Abstract
Malnutrition and cachexia affects the majority of cancer patients and significantly worsens their quality of life and prognosis. However, the diagnostic criteria of malnutrition and cachexia remain a topic under constant debate. To overcome this hurdle, diagnostic tools to objectively detect and quantify the loss of muscle and fat mass are needed. Computed tomography (CT)-based measurement is currently considered the golden standard. Bioelectrical impedance analysis (BIA) is an economical, non-invasive tool but it is seen controversial in patients with cancer and malnutrition because of possible estimation errors.
BIA and CT-based analysis of body mass compartments were performed 172 times in 118 cancer patients, within the nutrition program of our institution. Prevalence of malnutrition was determined according to the global leadership initiative on malnutrition criteria. Data obtained for muscle and fat mass from both BIA and CT were correlated using Pearson's ρ. All analyses were performed with an explorative significance level of 5%.
45.7% of the cohort were classified as “malnourished.” No significant differences were observed between the 2 groups regarding demographic data. Median body mass index, Karnofsky performance status, and nutritional risk score were lower in the malnourished group. Values for muscle and fat mass by BIA and CT were significantly lower in malnourished patients. Correlation of the measured parameters were highly significant between CT-based and BIA measurement. In the overall cohort, correlation of measured muscle mass values by CT and BIA was significant with Pearson's ρ = 0.794 (P < .01). Looking at patients without malnutrition only, Pearson's ρ was 0.754 (P < .01). The correlation of measured fat mass values was equally significant, with Pearson's ρ of 0.748 (P < .01) in the overall cohort and 0.771 (P < .01) in patients with malnutrition.
To our knowledge, this is the first study comparing BIA to CT-based body mass analysis in a large cohort of cancer patients with malnutrition. The results suggest that BIA is a valid diagnostic tool for the assessment of muscle and fat mass, even in patients with malnutrition, and could be implemented for the early detection and short-term follow-up of malnutrition and cachexia.
Keywords: bioelectrical impedance analysis, cachexia, computed tomography, fat mass assessment, malnutrition, muscle mass assessment
1. Introduction
1.1. Background/rationale
Malnutrition affects up to 80% of patients with cancer and leads to reduced treatment response and tolerance, survival, and quality of life.[1–5] Different types of cancers have variable effects on body weight and complexion. While patients with cancers of the oropharyngeal or digestive tract often lose weight due to feeding problems, cancer cachexia or muscle wasting are most frequently observed in patients with pancreatic- and lung cancer. The mechanisms that lead to cachexia are still poorly understood, but consensus is that is has to be regarded as a complex of multiple, interdependent patient- and tumor-specific components, such as metabolic and humoral changes as well as psychological issues, anorexia, fatigue, and adverse effects of anticancer therapies.[6] In surgical patients, malnutrition and cachexia negatively influences postoperative morbidity and the incidence of surgical complications.[7]
Recently, it has become clear that the most important clinical feature of cachexia is the excessive wasting of skeletal muscle mass (MM).[8,9] Current research has shown that low MM is associated with poor prognosis and postoperative outcomes in cancer patients and could be used as prognostic factor and for pre-surgical risk assessment.[9,10] However, the loss of MM can be difficult to diagnose and to quantify, especially in obese patients and early stages. Therefore, specific assessments of body composition are required to detect malnutrition and loss of MM in these cases.[8] The quantity of MM can be assessed by indirect or direct methods. Approved direct methods include dual energy x-ray absorptiometry (DXA), computed tomography (CT), and magnetic resonance imaging (MRI).[11]
DXA machines are widely used for the diagnosis of osteoporosis, however, using specific software, whole-body fat- and fat free mass (FM/FFM) can be estimated using the appendicular skeletal MM of the arms and legs, The accuracy of DXA is variable, depending on the patient's body thickness, size and hydration-status as well as the used hard- and software, making it difficult to compare DXA measurements between different settings.[11,12]
Measurement of MM and body composition by (CT) is considered the golden standard for the assessment in research settings.[13,14] Cancer patients usually undergo CT scans on a regular basis, which allow evaluation of body composition changes over time and in correlation with disease progression and treatment response. In addition, CT scans permit separation of specific compartments within FFM (skeletal muscle, non-skeletal muscle, organs, connective tissue) and FM (subcutaneous fat, visceral fat, intramuscular adipose tissue).[8,10,15] However, CT analysis requires a certain effort, for example, hospital/research setting, appropriate software and specifically trained personnel and is time-consuming.[11] Due to the amount of radiation and costs, CT is not suitable for short-term follow-up and repeated measurements e.g. in the framework of nutrition and exercise programs or clinical trials. The only method to assess FM and MM even more accurate is MRI analysis. Not only the image resolution is much higher than of CT scans, MRI can even quantify the lipid content within skeletal muscle. Furthermore, the images for MRI are not acquired using ionizing radiation making it suitable and safe for longitudinal studies. However, the use of MRI is very limited due to the high costs, long scan and processing times as well as the high level of technical expertise required.[11,14]
Approved indirect methods include anthropometry (eg, body mass index [BMI], arm and calf circumference and arm muscle cross-sectional area as a function of arm muscle circumference and skinfold thickness) air displacement plethysmography and bioelectrical impedance analysis (BIA).[16,17] Anthropometric methods are simple but lack precision.[17] Air displacement plethysmography is mostly used and validated in pediatric patients, requires specific hardware and is prone to measurement errors.[18] Therefore, BIA is often preferably used in clinical practice and research. BIA is a simple, portable, low-cost, and noninvasive method to measure body composition, based on the principle that electric flow is facilitated through hydrated tissue and extracellular water.[19,20] Raw values obtained by BIA are resistance (R) and reactance (Xc). R negatively correlates with the quantity of ionic solutions and Xc is directly related to the amount of soft tissue structures.[21] From these parameters total body FFM and FM can be estimated using population-specific regression equations.[11] The phase angle (PA) is calculated as arctan (Xc/R). PA values correspond with cellularity and quality of cell membrane integrity and were shown to correlate with morbidity, nutritional risk, and survival in cancer patients.[21–23] However, the BIA approach has previously been criticized to be unreliable in patients with malnutrition or cachexia, as they differ from the validation population in BMI, hydration, and training status.[20,21,24–26]
1.2. Objective
The objective of this study is to compare body composition measured by the preferably used indirect method BIA with the direct, reference method CT-analysis in a cohort of cancer patients. Specifically, the aim is to investigate if BIA is a valid diagnostic tool even in cancer patients with and without malnutrition and could thus be safely used for short-term follow-up (eg, in nutritional intervention trials) or in non-specialized/out-patient settings.
