Abstract
Background:
The aim of this meta-analysis was to evaluate the diagnostic value of lung ultrasound (LUS) in comparison to chest radiography (CXR) in children with pneumonia.
Methods:
Computer-based retrieval was performed on PubMed and EMBASE. Quality was evaluated according to the quality assessment of diagnostic accuracy studies-2, and Meta-Disc was adopted to perform meta-analysis. Heterogeneity was assessed using Q and I2 statistics. The pooled sensitivity, specificity, and diagnostic odds ratio (DOR) with 95% confidence intervals (CIs) as the primary outcomes were calculated for each index test.
Results:
Twenty two studies with a total of 2470 patients met the inclusion criteria. Our results showed that the pooled sensitivity, specificity, and DOR for children with pneumonia diagnosed by LUS were 0.95 (95% CI: 0.94 to 0.96), 0.90 (95% CI: 0.87 to 0.92), and 137.49 (95% CI: 60.21 to 313.98), respectively. The pooled sensitivity, specificity, and DOR for pediatric pneumonia diagnosed by CXR was 0.91 (95% CI: 0.90 to 0.93), 1.00 (95% CI: 0.99 to 1.00), and 369.66 (95% CI: 137.14 to 996.47), respectively. Four clinical signs, including pulmonary consolidation, positive air bronchogram, abnormal pleural line, and pleural effusion were most frequently observed using LUS in the screening of children with pneumonia.
Conclusions:
The available evidence suggests that LUS is a reliable, valuable, and alternative method to CXR for the diagnosis of pediatric pneumonia.
Keywords: children, lung, meta-analysis, pneumonia ultrasound
1. Introduction
Pneumonia is a common infectious disease in children and the main cause of death in children .[1] At present, the diagnosis of pneumonia in children mainly depends on medical history, clinical manifestations, and related auxiliary examinations (e.g., chest X-ray), which have played an important role in the diagnosis of pneumonia in children. However, chest radiography (CXR) has several limitations. In detail, the results of CXR are greatly affected by internal and external factors such as the child's posture and reporting physicians. CXR cannot be discerned when lung consolidation is <1.0 cm.[2] This may be due to the fact that chest radiographs are two-dimensional images of normal and abnormal lobes superimposed, making it difficult to observe small lesions.[3] Next, CXR is inconvenient and costly for children to be examined. Additionally, the sensitivity of radiation damage for children is at least 4 times that of adults.[4] Therefore, some scholars are actively exploring and eager to find an inspection method that can not only improve the accuracy of diagnosis of pneumonia, but also reduce exposure to ionizing radiation.
The lung is a gas-containing organ and has always been a blind spot for ultrasound. In recent years, with the continuous advancement of ultrasound diagnostic techniques, ultrasound images have been used to analyze pleural and lung tissue sonograms under pathological conditions. Therefore, it is possible to apply ultrasound to the diagnosis of pneumonia. In 1986, Weinbeg et al initially proposed the use of type B pulmonary ultrasound to evaluate pneumonia.[5] Due to the small size of the lungs in children, changes in the lungs can easily reach the pleura, making it easier to detect abnormal signs during lung ultrasonography.[6] A large number of studies have investigated the diagnostic yield of lung ultrasound (LUS) in children pneumonia. However, these studies not only had wide variation in sample size, but also conveyed inconclusive results. We therefore pre-stated rigorous inclusion criteria and conducted a meta-analysis involving available studies to systematically assess the diagnostic yield of LUS in children with pneumonia.
2. Methods
2.1. Search strategy and selection criteria
Computer-based retrieval was performed on PubMed and EMBASE from inception through October 2019 for eligible studies with the following keywords
“ultrasonography” or “ultrasound” and “pneumonia” and “children” or “childhood” or “pediatric”. All eligible trials were published in English. Bibliographies of all potential studies, such as reference lists, citation searches, and relevant systematic reviews, were searched by hand. The present study was supported by the Ethics Committee of Binzhou Medical University Hospital.
The present selection criteria were as follows:
-
1.
population: children or pediatric patients (age < 18 years) with pneumonia based on a combination of clinical data, laboratory results, and CXR;
-
2.
study design: comparing the diagnostic value of LUS vs CXR in the diagnosis of child pneumonia;
-
3.
sufficient data: reported data allowing calculation of the true-positive (TP), false-positive (FP), false-negative (FN), and true-negative (TN) values.
2.2. Data extraction and quality assessment
All data were extracted from all trials by 2 independent investigators (JHY and LP). The data included the first author, publication year, country, number of patients, age and sex of patients, LUS technique and operator, study design, blind, and pneumonia diagnostic criteria. Disagreements among authors were settled by discussion or a third investigator (YBG).
The quality of the studies was evaluated according to the quality assessment of diagnostic accuracy studies-2 (QUADAS-2).[7] The QUADAS-2 tool contains 4 key domains:
-
1.
patient selection,
-
2.
index test,
-
3.
reference standard, and
-
4.
flow and timing.
Each domain is assessed as “yes”, “unclear”, and “no” to judge risk of bias. Furthermore, the first 3 domains are also assessed as “high”, “Unclear”, and “low” concern to judge applicability. We rated the quality assessment and risk of bias using the RevMan 5.3.0 (Nordic Cochrane Centre). This evaluation information is detailed in Supplemental Digital Content (Fig. S1), which is contained in online appendices.
