Abstract
Second primary cancer is prevalent in patients with gastrointestinal (GI) cancer, for which lung cancer is the most common and associated with high lethality. Image screening for lung cancer was proved to be effective in early diagnosis and lower mortality. However, trials of screen for lung cancer generally excluded patients with a previous diagnosis of malignancy. The study aimed to investigate the outcome of second primary lung cancer and the factor that improve survival in patients with hepato-GI cancer.
A total of 276 patients with secondary lung cancer were found among 3723 newly-diagnosed lung cancer patients diagnosed in Chang Gung Memorial Hospital, between 2010 and 2014. Patients’ clinical characteristics, stages and survival were recorded and analyzed. The patients were separated into 2 groups: Group I was defined as lung cancer detected in original primary cancer clinic and group II patients defined as lung cancer detected in other medical places.
Sixty-nine cases with primary GI-hepatic and secondary lung cancer were diagnosed (42 (60.8%) in Group I and 27 (39.1%) in Group II). Although both groups had comparable primary cancer stages and treatment, more patients in Group I than Group II were diagnosed as early stage lung cancer (stage I-II: 40.5% vs 11.1%; P = .023). Group II had larger lung tumor sizes than Group I (4.7 vs 3.5 cm; P = .025). Group I showed better 5-year overall survival than Group II (P = .014, median survival: 27 vs 10 months). Among Group II, only 37% had received image follow up in clinic compared with 67% of Group I cases (P = .025). Patients with chest image follow up in clinics also had better 5-year overall survival (P = .043).
GI-hepatic cancer was the most common primary malignancy in the lung cancer cohort. Patients had better survival outcome when secondary lung cancer was diagnosed in original primary cancer clinic. Chest image screening strategy may contribute better survival in secondary lung cancer due to detection at an earlier stage.
Keywords: chest radiograph, gastrointestinal cancer, lung cancer, second primary cancer
1. Introduction
Multiple primary cancer is defined as 2 or more tumors occurring at different primary sites and should exclude recurrence or metastasis.[1] Possible reasons for an increased risk for second primary cancers in cancer survivors are genetic and behavioral risk factors and treatment of the first primary cancer.[2,3] Therapeutic progress has led to improvement in cancer survival. Because of the increased survival and common risk factors, cancer survivors are susceptible to developing synchronous or metachronous malignancy.[4] Multiple malignancies have become more prevalent in clinical practice worldwide.[5–10]
The second primary malignancies are most commonly diagnosed among survivors of lung, prostate, colorectal, and kidney cancers and the incidence of second primary lung cancer ranges from 0.5% to 10%.[4,11–13] In a US population-based study, lung cancer is the most commonly diagnosed second primary malignancy.[4] In addition, lung cancer is the leading cause of cancer mortality among patients with multiple primary malignancies (6% of the entire cohort and 12% of patients with a second primary malignancy died of lung cancer).[4]
Gastrointestinal (GI) cancer is the one of the most common cancers to develop a second primary lung cancer.[4,6,14] The number of GI cancer survivors has increased due to improvements in early diagnosis and treatment, therefore, second primary malignancy has become a threat to patient survival. Post-treatment surveillance, including radiological image screen, endoscopic evaluation or tumor markers, has been suggested to detect recurrence.[15,16] However, there is still no consensus about the surveillance strategy for second primary malignancy.
Although screening for lung cancer has been proved to be effective in early diagnosis and treatment,[17–20] there is still no study to prove if surveillance of second primary lung cancer may improve outcome. We conducted a cohort study including lung cancer between 2010 and 2014 in Chang Gung Memorial Hospital (CGMH) to see if the surveillance strategy of clinic may improve survival.
2. Material and method
2.1. Patients
In this retrospective cohort study, we included adult patients (≥ 20 years old) who were newly diagnosed with lung cancer between 2010 and 2014 in CGMH, Linkou branch. Among these patients, we identified those who previously had primary hepatic-GI cancer and then developed second primary lung cancer during this period. Patients with metastatic tumors from primary hepatic-GI to lungs were excluded from the study. We also excluded the patients whose second primary lung cancer was diagnosed less than 3 months after first primary cancer.[1] This study was approved by CGMH (IRB number: 201800039B0) and informed consent was not needed in this retrospective cohort study.
Patients were divided into 2 groups: group I patients who were diagnosed second primary lung cancer at primary hepato-GI clinic, where the first primary cancer was under follow-up and Group II was patients who had diagnosis of lung cancer at places other than primary hepato-GI clinic (e.g., patients may have respiratory distress and went to chest clinic or emergency department for help). We also defined chest radiography (CXR) group as patients receiving at least 1 CXR every year after first primary cancer was diagnosed and N-CXR referred to patients without CXR follow-up annually.
We collected patient's clinical characteristics, date of cancer diagnoses, cancer pathology types, lung cancer staging (American Joint Committee on Cancer 7th staging),[21] survival time, interval between the diagnosis of first and second primary cancers, and cancer therapy (surgery, radiation therapy, chemotherapy, or target therapy). December 31, 2018, was the census date for overall survival analysis. The frequencies of CXR and chest computed tomography (CT) during clinic follow-up or ward admission were also recorded.
2.2. Statistical analysis
Data are shown as mean (or median) ± standard deviation (or range) for continuous variables and as absolute and relative frequencies for categorical variables. To compare the different patients groups, the Student t test was used for continuous variables and the Fisher exact test or Chi-squared test was used for categorical variables. The Kaplan–Meier method with a log-rank test was used for survival analysis. Null hypotheses of no difference were rejected if P values were less than .05. All statistical tests were performed with SPSS version 18.0 for Windows (SPSS Inc., Chicago, IL).
