Abstract
The European cohort study has indicated about CD74 IgG-autoantibodies as potential marker for axial spondyloarthritis (axSpA) diagnosis. However, multiple studies have questioned the diagnostic value of various disease-specific autoantibodies in different ethnic groups. Here, we have tried to assess the diagnostic value of anti-CD74 IgG and IgA autoantibodies in axSpA patients from Chinese Han population.
The anti-CD74 IgG and IgA autoantibodies were analyzed using ELISA assay in a cohort of 97 axSpA patients, including 47 treatment-naïve axSpA patients never treated with steroids or immunosuppressants and 50 treated axSpA patients. The rheumatic disease control (RDC) group consisted of 40 rheumatoid arthritis, 25 systemic lupus erythematosus, 18 psoriatic arthritis patients, and 60 healthy controls (HC).
Our data demonstrated the presence of anti-CD74 IgA auto-antibodies in 25.8% of the axSpA patients, 30.1% of the RDC group patients and none in HC. Similarly, anti-CD74 IgG autoantibodies were observed in 23.7% of the axSpA patients, 18.1% of the RDC patients and 18.3% of the HC. The sensitivity, specificity, and accuracy of IgA autoantibodies were 21.3%, 82.5%, & 67.4%, respectively, while for IgG, it was 27.7%, 81.8%, and 68.4%, in treatment-naïve axSpA patients. Furthermore, weak positive relationship between anti-CD74 IgA autoantibodies and bath ankylosing spondylitis disease activity index ( r = 0.253, P = .012) and functional index (bath ankylosing spondylitis functional index; r = 0.257, P = .011) was observed.
Overall, our study demonstrated little clinical and predictive value of CD74 autoantibodies in the diagnosis of axSpA and its related manifestations, among Chinese Han population.
Keywords: anti-CD74, autoantibody, axial spondyloarthritis, biomarker
1. Introduction
Spondyloarthritis (SpA) consists of a group of inflammatory diseases that affect the axial and peripheral skeleton, including ankylosing spondylitis (AS), a prototypic form of SpA. It is commonly diagnosed in the presence of definitive sacroiliitis by conventional X-rays, and also as non-radiographic axial spondyloarthritis (axSpA), which can cause chronic pain, structural damage, and disability.[1] Currently, AS is diagnosed based on the modified New York Classification Criteria developed in 1984,[2] which predominantly relies on radiographic changes. But radiographic changes develop 6 to 10 years following the disease onset,[3] thereby results in diagnostic delay and prevents the early treatment in many patients. Henceforth, serum biomarkers have been indicated to have significant value in the management of axSpA patients, as they can be unequivocally identified before sacroiliitis detection with radiographic imaging, and thus are helpful in assessing and monitoring the disease progression.[4] Currently, the human leukocyte antigen (HLA) B27 is the most common biomarker used to diagnose axial SpA patients with 60% to 95% sensitivity and is a part of the core laboratory testing. However, HLA-B27 specificity is also often insufficient for diagnosing axSpA.[1,5]
CD74, also known as HLA class II histocompatibility antigen gamma chain or invariant chain expressed on the plasma membrane, is responsible for several biological processes, including the correct folding and transport of major histocompatibility complex class II molecules, the differentiation of B cells, and the inflammatory response as a part of the macrophage migratory inhibitory factor.[6] Baerlecken et al[7] first reported on the potential role of CD74 IgG-antibodies (anti-CD74 antibodies) in the diagnosis of SpA. Specifically, they verified the presence of anti-CD74 IgG antibodies in 67% of axSpA patients, along with 45% of psoriatic arthritis (PsA) patients without axial involvement, 11% of rheumatoid arthritis (RA) patients, 15% of systemic lupus erythematosus (SLE) patients, 2.5% of human immunodeficiency virus patients, and 0.8% of blood donors. Several research teams have successively reported the diagnostic value of anti-CD74 antibodies in axSpA, and indicated that it is associated with specific clinical manifestations.[8–11] Nevertheless, controversial results appeared in the research of de Winter et al[12] and Liu et al[13], who showed that anti-CD74 antibodies are inadequate for identifying axSpA patients.
In this context, some studies have also emphasized about differences in the diagnostic value of some disease-specific autoantibodies in different ethnic groups, like the presence of anti-soluble liver antigen/liver pancreas autoantibody in up to 58% of the autoimmune hepatitis patients in North America and Europe,[14,15] while showing very low frequency in Japanese[16] and Chinese[17] adult patients with autoimmune liver disease. Due to the different genetic susceptibility of AS in different ethnic groups, we aimed to confirm the diagnostic value of anti-CD74 IgG and IgA autoantibodies in Chinese Han patients with axSpA.