2.3. Statistical analysis
The present study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.[8] The DerSimonian-Laird random-effects model was used to calculate the data as a forest plot of pooled sensitivity, specificity, positive likelihood ratio (PLR) and negative likelihood ratio (NLP), and diagnostic odds ratio (DOR) with 95% confidence intervals (CIs) for LUS and CXR, respectively. The summary receiving operating characteristic (SROC) curve and the pooled diagnostic accuracy (Q∗ index) as well as the area under curve (AUC) were also measured. The SROC curve moves closer to the upper left corner of the larger area under the curve, which indicates that the accuracy of diagnostic tests is higher. Heterogeneity was evaluated using I2 statistics, and threshold effect was determined using the Spearman correlation coefficient.[9,10] If I2 > 50%, potential sources of heterogeneity were identified by sensitivity analyses. Furthermore, subgroup analyses were performed to explore observed heterogeneity and examine the influence of various exclusion criteria based on sample sizes (>100 vs ≤100), study design (prospective vs. retrospective), blind or non-blind study, LUS operator (expert vs non-expert), and ultrasound probe type (linear vs convex). All meta-analyses were performed using Meta-DiSc 1.4 (XI Cochrane Colloquium; Barcelona, Spain).[11] Publication bias was inspected using Deeks funnel plot,[12] which was analyzed using Stata 12.0 (Stata Corporation, College Station, TX, USA). A Z-test was performed to determine whether there was a statistical difference in the overall sensitivity and specificity between LUS and CXR. A two-sided P value of <.05 was considered to indicate statistical significance.
3. Results
3.1. Bibliographic search results
A total of 1605 relevant articles were identified from the initial search. After reviewing the titles and abstracts, 1555 were excluded for duplicate studies and for various reasons (e.g., case reports, editorials, reviews, and or not using both LUS and CXR). A detailed flowchart of the study selection is presented in Figure 1. Finally, the remaining 22 eligible studies with a total of 2470 patients were identified for the present meta-analysis.[2–4,13–31]
Figure 1.
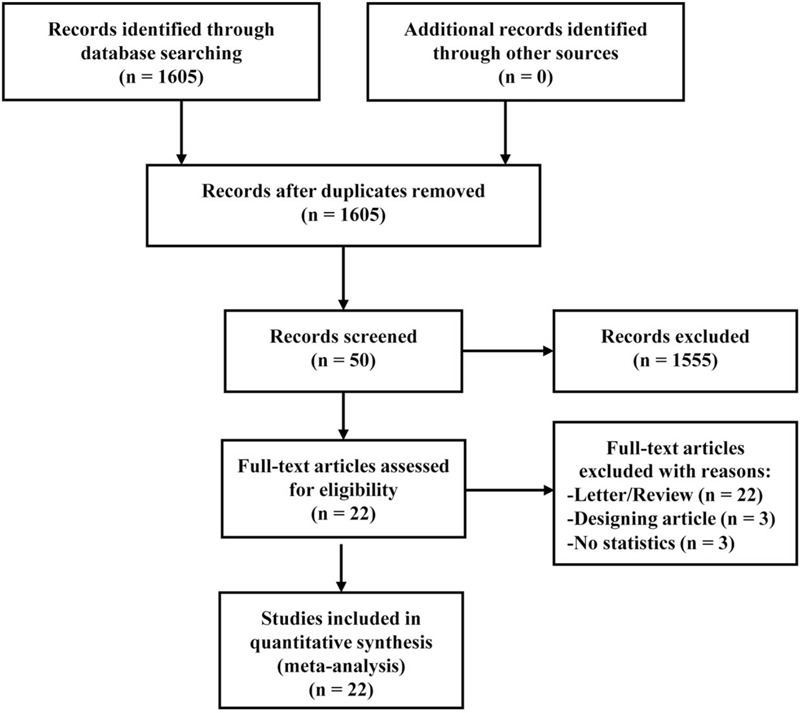
PRISMA flow diagram.
3.2. Study characteristics and quality assessment
The main characteristics of the retrieved studies are shown in Table 1. Table 1 shows that the sample size of 22 trials ranged from 47 to 222, and all studies were published between 2008 and 2018.[2–4,13–31] Of all the studies, only 2 studies[16,31] enrolled neonatal patients, and 3 studies[18,19,26] did not report gender situations. In terms of study design, 17 prospective studies[2–4,13,14,16,17,19,20,22,23,25–27,29–31] and 5 retrospective studies[15,18,21,24,28] were included in the present study. Next, 19 studies[2–4,13–20,22,23,25–30] used blind methods and 3 studies[21,24,31] used non-blind methods. Furthermore, ultrasonic procedures were performed by experts in 14 studies[3,13–16,18–21,23,24,28,30,31] and by non-experts, including primary or temporary trainers in 8 other 8 studies.[2,4,17,22,25–27,29] Finally, for the type of ultrasound probe, 11 studies[2,16–19,22,25,26,28,29,31] used the linear probe, 3 studies[3,15,27] used the convex probe, and 8 studies[4,13,14,20,21,23,24,30] used a linear probe together with a convex probe.
Table 1.
Characteristics of randomized controlled trials included in the meta-analysis.