2. Methods
2.1. Study design, setting, and participants
For this cross-sectional analysis, the prospective database of our center for nutrition and exercise for cancer patients (ESZK) was searched between April 2010 and May 2017. Subjects were eligible for this study if they underwent body composition measurements by BIA and in addition had undergone a routine diagnostic abdominal CT scan that was taken maximum +/– 45 days apart of the BIA measurement. No other eligibility criteria were applied. The manuscript was written in accordance with the STROBE Statement and Checklist.[27]
2.2. Variables and measurements
2.2.1. Definition of malnutrition/cachexia
According to the recent consensus of the Global Leadership Initiative on Malnutrition (GLIM) the definition of malnutrition is based on 3 phenotypic criteria (nonvolitional weight loss, low body mass index, and reduced MM) and 2 etiologic criteria (reduced food intake or assimilation, and inflammation or disease burden). To diagnose malnutrition at least 1 phenotypic criterion and 1 etiologic criterion should be present.[28] The etiologic criterion in our cohort was “cancer,” the phenotypic criterion was “unintentional weight loss >10% of body weight” at first presentation in our institution. If both criteria were fulfilled subjects were classified as “malnourished.”
2.2.2. Demographic data
The following descriptive data were obtained of all patients: age, gender, BMI, current weight, initial weight before onset of disease, Karnofsky performance status, and nutritional risk screening score. Furthermore, cancer type, type of treatment (chemotherapy, radiotherapy, surgery, combination) and intention of treatment (curative, palliative) were recorded.
2.2.3. Key laboratory parameters
To support the clinical picture of cachexia in the malnourished group the following laboratory parameters were analyzed if available: sodium [mmol/L]; potassium [mmol/L]; S-creatinine [mg/dL]; S-cholinesterase [U/L]; S-protein [g/dL]; S-albumin [g/dL]; C-reactive protein [mg/dL]; triglycerides [mg/dL]; S-glucose [mg/dL]; leucocytes [G/L]; hemoglobin [g/dL].
2.2.4. Measurements of body composition by BIA
BIA measurements were conducted according to the standard procedures described in literature.[29,30] Subjects had to be fasting (including no alcohol consumption within 12 hours) and resting (no exercise within the last 8 hours) and lie on a comfortable area free of drafts and electric heaters with limbs in 30° abduction position. BIA was performed with the BIA 101 anniversary SE vector impedance analyzer (Akern Bioresearch, Italy), using 50-kHz frequency. Resistance (R50), reactance (Xc50) and PA were obtained and FFM, FM, total body water, total body cell mass, and MM in [kg] and [%] of total body weight, were calculated by the BIA-software BodyGram V3.0 (Akern Bioresearch) as previously described.[31] Values obtained for FM and MM were standardized by body height resulting in the following outcome variables: BIA-fat mass index (BIA-FMI) [kg/m2]; BIA-muscle mass index (BIA-MMI) [kg/m2]; BIA PA [°].
2.2.5. Measurements of body composition by CT
CT-image analysis was performed on routinely obtained abdominal CT scans using the Slice-O-Matic software V 5.0 (Tomovision, Montreal, Canada). CT Hounsfield unit (HU) thresholds for different tissue types were used as follows: Skeletal muscle –29 to +150 HU, adipose tissue –180 to –30 HU. Total abdominal muscle area and fat area on 2 consecutive images at the level of lumbar vertebra 3 (L3) was calculated and standardized for body height, resulting in the CT muscle mass index (CT-MMI) [cm2/m2] and CT fat mass index (CT-FMI) [cm2/m2] which correlate with the whole-body muscle and fat mass as previously described.[8,15,32–34]Figure 1 shows an example of a CT slice on level L3 with measurements of skeletal muscle, subcutaneous fa, and visceral fat areas by HUs.
