Abstract
Background.
Strategies to extend the pool of organs include and promote the use of segmental liver grafts. While performing a living donor left lateral segment (LLS) liver transplant and in split procedures, the hepatic artery´s division becomes critical when a dominant segment 4 artery (S4A) emerges from the left hepatic artery (LHA). We aim to describe a novel technique that consists of performing microsurgical reconstruction from the pyloric artery (PA) to S4A.
Case Reports.
A 45-y-old living donor was evaluated to use his LLS as a graft for a pediatric recipient. During the procedure, a dominant S4A born from the LHA was dissected. To obtain an appropriate LHA length and diameter for the recipient, it was necessary to transect it. An extended right lobe split graft was used in a 61-y-old patient. The S4A born from LHA had to be sectioned during the split procedure. In both cases, segment 4 remained incompletely perfused. The PA was dissected with enough length to be rotated, to perform a microsurgical anastomosis to the S4A, recovering parenchyma’s color and Doppler signal while vascular permeability was demonstrated using CT scan. There was no biliary or cut surface complication.
Conclusions.
PA to S4A reconstruction is a simple and novel technique that can be used for LLS and extended right lobe split graft and might contribute to increase donor selection and reduce living donor and recipient S4A-related complications.
INTRODUCTION
The progressively increasing gap between patients waiting for liver transplant and the availability of cadaveric organs is still the main reason to continue using segmental grafts as organ source. Left lateral living donor liver transplantation has become the most commonly used graft for pediatric liver transplant worldwide as well as the preferred choice at the time of deciding to perform a split procedure.
The Couinaud and Bismuth anatomical classifications have been accepted as a standard to perform, describe, and name segmental organ allografts.1 Although the technical aspects of both procedures have been described and reported very well, there is still room for improvement. The latter is one of the reasons why we have decided to report on our novel contribution to the field.
It has been described that the shapes of liver segments, as well as their volume and vascular branching, have significant variability, increasing the challenges to be faced at the time of performing either living donor surgery or splitting. Segment 4 (S4) and its arterial supply have been a topic of debate because of the risk of developing ischemia and necrosis of the mentioned segment after split liver transplantation, after living donation, and after performing associating liver partition and portal vein ligation for staged hepatectomy procedures. Complications related to S4, in extended right lobe split graft (ERLG), can occur in up to 22% of cases and have been associated with a trend to reduced graft and patient survival.2 In recent publications, including the multicenter experience with split liver transplant in our country, the incidence of S4-related complications was up to 16%.3
The reported risks and all types of anatomical variations, which might be related to them, have been very well described. However, to the best of our knowledge, there is still a lack of publications offering possible technical solutions to either overcome or provide alternatives to ensure donor safety and lower risks to the recipient if the S4 artery (S4A) branch needs to be sacrificed in the donor.2-4
Therefore, we aim to describe the pyloric artery (PA) to S4A branch reconstruction as a novel technique, successfully offered for the first time, during a pediatric living donor liver transplant. Besides being recognized as a possible solution to reduce potential donor risks, this technique became an innovation to be used for ERLG in adult liver transplants.
Background: Arterial Blood Supply of Segment 4
The middle hepatic artery (MHA) supplies S4; therefore, a clear knowledge of the arterial pattern is key when planning to perform a living donor liver transplant, a split, or a liver resection.5-7 However, with current imaging technologies, it is easier to plan a living donor or a resection than an in situ or ex vivo split.
A recent publication by Alghamdi et al8 has described, for the first time, the differences in S4 blood supply based on the variable origins of its branches. They were able to establish that blood supply of S4 arises from the right hepatic artery (RHA), the MHA, or the left hepatic artery (LHA) in the following patterns: in 14% of the cases, S4 is supplied by the RHA and MHA, where in MHA is a branch of the RHA; in a second group (17%), S4 is supplied by RHA, MHA, and LHA, but the MHA originates from RHA. In a third group (28%), S4 is supplied by the RHA and MHA, but the MHA branches from the LHA; in 3%, S4 is supplied by the 3 arteries with a similar pattern to the one described above. In 28% of the cases, S4 is supplied by the RHA and LHA without the MHA presence. There are 3% of the cases with double MHA branches, which originate from the RHA and the LHA. Finally, in 7% of the cases, the LHA originates from the left gastric artery and supplies S4.