| LUS | |||||||||||||||
| Author, year country | Sample size | Boy/Girl | MeanAge (Range) | Study design | Blinding | Patients setting | LUS operator | Ultrasound system | Pneumonia diagnosis | LUS findings | CXR | TP | FP | FN | TN |
| Copetti, 2008, Italy | 79 | 37/42 | 5.1y (6 mo-16 y) | Prospective | Yes | Emergency Department | The samea expert operator | Esaote,convex probe (3.5–5 MHz), linear probe (7.5–10 MHz) | CXR | Consolidations | LUS | 60 | 0 | 0 | 19 |
| CXR | 53 | 0 | 7 | 19 | |||||||||||
| Iuri, 2009, Italy | 28 | 17\11 | 4.5y (4mo-17y) | Prospective | Yes | Paediatric emergency ward | Two radiologists | Philip, convex probe (2–5 MHz) and linear probe (5–12MHz) | CXR | Consolidations | LUS | 22 | 0 | 2 | 4 |
| CXR | 24 | 0 | 0 | 4 | |||||||||||
| Caiulo, 2013, Italy | 102 | 53/49 | 5y (1–16y) | Prospective | Yes | Pediatric department | An expert pediatric sonographer | Sono57500; Philips, Bothell, WA, convex probe (5MHz) | Physical and CXR | Consolidations, FBL, PLA | LUS | 88 | 0 | 1 | 13 |
| CXR | 81 | 0 | 8 | 13 | |||||||||||
| Shah, 2013, American | 200 | 112/88 | 3y (1-8y) | Prospective | Yes | Emergency departments | Trained clinicians | Sonosite, GS60, Siemensa, linear probe (7.5–10MHz) | CXR | Consolidations | LUS | 31 | 18 | 5 | 146 |
| CXR | 36 | 0 | 0 | 164 | |||||||||||
| Hadeel, 2013, Egypt | 75 | 36/39 | 3–26d | Prospective | NO | NICU | The same radiologist | Nemio XG SSA-580A, and a linear 7MHz | CXR | Consolidations | LUS | 68 | 0 | 7 | 0 |
| CXR | 64 | 0 | 11 | 0 | |||||||||||
| Esposito, 2014, Italy | 103 | 56/47 | 5.6y (1mo-14y) | Prospective | Yes | Pediatric ICU | Trained resident paediatrics | MyLab, convex probe (2.5–6.6MHz), linear probe (7.5–12MHz) | Physical and CR | Consolidations | LUS | 47 | 3 | 1 | 52 |
| CXR | 48 | 0 | 0 | 55 | |||||||||||
| Ho, 2014, Taiwan | 163 | 91/72 | 6.1y | Retrospective | Yes | Pediatric ward | Experienced pediatric pulmonologists | Sono57500, Philips, convex probe (5MHz) | BTS guideline | Consolidations, PE, FBL | LUS | 159 | 0 | 4 | 0 |
| CXR | 151 | 0 | 12 | 0 | |||||||||||
| liu, 2014, China | 80 | 43/37 | Newborn | Prospective | Yes | Department of Neonatology & NICU | A single examiner expert physician | GE Voluson E6 or E8, linear probe(9–12 MHz) | Physical and CXR | Consolidations | LUS | 40 | 0 | 0 | 40 |
| CXR | 40 | 0 | 0 | 40 | |||||||||||
| Reali, 2014, Italy | 107 | 61/46 | 4y (0–16y) | Prospective | Yes | Pediatric department | A pulmonologist and two residents | Mylab25; Esaote, Genoa and a linear probe (7.5–10MHz) | Physical and CXR | Consolidations, FBL | LUS | 76 | 1 | 5 | 25 |
| CXR | 66 | 2 | 15 | 24 | |||||||||||
| Iorio, 2015, Italy | 52 | NR | 3.5y (2–12.5y) | Retrospective | Yes | Pediatric ward | The same expert operator | Sonosite, linear probe (5–10MHz) | BTS guideline | Consolidations | LUS | 28 | 1 | 1 | 22 |
| CXR | 25 | 1 | 4 | 22 | |||||||||||
| Dianova, 2015, Russia | 154 | 87/67 | 0–18y | Prospective | Yes | Children's Teaching Hospital | Eperienced radiologist | Hitachi Vision Avius (Japan) and sonoscape s8Exp (China) with 4–11 mHz multifrequency linear probes and 4–11 mHz convex probes | Clinical, CXR, CT | Consolidation, FBL, atelectasis, PE | LUS | 147 | 0 | 7 | 0 |
| CXR | 126 | 0 | 28 | 0 | |||||||||||
| Urbankowska, 2015, Poland | 106 | NR | 52.5mo (1–213mo) | Prospective | Yes | Pediatric ward | The same pediatric sonographer | ALOKA, linear probe (3–7and5–9MH) | Physical and CXR | Consolidations | LUS | 71 | 0 | 5 | 30 |
| CXR | 76 | 0 | 0 | 30 | |||||||||||
| Guerra, 2016, Italy | 222 | 108/114 | 3mo–16y | Prospective | Yes | Paediatric department | Three paediatricians with specific LUS expertise | MyLAB25, Esaote, linear probe (7.5–10MHz), convex probe (3.5–5-MHz) | Clinical characteristis | Consolidations | LUS | 207 | 0 | 7 | 8 |
| CXR | 197 | 0 | 17 | 8 | |||||||||||
| Ianniello, 2016, Italy | 84 | 44/40 | 6y (3–16y) | Retrospective | NO | Emergency department | Professional sonographer | Siemens, convex probe 4MHz and linear probe (7.5–10 MHz) | Clinical, CXR | Consolidations, FBL, PE, air bronchograms | LUS | 60 | 0 | 1 | 23 |
| CXR | 47 | 0 | 14 | 23 | |||||||||||
| Samson, 2016, Spain | 200 | 116/84 | 29.5mo (18.5–52.5mo) | Prospective | Yes | Emergency department | Pediatricians with limited training | Sonosite, linear probe (6–15MHz) | Physical and CXR | Consolidations, PE, alveolar infiltrate | LUS | 74 | 6 | 11 | 109 |
