Abstract
Background:
Peripheral neurotoxicity (PN) is a frequent side effect of oxaliplatin treatment, and also is its dose-limiting toxicity. Studies have confirmed that ω-3 polyunsaturated fatty acids (ω-3 PUFAs) had a neuroprotective effect. However, the efficacy of ω-3 PUFAs on the prevention of oxaliplatin-related neurotoxicity remains unclear. We assessed the effect of ω-3 PUFAs on the neurotoxicity in colon cancer patients treated by oxaliplatin combined with capecitabine.
Methods:
In a randomized, double-blind, placebo-controlled study, 179 patients with colon cancer receiving oxaliplatin combined with capecitabine were recruited, and randomly assigned to take ω-3 PUFAs, 640 mg t.i.d during chemotherapy and 1 month after the end of the treatment or placebo. All patients were treated with chemotherapy for 6 treatment cycles. The incidence and severity of PN were evaluated, and the nerve conduction was measured before the onset of chemotherapy and 1 month after treatment. In addition, the quality of life was also accessed using Chinese version of European organization for research and treatment of cancer quality of life questionnaire.
Results:
The incidence of PN in the ω-3 PUFAs group and placebo group was 52.22% and 69.66%, respectively (P = .017). In addition, there was a significant difference in the severity of PN between the 2 groups (P = .017). In terms of motor and sensory nerve conduction, the sensory action potentials amplitude of sural nerve in the ω-3 PUFAs group and placebo group after chemotherapy treatment were (15.01 ± 3.14) and (13.00 ± 3.63) μ V respectively, suggesting there was a significant difference in the 2 groups (P = .000). In addition, the mean score of the global health-status/quality of life was obviously higher in the ω-3 PUFAs group than that in the placebo group.
Conclusion:
ω-3 PUFAs seem to reduce the incidence and severity of oxaliplatin-related neurotoxicity, and improve the quality of patients’ life, indicating it is expected to be a potential drug for the treatment of oxaliplatin-related neurotoxicity.
Keywords: ω-3 polyunsaturated fatty acids, chemotherapy, colon cancer, oxaliplatin-related neurotoxicity, quality of life
1. Introduction
Global cancer statistics have reported that over 1.8 million new colorectal cancer (CRC) cases and 881,000 deaths were estimated to occur in 2018, and the incidence and mortality of CRC was 6.1% and 9.2% in all cancer cases, respectively.[1] Therefore, it is an important to effectively control the development of CRC. Chemotherapy is a standard adjuvant treatment of CRC.[2] Oxaliplatin, a third generation platinum-based agent, is the principal chemotherapeutic drug for the treatment of CRC. Although oxaliplatin can significantly improve the patients’ overall survival,[3] the peripheral neurotoxicity (PN) caused by oxaliplatin is a frequent dose-limiting toxicity.[4,5] Some degree of PN occurs in nearly all patients with receiving oxaliplatin.[4] The mechanism of oxaliplatin induced neurotoxicity is unclear. Several studies have suggested that the occurrence of PN was related to the accumulation of oxaliplatin in the dorsal root ganglia.[6,7] In clinic, calcium and magnesium infusions are often used to treat oxaliplatin-related neurotoxicity,[8,9] but the curative effect does not well, and the most effective way is to suspend to use oxaliplatin.
ω-3 polyunsaturated fatty acids (ω-3 PUFAs) are essential fatty acids in the human body, including eicosatetraenoic acid (EPA), and docosahexenoic acid (DHA). Accumulated studies have shown that EPA and DHA were often used in the treatment of neurodegenerative diseases by exerting anti-oxidative, anti-inflammatory, and neurotrophic effects.[10,11] Moreover, DHA had an prevention role in treating peripheral neuropathy caused by bortezomib in patients with multiple myeloma.[12] However, little is known about the effect of ω-3 PUFAs on the treatment of oxaliplatin-related neurotoxicity. Herein, we had conducted a randomized, double-blind, placebo-controlled clinical trail to evaluate the efficacy of the ω-3 PUFAs on the treatment of neurotoxicity in patients receiving oxaliplatin combined with capecitabine.
2. Patients and methods
2.1. Patients
The protocol of this study was approved by the Medical Ethics Committee of Zhejiang Cancer Hospital (IRB-2015-216). Before the study was conducted, written informed consents of patients were obtained.