Figure 1.
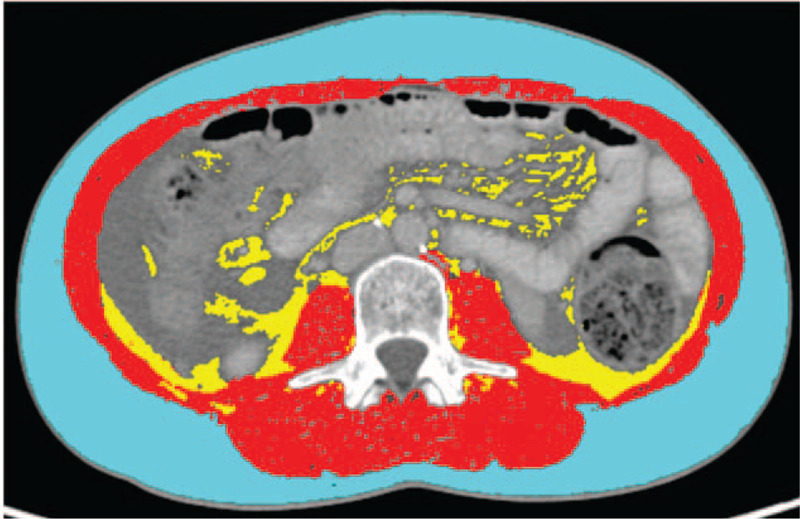
Example for CT scan analysis by the Slice-o-matic software. CT slice on level L3 with measurements of skeletal muscle, subcutaneous fat and visceral fat areas by Hounsfield units. Light blue outer ring: subcutaneous fat; Red inner ring: skeletal muscle; Yellow central area: visceral fat. CT = computed tomography.
2.3. Statistical analyses
The statistical analysis and linear correlation of data were performed with SPSS software V24.0 (IBM, Armonk, NY) and Prism V7.0 (Graphpad, San Diego, CA). Quantitative variables are expressed by median and interquartile range (P25-P75) due to asymmetric distributions. Qualitative variables are expressed by actual and relative frequencies. Differences between quantitative variables were accessed using the Mann–Whitney U test. Qualitative variables were compared using the chi-square test. Correlations were analyzed using Pearson's ρ. All analyses were performed with an explorative significance level of 5%. No adjustment for multiple comparisons was done.
3. Results
3.1. Participants/study size
Between April 2010 and May 2017, the body composition of 313 cancer patients with different types of malignancies was measured by BIA on 4 consecutive study visits (1252 measurements). Of these patients, 118 underwent a routine diagnostic abdominal CT scan that was eligible for body composition analysis +/– 45 days apart from the BIA measurement. An additional 54 matched BIA and CT measurements were available of the same patients on later visits (4–12 weeks apart from the initial visit). Finally, 172 matching CT and BIA measurements of 118 patients were included for the analysis.
3.2. Descriptive data
In our cohort, 54 of 118 patients experienced weight loss >10% and were thus classified as malnourished (45.7%) according to the GLIM criteria. Tables 1 and 2 show the characteristics of the included cases with or without malnutrition. Patients with malnutrition did not differ significantly from those without it in respect to age (median 56 years without malnutrition vs 63 years with malnutrition; P = .26) (see Table 1) and gender (see Table 2). Median BMI was significantly lower in the group with malnutrition, 22.5 versus 25.0 kg/m2 (P < .001). Initial weight before onset of disease was not different between the 2 groups, whereas the current weight on the day of visit was significantly lower in the group with malnutrition (median 65.7 vs 71.0 kg; P = .004). Correspondingly, the median Karnofsky performance status was significantly lower in the malnourished group (median 80% vs 90%) and the nutritional risk screening was significantly higher (median 3 points) than in patients without malnutrition (median 1 point) (P < .001).
Table 1.
Patient characteristics in 118 patients with or without malnutrition.
| No malnutrition (n = 64) | Malnutrition (n = 54) | ||||||
| Median | LQ | UQ | Median | LQ | UQ | P∗ | |
| Age [yr] | 56 | 47 | 68 | 63 | 49 | 68 | .236 |
| BMI [kg/m2] | 25.0 | 21.8 | 27.7 | 22.5 | 20.4 | 24.2 | <.001 |
| Current weight [kg] | 71.0 | 63.5 | 82.1 | 65.7 | 55.6 | 73.5 | .004 |
| Initial weight [kg] | 72.0 | 63.5 | 85.0 | 78.5 | 65.0 | 85.0 | .189 |
| Karnofsky status [%] | 90 | 90 | 90 | 80 | 80 | 90 | <.001 |
| Nutritional risk score | 1 | 1 | 1 | 3 | 1 | 4 | <.001 |
BMI = body mass index, LQ = lower quartile 25P, UQ = upper quartile 75P.
Man–Whitney U test, 95% CI alpha 5%.
Table 2.