2. Methods
2.1. Patient samples
Total 97 axSpA patients enrolled in our study were recruited from the Peking Union Medical College Hospital (PUMCH) axSpA registry database, between August 2012 and August 2015. Among these, 62 patients met the modified New York (mNY) criteria for AS diagnosis,[2] while the remaining 35 met the diagnosis criteria of AS for non-radiographic axial spondylitis.[18,19] The complete summary of patient characteristics is described in Table 1. In addition, the rheumatic disease control (RDC) group consisted of 40 patients diagnosed with RA (mean age 52.1 ± 11.8, male/female 15/25) based on the criteria outlined by the American College of Rheumatology and European League Against Rheumatism in 2010,[20] 25 patients with SLE (mean age 33.8 ± 10.6, male/female 8/17) diagnosed based on the 1997 revised classification criteria of the American College of Rheumatology,[21] 18 patients with PsA (mean age 40.3 ± 11.7, male/female 11/7) who met the CASPAR classification criteria,[22] and 60 healthy individuals (healthy controls (HCs), mean age 32.2 ± 6.6, male/female 40/12), without any symptoms of autoimmune disease, cardiovascular disease, cancer, metabolic disease, backache, or any other chronic disease. From each subject, 4 mL of blood was collected with the help of the BD vacutainer without anticoagulants. For the next 1 hour, the blood was allowed to clot and was later centrifuged at 4°C for 5 minutes at 3,000 rpm. The serum was collected and stored at –80°C. Before undertaking the final analysis, no sample was subjected to more than 1 freeze-thaw cycle. This study was approved by the Medical Ethics Committee of PUMCH (S-478), and all methods were carried out in accordance with the principles stated in the Declaration of Helsinki. All patients signed the informed consent form before enrollment in our study.
Table 1.
Summary of axial spondyloarthritis patient's clinical characteristics.
| Variables | AS | nr-axSpA | P |
| N | 62 | 35 | |
| Age (yr; mean ± SD) | 29.9 ± 9.5 | 30.9 ± 10.1 | .65 |
| Gender (M/F) | 52/10 | 26/9 | .25 |
| Treatment-naïve | 32 (51.61%) | 15 (42.86%) | .41 |
| Clinical features | |||
| Median disease duration (mo, P25–P75) | 33.5 (5.8–62.9) | 21.3 (4.1–53.8) | .87 |
| HLA-B27 positive (n, %) | 32/10 (76.19%) | 24/5 (82.76%) | .51 |
| Positive family history (n, %) | 13 (21.0%) | 10 (28.6%) | .40 |
| Smoking status (n, %) | 25 (40.3%) | 9 (25.7%) | .15 |
| BASDAI (median, P25–P75) | 2.6 (1.0–4.4) | 2.3 (1.4–4.8) | .58 |
| BASFI (median, P25–P75) | 1.1 (0.3–3.6) | 1.0 (0.3–3.5) | .70 |
| BASMI (median, P25–P75) | 2.0 (1.0–5.0) | 1.0 (1.0–2.0) | .00 |
AS = ankylosing spondylitis, BASDAI = Bath ankylosing spondylitis disease activity Index (scale 0–10), BASFI = Bath Ankylosing Spondylitis Functional Index (scale 0–10), BASMI = Bath Ankylosing Spondylitis Metrology Index (scale 0–10), HLA-B27 = human leukocyte antigen B27, nr-axSpA = non-radiographic axial spondyloarthritis, P25–P75 = percentile 25–75.
2.2. Measurements of anti-CD74 antibodies in serum
The anti-CD74 IgG and IgA auto-antibodies were measured in patient's serum samples using enzyme-linked immunosorbent assay kit, obtained from AESKU Diagnostics (Wendelsheim, Germany) according to the manufacturer's protocol. After collection and isolation, serum samples were further diluted to 1:100 a ratio with sample buffer. Next, 100 μL of each diluted serum sample was added to the antigen-coated wells of a microplate, along with the reference standards, negative controls, and positive control solutions. After 1 hour incubation at room temperature, the microplate was washed with 300 μL of washing buffer 3 times, and 100 μL of the conjugate was added to each well, followed by another incubation of 1 hour at room temperature. Subsequently, the microplate was washed with 300 μL of washing buffer 3 times before100 μL of the substrate was added into each well. Additionally, the microplate was again incubated for another 1 hour at room temperature. Finally, the optical density of each well was assessed using a microplate reader after adding 100 μL stop solution to each well. Cut-off values were determined strictly in accordance with the kit instructions. The cut-off values were 20 U/mL for anti-CD74 IgG and 25 U/mL for anti-CD74 IgA. All samples having an optical density higher than the cut-off value were categorized as positive. Moreover, the concentration of each antibody type was assessed, according to the respective standard curve.
