Abstract
In the present study, we aimed to investigate whether copeptin values on admission are related to left ventricle (LV) systolic function and its improvement at 6 months in ST-segment elevation myocardial infarction (STEMI) patients.
In this single-center, prospective observational study, we included 122 STEMI patients from January 2016 to November 2016. LV systolic functions in the form of global longitudinal strain (GLS) in addition to conventional echocardiography parameters were evaluated on admission and at 6-month. Serum copeptin levels were determined using an ultrasensitive immunofluorescence assay.
The study population was divided into 2 groups according to median values of copeptin. GLS was significantly lower in patients with high copeptin levels compared to those with low copeptin levels at early stage and 6-month (−16% (16–16.5) vs −15% (15–15.5), P < .001 and −18% (18–19) vs −16% (16–16.25), P < .001, respectively). Copeptin values were negatively correlated with an early and 6-month GLS (r = –0.459 at early stage and r = –0.662 at 6-month). In addition, we observed that copeptin values were negatively correlated with the improvement of GLS at 6-month follow-up (r = −0.458, P < .001 and r = −0.357, P = .005, respectively).
Serum copeptin levels in STEMI patients at the time of admission may predict early and 6-month LV systolic function assessed by two-dimensional GLS. To the best of our knowledge, this study is the first to specifically address the relationship between copeptin values and GLS in STEMI patients.
Keywords: copeptin, global longitudinal strain, ST-segment elevation myocardial infarction
1. Introduction
Acute coronary syndrome (ACS) is subcategory of coronary artery disease (CAD) that is one of the leading causes of mortality and morbidity worldwide.[1] According to a recent statistic of cardiovascular disease, approximately 1.8 million people in Europe lose their lives from CAD.[2]
In the management of ACS, an early diagnosis and prompt treatment including revascularization procedures and medical therapies are life-saving. Despite the effectiveness of such treatment modalities, heart failure (HF) remains a common occurrence following ACS, complicating up to 45% of all infarcts.[3] In the pathophysiology of postacute myocardial infarction HF, an adverse left ventricle (LV) remodeling is an underlying mechanism. An adverse LV remodeling is generally described by the presence of an enlarged LV cavity and/or reduced LV ejection fraction (EF). In patients with acute myocardial infarction, the size of infarction is usually related to the remodeling of the LV and a larger infarct size indicates a poor prognosis.[4]
Two-dimensional (2D) tracking-based function measurements may provide true regional and global information.[5] According to previous studies, speckle tracking based 2D strain provides better and more exhaustive information about systolic function than LV EF. In a previous study, 2D echocardiographic LV global longitudinal strain (GLS) has been also demonstrated to be well-correlated with cardiac magnetic resonance imaging (MRI) in the estimation of infarct size.[6]
Previous studies have shown that strain echocardiography is a predictor of left ventricular remodeling after STEMI, especially three-dimensional speckle tracking echocardiography (STE).[7]
Some neurohormones play an important role in the pathophysiology of ACS, and they are found to be useful both in diagnosis and predicting a poor prognosis.[8] Copeptin is such neurohormone that may be used as a marker of acute hemodynamic stress.[9,10] In previous studies, copeptin has been shown to be useful in the diagnosis of ACS, including ST-segment elevation myocardial infarction (STEMI).[10–12] In addition to its use in the diagnosis of STEMI, a cardiac MRI study found that copeptin values were associated with larger infarct sizes after 2 days from the diagnosis of STEMI.[13]
In this present study, we aimed to investigate whether copeptin values on admission are related to LV systolic function and its improvement at six months in STEMI patients.
2. Materials and methods
In this single-center, prospective observational study, we included 122 STEMI patients from January 2016 to November 2016. Blood samples were taken from the antecubital vein before coronary angiography from 150 patients who were initially admitted to our hospital with the diagnosis of STEMI and underwent primary percutaneous coronary intervention (PPCI). The samples were collected in ethylenediaminetetraacectic tubes, and then centrifuged for 10 minutes at 2000 × g within 30 minutes to obtain serum plasma. The serum plasma was then stored at −80°C until further analysis. After the patient inclusion in the study was terminated, the collected blood samples were studied simultaneously. Since the blood samples of 28 patients did not give a healthy result, those patients were excluded from the study. As a result, 122 patients were included in the study. Echocardiography examinations were done within 24 hours following the PPCI procedure and stored in the hardware system of the echocardiography machine. Measurements were averaged over 3 beats and were made by the same observer, who was blinded to the clinical data.
In our study, the exclusion criteria were; patients having a previous diagnosis of CAD, presented with Killip class 3 to 4 HF symptoms, having symptoms of more than 12 hours, had a right ventricular MI, a previous diagnosis of significant valvular heart disease, chronic renal dysfunction (estimated glomerular filtration rate <30 mL/min/1.73 m2 more than 3 months), hepatic failure, and those with active infection (s). The diagnosis of STEMI was accepted according to the current guidelines of the European Society of Cardiology of STEMI guideline.[14] In all of the patients, infarct-related artery (s) was successfully revascularized with PPCI. During 6-month follow-up, all patients were evaluated in terms of target vessel revascularization, myocardial reinfarction, and mortality. This study was conducted in accordance with the principle of the Declaration of Helsinki, and our hospital's ethics committee approved the design of the current study. All of the patients provided written informed consent before participating in the study.