| CXR | 85 | 0 | 0 | 115 | |||||||||||
| Boursiani, 2017, Greece | 69 | 27/42 | 6mo-12y | Prospective | Yes | Emergency department | Eperienced pediatric radiologist | Microconvex probe (5–8MHz), linear probe (5–12MHz), convex (3–5MHz) | clinical criteria and CXR | Consolidations, FBL, atelectasis, PE | LUS | 62 | 0 | 4 | 3 |
| CXR | 63 | 0 | 3 | 3 | |||||||||||
| Man, 2017, Romania | 81 | 42/39 | 6.5y | Retrospective | NO | Emergency department | Senior radiologist experienced | Accuvix V20, convex probe (7–11 MHz) and linear probe (3.5–5 MHz) | CXR | Consolidations | LUS | 57 | 15 | 5 | 4 |
| CXR | 72 | 0 | 0 | 9 | |||||||||||
| Claes, 2017, Belgium | 143 | 77/66 | 41mo (8d–14y) | Prospective | Yes | Emergency room | Basic ultrasound knowledge | Philips iU-22, linear probe (12–5 MHz) | CXR | Consolidations | LUS | 44 | 8 | 1 | 90 |
| CXR | 45 | 0 | 8 | 90 | |||||||||||
| Yilmaz, 2017, Turkey | 160 | NR | 1mo- 18y | Prospective | Yes | Pediatric emergency department | A single trained operator | SonoSite, linear probe (6–13MHz) | BTS guideline | Pleural irregularity, consolidation, FBL, PE, air bronchograms | LUS | 142 | 4 | 7 | 7 |
| CXR | 132 | 0 | 17 | 11 | |||||||||||
| Yadav, 2017, India | 118 | 55/63 | 26.22mo (2–59 mo) | Prospective | Yes | Pediatric emergency department | Trained pulmonary radiologist | GE, LOGIQ P5, microconvex | Physical and CXR | Consolidations, FBL, PLA | LUS | 105 | 0 | 13 | 0 |
| CXR | 101 | 0 | 0 | 17 | |||||||||||
| Iorio, 2018, Italy | 47 | 27/20 | 4y (1mo-12y) | Retrospective | Yes | Pediatric department | A skilled sonographer | Sonosite Micro Maxx Systems ecographic equipment with a 5-to10 MHz linear probe (L38e) | Medical records | Consolidations, PE | LUS | 47 | 0 | 0 | 0 |
| CXR | 38 | 0 | 9 | 0 | |||||||||||
| Lissaman, 2018, Australia | 97 | 47/48 | 1 mo to 18y | Prospective | Yes | Pediatric emergency department | A first-year paediatric emergency medicine fellow with specifical training | A Zonare z.one ultrasound using an L14–5w linear transducer | CXR | Consolidations, FBL, PLA, PE | LUS | 46 | 17 | 4 | 30 |
| CXR | 44 | 0 | 0 | 17 |
BTS = British Thoracic Society, CXR = chest radiography, ED = emergency department, FBL = focal B-lines, FN = false-negative, FP = false-positive, ICU = intensive care unit, LUS = lung ultrasound, NICU = neonatal intensive care unit, PE = pleural effusion, PLA = pleural line abnormality, TN = false-negative, TP = true-positive.
Two authors (JHY and LP) agreed on each item of the QUADAS-2. The risk-of-bias analyses suggested that 19 trials[2–4,14–23,25–29,31] were followed with low risk in terms of patient selection, index test, reference standard, flow, and timing. Three other studies[13,24,30] were followed with a high risk of the index test. In addition, all trials were followed with high concern regarding applicability. The detailed quality assessment of the 22 studies is illustrated in Figure S1.
3.3. Diagnostic accuracy of LUS and CXR
The overall diagnostic sensitivity was 0.95 (95% CI: 0.94 to 0.96; χ2 = 51.89; I2 = 59.5%; P = .0002) and 0.91 (95% CI: 0.68 to 0.82; χ2 = 61.49; I2 = 95.1%; P = .0000) (Fig. 2), and the overall diagnostic specificity was 0.90 (95% CI: 0. 87 to 0.92; χ2 = 116.76; I2 = 82%; P = .0000) and 1.00 (95% CI: 0.99 to 1.00; χ2 = 16.10; I2 = 0.0%; P = .7640) (Fig. 3) for children pneumonia diagnosed by LUS and CXR, respectively. Heterogeneity was significant in terms of pooled sensitivity for the 2 arms. Next, sensitivity analyses were performed to further explore the potential source of heterogeneity across studies. Further exclusion of any single study did not resolve the heterogeneity, and the pooled sensitivity ranged from 0.95 (95% CI: 0.94 to 0.96; χ2 = 43.71; I2 = 54.2%) to 0.95 (95% CI: 0.94 to 0.96; χ2 = 51.77; I2 = 61.4%), 0.91 (95% CI: 0.89 to 0.92; χ2 = 124.42; I2 = 83.9%) to 0.91 (95% CI: 0.90 to 0.93; χ2 = 142.63; I2 = 86.0%) for LUS and CXR, respectively. Next, threshold effect analysis showed that the Spearmans correlation coefficients were −0.390 (P = .073) and −0.421 (P = .051) for LUS and CXR, which suggested that no diagnostic threshold effect existed for pneumonia diagnoses. Moreover, the heterogeneity among studies could mainly result from clinical and methodological differences.
Figure 2.
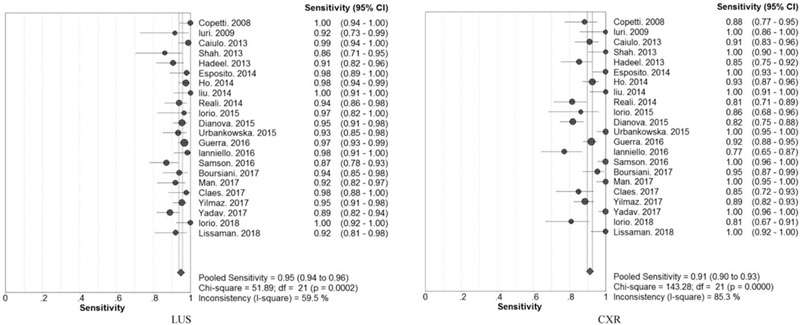
Forest plots of the pooled sensitivity for children pneumonia diagnosed by LUS and CXR.