MATERIALS AND METHODS
Ethics Statement
This report was approved by the Ethics Committee of the Favaloro Foundation University Hospital (ACTA 749), strictly under the current regulations of the Argentine Government, the Declaration of Helsinki by the World Medical Assembly (2013) and subsequent revisions, and the Document of the Americas on good clinical practices (OPS 2015).
Case Reports
Case 1
A 45-y-old male was chosen as an left lateral segment (LLS) living donor for his 1-y-old daughter with biliary atresia. His weight was 73 kg, and his height 174 cm (BMI 24.1 kg/m2). Pretransplant CT scan vascular reconstruction described normal arterial anatomy with an estimated volume of the LLS of 215 g (Figure 1). The procedure was performed understanding that the donor anatomical S4A variation was born from the LHA. To obtain an adequate length and diameter of the LHA for the engraftment, the S4A branch required to be sectioned, compromising the arterial supply of S4, confirmed by pulsed-wave Doppler ultrasound. Besides that, the donor was the patient’s only alternative, since by law (as a foreigner), he does not have access to a cadaveric donor.
FIGURE 1.
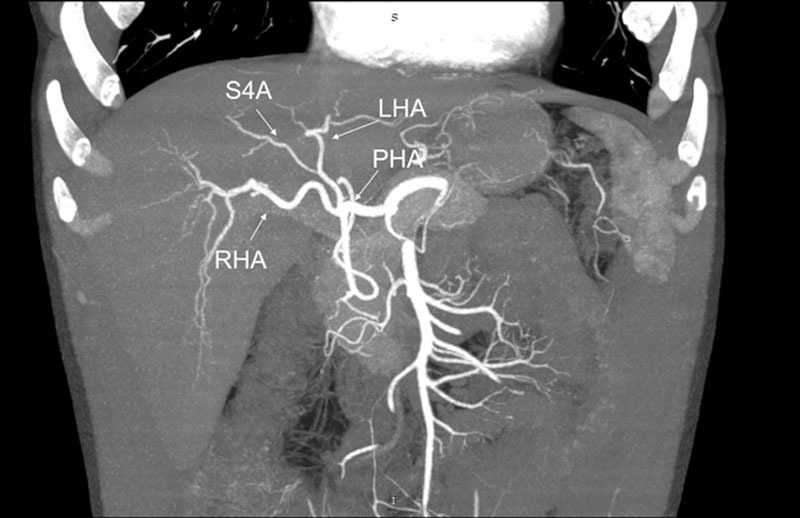
Preoperative CT scan of case 1. LHA, left hepatic artery; PHA, proper hepatic artery; RHA, right hepatic artery; S4A, segment 4 artery.
Therefore, we proposed and performed the following procedure to preserve donor safety: once the LLS was removed from the field, PA mobilization was performed using low intensity electrocautery. Dissection was started at the level of the hepatic artery and followed until the pylorus. Once it was completely dissected, the length was measured, and then a ruler was used to assess the length from the origin of the PA to the segment 4 artery. Upon confirming that the length and the diagonal rotation would allow us to reach the S4A, without tension, we proceeded to transect it and to perform a microsurgical end-to-end anastomosis, with 8-0 Prolene running suture, using 3.5× magnification glasses (Figure 2). After reperfusion, the parenchyma of the S4 recovered color and Doppler signal. The postoperative course was followed by using Doppler US during the first 3 d. For long-term follow-up, Doppler US was performed 3 mo posttransplant, and at the end of the first year, showing patency of the S4 reconstructed artery. The reconstruction between the stump of the recipient’s LHA and the remnant S4A was not performed because of the need to interpose a vascular graft because of the length between both structures.
FIGURE 2.