In this randomized, double-blind, placebo-controlled study, participants were recruited for the study from the department of integrated traditional Chinese and western medicine of Zhejiang Cancer Hospital from January 2017 to December 2019. The inclusion criteria will consist of: patients with stage III and IV colon cancer if they have to begin receiving oxaliplatin combined with capecitabine; ECOG sore ranged from 0 to 1; not having autoimmune disease, diabetes, or renal, hepatic, cardiac, and parathyroid disorders; not having peripheral neuropathy disease; not receiving any other chemotherapeutic drugs; not taking neuroprotective drugs, such as calcium and magnesium infusions; not taking ω-3 and vitamin/mineral supplements and other nutrition medications; not allergic to fish and fish products; and without history of having other cancers. Exclusion criteria will consist of: changes chemotherapy regimen; affected by any acute disease during the study; refuses to continue chemotherapy; unwilling to continue the study; also, patients who were less than 90% compliant with treatment will be excluded. Figure 1 shows a summary of the study design.
Figure 1.
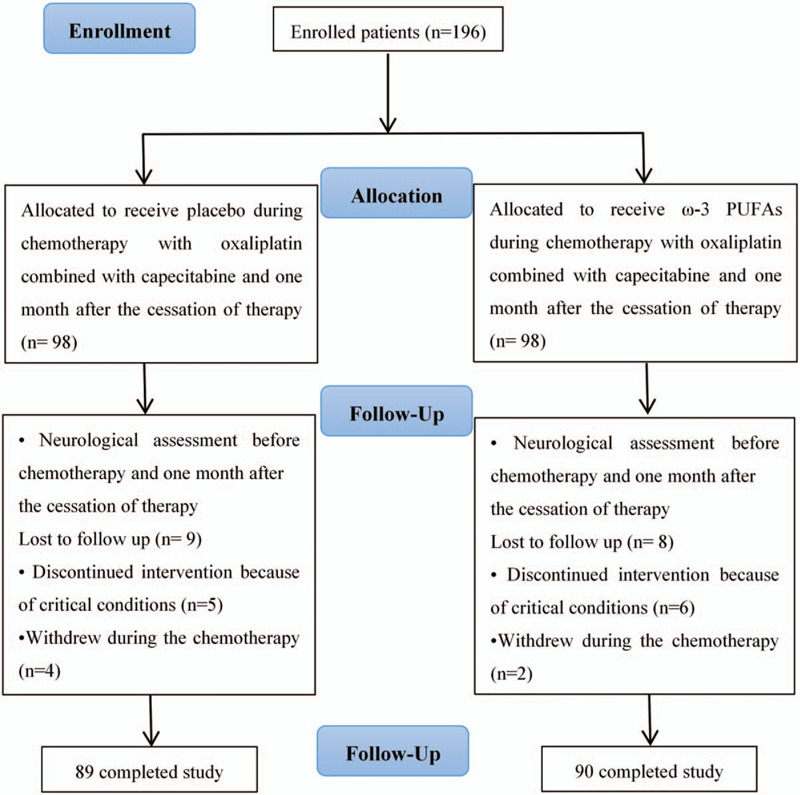
Diagram of randomization of patients with colon cancer to receive ω-3 polyunsaturated fatty acids or placebo.
2.2. Randomization and blinding
The randomization assignment will be performed using opaque envelopes with the numbers of the experiment groups. The participants who meet the criteria will be randomly allocated into 2 groups:
-
1)
640 mg ω-3 PUFAs (54% DHA, 10% EPA) 3 times a day during chemotherapy and 1 month after the end of therapy or
-
2)
placebo capsules, that were equal calorie and similar in appearance to ω-3 PUFAs capsules, and similarly administered. Noteworthy, the dose of ω-3 PUFAs was determined based on previous clinical study.[13] All capsules were produced from Zhejiang hailisheng Co., Ltd, China. For the regimen of chemotherapy: all patients received oxaliplatin 130 mg/m2 combined with capecitabine 1000 mg/m2 every 3 weeks for 6 cycles.
For blinding, a person not involved in the study will make the randomization list, assigning participants to the ω-3 PUFAs or placebo group. ω-3 PUFAs and placebo tablets will be placed into unlabeled identical capsules. All participants and investigators will be blinded to the random assignments.