Figure 3.
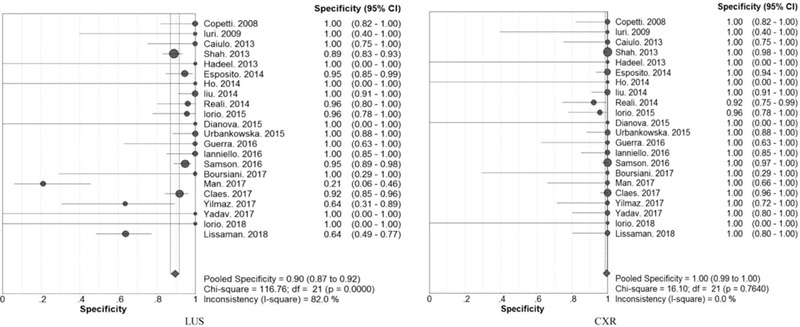
Forest plots of the pooled specificity for children pneumonia diagnosed by LUS and CXR.
The pooled PLR, NLR, and DOR were 8.67 (95% CI: 3.98 to 18.89), 0.07 (95%CI: 0.05 to 0.10), and 137.49 (95% CI: 60.21 to 313.98) for LUS, respectively. Correspondingly, the pooled PLR, NLR, and DOR were 19.96 (95% CI: 10.42 to 38.24), 0.09 (95%CI: 0.06 to 0.14), and 369.66 (95% CI: 137.14 to 996.47) for CXR, respectively. The above results are detailed in the Supplemental Digital Content (Fig. S2, Fig. S3, and Fig. S4). Additionally, the 2 SROC curves are presented in Figure 4, which shows that the AUC and Q∗ index with a standard error (SE) of 0.9817 (0.9405 ± 0.0122) and 0.9866 (0.9505 ± 0.0125) for LUS and CXR (Fig. 5), respectively.
Figure 4.
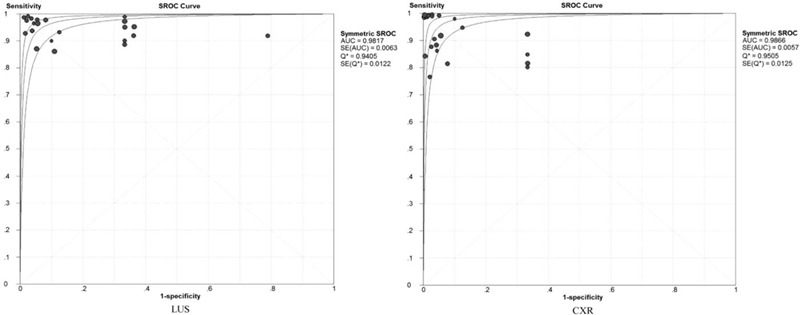
Summary receiving operating characteristic curve and Q∗ index for LUS and CXR.
Figure 5.
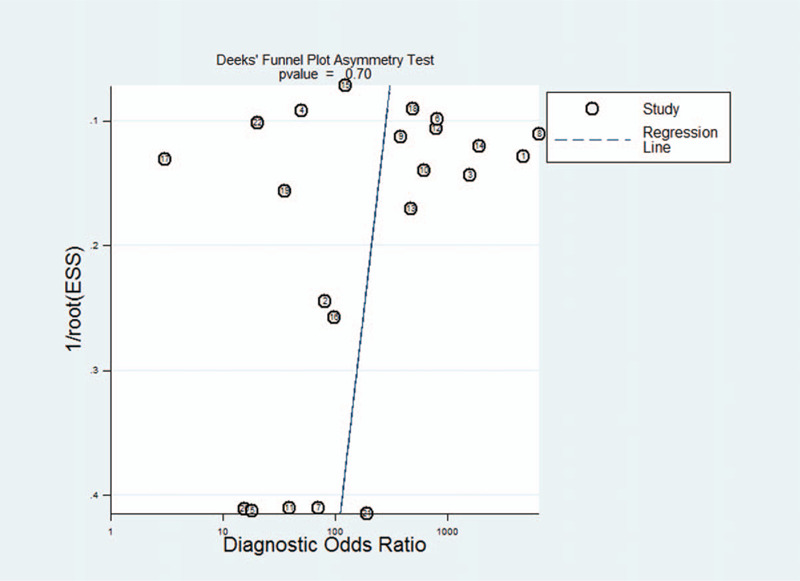
Publication bias.
Specifically, the Z-test for the overall sensitivity and specificity suggested that there was no statistical difference between LUS and CXR (all P > .05). In other words, LUS and CXR have similar sensitivity and specificity.
3.4. Subgroup analyses
We performed subgroup analyses using a random effects model to explore the heterogeneity of sensitivity and examine the influence of various exclusion criteria based on sample sizes (>100 vs ≤100), study design (prospective vs. retrospective), blind or non-blind study, LUS operator (expert vs non-expert), and ultrasound probe type (linear vs convex). Table 2 shows the detailed indication for subgroup analyses of LUS and CXR for the pooled sensitivity, specificity, and DOR in all eligible studies.
Table 2.