Patient characteristics in 118 patients with or without malnutrition.
| No malnutrition (n = 64) | Malnutrition (n = 54) | ||||
| n | (%) | n | (%) | P∗ | |
| Gender | |||||
| Male | 29 | 45.3% | 28 | 51.9% | .479 |
| Female | 35 | 54.7% | 26 | 48.1% | |
| Type of cancer | |||||
| Gastrointestinal | 27 | 42.2% | 36 | 66.7% | .081 |
| Gynecological | 13 | 20.3% | 5 | 9.3% | |
| Urogenital | 4 | 6.3% | 3 | 5.6% | |
| Head and neck | 2 | 3.1% | 4 | 7.4% | |
| Hematological | 8 | 12.5% | 1 | 1.9% | |
| Lungs and pleura | 4 | 6.3% | 2 | 3.7% | |
| Skin/Soft tissues | 2 | 3.1% | 2 | 3.7% | |
| CNS | 1 | 1.6% | 1 | 1.9% | |
| CUP | 3 | 4.7% | 0 | 0.0% | |
| Treatment intention | |||||
| Curative | 26 | 40.6% | 29 | 53.7% | .156 |
| Palliative | 38 | 59.4% | 25 | 46.3% | |
| Surgery | |||||
| No | 30 | 47.6% | 11 | 20.8% | .003 |
| Yes | 33 | 52.4% | 42 | 79.2% | |
| Current chemotherapy | |||||
| No | 29 | 45.3% | 35 | 66.0% | .025 |
| Yes | 35 | 54.7% | 18 | 34.0% | |
| History of chemotherapy | |||||
| No | 39 | 60.9% | 27 | 50.9% | .278 |
| Yes | 25 | 39.1% | 26 | 49.1% | |
| Radiotherapy | |||||
| No | 47 | 73.4% | 39 | 73.6% | .986 |
| Yes | 17 | 26.6% | 14 | 26.4% | |
CNS = central nervous system, CUP = cancers of unknown primary.
Chi-square test.
As shown in Table 2, cancer entities were mainly gastrointestinal and gynecological. However, the distribution between patients with or without malnutrition was not equal. In the malnutrition group more patients had gastrointestinal, head, and neck as well as skin or soft tissue cancers. In the group without malnutrition, more patients had gynecological and hematological malignancies as well as cancers of unknown primary. Treatment intention and type of oncological treatment (chemotherapy or radiotherapy) were equally distributed between the 2 groups. Patients with malnutrition were subject to surgery more often than patients without malnutrition (78.9% vs 57.7%; P = .02), which is explainable by the differences in cancer entities on the 1 hand, and the fact that palliative surgeries were included here on the other hand.
Table 3 shows key laboratory parameters in patients with or without malnutrition. As not all laboratory values were available for all patients the number (n) of analyzed values is given for each parameter. There were no significant differences between patients with or without malnutrition except for a lower median Hemoglobin of 12.4 vs 12.9 [mg/dL] (P = .02). Liver function indicated by median serum Cholinesterase level was not significantly different in patients without or with malnutrition 7413 versus 6608 U/L (P = .07). Kidney function was good in both groups with a median serum creatinine value of 0.8 mg/dL versus 0.9 mg/dL (P = .23). Median C-reactive protein levels as a marker of systemic inflammation were also not significantly different in the both groups 0.2 mg/dL versus 0.1 mg/dL (P = .14).
Table 3.
Key laboratory parameters in patients with or without malnutrition.
| No malnutrition | Malnutrition | ||||||||
| n | Median | LQ | UQ | n | Median | LQ | UQ | P∗ | |
| Sodium [mmol/L] | 55 | 140 | 138 | 142 | 47 | 141 | 140 | 142 | .07 |
| Potassium [mmol/L] | 55 | 4.4 | 4.1 | 4.7 | 47 | 4.4 | 4.1 | 4.6 | .77 |
| S-Creatinine [mg/dL] | 57 | 0,9 | 0.7 | 1.0 | 47 | 0.8 | 0.7 | 0.9 | .23 |
| S-Cholinesterase [U/L] | 44 | 7413 | 6492 | 8496 | 35 | 6608 | 5208 | 7683 | .07 |
| S-Protein [g/dL] | 48 | 7.0 | 6.7 | 7.3 | 37 | 6.8 | 6.4 | 7.2 | .24 |
| S-Albumin [g/dL] | 44 | 4.50 | 4.30 | 4.60 | 36 | 4.40 | 4.10 | 4.55 | .16 |
| CRP [mg/dL] | 48 | 0.2 | 0.1 | 0.7 | 37 | 0.1 | 0.1 | 0.6 | .14 |
| Triglycerides [mg/dL] | 44 | 124 | 84 | 185 | 35 | 115 | 98 | 174 | .87 |
| S-Glucose [mg/dL] | 42 | 95 | 89 | 110 | 33 | 95 | 87 | 114 | .50 |
| Leucocytes [G/L] | 54 | 5.63 | 4.75 | 7.09 | 45 | 6.18 | 4.38 | 8.32 | .72 |
| Hemoglobin [g/dL] | 54 | 12.9 | 12.1 | 13.6 | 45 | 12.4 | 11.1 | 13.1 | .02 |
CRP = C-reactive protein, LQ = lower quartile 25P, S = serum, UQ = upper quartile 75P.
