the 2 was found. Interaction for concomitant use of dexlansoprazole and clopidogrel in terms of PI may be caused by confounding effect. This study should improve decision-making for concomitant use of PPIs and clopidogrel.
Author contributions
Conceptualization: Sheng-Feng Lin, Pei-Chin Lin, Chih-Chun Chang, Wei-Lun Chang.
Data curation: Sheng-Feng Lin.
Formal analysis: Sheng-Feng Lin, Pei-Chin Lin, Wei-Lun Chang.
Investigation: Sheng-Feng Lin.
Methodology: Sheng-Feng Lin, Fang-Yeh Chu.
Software: Sheng-Feng Lin.
Supervision: Fang-Yeh Chu.
Validation: Fang-Yeh Chu.
Writing – original draft: Sheng-Feng Lin.
Writing – review & editing: Sheng-Feng Lin.
Supplementary Material
Footnotes
Abbreviations: AR = absolute risk, CI = confidence interval, COGENT = Clopidogrel and the Optimization of Gastrointestinal Events Trial, CYP = cytochrome P450, DAPT = dual antiplatelet therapy, LTPR = low on-treatment platelet reactivity, PI = percentage of platelet inhibition, PPI = proton pump inhibitors, PRU = P2Y12 reaction unit.
How to cite this article: Lin SF, Lin PC, Chang CC, Chang WL, Chu FY. Investigation of the interaction between proton pump inhibitors and clopidogrel using VerifyNow P2Y12 assay. Medicine. 2020;99:50(e23695).
Author SFL wrote the first draft of this manuscript. This study was supported by the Department of Clinical Pathology, Far Eastern Memorial Hospital, New Taipei, Taiwan. This study received no funding.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
Supplemental digital content is available for this article.
References
- [1].Kauffman G. Aspirin-induced gastric mucosal injury: lessons learned from animal models. Gastroenterology 1989;96: 2 Pt 2 Suppl: 606–14. [DOI] [PubMed] [Google Scholar]
- [2].Kawamura N, Ito Y, Sasaki M, et al. Low-dose aspirin-associated upper gastric and duodenal ulcers in Japanese patients with no previous history of peptic ulcers. BMC Res Notes 2013;6:455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cryer B, Mahaffey KW. Gastrointestinal ulcers, role of aspirin, and clinical outcomes: pathobiology, diagnosis, and treatment. J Multidiscip Healthc 2014;7:137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jiang XL, Samant S, Lesko LJ, et al. Clinical pharmacokinetics and pharmacodynamics of clopidogrel. Clin Pharmacokinet 2015;54:147–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].McQuaid KR, Laine L. Systematic review and meta-analysis of adverse events of low-dose aspirin and clopidogrel in randomized controlled trials. Am J Med 2006;119:624–38. [DOI] [PubMed] [Google Scholar]
- [6].Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2019. [DOI] [PubMed] [Google Scholar]
- [7].Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non-ST-Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation 2016;134:e123–55. [DOI] [PubMed] [Google Scholar]
- [8].Kheiri B, Osman M, Abdalla A, et al. Clopidogrel and aspirin after ischemic stroke or transient ischemic attack: an updated systematic review and meta-analysis of randomized clinical trials. J Thromb Thrombolysis 2019;47:233–47. doi:10.1007/s11239-018-1786-z. [DOI] [PubMed] [Google Scholar]
- [9].Hackam DG, Spence JD. Antiplatelet therapy in ischemic stroke and transient ischemic attack. Stroke 2019;50:773–8. [DOI] [PubMed] [Google Scholar]
- [10].Tan VP, Yan BP, Kiernan TJ, et al. Risk and management of upper gastrointestinal bleeding associated with prolonged dual-antiplatelet therapy after percutaneous coronary intervention. Cardiovasc Revasc Med 2009;10:36–44. [DOI] [PubMed] [Google Scholar]
- [11].Vallurupalli NG, Goldhaber SZ. Gastrointestinal complications of dual antiplatelet therapy. Circulation 2006;113:e655–8. [DOI] [PubMed] [Google Scholar]
- [12].Vaduganathan M, Bhatt DL, Cryer BL, et al. Proton-pump inhibitors reduce gastrointestinal events regardless of aspirin dose in patients requiring dual antiplatelet therapy. J Am Coll Cardiol 2016;67:1661–71. [DOI] [PubMed] [Google Scholar]
- [13].Bhatt DL, Cryer BL, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med 2010;363:1909–17. [DOI] [PubMed] [Google Scholar]