2.3. Measurements
Before the beginning of the intervention, a questionnaire about patients’ cancer history, medications, diseases, and probable supplement use will be recorded. According to previous study,[13] the neurotoxicity was measured at 1 month after the end of chemotherapy according to the classification criteria of neurotoxicity by the same neurologist.[14] Nerve conduction studies were conducted unilaterally (right side) by using a Nicolet/VIASYS Viking Quest EMG Machine based on the standard methods. The distal motor latency, peak to baseline amplitude of compound muscle action potentia, and motor cond uction velocity of the nerves in tibial, peroneal, and ulnar were measured as the motor nerve conduction assessment. The sensory action potential amplitude (a-SAP) and sensory conduction velocity were measured for peroneal, and ulnar nerves as the sensory nerve conduction measurements.
Besides, the quality of life was accessed using Chinese version of European organization for research and treatment of cancer quality of life questionnaire (EORTC QLQ-C30 questionnaire).[15] The EORTC QLQ-C30 questionnaire has 30 items arranged into 9 scales and 6 single items. The scales are divided into 5 function scales (physical, role, cognitive, emotional, and social functions); 3 symptom scales (fatigue, nausea, or vomiting, pain) and 1 global health-status/quality of life scale. The 6 single items address specific symptoms: dyspnoea, insomnia, appetite loss, constipation, diarrhoea, and financial problems. The questionnaire was officially translated into the Chinese language. The higher score of function scales and global health-status, the better the patient's quality of life; and the higher score of symptom scales, the worse the patient's quality of life. The questionnaire was officially translated into the Chinese language.
2.4. Statistical analysis
All date was presented as mean ± S.D. All analyses will be performed with IBM SPSS Statistics 19 (IBM Corp, Armonk, NY). The 2 groups of patients’ baseline characteristics were statistically evaluated using Chi-squared or Fisher's test. Analysis of covariance (ANCOVA) test was used to determine the differences of nerve conduction measurements between the 2 groups at the end of study, adjusting for baseline values and covariates. The difference of PN incidence and the severity of PN between the 2 study groups was estimated by using logistic regression and ordinal regression analysis, respectiely. P < .05 was considered statistically significant.
3. Results
3.1. Patient characteristics
196 patients were enrolled in the study and randomly assigned to receive ω-3 PUFAs (n = 98) or placebo (n = 98). 11 patients discontinued the study due to a critical health conditions (including grade 4 neutropenia and hand-foot syndrome, and severe liver function damage during chemotherapy) and 6 patients were unwilling to continue during the chemotherapy (lost to follow up, n = 17). So 179 colon cancer patients completed the study, and 90 patients in the ω-3 PUFAs group and 89 patients in the placebo group (Fig. 1). The ω-3 PUFAs group and the placebo group were comparable for gender, age, performance scores, clinical stages, temperature, respiratory rates, pulse, systolic blood pressure, and diastolic blood pressure. There was no statistical significant difference between the 2 groups. Patient characteristics are displayed in Table 1.
Table 1.
Comparison of clinical features between the 2 groups.
| Total Number (n = 179) | ω-3 PUFAs group (n = 90) | Placebo group (n = 89) | x2 | P | |
| Gender | 0.005 | .943 | |||
| Male | 93 (52.0%) | 47 (52.2%) | 46 (51.7%) | ||
| Female | 86 (48.0%) | 43 (47.8%) | 43 (48.3%) | ||
| Age | 2.011 | .156 | |||
| <65 | 85 (47.5%) | 38 (42.2%) | 47 (52.8%) | ||
| ≥65 | 94 (52.5%) | 52 (57.8%) | 42 (47.2%) | ||
| ECOG score | 0.137 | .712 | |||
| 2 | 84 (46.9%) | 41 (45.6%) | 43 (48.3%) | ||
| 3 | 95 (53.1%) | 49 (54.4%) | 46 (51.7%) | ||
| Clinical stage | 0.148 | .700 | |||
| III | 79 (44.1%) | 41 (45.6%) | 38 (42.7%) | ||
| IV | 100 (55.9%) | 49 (54.4%) | 51 (57.3%) | ||
| BMI | 18.85 ± 1.69 | 18.77 ± 1.52 | .755 | ||
| Temperature | 36.74 ± 0.402 | 36.77 ± 0.366 | .554 | ||
| Respiratory rates | 18.48 ± 2.833 | 18.38 ± 2.906 | .824 | ||
| Pulse (Times) | 77.778 ± 9.24 | 78.967 ± 8.090 | .361 | ||
| SBP (mm Hg) | 116.778 ± 15.020 | 116.494 ± 13.869 | .896 | ||
| DBP (mm Hg) | 77.378 ± 9.069 | 77.629 ± 8.790 | .851 |
DBP = Diastolic blood pressure, SBP = Systolic blood pressure.