Man–Whitney U test, 95% CI alpha 5%.
3.3. Outcome data
Results of 172 body mass assessments by BIA and CT are presented in Table 4. Patients with malnutrition had significantly lower values for muscle and fat mass measured by CT-MMI (42.28 vs 44.26 [cm2/m2]; P = .015) and CT-FMI (66.39 vs 77.51 [cm2/m2]; P = .014). The same observation can be made for muscle and fat mass measured by BIA MMI (9.68 vs 10.64 [kg/m2]; P = .005), and BIA-FMI (4.6 vs 5.1 [kg/m2]; P < .001). In addition, values for BIA PA were significantly lower in patients with malnutrition (5.09° vs 6.59°; P = .001).
Table 4.
CT and BIA body mass assessments with or without malnutrition.
| Without malnutrition (n = 97) | With malnutrition (n = 75) | Total (n = 172) | |||||
| Median | LQ | UQ | Median | LQ | UQ | P∗ | |
| CT-MMI [cm2/m2] | 44.26 | 40.76 | 49.52 | 42.28 | 36.80 | 47.52 | .015 |
| CT-FMI [cm2/m2] | 77.51 | 52.89 | 128.54 | 66.39 | 42.86 | 98.94 | .014 |
| BIA MMI [kg/m2] | 10.64 | 9.51 | 12.00 | 9.68 | 8.98 | 11.46 | .005 |
| BIA FMI [kg/m2] | 6.59 | 5.20 | 8.62 | 5.09 | 3.99 | 6.78 | <.001 |
| BIA Phase angle [°] | 5.10 | 4.50 | 5.60 | 4.60 | 4.20 | 5.10 | .001 |
BIA = bioelectrical impedance analysis, CT = computed tomography, FMI = fat mass index, FMI = fat mass Index, LQ = lower quartile 25P, MMI = muscle mass index, SMI = skeletal muscle index, UQ = upper quartile 75P.
Man–Whitney U test, 95%CI alpha 5%.
Table 5 shows the correlation of body mass assessments by BIA and CT in patients with or without malnutrition. Values for BIA-MMI and BIA-FMI were correlated with CT-MMI and CT-FMI. In the group of all patients, correlation of CT-MMI and BIA-MMI was very high with Pearson's ρ = 0.794 (P < .01). Looking at patients without malnutrition only, Pearson's ρ was 0.754 (P < .01), whereas in patients with malnutrition it was 0.832 (P < .01). The correlation of CT-FMI and BIA-FMI was equally high, with Pearson's ρ of 0.748 (P < .01) in the group of all patients. In patients without malnutrition Pearson's ρ was 0.748 (P < S.01) and in patients with malnutrition 0.771 (P < .01).
Table 5.
Pearson's correlation coefficients of BIA and CT Indices in patients with or without malnutrition.
| Total (n = 172) | Without malnutrition (n = 97) | With malnutrition (n = 75) | ||||
| CT FMI [cm2/m2] | CT MMI [cm2/m2] | CT FMI [cm2/m2] | CT MMI [cm2/m2] | CT FMI [cm2/m2] | CT MMI [cm2/m2] | |
| BIA FMI [kg/m2] | 0.748∗ | – | 0.721∗ | – | 0,771∗ | – |
| BIA MMI [kg/m2] | – | 0.794∗ | – | 0.754∗ | – | 0.832∗ |
BIA = bioelectrical impedance analysis, CT = computed tomography, FMI = fat mass index, MMI = muscle mass index.
The correlation is significant on a level of 0.01 (2-tailed).
4. Discussion
4.1. Key results
We chose to investigate BIA in cancer patients with malnutrition, as it is the indirect method which is preferably used and has been validated in populations of cancer before. BIA is an easy, noninvasive measuring technique of body composition, providing rapid results with high reproducibility and requires little training to use the portable equipment. Furthermore, it is less expensive than other standard measuring techniques such as DXA, CT, and MRI imaging.[4] Analysis of CT scans was chosen as a reference method over DXA and MRI, as it is routinely performed in cancer patients, requiring no additional examinations for the patients. Furthermore, CT and MRI are more accurate than DXA.[35]
Grundman et al performed a high-quality review of 27 original research articles related to BIA measures in cancer patients in 2015.[22] They concluded that the use of BIA measures benefits in the prevention, diagnosis, prognosis, and outcomes related to nutritional interventions in cancer patients. They further suggest careful interpretation of results in the context of the individual patient rather than comparison with population data to take into account the limitations of BIA measures, that is, the high interpatient variability.[22] Other authors have stated that while BIA is a valuable tool in healthy populations, it is a potentially inaccurate measure of body composition in cancer patients with altered hydration and nutritional status.[4,33] For example, previous studies using BIA in breast cancer patients with lymphedema, showed that the method was inaccurate as a consequence of the fluid accumulation.[4] If BIA is used in such specific cancer cohorts, specific regression equations for BIA have to be chosen. Another approach to overcome this hurdle, is using bioelectrical impedance vector analysis which uses the plot of resistance and reactance normalized per height, in subjects with altered hydration status as was reviewed by Norman et al.[23] However, we suggest that in such specific patient cohorts, BIA data has to be interpreted with special care, and ideally a second tool (ie, CT) is used to validate the results.