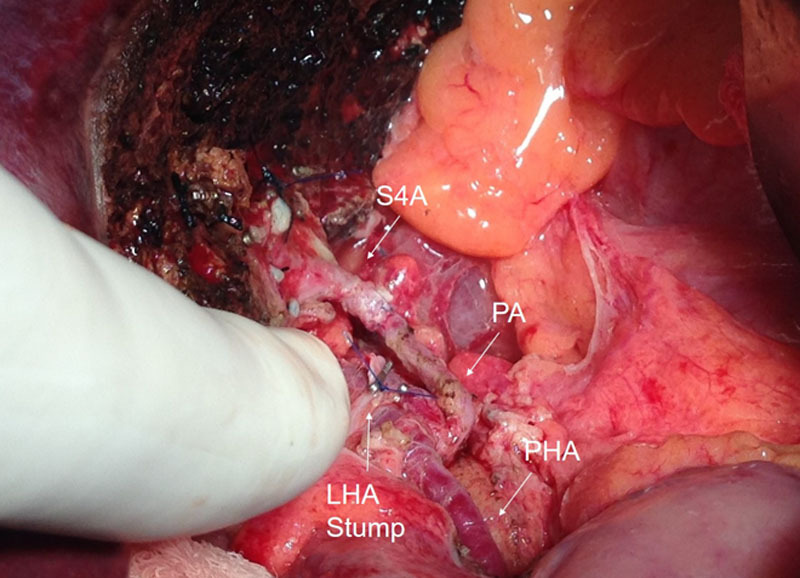
Intraoperative picture of the arterial reconstruction of case 1. LHA Stump, left hepatic artery stump; PA, pyloric artery; PHA, proper hepatic artery; S4A, segment 4 artery.
Case 2
A 61-y-old female (weight: 69 kg, height: 153 cm, BMI: 29.4), with HCV (genotype 1a) cirrhosis (Child-Pugh B), with a real MELD score of 14, but with a supplemental MELD of 24, granted for being diagnosed with hepatocellular carcinoma (Milan criteria), was listed. As a result of progressive liver disease, she had several risk factors: arterial hypertension, von Willebrand disease, ascites, encephalopathy, and spontaneous bacterial peritonitis. She had been on a waiting list for 48 mo, when a 20-y-old donor (weight 70 kg, height: 174 cm), with trauma as a cause of death, without inotropic requirements for maintenance, was offered after an in situ split procedure.
Once the organ was received, and during the back-table, an S4A was found transected, as a result of the in situ splitting. The ERLG was brought to the operative field, and after performing the portal and hepatic artery reconstruction (donor RHA was anastomosed to the recipient RHA with a running 8-0 Prolene), we observed that S4 remained incompletely perfused, and the lack of arterial inflow was confirmed by pulsed-wave Doppler ultrasound. Therefore, and based on the technique applied in case 1, as the recipient’s left and right hepatic arteries were employed to build up the cuff used to perform the anastomosis with the remaining hepatic artery from the ERLG, we decided to dissect the recipient’s hepatic artery up to the PA. Then, the PA was dissected up to an acceptable length to reach the stump of donors’ S4A.
An 8-0 Prolene running anastomosis using 3.5× magnifying glasses was performed (Figure 3). As soon as the anastomosis was completed and the arterial flow restored, the color of S4 returned to normal, and the pulsed-wave Doppler signal had a normal pattern. Throughout the postoperative course, Doppler ultrasound tests were done during the first 3 d and a CT scan on the seventh postoperative day.
FIGURE 3.