2.1. Copeptin analysis
All blood samples were collected on admission before coronary angiography. The samples were collected in ethylenediaminetetraacectic tubes, and then centrifuged for 10 minutes at 2000 × g within 30 minutes to obtain serum plasma. The serum plasma was then stored at −80°C until further analysis. Copeptin values were measured from all stored plasma samples simultaneously. Plasma copeptin concentrations were determined using a sandwich immunoluminometric assay (Thermo Scientific, Copeptin ultrasensitive, Kryptor assay). The assay had a detection limit of 0.9 pmol/L and a functional assay sensitivity of <2 pmol/L. Copeptin concentrations of 10 pmol/L or more were considered to be a positive. This cut-off value was chosen from a previous study that analyzed various diagnostic cut-off values in ACS patients.[10]
2.2. Echocardiography
All of the patients underwent an initial transthoracic echocardiographic (TTE) examination within 24 hours following admission to hospital. At 6 months, second TTE evaluation was performed. All TTE examination was performed using the Philips Epic 5 (Philips Healthcare, Andover, USA) device using a 1 to 5 MHz transducer. LV and left atrial diameters measurements were obtained from the M-mode images in the parasternal long-axis view.[15] Peak tricuspid regurgitation velocities were obtained using a continuous wave Doppler technique, and the modified Bernoulli equation was used to estimate the pulmonary systolic artery pressure. The modified Simpson method was used for the estimation of LV EF using the apical 2-chamber and 4-chamber view.[15] From the apical window view, a 2-mm pulsed Doppler sample volume was placed at the tip of the mitral valve, and mitral flow velocities of three cardiac cycles were recorded by obtaining peak velocities of the early diastolic trans-mitral flow (E) and late diastolic trans-mitral flow (A). In addition, the early diastolic lateral mitral annulus velocity (E’ lateral), atrial contraction (A’ lateral) velocity, and lateral systolic (S) myocardial velocity were measured using the pulsed wave Doppler in the TDI imaging.
2.3. Analysis of longitudinal 2D strain and strain rate
Echocardiography images showing GLS were obtained from the standard apical 4-chamber, 3-chamber, and 2-chamber views from the LV apex. Three cardiac cycles were stored for each view, and all the data were analyzed using an offline inbuilt program (Q-Lab., Version 10.1). The frame rates used for GLS analysis were between 40 and 80 frames/s.[16] By using conventional 2D gray scale echocardiographic images, the activity of the speckles was tracked throughout the myocardial tissue. The regions of interest were manually outlined by marking the endocardial borders at the mitral annulus level as well as at the apex of each digital loop. The epicardial surface was automatically generated by the software system. After any desired manual adjustments, the regions of interest was divided into 6 segments. Each segment was then scored automatically by the software. The peak systolic strain values in an 18-segment LV model were used.[16] The results of all three planes were then combined in a single bulls-eye summary that yielded the GLS. Measurements were repeated at least three times, and the average measurements were obtained. Reproducibility was assessed by repeated measurements in a subset of patients with an average of coefficient of variation for GLS of less than 10%. The intraoperator variability for GLS was 0.82.
2.4. Statistical analysis
All statistical analyses were performed using SPSS Version 23 (IBM Corp; Armonk, USA). Categorical data were presented as numbers and percentages. Continuous variables were presented as mean ± standard deviation when normally distributed, otherwise median and interquartile ranges (IQR) was used for continuous variables without normal distribution.
The Kolmogorov-Smirnov test was performed to test the normality of data. For variables without normally distributed, non-parametric statistical methods were used. Mann–Whitney U test was performed to compare 2 independent groups. When the number of independent groups was greater than two, the Kruskal-Wallis test was performed to compare the groups. Relations between independent numerical variables were assessed using Spearman's correlation coefficient.
3. Results
Clinical characteristics and laboratory results of all patients are summarized in Table 1. The mean age of the study population was 57.6 ± 10.7 years, 73.8% of patients were male. The median door-to-balloon time was 60 (IQR = 50.7–73.2) minutes, and median symptom onset-to-balloon time was 90 (IQR = 60–240) minutes in the study. We observed that median copeptin concentration was 69.13 pmol/L (IQR = 38.8–156.1 pmol/L) in the present study.
Table 1.
Baseline demographic, laboratory and echocardiographic properties of all patients.
| Age, yrs | 57.6 ± 10.7 |
| Male gender, n (%) | 90 (73.8) |
| Hypertension, n (%) | 26 (21.3) |
| Hyperlipidemia, n (%) | 34 (27.9) |
| Diabetes Mellitus, n (%) | 28 (%23) |
| Smoking status, n (%) | 100 (82) |
| Anterior MI, n (%) | 56 (49) |
| Admission time, n (%) | |
| <30 min | 16 (13.1) |
| 30–90 min | 46(37.7) |
| 1.5–6 hrs | 46 (37.7) |
| 6–12 hrs | 14 (11.5) |
| Total occlusion of the IRA, n (%) | 86 (70.5) |
| Number of diseased vessels | |
| One vessel, n (%) | 68 (55.7) |
| Two vessels, n (%) | 42 (34.4) |
| Three vessels, n (%) | 12 (9.8) |
| eGFR, ml/min/1.73 m2 | 79 ± 8.1 |
| CK-MB median, U/l | 59 (19–180.5) |
| Troponin I, ng/l | 0.17 (0.01–8.1) |
| NT-proBNP, ng/l | 99 (62–199) |
| Copeptin, pmol/l | 69.13 (38.8–156.1) |
| LVEF, % | 45 (40–46.5) |
| LV GLS, % | 16 (15–16) |
Continuous variables are presented mean ± standard deviation or median. Nominal variables are presented with frequency and percentage.