3.2. Peripheral neuropathy
According to the classification criteria of neurotoxicity, the severity of PN in the patients of ω-3 PUFAs group was as follows: 43 patients (47.8%) did not develop PN and 47 patients (52.2%) manifested some grade of PN: 25 patients (27.8%) developed stage I of PN; 11 patients (12.2%) developed stage II of PN; 6 patients (6.7%) developed stage III of PN; and 5 patients (5.6%) developed stage IV of PN (Table 2).
Table 2.
Oxaliplatin-induced peripheral neuropathy in the study groups.
| ω-3 PUFAs group | Placebo group | x2 | P | |
| 0 | 43 (47.8%) | 27 (30.3%) | ||
| I | 25 (27.8%) | 22 (24.7%) | ||
| II | 11 (12.2%) | 11 (12.4%) | ||
| III | 6 (6.7%) | 17 (19.1%) | ||
| IV | 5 (5.6%) | 12 (13.5%) | ||
| No PN | 43 (47.8%) | 27 (30.3%) | ||
| Number of PN | 47 (52.2%) | 62 (69.7%) | ||
| Total | 90 (100%) | 89 (100%) | ||
| Incidence of PN | 5.716 | .017 | ||
| Severity of PN | 11.987 | .017 |
PN = peripheral neurotoxicity.
In placebo group, PN was not observed in 27 patients (30.3%), while 22 patients (24.7%) developed stage I of PN; 11 patients (12.4%) developed stage II of PN; and 17 patients (19.1%) developed stage III of PN. Also, stage IV of PN was seen in 12 patient (13.5%) (Table 2). These results indicated that there was a significant difference between the ω-3 PUFAs group and the placebo group in PN (P = .017).
3.3. Nerve conduction study parameters
The differences of quantitative values between 2 study groups were measured by using analysis of covariance adjusting baseline measurements. In the study, there was a significant difference in sural a-SAP between the ω-3 PUFAs group and the placebo group (P = .000) with a sharp decrease of sural a-SAP in the placebo group. Furthermore, the other differences of nerve conduction study parameters has not statistical significance between the 2 groups. (Tables 3 and 4).
Table 3.
Motor nerve conduction measurements.
| Group | Pre-chemotherapy | Post-chemotherapy | P∗ | |
| Tibial nerve | ||||
| DML (ms) | ω-3 PUFAs | 16.75 (1.93) | 17.90 (2.33) | .000∗ |
| Placebo | 16.62 (2.34) | 17.29 (2.33) | .037∗ | |
| P# | .646 | .079 | ||
| a-CMAP (mV) | ω-3 PUFAs | 11.20 (1.84) | 11.63 (2.50) | .092 |
| Placebo | 11.68 (2.49) | 11.34 (2.56) | .372 | |
| P# | .061 | .447 | ||
| MCV (m/s) | ω-3 PUFAs | 46.92 (5.83) | 46.47 (5.70) | .595 |
| Placebo | 46.86 (5.60) | 46.59 (5.48) | .745 | |
| P# | .939 | .884 | ||
| Peroneal nerve | ||||
| DML (ms) | ω-3 PUFAs | 15.61 (2.46) | 15.607 (1.972) | .99 |
| Placebo | 15.71 (2.73) | 15.658 (2.25) | .881 | |
| P# | .791 | .872 | ||
| a-CMAP (mV) | ω-3 PUFAs | 8.25 (2.96) | 8.06 (2.83) | .658 |
| Placebo | 7.90 (2.83) | 7.88 (2.73) | .948 | |
| P# | .42 | .655 | ||
| MCV (m/s) | ω-3 PUFAs | 47.61 (7.80) | 46.98 (6.71) | .558 |
| Placebo | 47.27 (6.59) | 46.01 (7.77) | .245 | |
| P# | .751 | .375 | ||
| Ulnar nerve | ||||
| DML (ms) | ω-3 PUFAs | 11.65 (4.46) | 11.94 (4.20) | .655 |
| Placebo | 12.27 (4.21) | 12.44 (4.18) | .782 | |
| P# | .343 | .424 | ||
| a-CMAP (mV) | ω-3 PUFAs | 17.18 (4.81) | 17.29 (4.01) | .873 |
| Placebo | 17.30 (4.17) | 17.39 (4.08) | .886 | |
| P# | .858 | .864 | ||
| MCV (m/s) | ω-3 PUFAs | 54.16 (11.11) | 52.50 (10.38) | .303 |
| Placebo | 52.78 (12.31) | 52.32 (11.27) | .667 | |
| P# | .434 | .909 | ||
a-CMAP = amplitude of compound muscle action potential, DML = distal motor latency, MCV = motor conduction velocity.