To our knowledge, this is the first study explicitly correlating body mass assessments by BIA to CT-based analysis in a large cohort of cancer patients with and without malnutrition, defined by the GLIM criteria.[36] We were able to demonstrate that BIA-values for MM and FM correlate significantly with values from CT-analysis independent of the patient's nutritional status. Our data strongly suggest that if CT-scans are not available or feasible, BIA may be used safely for the analysis of body composition in cancer patients with and without malnutrition.
As previously observed in other studies, except for hemoglobin, laboratory values did not differ significantly between malnourished and non-malnourished patients and are thus not useful to establish a diagnosis of malnutrition or cachexia in clinical routine.[3] Although anemia seems to be consistently associated with malnutrition, it is unspecific, especially in cancer patients.
4.2. Limitations
Limitations of the present study include a single-center study design. Furthermore, data were collected from patients of which a CT scan on level L3 was available, resulting in a reduced sample size. The clinical and BIA data was collected prospectively in the setting of our nutrition and exercise program. The CT scans, however, were done in clinical routine diagnostics. Only subjects that had matching CT including level L3 and BIA data were then retrospectively selected for the trial, and the CT body mass analysis performed. Future trials should preferably be conducted in a multicenter design and include a larger sample size. Furthermore, when CT scans are performed in routine oncological diagnostics, it should be made sure they include Level L3, so that body mass assessments can be retrospectively analyzed in larger cohorts.
4.3. Interpretation
If CT is not available or not feasible, BIA can be safely and cost-effectively used to complete the clinical picture of a patient and evaluate longitudinal changes in body composition due to disease progression or investigative treatment effects. Our findings should enhance future research, especially prospectively designed studies, aimed at facilitating the diagnosis of malnutrition and cachexia in cancer patients and further rising the awareness of this critical medical condition.
4.4. Generalizability
The diagnostic criteria of malnutrition and cachexia remain a topic under constant debate. The recently published international consensus by the GLIM, included 3 phenotypic criteria (non-volitional weight loss, low body mass index, and reduced MM) and 2 etiologic criteria (reduced food intake or assimilation, and inflammation or disease burden).[36] To diagnose malnutrition at least 1 phenotypic criterion and 1 etiologic criterion should be present.[28] Involuntary weight loss and reduced MM are the phenotypic criteria with the strongest evidence according to the GLIM consensus. However, there is no consensus regarding which is the best measurement to define reduced MM, particularly in clinical settings. Generally applicable cut-off values for malnutrition or cachexia/sarcopenia by both BIA and CT are not available since they depend on sex, ethnicity, age, and BMI.[37] The currently used terminology in literature can be quite confusing and the suggested cut-off values vary largely as was reviewed before.[37,38] In addition, BIA cut-off values are valid only for the population in which the BIA equations were validated, using the same BIA device and software. Using optimal stratification methodology, numerous studies have published cut off values for CT-MMI associated with mortality in cancer cohorts, ranging from 36 to 55.8 cm2/m2 for men and 29 to 46.6 cm2/m2 for women.[37]
The same discussion applies to the cut-off values for the amount of weight-loss relative to time. The criteria of the International Cancer Cachexia Consensus from 2011 proposed the diagnostic criterion for cachexia was weight loss >5%, or weight loss >2% in individuals already showing depletion according to current body-mass index (BMI <20 kg/m2) or skeletal MM (sarcopenia). Current research shows that these criteria rather overestimate the prevalence of cachexia.[16,39,40] In comparison, the GLIM criteria suggest weight loss >5% within the last 6 months or >10% beyond 6 months. However, in our experience all of these criteria are rarely fail-safe in clinical practice, since they rely on a specific amount of weight loss in a specific time frame, which many patients do not recall exactly. Therefore, we currently discourage the exclusive use of specific cut-off values of the amount of weight loss, BIA, and CT assessments for diagnosing malnutrition or cachexia. In our opinion the diagnosis has to be derived from the full clinical picture of the patient individually, including detailed anamnesis, clinical presentation, anthropometry, laboratory values and ideally CT analysis, and/or BIA analysis for support.
Acknowledgments
The authors would like to thank all patients for participating in this study and all staff members of the ESZK for their support. The authors also thank Dr. Victoria Kehl for her advice on the statistical methods.
Author contributions
Conceptualization: Tara Catharina Mueller, Olga Prokopchuk, Helmut Friess, Marc E. Martignoni.
Data curation: Tara Catharina Mueller.
Formal analysis: Tara Catharina Mueller, Marc E. Martignoni.
Investigation: Tara Catharina Mueller, Lilly Reik.
Methodology: Tara Catharina Mueller.
Project administration: Tara Catharina Mueller, Helmut Friess, Marc E. Martignoni.
Resources: Marc E. Martignoni.
Software: Lilly Reik.
Supervision: Helmut Friess, Marc E. Martignoni.
Validation: Tara Catharina Mueller, Olga Prokopchuk, Marc E. Martignoni.
Writing – original draft: Tara Catharina Mueller.
Writing – review & editing: Lilly Reik, Olga Prokopchuk, Helmut Friess, Marc E. Martignoni.