CK-MB = creatinine kinase myocardial band, EF = Ejection fraction, eGFR = estimated glomerular filtration rate, GLS = global longitudinal strain, IRA = infarct related artery, LV = Left ventricle, MI = myocardial infarction, NT-proBNP = N terminal brain natriuretic peptide.
We divided the study population into 2 groups according to median values of copeptin and compared laboratory and echocardiographic parameters. LV end-diastolic diameter and LV end-systolic diameter were significantly greater in patients with higher copeptin levels. When patients with and without high copeptin levels compared in terms of diastolic functions, the mitral diastolic E and A wave and E/A were not statistically different. However, when groups were compared in terms of E/E’m ratio, median E/E’m value of higher copeptin group was 10.2 (8.4–13.2), median E/E’m value of lower copeptin group was 8.6 (7.1–11.1), this difference was statistically significant (P = 0.014). In terms of laboratory findings, we found that only hemoglobin levels were different between the groups. On the other hand, other laboratory findings were similar (Table 2).
Table 2.
Baseline characteristics, laboratory and echocardiography results of all patients according to median copeptin levels.
| Copeptin level >69.13 pmol/L (n = 60) | Copeptin level <69.13 pmol/L (n = 62) | P value | |
| Age, yrs | 58.5 ± 11.6 | 56.8 ± 9.8 | .534∗ |
| Female gender, n(%) | 20 (33.3) | 12 (19.4) | .171† |
| Hypertension, n(%) | 12 (20) | 14 (22.6) | .527† |
| Diabetes mellitus, n(%) | 12 (20) | 16(25.8) | .408† |
| Hyperlipidemia, n(%) | 16 (26.7) | 18 (29) | .532† |
| Smoking, n(%) | 50 (83.3) | 50 (80.6) | .524† |
| Anterior MI, n(%) | 26 (43.3) | 30 (48.4) | .445† |
| Systolic blood pressure, mmHg | 120 (110–126) | 120 (110–130) | .137‡ |
| Diastolic blood pressure, mmHg | 77.5 (70–80) | 80 (70–85) | .449‡ |
| Heart rate, beat/min | 87.5 (80–90) | 82 (80–90) | .142‡ |
| Echocardiography parameters | |||
| LVEDD, mm | 49.5 (47–52) | 47 (45–50) | .007‡ |
| LVESD, mm | 28.2 (25.9–31.4) | 25.3 (24.7–27.5) | .006‡ |
| LVEF, % | 41 (35–48) | 45 (45–46) | .053‡ |
| E (m/s) | 0.7 (0.6–0.9) | 0.7 (0.5–0.8) | .290‡ |
| A (m/s) | 0.65 (0.53–0.71) | 0.60 (0.5–0.7) | .145‡ |
| E/A | 1.16 (0.85–1.5) | 1.25 (0.83–1.44) | .862‡ |
| E’m peak velocity (cm/s) | 7.1 (5.6–8.1) | 8.1 (6.3–9.4) | .036‡ |
| A’m peak velocity (cm/s) | 9 (6.7–11) | 10.2 (8.7–12) | .060‡ |
| S m peak velocity (cm/s) | 6.7 (5.5–7.4) | 7 (6–8) | .051‡ |
| E/E’m | 10.2 (8.4–13.2) | 8.6 (7.1–11.1) | .014‡ |
| Laboratory parameters | |||
| Hemoglobin, g/dL | 14.3 (13–15.3) | 15.4 (14.4–16) | .006‡ |
| WBC, cells/mL | 10.55 (8.6–11.9) | 10.5 (8.8–13.2) | .681‡ |
| Platelet count, cells/mL | 256 (207–299) | 231 (182–266) | .071‡ |
| Fasting glucose, mg/dl | 92.5 (86.7–98.5) | 90 (87–97) | .238‡ |
| Creatinine, mg /dl | 1.05 (0.85–1.2) | 0.94 (0.83–1.07) | .220‡ |
| AST, U/L | 127 (43.7–205) | 72 (31–187) | .226‡ |
| Troponin I, ng /dL | 0.05 (0.01–18.3) | 0.18 (0.01–6.2) | .811‡ |
| CK-MB, ng /dL | 36.5 (18.5–128) | 64 (19–201) | .302‡ |
| Total cholesterol, mg/dL | 178.5 (151.7–217) | 183 (149–208) | .834‡ |
| LDL cholesterol, mg /dL | 136.5 (116.7–159) | 142 (117–156) | .874‡ |
| HDL cholesterol, mg /dL | 41.5 (35–47.5) | 38 (36–42) | .138‡ |
| Triglyceride, mg/dL | 137 (73.5–177) | 117 (106–175) | .579‡ |
| Copeptin, pmol/l | 156.1 (115–223) | 40.5 (16.4–57) | <.001‡ |
Continuous variables are presented mean ± standard deviation or median. Nominal variables are presented with frequency and percentage.