One month after the cessation of chemotherapy.
P value is reported based on the analysis of covariance.
Table 4.
Sensory nerve conduction measurements.
| Group | Pre-chemotherapy | Post-chemotherapy∗ | P-value# | |
| Sural nerve | ||||
| a-SAP (μ V) | ω-3 PUFAs | 14.78 (3.21) | 15.01 (3.14) | .638 |
| Placebo | 14.97 (4.08) | 13.00 (3.63) | .001∗ | |
| P# | .732 | .000# | ||
| SCV (m/s) | ω-3 PUFAs | 50.89 (10.82) | 50.75 (11.74) | .932 |
| Placebo | 50.96 (9.91) | 51.06 (9.58) | .942 | |
| P# | .967 | .844 | ||
| Ulnar nerve | ||||
| a-SAP (μ V) | ω-3 PUFAs | 36.90 (9.31) | 32.23 (9.75) | .001∗ |
| Placebo | 34.76 (12.4) | 29.36 (12.02) | .004∗ | |
| P# | .192 | .08 | ||
| SCV (m/s) | ω-3 PUFAs | 52.41 (10.47) | 52.10 (9.85) | .835 |
| Placebo | 51.48 (11.64) | 51.51 (10.84) | .984 | |
| P# | .573 | .707 | ||
a-SAP = sensory action potential amplitude, SCV = sensory conduction velocity.
One month after the cessation of chemotherapy.
P value is reported based on the analysis of covariance.
3.4. Scale and item scores in the EORTC QLQ-C30 questionnaire
The scores of EORTC QLQ-C30 for all scales and items was shown in Table 5. In this study, the mean score of the global health-status/quality of life was obviously higher in the ω-3 PUFAs group than that in the placebo group (P = .032). For measurement of function scales, a significant reduction in physical function score was found in the placebo group than that in the ω-3 PUFAs group (P = .046). In addition, compared to the ω-3 PUFAs group, the mean score of appetite loss was higher in the placebo group (P = .025).
Table 5.
The scores of all scales and items for Quality of Life Questionnaire-C30 in patients after chemotherapy.
| ω-3 PUFAs | Placebo | P | |
| Global health status | 62 ± 10.45 | 43 ± 12.32 | .032 |
| Physical function | 48 ± 9.69 | 30 ± 10.39 | .046 |
| Role function | 35 ± 10.35 | 28 ± 15.52 | .124 |
| Emotional function | 40 ± 10.58 | 38 ± 13.25 | .842 |
| Cognitive function | 56 ± 14.92 | 53 ± 13.44 | .245 |
| Social function | 45 ± 11.42 | 39 ± 15.77 | .675 |
| Fatigue | 52 ± 15.23 | 55 ± 14.78 | .156 |
| Nausea/vomiting | 54 ± 18.54 | 63 ± 19.47 | .078 |
| Pain | 62 ± 17.36 | 65 ± 15.25 | .956 |
| Dispnea | 42 ± 9.28 | 48 ± 8.22 | .652 |
| Insomnia | 66 ± 10.78 | 63 ± 7.86 | .078 |
| Appetite loss | 62 ± 9.34 | 79 ± 14.39 | .025 |
| Constipation | 42 ± 8.79 | 49 ± 12.71 | .534 |
| Diarrhoea | 69 ± 8.67 | 71 ± 10.62 | .658 |
| Financial problems | 68 ± 7.44 | 70 ± 10.33 | .466 |
ω-3 PUFAs = ω-3 polyunsaturated fatty acids.