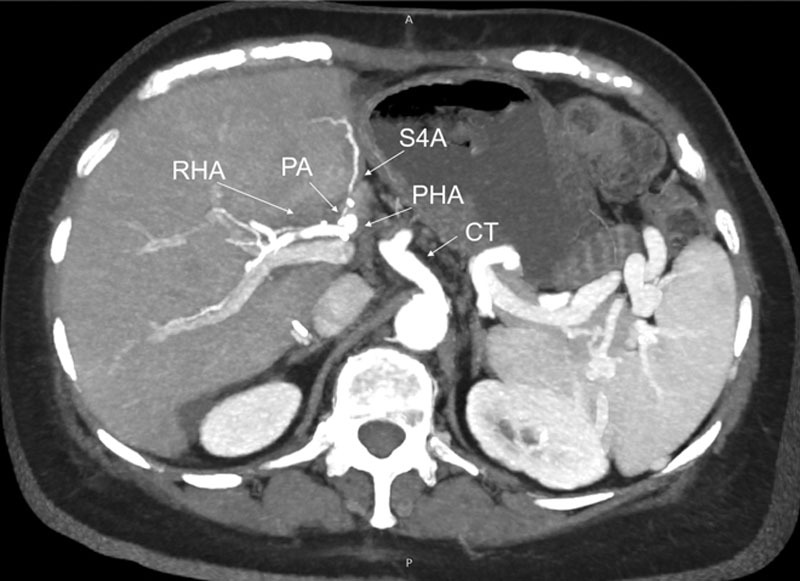
Postoperative CT scan of case 2. CT, celiac trunk; PA, pyloric artery; PHA, proper hepatic artery; RHA, right hepatic artery; S4A, segment 4 artery.
In the long-term follow-up, CT scan angiograms obtained 3 and 12 mo after the procedure confirmed that the reconstruction remained open.
DISCUSSION
Techniques for donor liver partition and LLS procurement for living donors have not undergone significant modifications or innovations since their original descriptions.9,10 Over the past decade, significant attention has been paid to the role of imaging and all anatomical aspects of the liver. Multiple manuscripts have described the accuracy of CT scan and MRI to assess arterial, portal, and hepatic veins anatomy; their variations; and precise liver volume. Although the S4A can and has been ligated in most extended right lobe split graft cases,4 some authors consider that its presence remains as a relative contraindication to donate the LLS.11 Because of current microvascular surgical techniques, anatomical variations in a potential donor are an infrequent reason for exclusion.12 In some cases, despite adequate pretransplant images, or in split cases, because of the lack of them, surgeons have to face the problem “in situ,” and thoughtful decisions need to be made. Therefore, under specific circumstances, reconstruction might be needed or considered. Thus far, no article has described alternatives to restore the flow of S4 if the artery needs to be divided or sacrificed as part of the segmental graft procurement.
Different reports have shown significant improvements in the overall and long-term results of ERLG split, compared with whole liver grafts and split LLS.13-16 Therefore, split livers should no longer be considered as marginal grafts.17-19 The same reports mention that although the global complication rate for these procedures is close to 30%,20,21 they do not impact short- or long-term graft or patient survival. Although S4-related complications secondary to atrophy, biliary leaks, or cut surface collections are well described, they have not been thoroughly reported (Table 1). As an example of this statement, Sepulveda et al have described that the appearance of S4 at the end of the engraftment was associated with worse graft survival; but on the other hand, Maggi et al24 have found no differences in the outcome of patients with S4 inadequately perfused. In a recent multicenter study done in Argentina (which includes data from our program), biliary complications associated with S4 necrosis were among the most frequent complications of the ERLG. Although the occurrence of complications was high and likely to be associated with a dominant use of the ex vivo technique, it had no statistically significant impact on patient or graft survival.3 However, to the best of our knowledge, there are no reports describing how many S4A required to be ligated from the total number of split or living donors performed and the impact resulting from this action. Therefore, when a living donor liver transplant is done, our commitment is to reduce the risk of any complication that might impact the living donor´s quality or quantity of life.
TABLE 1.