A’m = late diastolic myocardial peak velocity of mitral lateral annulus, A = late diastolic peak velocity, AST = aspartate aminotransferase, CK-MB = creatinine kinase myocardial band, E’m = early diastolic myocardial peak velocity of mitral lateral annulus, E = early diastolic peak velocity, EF = Ejection fraction, EF = Ejection fraction, HDL = CK-MB = creatinine kinase myocardial band, HDL = High-density lipoprotein, LDL = Low-density lipoprotein, LV = Left ventricle, LVEDD = Left ventricle end-diastolic diameter, LVESD = Left ventricle end-systolic diameter, S m = peak systolic velocity of mitral lateral annulus, WBC = White blood cell.
Student T test.
Pearson Chi-square.
Mann–Whitney U test.
In our study, we divided patients into different groups according to the time of onset of symptoms. We observed that the highest median copeptin value was observed in the 90 to 360 minutes. interval group (Table 3).
Table 3.
Copeptin values according to symptoms onset.
| Symptoms onset | Median copeptin value | IQR | P value |
| Copeptin, pmol/l | |||
| <30 min (n = 16 patients) | 64.14 | 42–108 | |
| = 30–90 min (n = 46 patients) | 70.07 | 54–157 | .133 |
| >90–360 min (n = 46 patients) | 99.09 | 21–215 | |
| >6–12 hrs (n = 14 patients) | 19.80 | 8–57 |
IQR = interquartile range.
When patients’ copeptin levels were compared according to the infarct region as an anterior or nonanterior, we noted that there was no difference in terms of copeptin values (Fig. 1). In addition, we compared copeptin levels according to the patency of the infarct related artery and median copeptin values were similar between the groups (Fig. 2).
Figure 1.
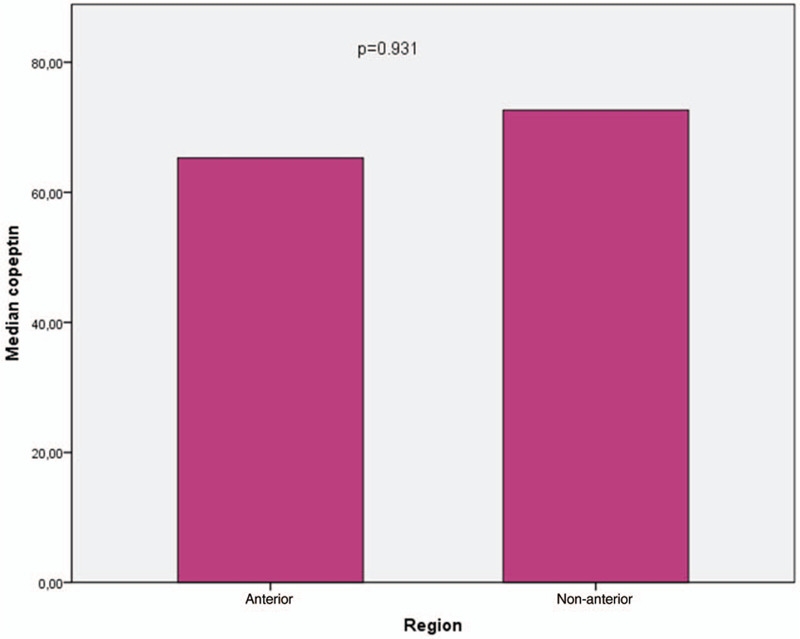
Comparison of copeptin values according to MI region.
Figure 2.
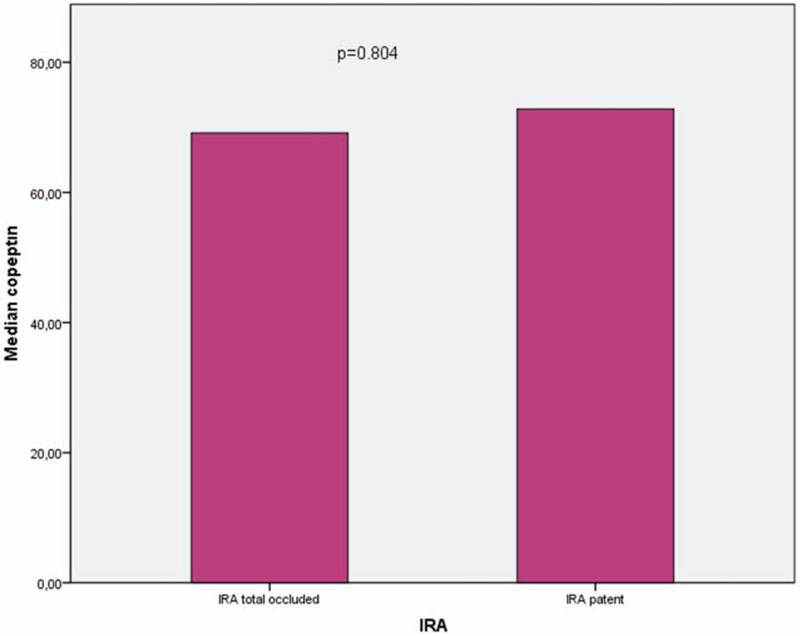
Relationship between copeptin and infarct-related artery patency before primary percutaneous coronary intervention.