Footnotes
Abbreviations: 95% CI = 95% confidence interval, BIA = bioelectrical impedance analysis, BMI = body mass index, CNS = central nervous system, CT = computed tomography, DXA = = Ernähungs- und sport Zentrum für Krebspatienten (nutrition and exercise center for cancer patients), FFM = fat free mass, FM = fat mass, FMI = fat mass index, GLIM = Global Leadership Initiative on Malnutrition, HU = Hounsfield unit, L3 = lumbar vertebra 3, LQ = lower quartile, MM = muscle mass, MRI = magnetic resonance imaging, PA = phase angle, TBW = total body water, UQ = upper quartile.
How to cite this article: Mueller TC, Reik L, Prokopchuk O, Friess H, Martignoni ME. Measurement of body mass by bioelectrical impedance analysis and computed tomography in cancer patients with malnutrition – a cross-sectional observational study. Medicine. 2020;99:50(e23642).
All patients provided informed written consent for analysis of their pseudonymized data before inclusion into the study. No additional, study specific examinations or CT-scans were performed. The prospective collection of data, analysis, and publication of study results within the study registry of the ESZK project has been approved by the Ethics Committee of the “Klinikum Rechts der Isar der Technischen Universität München” Number 460/16S on the October 25th, 2016.
No funding was received. OP was supported by a “clinical leave grant” from the German Center of Infection Research (DZIF) (grant number TI07.001).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Rier HN, Jager A, Sleijfer S, et al. The prevalence and prognostic value of low muscle mass in cancer patients: a review of the literature. Oncologist 2016;21:1396–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Donohoe CL, Ryan AM, Reynolds JV. Cancer cachexia: mechanisms and clinical implications. Gastroenterol Res Pract 2011;2011:601434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Schwarz S, Prokopchuk O, Esefeld K, et al. The clinical picture of cachexia: a mosaic of different parameters (experience of 503 patients). BMC Cancer 2017;17:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Di Sebastiano KM, Mourtzakis M. A critical evaluation of body composition modalities used to assess adipose and skeletal muscle tissue in cancer. Appl Physiol Nutr Metab 2012;37:811–21. [DOI] [PubMed] [Google Scholar]
- [5].Vashi PG, Dahlk S, Popiel B, et al. A longitudinal study investigating quality of life and nutritional outcomes in advanced cancer patients receiving home parenteral nutrition. BMC Cancer 2014;14:593–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mueller TC, Burmeister MA, Bachmann J, et al. Cachexia and pancreatic cancer: are there treatment options? World J Gastroenterol 2014;20:9361–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mason MC, Garcia JM, Sansgiry S, et al. Preoperative cancer cachexia and short-term outcomes following surgery. J Surg Res 2016;205:398–406. [DOI] [PubMed] [Google Scholar]
- [8].Baracos VE, Reiman T, Mourtzakis M, et al. Body composition in patients with non-small cell lung cancer: a contemporary view of cancer cachexia with the use of computed tomography image analysis. Am J Clin Nutr 2010;91:1133s–7s. [DOI] [PubMed] [Google Scholar]
- [9].Antoun S, Rossoni C, Lanoy E. What's next in using CT scans to better understand cachexia? Curr Opin Support Palliat Care 2018;12:427–33. [DOI] [PubMed] [Google Scholar]
- [10].Gibson DJ, Burden ST, Strauss BJ, et al. The role of computed tomography in evaluating body composition and the influence of reduced muscle mass on clinical outcome in abdominal malignancy: a systematic review. Eur J Clin Nutr 2015;69:1079–86. [DOI] [PubMed] [Google Scholar]
- [11].Prado CM, Heymsfield SB. Lean tissue imaging: a new era for nutritional assessment and intervention. JPEN J Parenter Enteral Nutr 2014;38:940–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Marra M, Sammarco R, De Lorenzo A, et al. Assessment of body composition in health and disease using bioelectrical impedance analysis (BIA) and dual energy x-ray absorptiometry (DXA): a critical overview. Contrast Media Mol Imaging 2019;2019:3548284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ni Bhuachalla EB, Daly LE, Power DG, et al. Computed tomography diagnosed cachexia and sarcopenia in 725 oncology patients: is nutritional screening capturing hidden malnutrition? J Cachexia Sarcopenia Muscle 2018;9:295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Albano D, Messina C, Vitale J, et al. Imaging of sarcopenia: old evidence and new insights. Eur Radiol 2020;30:2199–208. [DOI] [PubMed] [Google Scholar]
- [15].Antoun S, Birdsell L, Sawyer MB, et al. Association of skeletal muscle wasting with treatment with sorafenib in patients with advanced renal cell carcinoma: results from a placebo-controlled study. J Clin Oncol 2010;28:1054–60. [DOI] [PubMed] [Google Scholar]
- [16].Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–95. [DOI] [PubMed] [Google Scholar]