4. Discussion
Oxaliplatin is one the standard adjuvant chemotherapy drug of colon cancer, and also of the standard palliative treatment for metastases. However, peripheral sensory neurotoxicity is a common toxic effect of oxaliplatin, which is often limited the use of oxaliplatin doses and affected the efficacy of the chemotherapy treatment.[9] Oxaliplatin-related PN includes acute PN and chronic PN.[16] Acute PN often occurs during the treatment, which is characterized by rapid onset of paresthesia of peripheral nerves that are sensitive to cold stimuli, such as numbness or pain in extremities and oropharynx.[17] Chronic peripheral neuropathy is a delayed peripheral neuropathy that occurs after repeated oxaliplatin administration, and is manifested as loss of sensation and ataxia. It was estimated that most patients who received this chemotherapeutic agent developed dose-dependent neurotoxicity.[4] Therefore, oxaliplatin-related PN affected the patients’ quality of life obviously, and it is important to prevent oxaliplatin-related neurotoxicity effectively.
The aim of the current randomized, double-blind, placebo-controlled study was to confirm the efficacy of ω-3 PUFAs in prophylaxis against oxaliplatin induced PN. According to our knowledge, this is the first time to confirm the efficacy of ω-3 in reducing the incidence and severity of oxaliplatin induced PN. In the study, there was a significant difference in the incidence of oxaliplatin-related PN between the 2 groups that 47.8% of patients taking ω-3 PUFAs did not develop PN while incidence was 30.3% in the placebo group, indicating that ω-3 PUFAs had neuroprotective effects and decreased the incidence of oxaliplatin-related neurotoxicity considerably. Our results are similar to previous studies that have discovered ω-3 PUFAs reduced the incidence of PN in diabetic neuropathy.[18] In addition, neurophysiologic studies improved the accuracy and precision of PN evaluation and helped to identify the severity of PN in patients with oxaliplatin. Thus, we used the classification criteria of neurotoxicity that reported previous study to identify the severity of PN in patients,[14] we found that there was also a significant difference in the severity of PN between the 2 groups that ω-3 PUFAs reduced the severity of PN remarkably. These results are in accordance with previous studies that have reported the efficacy of ω-3 PUFAs in degenerative diseases. Study showed that ω-3 PUFAs could attenuate the severity of neuropathy by inhibiting the inflammatory response in patients with degenerative diseases.[11] Meanwhile, a combined EPA and DHA oral administration accelerated nerve regeneration and prevented neuropathic pain though its anti-neuroinflammatory activity in mice with peripheral nerve injury.[19] Also, accumulating evidence has shown that ω-3 PUFAs alleviated neuropathic pain by suppressing neuroinflammation and oxidative stress.[20,21] In addition, study also reported fish oil treatment can provide some beneficial effects against cisplatin-induced peripheral neuropathy by changing latencies through anti-oxidant and anti-inflammatory, which may lead to myelinated axon regeneration.[22] According to these results, we proposed that ω-3 PUFAs might prevent the incidence and severity of PN via inhibiting neuroinflammatory response.
In the current study, the nerve conduction was further analyzed, and a considerable difference was found in sural nerve a-SAP between the 2 groups that a sharp decrease in the placebo group. Similarly, Ghoreishi et al had reported that omega-3 fatty acids were protective against paclitaxel-induced peripheral neuropathy might be related to prevent the significant decrease of sural nerve a-SAP. Besides, Argyriou et al found that the decrease sural a-SAP >50% of the baseline value, as being the sole, significant predictor of worse neurological outcome in the cisplatin- and paclitaxel-based chemotherapy induced peripheral neuropathy.[23] Therefore, our results indicated that ω-3 PUFAs attenuate oxaliplatin induced PN may be also associated with remission of sural nerve a-SAP reduction.
Numerous studies have confirmed that chemotherapy-induced PN affected patient's quality of life, including patients’ emotional and functional domains.[24,25] Therefore, based on the efficacy of ω-3 PUFAs in prevention of oxaliplatin-related PN, we further measured the patients’ quality of life. The mean scores of the global health-status/quality of life and physical function were higher after ω-3 PUFAs treatment in patients with oxaliplatin combined with capecitabine. Meanwhile, the score of appetite loss was reduced significantly in the ω-3 PUFAs group. These changes may be related to the attenuation of oxaliplatin-related PN by ω-3 PUFAs administration. Thus, these results indicated that ω-3 PUFAs improved the patients’ quality of life via alleviating PN in colon cancer patients with oxaliplatin combined with capecitabine.
However, there were some limitations in the design of this randomized, double-blind, placebo-controlled study. Lack of long-term follow-up of measurement results is a potential limitation of this study. Additionally, relatively small sample size and single-center study were another possible limitations of the current trial.