Echocardiographic examination of the patients was repeated at 6th months control. The echocardiographic parameters of each group at early and 6-month are shown in Table 4. The early GLS values of patients with low copeptin levels were greater compared to those with high copeptin levels (−16% (−16 to 16.5) vs −15% (−15 to 15.5), P < .001, respectively). At 6-month, we observed that this finding was also similar between the groups (−18% (−18 to 19) vs −16% (−16 to 16.25), P < .001, respectively). Also, there was a greater improvement of GLS at 6-month in patients with low copeptin levels compared to those with high copeptin levels (−2% (−1 to 2) vs −1% (−1 to 1), P = .001, respectively). These echocardiographic results is also shown in Figure 3.
Table 4.
Comparison of echocardiographic parameters according to copeptin levels.
| Copeptin level >69.13 pmol/L (n = 60) | Copeptin level <69.13 pmol/L (n = 62) | P value | |
| LVEDD at admission, mm | 49.5 (47–52) | 47 (45–50) | .007∗ |
| LVEDD at 6-mo, mm | 47.5 (45–50) | 45 (43–48) | .007∗ |
| LVESD at admission, mm | 28.2 (25.9–31.4) | 25.3 (24.7–27.5) | .006∗ |
| LVESD at 6-mo, mm | 26.8 (24.3–30.1) | 23 (21.6–24.8) | <.001∗ |
| EF at admission, % | 41 (35–48) | 45 (45–46) | .054∗ |
| EF at 6-mo, % | 41.5 (35–48.25) | 49 (45–51) | <.003∗ |
| ΔEF, % | 0 (0–1) | 4 (2–5) | <.001∗ |
| GLS at admission, % | 15 (15–15.5) | 16 (16–16.5) | <.001∗ |
| GLS at 6-mo, % | 16 (16–16.25) | 18 (18–19) | <.001∗ |
| Δ GLS, % | 1 (1–1) | 2% (1–2) | .001∗ |
Δ= change in 6 months.
EF = ejection fraction, GLS = global longitudinal strain, LVDD = left ventricle end-diastolic diameter, LVESD = left ventricle end-systolic diameter.
Mann–Whitney U test.
Figure 3.
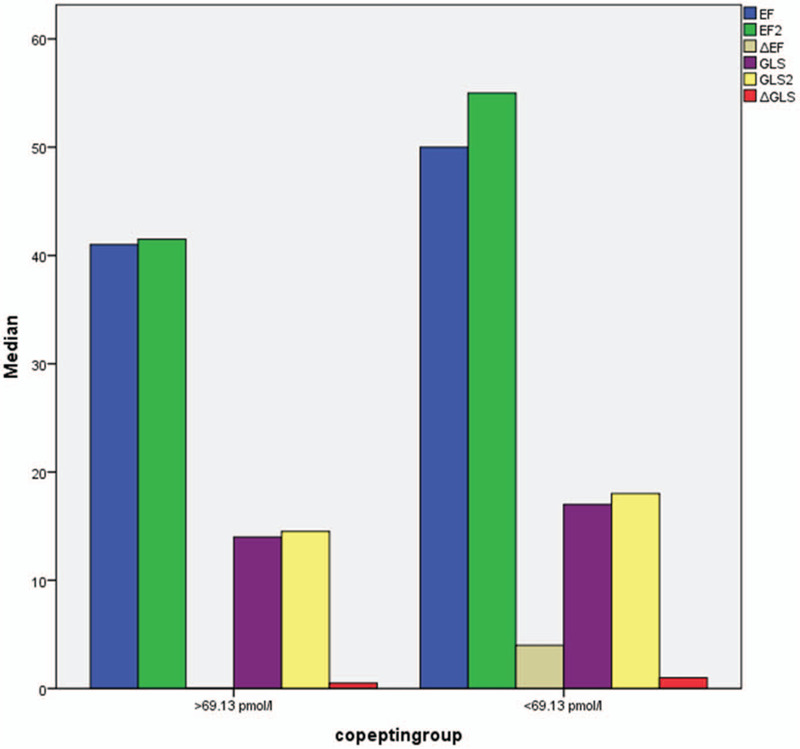
Comparison of echocardiographic parameters of the study groups according to copeptin levels.
The associated study parameters with the copeptin value were analyzed with the correlation analysis. The results of the correlation analysis are shown in Table 5. Copeptin value was found to be positively correlated with the left ventricular diameters and negatively correlated with the GLS and LVEF.
Table 5.
Correlation between copeptin levels and clinical parameters.
| r | P value | |
| Left ventricle end-diastolic diameter | 0.348 | .006 |
| Left ventricle end-systolic diameter | 0.404 | .001 |
| Left ventricle EF | −0.299 | .019 |
| Left ventricle GLS | −0.459 | <.001 |
| Time from symptom to revascularization | −0.143 | .271 |
| Troponin I | 0.009 | .945 |
| CK-MB | −0.079 | .546 |
r = Spearman-rho correlation coefficient.
CK-MB = creatinine kinase myocardial band, EF = Ejection fraction, GLS = global longitudinal strain.