Subgroup analyses of the eligible studies for the pooled sensitivity, specificity, and DOR based on various exclusion criteria.
| Pooled sensitivity (95%CI), I2 | Pooled specificity (95%CI), I2 | Pooled DOR (95%CI), I2 | |||||
| Various exclusion criteria | n/N | LUS | CXR | LUS | CXR | LUS | CXR |
| All included trials | 2470/22 | 0.95 (0.94–0.96), 59.5% | 0.91 (0.90–0.93), 85.3% | 0.90 (0.87–0.92), 82.0% | 1.00 (0.99–1.00), 0.0% | 137.49 (60.21–313.98), 65.2% | 369.66 (137.14–996,47), 52.9% |
| Number of patients ≤ 100 | 692/10 | 0.95 (0.93–0.97), 58.9% | 0.91 (0.88–0.93), 83.5% | 0.82 (0.75–0.87), 89.0% | 0.99 (0.96–1.00), 0.0% | 136.29 (25.33–733.44), 75.4% | 213.77 (61.34–745.06), 30.2% |
| Number of patients > 100 | 1778/12 | 0.95 (0.93–0.96), 62.9% | 0.92 (0.90–0.93), 87.5% | 0.92 (0.90–0.94), 45.2% | 1.00 (0.99–1.00), 9.9% | 147.76 (67.89–321.58), 39.4% | 599.85 (131.40–2738.44), 64.8% |
| Prospective study | 2043/17 | 0.94 (0.93–0.95), 60.0% | 0.92 (0.90–0.93), 85.8% | 0.91 (0.89–0.93), 73.2% | 1.00 (0.99–1.00), 0.0% | 144.24 (65.74–316.49), 53.2% | 572.95 (175.63–1869.11), 56.1% |
| Retrospective study | 427/5 | 0.97 (0.95–0.98), 45.2% | 0.90 (0.86–0.92), 86.2% | 0.76 (0.64–0.85), 91.2% | 0.98 (0.90–1.00), 0.0% | 116.26 (6.02–2244.15), 81.5% | 97.73 (19.19–497.66), 27.7% |
| Blind study | 2230/19 | 0.95 (0.94–0.96), 61.4% | 0.92 (0.90–0.93), 84.4% | 0.91 (0.89–0.93), 70.3% | 1.00 (0.99–1.00), 0.0% | 159.07 (76.26–331.80), 48.4% | 429.71 (145.65–1267.82), 54.9% |
| Non-blind study | 240/3 | 0.93 (0.89–0.96), 54.9% | 0.88 (0.83–0.92), 91.9% | 0.65 (0.49–0.79), 94.4% | 1.00 (0.89–1.00), 0.0% | 40.55 (0.65–2525.41), 86.1% | 147.60 (8.53–2553.79), 52.5% |
| Expert operator | 1342/14 | 0.96 (0.95–0.97), 50.2% | 0.90 (0.89–0.92), 82.3% | 0.91 (0.86–0.95), 83.9% | 0.99 (0.97–1.00), 0.0% | 220.82 (49.86–977.93), 69.5% | 160.33 (55.85–460.24), 32.1% |
| Non-expert operator | 1128/8 | 0.92 (0.90–0.94), 49.9% | 0.93 (0.91–0.95), 89.2% | 0.89 (0.86–0.92), 80.0% | 1.00 (0.99–1,00), 41.4% | 89.52 (36.64–2218.73), 59.9% | 1820.35 (250.85–13209.84), 68.4% |
| Ultrasound linear probe | 1267/11 | 0.94 (0.91–0.95), 56.6% | 0.91 (0.89–0.93), 85.9% | 0.90 (0.87–0.92), 80.0% | 0.99 (0.98–1.00), 31.3% | 120.02 (49.65–290.13), 56.7% | 528.92 (103.66–2698.84), 67.9% |
| Ultrasound convex probe | 383/3 | 0.95 (0.92–0.97), 85.2% | 0.94 (0.91–0.97), 85.9% | 1.00 (0.77–1.00), 0.0% | 1.00 (0.89–1.00), 0.0% | 127.57 (8.17–1992.19), 48.9% | 308.91 (15.84–6023.74), 56.0% |
| Linear + convex probe | 820/8 | 0.96 (0.94–0.97), 34.1% | 0.90 (0.88–0.92), 86.6% | 0.86 (0.79–0.92), 88.7% | 1.00 (0.97–1.00), 0.0% | 176.12 (21.46–1445.75), 78.7% | 260.70 (68.90–986.34), 21.9% |
CI = confidence interval, CXR = chest radiography, DOR = diagnostic odds ratio, LUS = lung ultrasound, n = patient number, N = study number.
3.5. Publication bias
Deeks funnel plot asymmetry test was used to evaluate the final set of studies for potential publication bias. The slope coefficient was associated with a P value of .70, which suggested symmetry in the data and no publication bias (Fig. 5).
4. Discussion
The current meta-analysis including 22 studies was conducted to systematically evaluate the diagnostic value of LUS in comparison to CXR in children with pneumonia. Our results indicate that LUS is a reliable, valuable, and alternative method to CXR and could be considered as a first-line imaging modality for the diagnosis of pediatric pneumonia.
To date, several systematic reviews and meta-analyses have investigated the diagnostic value of LUS in children pneumonia.[32–35] However, these meta-analyses only described data on LUS, unilaterally analyzed the diagnostic value of LUS, and did not analyze the diagnostic value of CXR for children with pneumonia. From this point of view, the above meta-analyses did not systematically compare the diagnostic value of LUS and CXR, and they were also limited in the literature. Considering the above limitations, we carried out the present meta-analysis combining existing studies to increase the sample size, strengthen our analyses, and produce more robust results to compare the diagnostic value of LUS in comparison to CXR in children with pneumonia.