- [17].Cooper C, Fielding R, Visser M, et al. Tools in the assessment of sarcopenia. Calcif Tissue Int 2013;93:201–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Fields DA, Higgins PB, Radley D. Air-displacement plethysmography: here to stay. Curr Opin Clin Nutr Metab Care 2005;8:624–9. [DOI] [PubMed] [Google Scholar]
- [19].Nicoletti CF, Camelo JS, Jr, dos Santos JE, et al. Bioelectrical impedance vector analysis in obese women before and after bariatric surgery: changes in body composition. Nutrition (Burbank, Los Angeles County, Calif) 2014;30:569–74. [DOI] [PubMed] [Google Scholar]
- [20].Castillo-Martinez L, Colin-Ramirez E, Orea-Tejeda A, et al. Cachexia assessed by bioimpedance vector analysis as a prognostic indicator in chronic stable heart failure patients. Nutrition (Burbank, Los Angeles County, Calif) 2012;28:886–91. [DOI] [PubMed] [Google Scholar]
- [21].Cova I, Pomati S, Maggiore L, et al. Nutritional status and body composition by bioelectrical impedance vector analysis: A cross sectional study in mild cognitive impairment and Alzheimer's disease. PLoS One 2017;12:e0171331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Grundmann O, Yoon SL, Williams JJ. The value of bioelectrical impedance analysis and phase angle in the evaluation of malnutrition and quality of life in cancer patients--a comprehensive review. Eur J Clin Nutr 2015;69:1290–7. [DOI] [PubMed] [Google Scholar]
- [23].Norman K, Stobaus N, Pirlich M, et al. Bioelectrical phase angle and impedance vector analysis--clinical relevance and applicability of impedance parameters. Clin Nutr 2012;31:854–61. [DOI] [PubMed] [Google Scholar]
- [24].Gaba A, Kapus O, Cuberek R, et al. Comparison of multi- and single-frequency bioelectrical impedance analysis with dual-energy X-ray absorptiometry for assessment of body composition in post-menopausal women: effects of body mass index and accelerometer-determined physical activity. J Hum Nutr Diet 2015;28:390–400. [DOI] [PubMed] [Google Scholar]
- [25].Buckinx F, Reginster J-Y, Dardenne N, et al. Concordance between muscle mass assessed by bioelectrical impedance analysis and by dual energy X-ray absorptiometry: a cross-sectional study. BMC Musculoskelet Disord 2015;16:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Beaudart C, Reginster JY, Slomian J, et al. Estimation of sarcopenia prevalence using various assessment tools. Exp Gerontol 2015;61:31–7. [DOI] [PubMed] [Google Scholar]
- [27].Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. PLoS Med 2007;4:e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Cederholm T, Jensen GL, Correia M, et al. GLIM criteria for the diagnosis of malnutrition - a consensus report from the global clinical nutrition community. Clin Nutr 2019;38:1–9. [DOI] [PubMed] [Google Scholar]
- [29].Kyle UG, Bosaeus I, De Lorenzo AD, et al. Bioelectrical impedance analysis—part II: utilization in clinical practice. Clin Nutr 2004;23:1430–53. [DOI] [PubMed] [Google Scholar]
- [30].Walter-Kroker A, Kroker A, Mattiucci-Guehlke M, et al. A practical guide to bioelectrical impedance analysis using the example of chronic obstructive pulmonary disease. Nutr J 2011;10:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Piccoli A, Rossi B, Pillon L, et al. A new method for monitoring body fluid variation by bioimpedance analysis: the RXc graph. Kidney Int 1994;46:534–9. [DOI] [PubMed] [Google Scholar]
- [32].Prado CMM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 2008;9:629–35. [DOI] [PubMed] [Google Scholar]
- [33].Mourtzakis M, Prado CM, Lieffers JR, et al. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 2008;33:997–1006. [DOI] [PubMed] [Google Scholar]
- [34].Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 2013;31:1539–47. [DOI] [PubMed] [Google Scholar]
- [35].Messina C, Albano D, Gitto S, et al. Body composition with dual energy X-ray absorptiometry: from basics to new tools. Quant Imaging Med Surg 2020;10:1687–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Cederholm T, Jensen GL, Correia M, et al. GLIM criteria for the diagnosis of malnutrition - a consensus report from the global clinical nutrition community. J Cachexia Sarcopenia Muscle 2019;10:207–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Daly LE, Prado CM, Ryan AM. A window beneath the skin: how computed tomography assessment of body composition can assist in the identification of hidden wasting conditions in oncology that profoundly impact outcomes. Proc Nutr Soc 2018;77:135–51. [DOI] [PubMed] [Google Scholar]
- [38].Gonzalez MC, Heymsfield SB. Bioelectrical impedance analysis for diagnosing sarcopenia and cachexia: what are we really estimating? J Cachexia Sarcopenia Muscle 2017;8:187–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Blum D, Stene GB, Solheim TS, et al. Validation of the consensus-definition for cancer cachexia and evaluation of a classification model--a study based on data from an international multicentre project (EPCRC-CSA). Ann Oncol 2014;25:1635–42. [DOI] [PubMed] [Google Scholar]
- [40].van der Werf A, van Bokhorst QNE, de van der Schueren MAE, et al. Cancer cachexia: identification by clinical assessment versus international consensus criteria in patients with metastatic colorectal cancer. Nutr Cancer 2018;70:1322–9. [DOI] [PubMed] [Google Scholar]