Correlation between copeptin levels and early and 6th month LV EF and GLS is shown in Table 6. Copeptin values were negatively correlated with early and 6-month LV EF (r = –0.299 at early stage and r = –0.410 at 6-month) and GLS (r = –0.459 at early stage and r = –0.662 at 6-month). In addition, we observed that copeptin values were negatively correlated with the improvement of LV EF and GLS at 6-month follow-up (r = −0.458, P < .001 and r = −0.357, P = 0.005, respectively).
Table 6.
Correlation between copeptin and echocardiographic parameters at baseline and 6-month.
| Baseline | 6-month | Δ | ||||
| r | P value | r | P value | r | P value | |
| LV EF, % | −0.299 | .019 | −0.410 | .001 | −0.458 | .001 |
| LV GLS, % | −0.459 | <.001 | −0.662 | .001 | −0.357 | .005 |
r = Spearman-rho correlation coefficient; Δ=difference between baseline and 6-month.
EF = ejection fraction, GLS = global longitudinal strain, LV = Left ventricle.
During 6-month follow-up, we did not observe any significant clinical adverse event including target vessel revascularization and myocardial reinfarction as well as any death.
4. Discussion
In this prospective observational study, we demonstrated that plasma copeptin levels measured after the diagnosis of STEMI were associated with worse LV systolic function assessed by strain echocardiography. To the best of our knowledge, this study is the first to specifically address the relationship between copeptin values and GLS in STEMI patients.
Copeptin is an arginine vasopressin-associated glycopeptide that reflects the concentration of vasopressin in plasma.[17] Copeptin is more stable than arginine vasopressin and it is more useful in water homeostasis-related conditions. In the acute setting of MI, copeptin levels rise due to the decreasing of cardiac output stimulates cardiac and aortic baroreceptors, and also endogenous stress activates the vasopressin system.[17]
As the previous literature information, creatine kinase and troponin, which are the classic cardiac markers, are known to be associated with infarct size.[18] We do not have sufficient information about copeptin in this regard. Only in a cardiac MRI study copeptin values were found to be associated with larger infarct sizes after 2 days from the diagnosis of STEMI.[6] Copeptin is expected to increase in MI, but there is no information about the relationship between copeptin value and left ventricular systolic functions. Since copeptin is a marker that has been used recently, our knowledge in the field of CAD is not sufficient.
In our study, we observed that the highest copeptin values were in patients who were admitted within 90 to 360 minutes after the symptom onset, while the lowest values were in late-presenting patients. These findings were compatible with the temporal release pattern of copeptin demonstrated by Liebetrau et al.[19]
We evaluated left ventricular systolic functions with 2D STE in addition to conventional echocardiography parameters. The 2D strain obtained with STE provides better information about LV systolic function than LV EF, especially for cardiac events[20] and LV remodeling after acute MI.[21,22] Previous studies have revealed that GLS is better in reflecting the extent of infarct size and residual LV systolic function than LV EF.[23] It has also been shown that GLS is strongly correlated with cardiac MRI and SPECT findings.[24–27] Hence, we measured the GLS of all patients shortly after PPCI and at 6-month later after diagnosis of STEMI.
Since the GLS is better validated with cardiac MRI than the regional strain, which is the gold standard imaging method, we used it as a strain analysis method.[28] In addition, we did not choose the regional strain echocardiography technique because we had a heterogeneous patient population consisting of myocardial infarction patients affected by different regions of the myocardium.
Cardiac MRI is the ultimate test for evaluating myocardial functions, especially infarct size, but it is not as accessible as echocardiography and costs are higher. We could not compare the 2 techniques since we did not have the possibility of cardiac MR.
In our study, when we divided the patients into 2 groups, as below and above the median, according to the median copeptin value, and analyzed their data, it was observed that the group with low copeptin had better LV systolic functions.
When the patients were re-evaluated in the sixth month, the improvement of GLS was better in the low copeptin group than the high copeptin group.
These results suggest that the copeptin is negatively associated with left ventricular systolic functions. Copeptin related parameters were determined as GLS, LV EF, and left ventricular diameters and the strongest negative relationship was found with GLS. We think that this result has been achieved since GLS shows systolic functions better than EF.
In our study, troponin and creatine kinase values did not increase so significantly. this may be due to door to balloon time was optimal and symptom duration was not very long. We think that copeptin may be a more sensitive marker than these markers.
The difference of our study from previous studies is that it shows that the admission copeptin value in STEMI is predictive of left ventricular functions. Previously, in a cardiac MRI study, copeptin was shown to be informative about the infarct area, but in our study, it was shown that copeptin also predicts systolic functions determined by 2D STE.
Since infarct size and systolic functions are parameters related to prognosis This finding suggests that copeptin is not only a parameter used in the diagnosis and exclusion of myocardial infarction,[29] but also could be used as a prognosis predictor.
However, in our study, it is not possible to reach such a conclusion precisely because we did not follow patients in terms of clinical outcomes. However, we think that it is informative in terms of planning future studies.
4.1. Study limitations
This study had some limitations. There are many parameters that determine left ventricular systolic functions in STEMI. Which is the most important of the infarct-related artery. In our study, 45% of the patients consisted of individuals with anterior MI. Lack of a homogeneous group in terms of infarct location is a limitation. Another limitation is that we did not follow patients for clinical outcomes. Lastly, further studies with a larger sample size and a confirmatory technique such as cardiac MRI are necessary to confirm our findings.