2.3. Statistical analysis
All of the differences between axSpA and control groups were analyzed using the Student t test for continuous variables, and χ2 or Fisher exact test for proportions. Moreover, the anti-CD74 antibodies concentrations in the same axSpA patient, before and after treatment were analyzed using the paired t test. The sensitivity, specificity and area under the curve (AUC) for anti-CD74 antibodies were calculated as measures of diagnostic accuracy. Receiver operating characteristic curves were used to calculate the AUC. The investigation of the associations between anti-CD74 antibodies and different clinical variables in axSpA patients were conducted using the χ2 or Fisher exact test. The correlation between concentrations of anti-CD74 antibodies and SpA-related indexes (including bath ankylosing spondylitis disease activity index (BASDAI), bath ankylosing spondylitis functional index (BASFI), and Bath ankylosing spondylitis metrology index (BASMI)) were assessed using the Spearman's analysis. The SPSS statistical software package (version 16.0, IBM, Chicago, IL) was used for conducting all statistical analyses, and 2-tailed P-values of <.05 represented statistical significance.
3. Results
3.1. Anti-CD74 IgA & IgG auto-antibodies analysis in axSpA patients
Based on our analysis, axSpA patients displayed significantly higher mean concentration of anti-CD74 IgA antibody (25.7 ± 39.1 U/mL) in comparison to HC group (9.0 ± 3.6 U/mL; P = .000), but not against RDC group (27.3 ± 39.25 U/mL; P = .78). Also, the positive average rate of anti-CD74 IgA antibody in axSpA patients was significantly higher (25.8%) than HC group (0.00%, P = .000). However, there was no significant difference in the positive rate between axSpA and RDC groups (30.1%, P = .36). The frequency of anti-CD74 IgA antibodies in patients with RA, SLE, PsA was 42.5%, 28.0%, and 5.6% respectively. Surprisingly, there were no significant difference in anti-CD74 IgG antibody concentrations between axSpA (22.3 ± 42.8 U/mL), RDC (14.7 ± 43.3 U/mL; P = .13) and HC groups (19.8 ± 33.0 U/mL; P = .70). The positive rate of anti-CD74 IgG antibody in 97 axSpA patients was 23.7% with no significant difference from RDC group (18.1%, P = .36) or HC group (18.3%, P = .43). The frequency of anti-CD74 IgG antibodies in patients with RA, SLE, PsA was 15.0%, 24.0%, and 16.7% respectively. The quantification summary of anti-CD74 IgA and IgG antibodies serum concentration, in axSpA patients, RDC, and HC, has been shown in Figure 1.
Figure 1.
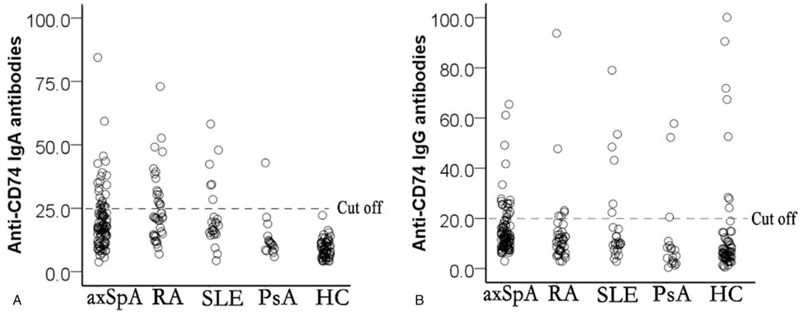
Estimation of antibody concentration in the serum of axial spondyloarthritis patients, rheumatic disease, and healthy controls; (A) anti-CD74 IgA and (B) anti-CD74 IgG.
3.2. Diagnostic performance analysis of anti-CD74 IgG & IgA autoantibodies in axSpA patients
We performed receiver operating characteristic curves curve analysis to determine the diagnostic accuracy of anti-CD74 IgG and IgA in axSpA patients. The AUC of anti-CD74 IgG between axSpA patients and controls was 0.673 (95% CI: 0.606–0.739; P<.001), sensitivity was 46.94% (95% CI: 0.328–0.616) and specificity was 59.11% (95% CI: 0.516–0.642). Similarly, the AUC of anti-CD74 IgA between axSpA patients and controls was 0.666 (95% CI: 0.598–0.734; P < .001), sensitivity was 50.98% (95% CI: 0.368–0.650) and specificity was 60.33% (95% CI: 0.527–0.674). Further analysis of anti-CD74 autoantibodies between treatment-naïve and treated axSpA patients revealed AUC of 0.520 (95% CI: 0.403–0.637), sensitivity of 56.52% (95% CI: 0.348–0.761), and specificity of 54.05% (95% CI: 0.421–0.656) for IgG, while AUC of 0.439 (95% CI: 0.324–0.554), sensitivity of 42.32% (95% CI: 0.240–0.628) and specificity of 49.30% (95% CI: 0.373–0.613). Overall our data, as shown in Figure 2, indicated no significant differences in the diagnostic ability of anti-CD74 IgG and IgA autoantibodies in axSpA Chinese patients.