5. Conclusion
This double-blind, randomized study illustrated that ω-3 PUFAs may be potential neuroprotective agent for treatment of oxaliplatin-related neurotoxicity. Another double-blind, multi-center, placebo-controlled randomized clinical study is needed to confirm these results. Moreover, we will further measure the incidence and severity of PN and quality of life at 6 months or 1 year after the end of chemotherapy as indicators of long-term efficacy of ω-3 PUFAs. Besides, the mechanism of ω-3 PUFAs on the prevention of oxaliplatin-related neurotoxicity should be further studied.
Author contributions
Conceptualization: Qinghua Yao, Xinjie Zhang, Haitao Chen, Liping Zhang.
Data curation: Xinjie Zhang, Haitao Chen, Yi Lu, Wang Yao, Runzhe Zhang.
Formal analysis: Xinjie Zhang, Yi Lu.
Funding acquisition: Qinghua Yao.
Investigation: Yi Lu.
Methodology: Haitao Chen.
Project administration: Qinghua Yao, Liping Zhang.
Software: Haitao Chen, Chao Xu, Wang Yao, Lu Xu.
Supervision: Chao Xu, Lu Xu, Liping Zhang.
Writing – original draft: Xinjie Zhang, Haitao Chen.
Writing – review & editing: Qinghua Yao, Yi Lu, Liping Zhang.
Footnotes
Abbreviations: ω-3 PUFAs = ω-3 polyunsaturated fatty acids, a-SAP = sensory action potential amplitude, CRC = colorectal cancer, DHA = docosahexenoic acid, EORTC QLQ-C30 questionnaire = Chinese version of European organization for research and treatment of cancer quality of life questionnaire, EPA = eicosatetraenoic acid, PN = peripheral neurotoxicity.
How to cite this article: Zhang X, Chen H, Lu Y, Xu C, Yao W, Xu L, Zhang R, Zhang L, Yao Q. Prevention of oxaliplatin-related neurotoxicity by ω-3 PUFAs: a double-blind randomized study of patients receiving oxaliplatin combined with capecitabine for colon cancer. Medicine. 2020;99:50(e23564).
XZ, HC, and YL contributed equally to this work.
The research was supported by Zhejiang Medical and Health Science and Technology Program (No. 2016DTA001) and Chinese Medicine Research Program of Zhejiang Province (No. 2020ZA018).
The authors have no conflicts of interest to disclose.
The data that support the findings of this study are available from a third party, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available.
References
- [1].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [2].Benson AB, Venook AP, Al-Hawary MM, et al. NCCN guidelines insights: colon cancer, version 2.2018. J Natl Compr Canc Netw 2018;16:359–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Comella P, Lorusso V, Maiorino L, et al. Oxaliplatin, irinotecan, and fluorouracil/folinic acid in advanced gastric cancer: a multicenter phase II trial of the southern Italy cooperative oncology group. Cancer Chemother Pharmacol 2009;64:893–9. [DOI] [PubMed] [Google Scholar]
- [4].Griffith KA, Zhu S, Johantgen M, et al. Oxaliplatin-induced peripheral neuropathy and identification of unique severity groups in colorectal cancer. J Pain Symptom Manage 2017;54:701–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Yildirim N, Cengiz M. Predictive clinical factors of chronic peripheral neuropathy induced by oxaliplatin. Support Care Cancer 2020;28:4781–8. [DOI] [PubMed] [Google Scholar]
- [6].Beijers AJ, Mols F, Vreugdenhil G. A systematic review on chronic oxaliplatin-induced peripheral neuropathy and the relation with oxaliplatin administration. Support Care Cancer 2014;22:1999–2007. [DOI] [PubMed] [Google Scholar]
- [7].Park SB, Lin CS, Krishnan AV, et al. Dose effects of oxaliplatin on persistent and transient Na+ conductances and the development of neurotoxicity. PLoS One 2011;6:e18469.doi: 10.1371/journal.pone.0018469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gamelin L, Boisdron-Celle M, Delva R, et al. Prevention of oxaliplatin-related neurotoxicity by calcium and magnesium infusions: a retrospective study of 161 patients receiving oxaliplatin combined with 5-fluorouracil and leucovorin for advanced colorectal cancer. Clin Cancer Res 2004;10:4055–61. [DOI] [PubMed] [Google Scholar]