5. Conclusions
Based on our results, we showed that copeptin values on admission are related to LV systolic function and its improvement at 6 months in STEMI patients.
Planning prospective studies, especially including clinical outcome data, confirmed by cardiac MRI, which is the gold standard imaging method to demonstrate cardiac functions, will be helpful in illuminating the prognostic importance of copeptin.
Acknowledgment
The authors thank their clinic nurses for their support.
Author contributions
Conceptualization: Hilal Erken Pamukcu, Faruk Aydinyilmaz, İlkin Guliyev, Saadet Demirtaş İnci, Nail Burak Özbeyaz, Ali Nallbani.
Data curation: Mehmet Ali Felekoğlu, Engin Algül, Faruk Aydinyilmaz, İlkin Guliyev, Nail Burak Özbeyaz, Ali Nallbani.
Formal analysis: Hilal Erken Pamukcu, Mehmet Ali Felekoğlu, Haluk Furkan Şahan, Faruk Aydinyilmaz.
Funding acquisition: Hilal Erken Pamukcu, Haluk Furkan Şahan.
Investigation: Hilal Erken Pamukcu, Engin Algül, İlkin Guliyev, Nail Burak Özbeyaz.
Methodology: Hilal Erken Pamukcu, Engin Algül, Haluk Furkan Şahan, Faruk Aydinyilmaz, İlkin Guliyev, Nail Burak Özbeyaz.
Project administration: Hilal Erken Pamukcu, Haluk Furkan Şahan, Ali Nallbani.
Resources: Hilal Erken Pamukcu, Mehmet Ali Felekoğlu, Haluk Furkan Şahan, Ali Nallbani.
Software: Hilal Erken Pamukcu, Saadet Demirtaş İnci, Ali Nallbani.
Supervision: Hilal Erken Pamukcu, Saadet Demirtaş İnci.
Validation: Hilal Erken Pamukcu.
Visualization: Hilal Erken Pamukcu, Mehmet Ali Felekoğlu, Faruk Aydinyilmaz.
Writing – original draft: Hilal Erken Pamukcu, Saadet Demirtaş İnci.
Writing – review & editing: Hilal Erken Pamukcu, Nail Burak Özbeyaz, Ali Nallbani.
Footnotes
Abbreviations: 2D = two-dimensional, ACS = acute coronary syndrome, CAD = coronary artery disease, EF = ejection fraction, GLS = global longitudinal strain, HF = heart failure, IQR = interquartile ranges, LV = left ventricle, MRI = magnetic resonance imaging, PPCI = primary percutaneous coronary intervention, ROI = regions of interest, STE = speckle tracking echocardiography, STEMI = ST-segment elevation myocardial infarction, TTE = transthoracic echocardiographic.
How to cite this article: Pamukcu HE, Felekoğlu MA, Algül E, Şahan HF, Aydinyilmaz F, Guliyev İ, İnci SD, Özbeyaz NB, Nallbani A. Copeptin levels predict left ventricular systolic function in STEMI patients: a 2D speckle tracking echocardiography-based prospective observational study. Medicine. 2020;99:50(e23514).
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Steg PG, James SK, et al. Task Force on the management of STseamiotESoC. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012;33:2569–619. [DOI] [PubMed] [Google Scholar]
- [2].Timmis A, Townsend N, Gale C, et al. European society of cardiology: cardiovascular disease statistics 2017. Eur Heart J 2018;39:508–79. [DOI] [PubMed] [Google Scholar]
- [3].Bhatt AS, Ambrosy AP, Velazquez EJ. Adverse Remodeling and Reverse Remodeling After Myocardial Infarction. Current cardiology reports 2017;19:71. [DOI] [PubMed] [Google Scholar]
- [4].Wu E, Ortiz JT, Tejedor P, et al. Infarct size by contrast enhanced cardiac magnetic resonance is a stronger predictor of outcomes than left ventricular ejection fraction or end-systolic volume index: prospective cohort study. Heart 2008;94:730–6. [DOI] [PubMed] [Google Scholar]
- [5].Galiuto LBL, Fox K, Sicari R, et al. Quantification of left ventricular function and synchrony using tissue Doppler, strain imaging, and speckle tracking. In: Voigt JU, ed. EAE Textbook of Echocardiography. Oxford, England: Oxford University Press; 2011:82–96. [Google Scholar]
- [6].Dimitriu-Leen AC, Scholte AJ, Katsanos S, et al. Influence of myocardial ischemia extent on left ventricular global longitudinal strain in patients after ST-Segment elevation myocardial infarction. Am J Cardiol 2017;119:1–6. [DOI] [PubMed] [Google Scholar]
- [7].Xu L, Huang X, Ma J, et al. Value of three-dimensional strain parameters for predicting left ventricular remodeling after ST-elevation myocardial infarction. Int J Cardiovasc Imaging 2017;33:663–73. [DOI] [PubMed] [Google Scholar]
- [8].Yalta K, Sivri N, Yalta T, et al. Copeptin (C-terminal provasopressin): a promising marker of arrhythmogenesis in arrhythmia prone subjects? Int J Cardiol 2011;148:105. [DOI] [PubMed] [Google Scholar]
- [9].Morgenthaler NG, Struck J, Alonso C, et al. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem 2006;52:112–9. [DOI] [PubMed] [Google Scholar]