Figure 2.
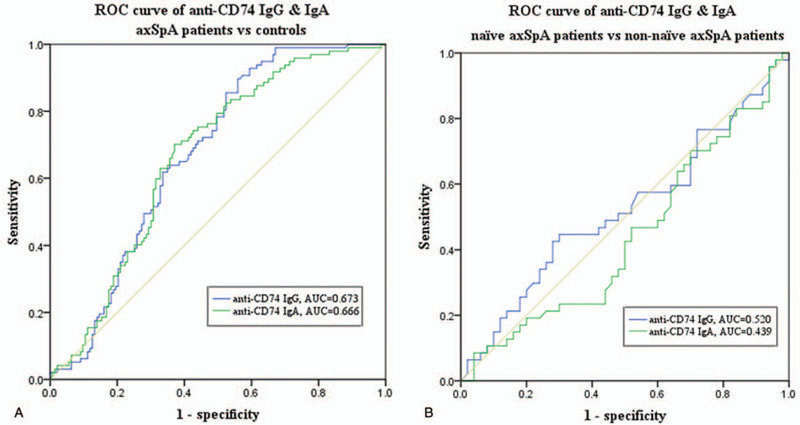
Receiver operating characteristic curve analysis of anti-CD74 IgG & IgA. (A) Estimation of anti-CD74 IgG and IgA area under the curves between axial spondyloarthritis patients and controls. (B) Estimation of anti-CD74 IgG and IgA area under the curves between treatment-naïve and treated axial spondyloarthritis patients. AUC = area under the curve.
3.3. Dual-Positivity analyses of anti-CD74 IgA & IgG antibodies in axSpA patients
Among the 47 treatment-naïve axSpA and 50 treated axSpA patients, only 3 patients from each group tested positive for 2 autoantibodies. The positivity of the anti-CD74 IgA antibody alone was only 17.0% in the treatment-naïve axSpA group, and was lower than 24.0% in treated axSpA group. However, this difference was not significant (χ2 = 1.28, P = .26). In contrast, the positivity of anti-CD74 IgG alone was 21.3% in the treatment-naïve axSpA group, and was higher than 14.0% observed in treated axSpA group. However, again the difference was not significant (χ2 = 0.89, P = .35). Overall, dual-positivity for both anti-CD74 IgA and IgG was nearly identical in both treatment-naïve and treated axSpA patients at 6.4% and 6.0%, respectively (Fig. 3).
Figure 3.
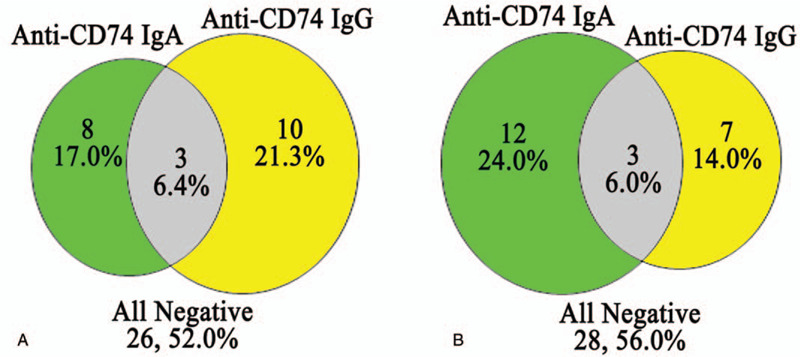
Dual-positivity analysis of anti-CD74 IgA and IgG antibodies in axial spondyloarthritis (axSpA) patients. Panel A, show data in 47 treatment-naïve axSpA patients, while panel B display data from 50 treated axSpA patients.
3.4. Assessment of anti-CD74 IgA and IgG antibody concentrations in axSpA patients, before and after treatment
From the 97 total axSpA patients, we only analyzed anti-CD74 IgA and IgG antibody concentrations in the serum of 21 patients, before and after treatment (Fig. 4). Following logarithmic transformation of anti-CD74 IgA and IgG antibody concentrations, the values were analyzed using Student paired t tests. It was observed that concentrations of anti-CD74 IgG antibodies in the serum of treated axSpA patients were significantly lower than before treatment (t = 3.94, P = .001). However, no significant differences were observed for the concentrations of anti-CD74 IgA antibodies in the serum of axSpA patients before and after treatment (t = 1.88, P = .07).
Figure 4.