- [9].Knijn N, Tol J, Koopman M, et al. The effect of prophylactic calcium and magnesium infusions on the incidence of neurotoxicity and clinical outcome of oxaliplatin-based systemic treatment in advanced colorectal cancer patients. Eur J Cancer 2011;47:369–74. [DOI] [PubMed] [Google Scholar]
- [10].Zhang YP, Brown RE, Zhang PC, et al. DHA, EPA and their combination at various ratios differently modulated Abeta25-35-induced neurotoxicity in SH-SY5Y cells. Prostaglandins Leukot Essent Fatty Acids 2018;136:85–94. [DOI] [PubMed] [Google Scholar]
- [11].Lorente-Cebrian S, Costa AG, Navas-Carretero S, et al. An update on the role of omega-3 fatty acids on inflammatory and degenerative diseases. J Physiol Biochem 2015;71:341–9. [DOI] [PubMed] [Google Scholar]
- [12].Maschio M, Zarabla A, Maialetti A, et al. Prevention of bortezomib-related peripheral neuropathy with docosahexaenoic acid and alpha-lipoic acid in patients with multiple myeloma: preliminary data. Integr Cancer Ther 2018;17:1115–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ghoreishi Z, Esfahani A, Djazayeri A, et al. Omega-3 fatty acids are protective against paclitaxel-induced peripheral neuropathy: a randomized double-blind placebo controlled trial. BMC Cancer 2012;12:355.doi: 10.1186/1471-2407-12-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gamelin E, Gamelin L, Bossi L, et al. Clinical aspects and molecular basis of oxaliplatin neurotoxicity: current management and development of preventive measures. Semin Oncol 2002;29:21–33. [DOI] [PubMed] [Google Scholar]
- [15].Velenik V, Secerov-Ermenc A, But-Hadzic J, et al. Health-related quality of life assessed by the EORTC QLQ-C30 questionnaire in the general Slovenian population. Radiol Oncol 2017;51:342–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Raphael MJ, Fischer HD, Fung K, et al. Neurotoxicity outcomes in a population-based cohort of elderly patients treated with adjuvant oxaliplatin for colorectal cancer. Clin Colorectal Cancer 2017;16:397–404. [DOI] [PubMed] [Google Scholar]
- [17].Argyriou AA, Cavaletti G, Briani C, et al. Clinical pattern and associations of oxaliplatin acute neurotoxicity: a prospective study in 170 patients with colorectal cancer. Cancer 2013;119:438–44. [DOI] [PubMed] [Google Scholar]
- [18].Head KA. Peripheral neuropathy: pathogenic mechanisms and alternative therapies. Altern Med Rev 2006;11:294–329. [PubMed] [Google Scholar]
- [19].Silva RV, Oliveira JT, Santos B, et al. Long-chain omega-3 fatty acids supplementation accelerates nerve regeneration and prevents neuropathic pain behavior in mice. Front Pharmacol 2017;8:723.doi: 10.3389/fphar.2017.00723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Huang CT, Tsai YJ. Docosahexaenoic acid confers analgesic effects after median nerve injury via inhibition of C-Jun N-terminal kinase activation in microglia. J Nutr Biochem 2016;29:97–106. [DOI] [PubMed] [Google Scholar]
- [21].Galan-Arriero I, Serrano-Munoz D, Gomez-Soriano J, et al. The role of omega-3 and omega-9 fatty acids for the treatment of neuropathic pain after neurotrauma. Biochim Biophys Acta Biomembr 2017;1859:1629–35. [DOI] [PubMed] [Google Scholar]
- [22].Kamisli S, Ciftci O, Cetin A, et al. Fish oil protects the peripheral and central nervous systems against cisplatin-induced neurotoxicity. Nutr Neurosci 2013;17:116–26. [DOI] [PubMed] [Google Scholar]
- [23].Argyriou AA, Polychronopoulos P, Koutras A, et al. Peripheral neuropathy induced by administration of cisplatin- and paclitaxel-based chemotherapy. Could it be predicted? Support Care Cancer 2005;13:647–51. [DOI] [PubMed] [Google Scholar]
- [24].Matsuoka H, Nakamura K, Matsubara Y, et al. The influence of chemotherapy-induced peripheral neuropathy on quality of life of gynecologic cancer survivors. Int J Gynecol Cancer 2018;28:1394–402. [DOI] [PubMed] [Google Scholar]
- [25].Jang CE, Jung MS, Sohn EH, et al. The evaluation of changes in peripheral neuropathy and quality-of-life using low-frequency electrostimulation in patients treated with chemotherapy for breast cancer: a study protocol. Trials 2018;19:526.doi: 10.1186/s13063-018-2874-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