- [10].Keller T, Tzikas S, Zeller T, et al. Copeptin improves early diagnosis of acute myocardial infarction. J Am Coll Cardiol 2010;55:2096–106. [DOI] [PubMed] [Google Scholar]
- [11].Reichlin T, Hochholzer W, Stelzig C, et al. Incremental value of copeptin for rapid rule out of acute myocardial infarction. J Am Coll Cardiol 2009;54:60–8. [DOI] [PubMed] [Google Scholar]
- [12].Marston NA, Shah KS, Mueller C, et al. Serial sampling of copeptin levels improves diagnosis and risk stratification in patients presenting with chest pain: results from the CHOPIN trial. Emerg Med J 2016;33:23–9. [DOI] [PubMed] [Google Scholar]
- [13].Reinstadler SJ, Klug G, Feistritzer HJ, et al. Association of copeptin with myocardial infarct size and myocardial function after ST segment elevation myocardial infarction. Heart 2013;99:1525–9. [DOI] [PubMed] [Google Scholar]
- [14].Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Eur Heart J 2012;33:2551–67. [DOI] [PubMed] [Google Scholar]
- [15].Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233–70. [DOI] [PubMed] [Google Scholar]
- [16].Voigt JU, Pedrizzetti G, Lysyansky P, et al. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. J Am Soc Echocardiogr 2015;28:183–93. [DOI] [PubMed] [Google Scholar]
- [17].Lukaszyk E, Malyszko J. Copeptin: pathophysiology and potential clinical impact. Adv Med Sci 2015;60:335–41. [DOI] [PubMed] [Google Scholar]
- [18].Turer AT, Mahaffey KW, Gallup D, et al. Enzyme estimates of infarct size correlate with functional and clinical outcomes in the setting of ST-segment elevation myocardial infarction. Curr Control Trials Cardiovasc Med 2005;6:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Liebetrau C, Nef H, Szardien S, et al. Release kinetics of copeptin in patients undergoing transcoronary ablation of septal hypertrophy. Clin Chem 2013;59:566–9. [DOI] [PubMed] [Google Scholar]
- [20].Ersboll M, Valeur N, Mogensen UM, et al. Prediction of all-cause mortality and heart failure admissions from global left ventricular longitudinal strain in patients with acute myocardial infarction and preserved left ventricular ejection fraction. J Am Coll Cardiol 2013;61:2365–73. [DOI] [PubMed] [Google Scholar]
- [21].Park YH, Kang SJ, Song JK, et al. Prognostic value of longitudinal strain after primary reperfusion therapy in patients with anterior-wall acute myocardial infarction. J Am Soc Echocardiogr 2008;21:262–7. [DOI] [PubMed] [Google Scholar]
- [22].Stanton T, Leano R, Marwick TH. Prediction of all-cause mortality from global longitudinal speckle strain: comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging 2009;2:356–64. [DOI] [PubMed] [Google Scholar]
- [23].Delgado V, Mollema SA, Ypenburg C, et al. Relation between global left ventricular longitudinal strain assessed with novel automated function imaging and biplane left ventricular ejection fraction in patients with coronary artery disease. J Am Soc Echocardiogr 2008;21:1244–50. [DOI] [PubMed] [Google Scholar]
- [24].Zhu W, Liu W, Tong Y, et al. Three-dimensional speckle tracking echocardiography for the evaluation of the infarct size and segmental transmural involvement in patients with acute myocardial infarction. Echocardiography 2014;31:58–66. [DOI] [PubMed] [Google Scholar]
- [25].Gjesdal O, Helle-Valle T, Hopp E, et al. Noninvasive separation of large, medium, and small myocardial infarcts in survivors of reperfused ST-elevation myocardial infarction: a comprehensive tissue Doppler and speckle-tracking echocardiography study. Circ Cardiovasc Imaging 2008;1:189–96. 182 p following 196. [DOI] [PubMed] [Google Scholar]
- [26].Munk K, Andersen NH, Nielsen SS, et al. Global longitudinal strain by speckle tracking for infarct size estimation. Eur J Echocardiogr 2011;12:156–65. [DOI] [PubMed] [Google Scholar]
- [27].Wang Q, Huang D, Zhang L, et al. Assessment of myocardial infarct size by three-dimensional and two-dimensional speckle tracking echocardiography: a comparative study to single photon emission computed tomography. Echocardiography 2015;32:1539–46. [DOI] [PubMed] [Google Scholar]
- [28].Amzulescu MS, Langet H, Saloux E, et al. Head-to-head comparison of global and regional two-dimensional speckle tracking strain versus cardiac magnetic resonance tagging in a multicenter validation study. Circ Cardiovasc Imaging 2017;10:5–7. [DOI] [PubMed] [Google Scholar]
- [29].Jacobs LH, van Borren M, Gemen E, et al. Rapidly rule out acute myocardial infarction by combining copeptin and heart-type fatty acid-binding protein with cardiac troponin. Ann Clin Biochem 2015;52(Pt 5):550–61. [DOI] [PubMed] [Google Scholar]