In the present study, we mainly focused on evaluating the diagnostic value of LUS in comparison to CXR in pediatric pneumonia. Our results showed that the pooled sensitivity was 0.95, 0.91, specificity was 0.90, and 1.00, DOR was 137.49 and 369.66, and AUC was 0.9817 and 0.9866 for LUS and CXR, respectively. The Z-test results suggested that there was no statistical difference in the pooled sensitivity and specificity between LUS and CXR (all P > .05), which suggested that the sensitivity and specificity of LUS were not inferior to those of CXR. Additionally, the 2 SROCs of LUS and CXR are presented in Figure 4, which suggests that both LUS and CXR have a fairly high diagnostic accuracy. Next, our sensitivity analyses did not significantly alter the heterogeneity among studies for pooled sensitivity. Threshold effect analysis showed that no diagnostic threshold effect existed for pneumonia diagnoses, which indicated that the heterogeneity among studies could be seen as a result of clinical and methodological differences. Moreover, the results of subgroup analyses indicated that LUS may appear to be slightly higher than CXR, but the difference was not statistically significant. Overall, those prospective blind studies with expert operators should be more specific for LUS. It should be noted that an ultrasound convex probe helps to improve the sensitivity and specificity of LUS diagnosis in pediatric pneumonia. However, more studies are needed to investigate these topics of interest. Finally, 4 clinical signs, including pulmonary consolidation, positive air bronchogram, abnormal pleural line, and pleural effusion, were most frequently observed using LUS in the screening of children pneumonia. Further research should focus on these diagnostic signs of LUS for pediatric pneumonia.
To be sure, there were several limitations to our study. First, the child patients were heterogeneous with different regions, different ages, and sex ratios. The experience of LUS operators was not consistent and may interfere with the accuracy of pneumonia diagnosis. Second, the design of the study was different, including blind methods and prospective or retrospective studies. The ultrasound system was not consistent and may interfere with the LUS operators judgment. Third, the sample size was different, and some studies with a wide variation in sample size were incorporated into our analysis. Overestimation of the diagnostic value is most likely to occur in smaller than in larger studies. Finally, several unpublished or missing data may increase the risk of bias.
5. Conclusions
In summary, our results suggest that LUS is a reliable, valuable, and alternative tool to CXR for children with suspected pneumonia, and LUS should be considered as a first-line imaging modality for the diagnosis of pediatric pneumonia. However, considering the significant heterogeneity found across the individual studies, further more methodologically rigorous studies are needed to focus on the diagnostic accuracy of LUS in pediatric pneumonia.
Acknowledgments
We really appreciated the insightful suggestions from the reviewers and editors.
Author contributions
Conceptualization: Lei Pan.
Data curation: Na Yu.
Formal analysis: Lei Pan.
Funding acquisition: Lei Pan.
Investigation: Yan-Bing Gao.
Methodology: Jun-Hong Yan.
Project administration: Jun-Hong Yan.
Software: Lei Pan.
Supervision: Lei Pan.
Visualization: Yan-Bing Gao.
Writing – original draft: Jun-Hong Yan.
Writing – review & editing: Yue-Heng Wang, Lei Pan.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: AUC = the areas under curve, CI = confidence interval, CT = Computed Tomography, CXR = chest radiography, DOR = diagnostic odds ratio, DOR = diagnostic odds ratio, FN = false-negative, FP = false-positive, LUS = lung ultrasound, NLP = negative likelihood ratio, PLR = positive likelihood ratio, QUADAS-2 = the quality assessment of diagnostic accuracy studies-2, SROC = the summary receiving operating characteristic, TN = true-negative, TP = true-positive.
How to cite this article: Yan JH, Yu N, Wang YH, Gao YB, Pan L. Lung ultrasound vs chest radiography in the diagnosis of children pneumonia: Systematic evidence. Medicine. 2020;99:50(e23671).
J-HY, NY, and Y-HW contributed equally to this work.
This work was supported by the Science and Technology Plan Project of Binzhou Medical University (No. BY2017KJ30), Health and Family Planning Commission of Shandong Province (No. 2017WS366), and Traditional Chinese Medicine Technology Development Plan of Shandong Province (No. 2019-0503).
The present study is only a meta-analysis without involving Human Participants and/or Animals. This research has been supported by the Ethics committee of Binzhou Medical University Hospital.
The authors declare that they have no conflict of interest.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental digital content is available for this article.