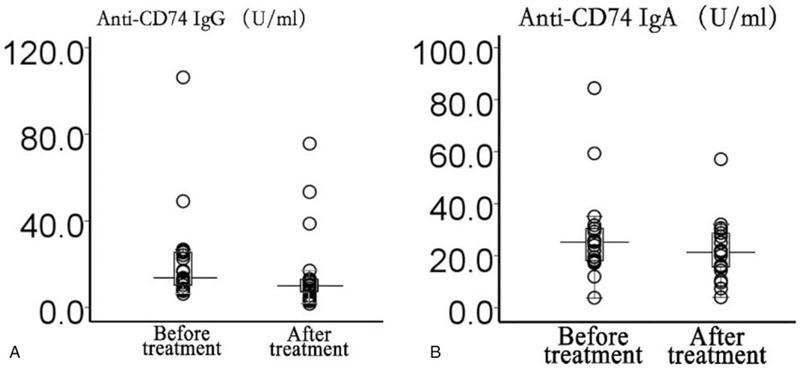
Antibody concentration assessment in 21 treatment-naïve axial spondyloarthritis patients, before and after treatment; (A) anti-CD74 IgG, and (B) anti-CD74 IgA.
3.5. Association analysis between axSpA-related clinical features and anti-CD74 antibodies
Among the various clinical manifestations presented by axSpA patients (Table 2), anti-CD74 IgA antibodies showed significant association with HLA-B27 positivity (χ2 = 4.57, P = .03). Other clinical features, including family history and smoking status, were not associated with the presence of anti-CD74 IgG or IgA antibodies. However, our study confirmed a positive relationship of anti-CD74 IgA antibodies concentration with BASDAI (r = 0.253, P = .012) and BASFI (r = 0.257, P = .011).
Table 2.
Association between axial spondyloarthritis-related clinical features and anti-CD74 antibody levels.
| Anti-CD74 IgG | Anti-CD74 IgA | ||||||
| Clinical features | Positivity | χ2 or r | P | Positivity | χ2 or r | P | |
| HLA-B27 positive | Yes | 21.4% | 0.92 | .34 | 19.6% | 4.57 | .03 |
| No | 33.3% | 46.7% | |||||
| Family history | Yes | 26.1% | 0.09 | .76 | 17.4% | 0.61 | .44 |
| No | 23.0% | 28.4% | |||||
| Smoking status | Yes | 26.5% | 0.22 | .64 | 35.3% | 2.48 | .12 |
| No | 22.2% | 20.6% | |||||
| BASDAI | 0.083 | .417 | 0.253 | .012 | |||
| BASFI | 0.095 | .357 | 0.257 | .011 | |||
| BASMI | −0.051 | .620 | 0.075 | .465 | |||
BASDAI = bath ankylosing spondylitis disease activity index, BASFI = bath ankylosing spondylitis functional index, BASMI = Bath ankylosing spondylitis metrology index, HLA-B27 = human leukocyte antigen B27.
4. Discussion
The recently introduced term, axial SpA (axSpA),[18,19] is one of the most common autoimmune inflammatory disease with diverse clinical presentation. It is typically characterized by inflammatory chronic back pain, stiffness, and ankylosis of the spinal joints. Its incidence rate is usually higher in males. The early and correct axSpA diagnosis, along with aggressive intervention is crucial to reduce the potentially destructive effects of this disease. Recently, public awareness about axSpA, advanced training of rheumatologists, the evolution of diagnosis criteria, and development of radiographic techniques have all contributed to the improved patient outcomes. However, the incidences of delayed or incorrect diagnoses remain too frequent due to the considerable delay of 7 to 10 years between the onset of inflammatory back pain and axial SpA diagnosis.[23,24]
In this study, anti-CD74 IgA and IgG autoantibodies were analysed using ELISA assay in axSpA cohort and other autoimmune diseases patients, along with healthy volunteers. We further divided the axSpA patients into treatment-naïve and treated axSpA groups. Low positivity of anti-CD74 IgA was observed in the axSpA patients, with 23.4% in the treatment-naïve and 30.0% in treated axSpA groups, which was consistent with results from an earlier published study.[12] In addition, we noted that the positive rate of anti-CD74 IgA in the treatment-naïve, treated, and unselected total axSpA patients were 23.4%, 30.0%, and 25.8% respectively, which indicated that the positive rate of anti-CD74 in axSpA patients varied among different patients. However, compared to HC group, the concentration and positivity rate of anti-CD74 IgA autoantibodies were significantly higher in axSpA group, yet no significant difference was observed between axSpA and RDC groups, thereby indicating little diagnostic value of anti-CD74 IgA autoantibodies in axSpA. Similarly, the anti-CD74 IgG auto-antibodies showed no significant difference in the concentrations and positive rates among axSpA, RDC and HC groups, suggesting that anti-CD74 autoantibody is not useful as a diagnostic marker for axSpA Chinese patients. Our results demonstrate that the positive rate of the PsA anti-CD74 antibody has the lowest level in RDCs. PsA is most closely related to SpA, with similarities in their pathogenesis, clinical manifestations, and treatments.[25,26] Hence, CD74 autoantibodies cannot distinguish axSpA or PsA from rheumatic diseases. Further research needs to clarify the clinical utility of anti-CD74 antibodies to SpA, especially axSpA.