Results reported about S4 complications
| Year | No. ERLG | S4 necrosis [N (%)] | S4 biliary complications[N (%)] | S4A described [N, (%)] | |
|---|---|---|---|---|---|
| Renz et al18 | 2004 | 152 | 1 (0.6) | N/A | N/A |
| Wilms et al15 | 2006 | 70 | N/A | N/A | N/A |
| Maggi et al24 | 2010 | 28 | 1 (2.7) | 8 (28) | N/A |
| Sepulveda et al2 | 2012 | 36 | 2 (5) | 8 (22) | 1 (2.7)a |
| Doyle et al14 | 2013 | 18 | N/A | 2 (11) | N/A |
| Hashimoto et al22 | 2014 | 25 | N/A | 12 (48) | N/A |
| Halac et al3 | 2016 | 51 | 8 (15) | 2 (1) | N/A |
| Battula et al23 | 2016 | 226 | N/A | 28 (12) | N/A |
| Gambaro et al13 | 2017 | 15 | N/A | 3 (20) | N/A |
aS4A was reconstructed with splenic artery.
ERLG, extended right lobe split graft; N/A, data not reported; S4, segment 4; S4A, S4 artery.
To date, there are no reports that aim to overcome the inadequate or absent S4A inflow at the end of an LLS living donor procurement or at the end of the engraftment of an ERLG. Although the use of microsurgical reconstruction has been proposed to improve long-term patency of arterial anastomosis, no article has included the PA as an alternative to restore the flow of S4.4,25,26 Hence, we report this technique to minimize the risk of S4 biliary complications secondary to progressive segmental ischemia and improve or favor adequate liver regeneration of the revascularized segment. The use of the PA also allows for preserving the normal flow of the main arterial anastomosis to the liver. Once the feasibility of performing the proposed technique is confirmed, the procedure requires approximately 20 min, representing only 5% of the total surgical time needed. Regarding the concern about the possibility of having a postoperative artery thrombosis of the PA-S4A anastomosis, if it happens, the evolution would be similar to the cases were the S4A ligated. Since it is independent of the main hepatic artery anastomosis, it will not jeopardize its patency.
In summary, this procedure is a simple and novel technique that can be used for LLS and ERLG. Its use would contribute both to increase donor selection and to reduce living donor and recipient S4-related complications.
Footnotes
Published online 15 December, 2020.
The authors declare no funding or conflicts of interest.
A.F. contributed to the design and writing of the paper. L.M.M. and H.P. contributed to the acquisition and interpretation of the data recollection. D.A.R., V. D., S.Y., and P.A.F. contributed to the drafting and critical revision of the article. P.B.S. contributed to the design and critical revision of the study. G.E.G contributed to the study´s design, writing, and the final revision of the paper.
REFERENCES
- 1.Strasberg S, Belghiti J, Clavien P-A, et al. The Brisbane 2000 terminology of liver anatomy and resections. HPB (Oxford). 2000; 2:333–9. [Google Scholar]
- 2.Sepulveda A, Scatton O, Tranchart H, et al. Split liver transplantation using extended right grafts: the natural history of segment 4 and its impact on early postoperative outcomes. Liver Transpl. 2012; 18:413–422. [DOI] [PubMed] [Google Scholar]
- 3.Halac E, Dip M, Quiñonez E, et al. Split liver transplantation: report of right and left graft outcomes from a multicenter Argentinean group. Liver Transpl. 2016; 22:63–70. [DOI] [PubMed] [Google Scholar]
- 4.Renz JF, Yersiz H, Reichert PR, et al. Split-liver transplantation: a review. Am J Transplant. 2003; 3:1323–1335. [DOI] [PubMed] [Google Scholar]
- 5.Chan SC, Lo CM, Liu CL, et al. Tailoring donor hepatectomy per segment 4 venous drainage in right lobe live donor liver transplantation. Liver Transpl. 2004; 10:755–762. [DOI] [PubMed] [Google Scholar]
- 6.Fan ST, Lo CM, Liu CL, et al. Safety and necessity of including the middle hepatic vein in the right lobe graft in adult-to-adult live donor liver transplantation. Ann Surg. 2003; 238:137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Renz JF, Reichert PR, Emond JC. Biliary anatomy as applied to pediatric living donor and split-liver transplantation. Liver Transpl. 2000; 6:801–804. [DOI] [PubMed] [Google Scholar]