References
- [1].Sminkey L. World report on child injury prevention. Injury Prevention 2008;14:69. [DOI] [PubMed] [Google Scholar]
- [2].Shah VP, Tunik MG, Tsung JW. Prospective evaluation of point-of-care ultrasonography for the diagnosis of pneumonia in children and young adults. JAMA Pediatrics 2013;167:119–25. [DOI] [PubMed] [Google Scholar]
- [3].Caiulo VA, Gargani L, Caiulo S, et al. Lung ultrasound characteristics of community-acquired pneumonia in hospitalized children. Pediatric Pulmonol 2013;48:280–7. [DOI] [PubMed] [Google Scholar]
- [4].Esposito S, Papa SS, Borzani I, et al. Performance of lung ultrasonography in children with community-acquired pneumonia. Italian J Pediatrics 2014;40:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Weinberg B, Diakoumakis EE, Kass EG, et al. The air bronchogram: sonographic demonstration. AJR. Am J Roentgenol 1986;147:593–5. [DOI] [PubMed] [Google Scholar]
- [6].Gargani L, Volpicelli G. How I do it: lung ultrasound. Cardiovascular Ultrasound 2014;12:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Internal Med 2011;155:529–36. [DOI] [PubMed] [Google Scholar]
- [8].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [10].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zamora J, Abraira V, Muriel A, et al. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol 2006;6:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol 2005;58:882–93. [DOI] [PubMed] [Google Scholar]
- [13].Copetti R, Cattarossi L. Ultrasound diagnosis of pneumonia in children. La Radiologia Medica 2008;113:190–8. [DOI] [PubMed] [Google Scholar]
- [14].Iuri D, De Candia A, Bazzocchi M. Evaluation of the lung in children with suspected pneumonia: usefulness of ultrasonography. La Radiologia Medica 2009;114:321–30. [DOI] [PubMed] [Google Scholar]
- [15].Ho MC, Ker CR, Hsu JH, et al. Usefulness of lung ultrasound in the diagnosis of community-acquired pneumonia in children. Pediatrics Neonatol 2015;56:40–5. [DOI] [PubMed] [Google Scholar]
- [16].Liu J, Liu F, Liu Y, et al. Lung ultrasonography for the diagnosis of severe neonatal pneumonia. Chest 2014;146:383–8. [DOI] [PubMed] [Google Scholar]
- [17].Reali F, Sferrazza Papa GF, Carlucci P, et al. Can lung ultrasound replace chest radiography for the diagnosis of pneumonia in hospitalized children? Respirat Int Rev Thoracic Dis 2014;88:112–5. [DOI] [PubMed] [Google Scholar]
- [18].Iorio G, Capasso M, De Luca G, et al. Lung ultrasound in the diagnosis of pneumonia in children: proposal for a new diagnostic algorithm. Peer J 2015;3:e1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Urbankowska E, Krenke K, Drobczynski L, et al. Lung ultrasound in the diagnosis and monitoring of community acquired pneumonia in children. Respirat Med 2015;109:1207–12. [DOI] [PubMed] [Google Scholar]
- [20].Guerra M, Crichiutti G, Pecile P, et al. Ultrasound detection of pneumonia in febrile children with respiratory distress: a prospective study. Europ J Pediatrics 2016;175:163–70. [DOI] [PubMed] [Google Scholar]
- [21].Ianniello S, Piccolo CL, Buquicchio GL, et al. First-line diagnosis of paediatric pneumonia in emergency: lung ultrasound (LUS) in addition to chest-X-ray (CXR) and its role in follow-up. Br J Radiol 2016;89:20150998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Samson F, Gorostiza I, Gonzalez A, et al. Prospective evaluation of clinical lung ultrasonography in the diagnosis of community-acquired pneumonia in a pediatric emergency department. Europ J Emerg Med 2018;25:65–70. [DOI] [PubMed] [Google Scholar]
- [23].Boursiani C, Tsolia M, Koumanidou C, et al. Lung ultrasound as first-line examination for the diagnosis of community-acquired pneumonia in children. Pediatric Emerg Care 2017;33:62–6. [DOI] [PubMed] [Google Scholar]
- [24].Man SC, Fufezan O, Sas V, et al. Performance of lung ultrasonography for the diagnosis of communityacquired pneumonia in hospitalized children. Med Ultrasonography 2017;19:276–81. [DOI] [PubMed] [Google Scholar]
- [25].Claes AS, Clapuyt P, Menten R, et al. Performance of chest ultrasound in pediatric pneumonia. Europ J Radiol 2017;88:82–7. [DOI] [PubMed] [Google Scholar]
- [26].Yilmaz HL, Ozkaya AK, Sari Gokay S, et al. Point-of-care lung ultrasound in children with community acquired pneumonia. Am J Emerg Med 2017;35:964–9. [DOI] [PubMed] [Google Scholar]
- [27].Yadav KK, Awasthi S, Parihar A. Lung ultrasound is comparable with chest roentgenogram for diagnosis of community-acquired pneumonia in hospitalised children. Indian J Pediatrics 2017;84:499–504. [DOI] [PubMed] [Google Scholar]
- [28].Iorio G, Capasso M, Prisco S, et al. Lung ultrasound findings undetectable by chest radiography in children with community-acquired pneumonia. Ultrasound Medi Biol 2018;44:1687–93. [DOI] [PubMed] [Google Scholar]
- [29].Lissaman C, Kanjanauptom P, Ong C, et al. Prospective observational study of point-of-care ultrasound for diagnosing pneumonia. Arch Dis Childhood 2019;104:12–8. [DOI] [PubMed] [Google Scholar]
- [30].Dolgikh OV, Krivtsov AM, Starkova KG, et al. Immune and genetic changes in workers exposed to industrial noise and dust. Med Tr Prom Ekol 2015;21–5. [PubMed] [Google Scholar]
- [31].Bonnen PE, Yarham JW, Besse A, et al. Mutations in FBXL4 cause mitochondrial encephalopathy and a disorder of mitochondrial DNA maintenance. Am J Human Genetics 2013;93:471–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Najgrodzka P, Buda N, Zamojska A, et al. Lung ultrasonography in the diagnosis of pneumonia in children-a metaanalysis and a review of pediatric lung imaging. Ultrasound Quarterly 2019;doi:10.1097/RUQ.0000000000000411. [DOI] [PubMed] [Google Scholar]
- [33].Orso D, Ban A, Guglielmo N. Lung ultrasound in diagnosing pneumonia in childhood: a systematic review and meta-analysis. J Ultrasound 2018;21:183–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Pereda MA, Chavez MA, Hooper-Miele CC, et al. Lung ultrasound for the diagnosis of pneumonia in children: a meta-analysis. Pediatrics 2015;135:714–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Xin H, Li J, Hu HY. Is lung ultrasound useful for diagnosing pneumonia in children: a meta-analysis and systematic review. Ultrasound Quarterly 2018;34:3–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