Similar conclusions have been obtained by Liu et al[13] and de Winter et al,[12] who found that anti-CD74 autoantibodies are not good biomarkers for the diagnosis of SpA in Asian and European people, especially in patients under 45 years old presenting with early back pain.[12] As mentioned earlier, several studies have assessed the diagnostic value of anti-CD74 antibodies for axSpA in European and Middle Eastern patients, finding that antibodies can be used as a new serological marker of axSpA.[7–11,27] Moreover, the latest research reveals that CD74 is a T cell antigen in SpA, eliciting Th1 and Th17 responses, which may be relevant in SpA disease pathogenesis.[27] axSpA is a disease of genetic susceptibility.[28–31] As these contradictory results show, the prevalence of anti-CD74 antibodies may be linked to differences in racial or geographical predilection. Moreover, given the MHC association of this disease,[32] there is no good reason to expect an autoantibody presence; however, this represents an area that needs further investigation.
In parallel, the dual-positivity of anti-CD74 IgA and IgG autoantibodies was also investigated in axSpA patients. We found that 52.0% and 56.0% of treatment-naïve and treated axSpA patients had no anti-CD74 IgA or IgG autoantibodies. Only 6.4% of the 47 treatment-naïve axSpA and 6.0% of the 50 treated axSpA patients were positive for anti-CD74 IgA and IgG autoantibodies. In addition, no significant difference in the positivity for anti-CD74 IgG alone or anti-CD74 IgA alone in the treatment-naïve and treated axSpA patients was observed. Paired samples from 21 patients analyzed before and after the treatment, showed that concentrations of anti-CD74 IgG antibodies in the serum of treated axSpA patients were significantly decreased after treatment. However, this trend was not observed for anti-CD74 IgA autoantibodies. As a matter of utility, there is substantial overlap between the pre- and post-treatment measures, and further study is needed to reveal the association between anti-CD74 antibodies and treatment.
Additionally, we assessed the association between anti-CD74 autoantibodies and axSpA-related clinical features, including HLA-B27 positivity, family history, smoking status, and disease activity index, as no previous studies have analyzed these relationships. However, our data showed that HLA-B27, family history, smoking status, BASDAI, BASFI, and BASMI scores did not show any association with anti-CD74 IgG antibodies. In regards to anti-CD74 IgA autoantibodies, the positivity was only associated with HLA-B27 positivity (χ2 = 4.57, P = .03). A weak positive relationship between their concentrations and BASDAI (r = 0.253, P = .012) and BASFI (r = 0.257, P = .011) scores were found. Different from the study of Riechers et al,[11] the positive rate of anti-CD74 IgA antibodies in their European cohort was as high as 47%, and the combination of anti-CD74 IgA and HLA-B27 provided post-test probabilities of 80.2% for distinguishing axSpA in chronic back pain (CBP) patients. Thus, our results are not sufficient to support anti-CD74 IgA autoantibodies as a useful tool for diagnosing axSpA.
Importantly, despite the outcome of our study, it has several strengths. First, this is the study analyzing anti-CD74 auto-antibodies in Chinese axSpA patients. Second, to avoid the effect of treatment on serum levels of anti-CD74 autoantibodies, we included treatment-naïve axSpA patients. Third, we performed a comparative dynamic analysis of anti-CD74 antibodies in same axSpA patients before and after treatment, along with analyzing the relationship between anti-CD74 antibodies concentrations and disease activity indexes, including BASDAI, BASFI, and BASMI. Nonetheless, the current study has some limitations, such as its relatively small sample size, and data being collected from a single center.
5. Conclusions
In summary, we have comprehensively analyzed the significance of anti-CD74 antibodies in the serum of Chinese Han patients with axSpA, especially in treatment-naïve patients. Our data indicated little clinical value of anti-CD74 autoantibodies, as the positivity of IgA and IgG autoantibodies was only 23.4% and 27.7% in treatment-naïve, while 30.0% and 20.0% in treated axSpA Chinese patients, respectively. Furthermore, our study also showed limited association between anti-CD74 antibodies and specific axSpA clinical features or disease activity indexes. Thus, the utility of anti-CD74 autoantibodies as diagnostic and predictive markers for axSpA diagnosis could not be validated in Chinese axSpA patients. However, due to the small sample size in our study, it would be interesting to conduct additional longitudinal, multicenter studies to further validate if anti-CD74 autoantibodies have any diagnostic and prognostic role in axSpA patients.
Author contributions
All authors were involved in the design of the study. CJH, MTL, and XL were involved in the collection of blood samples, experimental procedures, statistical analysis, and writing of the manuscript. MTL, LYP, SZZ, XML, JMS, and XFZ were involved in the recruitment of patients and evaluation of clinical data. All authors gave intellectual input to the study and approved the final version of the manuscript.