- 8.Alghamdi T, Viebahn C, Justinger C, et al. Arterial blood supply of liver segment IV and its possible surgical consequences. Am J Transplant. 2017; 17:1064–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bismuth H, Morino M, Castaing D, et al. Emergency orthotopic liver transplantation in two patients using one donor liver. Br J Surg. 1989; 76:722–724. [DOI] [PubMed] [Google Scholar]
- 10.Pichlmayr R, Ringe B, Gubernatis G, et al. Transplantation of a donor liver to 2 recipients (splitting transplantation)—a new method in the further development of segmental liver transplantation. Langenbecks archiv für chirurgie. 1988; 373:127–130. [PubMed] [Google Scholar]
- 11.Catalano OA, Singh AH, Uppot RN, et al. Vascular and biliary variants in the liver: implications for liver surgery. Radiographics. 2008; 28:359–378. [DOI] [PubMed] [Google Scholar]
- 12.Hennedige T, Anil G, Madhavan K. Expectations from imaging for pre-transplant evaluation of living donor liver transplantation. World J Radiol. 2014; 6:693–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gambaro SE, Romero P, Pedraza N, et al. Right extended split liver transplantation compared with whole liver transplantation: lessons learned at a single center in Latin America-Results from a match case-control study. Transplant Proc. 2017; 49:2122–2128. [DOI] [PubMed] [Google Scholar]
- 14.Doyle MB, Maynard E, Lin Y, et al. Outcomes with split liver transplantation are equivalent to those with whole organ transplantation. J Am Coll Surg. 2013; 217:102–112. Discussion 113. [DOI] [PubMed] [Google Scholar]
- 15.Wilms C, Walter J, Kaptein M, et al. Long-term outcome of split liver transplantation using right extended grafts in adulthood: a matched pair analysis. Ann Surg. 2006; 244:865–872. Discussion 872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spada M, Cescon M, Aluffi A, et al. Use of extended right grafts from in situ split livers in adult liver transplantation: a comparison with whole-liver transplants. Transplant Proc. 2005; 37:1164–1166. [DOI] [PubMed] [Google Scholar]
- 17.Moussaoui D, Toso C, Nowacka A, et al. Early complications after liver transplantation in children and adults: are split grafts equal to each other and equal to whole livers? Pediatr Transpl. 2017; 21:e12908. [DOI] [PubMed] [Google Scholar]
- 18.Renz JF, Emond JC, Yersiz H, et al. Split-liver transplantation in the United States: outcomes of a national survey. Ann Surg. 2004; 239:172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emond JC, Freeman RB, Jr, Renz JF, et al. Optimizing the use of donated cadaver livers: analysis and policy development to increase the application of split-liver transplantation. Liver Transpl. 2002; 8:863–872. [DOI] [PubMed] [Google Scholar]
- 20.Yersiz H, Cameron AM, Carmody I, et al. Split liver transplantation. Transplant Proc. 2006; 38:602–603. [DOI] [PubMed] [Google Scholar]
- 21.Emond JC, Whitington PF, Thistlethwaite JR, et al. Transplantation of two patients with one liver. Analysis of a preliminary experience with “split-liver” grafting. Ann Surg. 1990; 212:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hashimoto K, Fujiki M, Quintini C, et al. Split liver transplantation in adults. World J Gastroenterol. 2016; 22:7500–7506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Battula NR, Platto M, Anbarasan R, et al. Intention to split policy: a successful strategy in a combined pediatric and adult liver transplant center. Ann Surg. 2017; 265:1009–1015. [DOI] [PubMed] [Google Scholar]
- 24.Maggi U, Caccamo L, Reggiani P, et al. Hypoperfusion of segment 4 in right in situ split-liver transplantation. Transpl Proc. 2010; 42:1240–1243. [DOI] [PubMed] [Google Scholar]
- 25.Reichert PR, Renz JF, D’Albuquerque LA, et al. Surgical anatomy of the left lateral segment as applied to living-donor and split-liver transplantation: a clinicopathologic study. Ann Surg. 2000; 232:658–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emre S, Umman V. Split liver transplantation: an overview. Transplant Proc. 2011; 43:884–887. [DOI] [PubMed] [Google Scholar]