- [14].Sangkuhl K, Klein TE, Altman RB. Clopidogrel pathway. Pharmacogenet Genomics 2010;20:463–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].El Rouby N, Lima JJ, Johnson JA. Proton pump inhibitors: from CYP2C19 pharmacogenetics to precision medicine. Expert Opin Drug Metab Toxicol 2018;14:447–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].O’Donoghue ML, Braunwald E, Antman EM, et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet 2009;374:989–97. [DOI] [PubMed] [Google Scholar]
- [17].Goodman SG, Clare R, Pieper KS, et al. Association of proton pump inhibitor use on cardiovascular outcomes with clopidogrel and ticagrelor: insights from the platelet inhibition and patient outcomes trial. Circulation 2012;125:978–86. [DOI] [PubMed] [Google Scholar]
- [18].Jeong YH, Bliden KP, Antonino MJ, et al. Usefulness of the VerifyNow P2Y12 assay to evaluate the antiplatelet effects of ticagrelor and clopidogrel therapies. Am Heart J 2012;164:35–42. [DOI] [PubMed] [Google Scholar]
- [19].Lordkipanidzé M, Pharand C, Nguyen TA, et al. Assessment of VerifyNow P2Y12 assay accuracy in evaluating clopidogrel-induced platelet inhibition. Ther Drug Monit 2008;30:372–8. [DOI] [PubMed] [Google Scholar]
- [20].Piccolo R, Galasso G, De Luca G, et al. Relationship between changes in platelet reactivity and ischemic events following percutaneous coronary intervention: a meta-regression analysis of 30 randomized trials. Atherosclerosis 2014;234:176–84. [DOI] [PubMed] [Google Scholar]
- [21].Kim JY, Lee K, Shin M, et al. Cilostazol could ameliorate platelet responsiveness to clopidogrel in patients undergoing primary percutaneous coronary intervention. Circ J 2007;71:1867–72. [DOI] [PubMed] [Google Scholar]
- [22].Kim BK, Oh SJ, Yoon SJ, et al. A randomized study assessing the effects of pretreatment with cilostazol on periprocedural myonecrosis after percutaneous coronary intervention. Yonsei Med J 2011;52:717–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Price MJ, Endemann S, Gollapudi RR, et al. Prognostic significance of post-clopidogrel platelet reactivity assessed by a point-of-care assay on thrombotic events after drug-eluting stent implantation. Eur Heart J 2008;29:992–1000. [DOI] [PubMed] [Google Scholar]
- [24].Alexopoulos D, Dimitropoulos G, Davlouros P, et al. Prasugrel overcomes high on-clopidogrel platelet reactivity post-stenting more effectively than high-dose (150-mg) clopidogrel: the importance of CYP2C19∗2 genotyping. JACC Cardiovasc Interv 2011;4:403–10. [DOI] [PubMed] [Google Scholar]
- [25].Roberts JD, Wells GA, Le May MR, et al. Point-of-care genetic testing for personalisation of antiplatelet treatment (RAPID GENE): a prospective, randomised, proof-of-concept trial. Lancet 2012;379:1705–11. [DOI] [PubMed] [Google Scholar]
- [26].Nakamura M, Isshiki T, Kimura T, et al. Optimal cutoff value of P2Y12 reaction units to prevent major adverse cardiovascular events in the acute periprocedural period: post-hoc analysis of the randomized PRASFIT-ACS study. Int J Cardiol 2015;182:541–8. [DOI] [PubMed] [Google Scholar]
- [27].Yu LY, Sun LN, Zhang XH, et al. A review of the novel application and potential adverse effects of proton pump inhibitors. Adv Ther 2017;34:1070–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Horn J. Review article: relationship between the metabolism and efficacy of proton pump inhibitors--focus on rabeprazole. Aliment Pharmacol Ther 2004;20: Suppl 6: 11–9. [DOI] [PubMed] [Google Scholar]
- [29].Grabowski B, Lee RD. Absorption, distribution, metabolism and excretion of [14C]dexlansoprazole in healthy male subjects. Clin Drug Investig 2012;32:319–32. [DOI] [PubMed] [Google Scholar]
- [30].Johnson JA, Cavallari LH. Pharmacogenetics and cardiovascular disease--implications for personalized medicine. Pharmacol Rev 2013;65:987–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Brookhart MA, Schneeweiss S, Rothman KJ, et al. Variable selection for propensity score models. Am J Epidemiol 2006;163:1149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].McMurry TL, Hu Y, Blackstone EH, et al. Propensity scores: Methods, considerations, and applications in the Journal of Thoracic and Cardiovascular Surgery. J Thorac Cardiovasc Surg 2015;150:14–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