Conceptualization: Chao-Jun Hu.
Data curation: Chao-Jun Hu, Meng-Tao Li, Xi Li, Lin-Yi Peng, Shang-Zhu Zhang, Xiao-Mei Leng, Jin-Mei Su.
Formal analysis: Chao-Jun Hu, Xi Li, Shang-Zhu Zhang.
Funding acquisition: Chao-Jun Hu.
Investigation: Chao-Jun Hu, Xi Li, Shang-Zhu Zhang, Xiao-Mei Leng, Jin-Mei Su.
Methodology: Chao-Jun Hu, Xi Li, Jin-Mei Su.
Project administration: Chao-Jun Hu, Meng-Tao Li, Xiao-Feng Zeng.
Resources: Chao-Jun Hu, Lin-Yi Peng, Xiao-Mei Leng, Jin-Mei Su.
Software: Chao-Jun Hu.
Supervision: Chao-Jun Hu, Meng-Tao Li, Xiao-Feng Zeng.
Validation: Chao-Jun Hu, Meng-Tao Li, Xi Li, Xiao-Feng Zeng.
Visualization: Chao-Jun Hu, Meng-Tao Li, Xiao-Feng Zeng.
Writing – original draft: Chao-Jun Hu, Xi Li.
Writing – review & editing: Chao-Jun Hu, Xi Li, Xiao-Feng Zeng.
Footnotes
Abbreviations: AS = ankylosing spondylitis, AUC = area under the curve, axSpA = axial spondyloarthritis, BASDAI = bath ankylosing spondylitis disease activity index, BASFI = bath ankylosing spondylitis functional index, BASMI = bath ankylosing spondylitis metrology index, HC = healthy controls, HLA = human leukocyte antigen, PsA = psoriatic arthritis, RA = rheumatoid arthritis, RDC = rheumatic disease control, SLE = systemic lupus erythematosus, SpA = Spondyloarthritis.
How to cite this article: Hu C-J, Li M-T, Li X, Peng L-Y, Zhang S-Z, Leng X-M, Su J-M, Zeng X-F. CD74 auto-antibodies display little clinical value in Chinese Han population with axial spondyloarthritis. Medicine. 2020;99:50(e23433).
C-JH, M-TL, and XL contributed equally to this work and are the co-first authors.
This study was supported by the National Natural Science Foundation of China (81771780 and 81302610), the Chinese National Key Research R&D Program (2019YFC0840603), CAMS initiative for innovative medicine (2017-I2M-3-001), and the Youth Research Foundation of Peking Union Medical College (3332015091).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Dougados M, Baeten D. Spondyloarthritis. Lancet 2011;377:2127–37. [DOI] [PubMed] [Google Scholar]
- [2].van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 1984;27:361–8. [DOI] [PubMed] [Google Scholar]
- [3].Poddubnyy D, Rudwaleit M. Early spondyloarthritis. Rheum Dis Clin North Am 2012;38:387–403. [DOI] [PubMed] [Google Scholar]
- [4].Maksymowych WP. Biomarkers for diagnosis of axial spondyloarthritis, disease activity, prognosis, and prediction of response to therapy. Front Immunol 2019;10:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].van Tubergen A. The changing clinical picture and epidemiology of spondyloarthritis. Nat Rev Rheumatol 2015;11:110–8. [DOI] [PubMed] [Google Scholar]
- [6].Borghese F, Clanchy FI. CD74: an emerging opportunity as a therapeutic target in cancer and autoimmune disease. Expert Opin Ther Targets 2011;15:237–51. [DOI] [PubMed] [Google Scholar]
- [7].Baerlecken NT, Nothdorft S, Stummvoll GH, et al. Autoantibodies against CD74 in spondyloarthritis. Ann Rheum Dis 2014;73:1211–4. [DOI] [PubMed] [Google Scholar]
- [8].Baraliakos X, Baerlecken N, Witte T, et al. High prevalence of anti-CD74 antibodies specific for the HLA class II-associated invariant chain peptide (CLIP) in patients with axial spondyloarthritis. Ann Rheum Dis 2014;73:1079–82. [DOI] [PubMed] [Google Scholar]
- [9].Witte T, Köhler M, Georgi J, et al. IgA antibodies against CD74 are associated with structural damage in the axial skeleton in patients with axial spondyloarthritis. Clin Exp Rheumatol 2020;Mar 28. Epub ahead of print. [PubMed] [Google Scholar]
- [10].Ziade NR, Mallak I, Merheb G, et al. Added value of anti-CD74 autoantibodies in axial spondyloarthritis in a population with low HLA-B27 prevalence. Front Immunol 2019;10:574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Riechers E, Baerlecken N, Baraliakos X, et al. Sensitivity and specificity of autoantibodies against CD74 in nonradiographic axial spondyloarthritis. Arthritis Rheumatol 2019;71:729–35. [DOI] [PubMed] [Google Scholar]
- [12].de Winter JJ, van de Sande MG, Baerlecken N, et al. Anti-CD74 antibodies have no diagnostic value in early axial spondyloarthritis: data from the spondyloarthritis caught early (SPACE) cohort. Arthritis Res Ther 2018;20:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Liu Y, Liao X, Shi G. Autoantibodies in spondyloarthritis, focusing on anti-CD74 antibodies. Front Immunol 2019;10:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Efe C, Ozaslan E, Wahlin S, et al. Antibodies to soluble liver antigen in patients with various liver diseases: a multicentre study. Liver Int 2013;33:190–6. [DOI] [PubMed] [Google Scholar]
- [15].Ma Y, Okamoto M, Thomas MG, et al. Antibodies to conformational epitopes of soluble liver antigen define a severe form of autoimmune liver disease. Hepatology 2002;35:658–64. [DOI] [PubMed] [Google Scholar]
- [16].Miyakawa H, Kawashima Y, Kitazawa E, et al. Low frequency of anti-SLA/LP autoantibody in Japanese adult patients with autoimmune liver diseases: analysis with recombinant antigen assay. J Autoimmun 2003;21:77–82. [DOI] [PubMed] [Google Scholar]
- [17].Hu C, Deng C, Song G, et al. Prevalence of autoimmune liver disease related autoantibodies in Chinese patients with primary biliary cirrhosis. Dig Dis Sci 2011;56:3357–63. [DOI] [PubMed] [Google Scholar]
- [18].Rudwaleit M, Landewe R, van der Heijde D, et al. The development of assessment of spondyloarthritis international society classification criteria for axial spondyloarthritis (part I): classification of paper patients by expert opinion including uncertainty appraisal. Ann Rheum Dis 2009;68:770–6. [DOI] [PubMed] [Google Scholar]
- [19].Rudwaleit M, van der Heijde D, Landewe R, et al. The development of assessment of spondyloarthritis international society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009;68:777–83. [DOI] [PubMed] [Google Scholar]
- [20].Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American college of rheumatology/European league against rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. [DOI] [PubMed] [Google Scholar]
- [21].Hochberg MC. Updating the American college of rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725. [DOI] [PubMed] [Google Scholar]
- [22].Taylor W, Gladman D, Helliwell P, et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006;54:2665–73. [DOI] [PubMed] [Google Scholar]
- [23].Deodhar A, Mittal M, Reilly P, et al. Ankylosing spondylitis diagnosis in US patients with back pain: identifying providers involved and factors associated with rheumatology referral delay. Clin Rheumatol 2016;35:1769–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ibn Yacoub Y, Amine B, Laatiris A, et al. Relationship between diagnosis delay and disease features in Moroccan patients with ankylosing spondylitis. Rheumatol Int 2012;32:357–60. [DOI] [PubMed] [Google Scholar]
- [25].Dhillon S. Certolizumab pegol: a review of its use in patients with axial spondyloarthritis or psoriatic arthritis. Drugs 2014;74:999–1016. [DOI] [PubMed] [Google Scholar]
- [26].Olivieri I, D’Angelo S, Padula A, et al. Can we reduce the dosage of biologics in spondyloarthritis? Autoimmun Rev 2013;12:691–3. [DOI] [PubMed] [Google Scholar]
- [27].Sogkas G, Klose K, Baerlecken N, et al. CD74 is a T cell antigen in spondyloarthritis. Clin Exp Rheumatol 2020;38:195–202. [PubMed] [Google Scholar]
- [28].Schlosstein L, Terasaki PI, Bluestone R, et al. High association of an HL-A antigen, W27, with ankylosing spondylitis. N Engl J Med 1973;288:704–6. [DOI] [PubMed] [Google Scholar]
- [29].Brown MA, Laval SH, Brophy S, et al. Recurrence risk modelling of the genetic susceptibility to ankylosing spondylitis. Ann Rheum Dis 2000;59:883–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Geirsson AJ, Kristjansson K, Gudbjornsson B. A strong familiality of ankylosing spondylitis through several generations. Ann Rheum Dis 2010;69:1346–8. [DOI] [PubMed] [Google Scholar]
- [31].Khan MA. An update on the genetic polymorphism of HLA-B∗27 With 213 alleles encompassing 160 subtypes (and still counting). Curr Rheumatol Rep 2017;19:9. [DOI] [PubMed] [Google Scholar]
- [32].Ehrenfeld M. Spondyloarthropathies. Best Pract Res Clin Rheumatol 2012;26:135–45. [DOI] [PubMed] [Google Scholar]