3. Results
There were 3723 newly diagnosed lung cancer patients in CGMH, Linkou branch during 2010 to 2014. Two hundred forty-three patients had a history of other malignancy and then developed second primary lung cancer (Fig. 1). Among these patients with second primary lung cancer, 69 (28.4%) had hepato-GI cancer, followed by head and neck cancer (23.5%) and urology cancer (16.9%). The distribution of GI and hepatic cancers was in Figure 2. Colon cancer was the most frequent (35.7%), followed by liver cancer (25.7%) and stomach cancer (14.3%) (Fig. 2). These patients with second primary lung cancer were divided into 2 groups according to where lung cancer was diagnosed. Group I was patients who were diagnosed second primary lung cancer at initial clinic follow up for primary hepato-GI cancer. Group II was patients who had diagnosis of lung cancer at places other than primary hepato-GI clinic.
Figure 1.
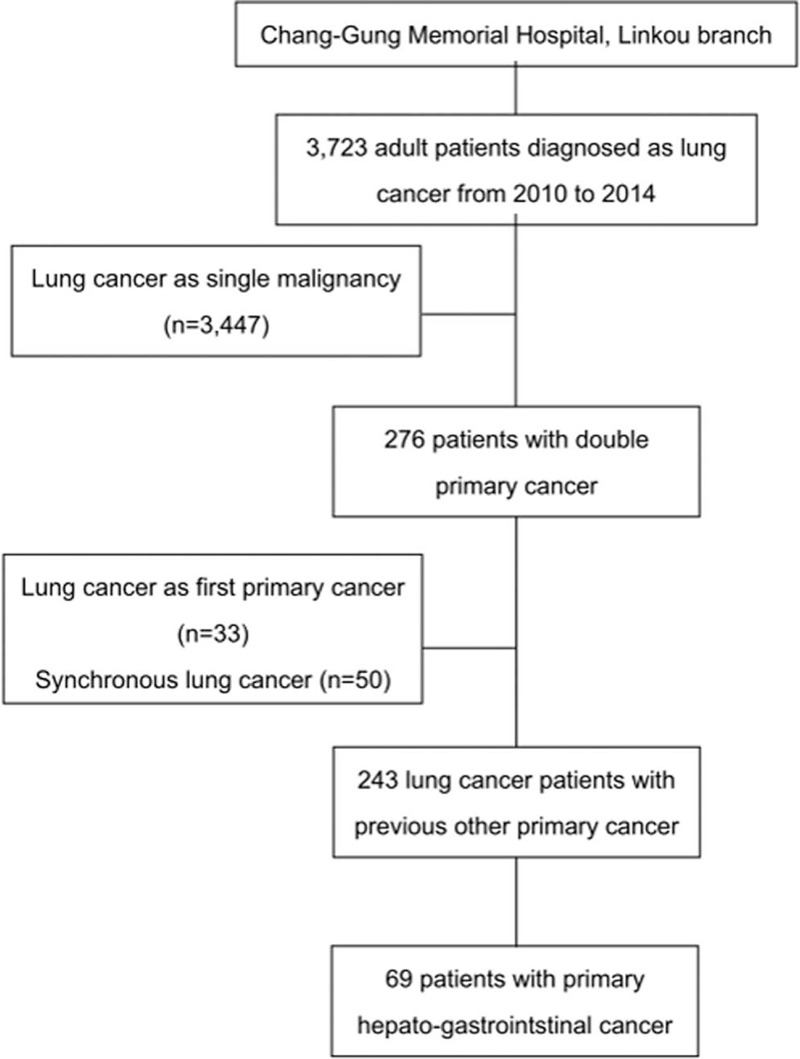
Flow diagram.
Figure 2.
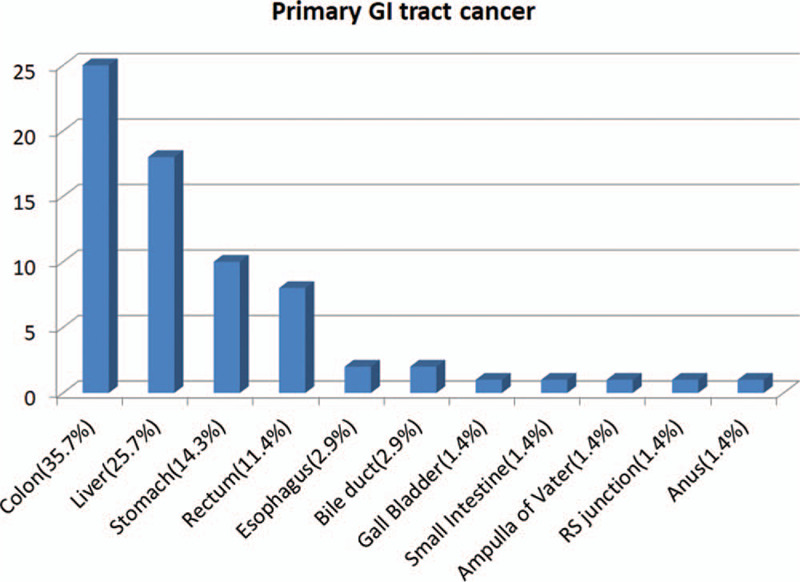
Distribution of first primary hepato-gastrointestinal cancer types.
The clinical characteristics of these 2 groups were listed in Table 1. There was no significant difference in age, gender and treatment of first cancer. The distribution of adenocarcinoma, squamous cell, and small cell carcinoma were similar in both groups. The tumor dimension in group II was significantly larger than group I (4.7 vs 3.5 cm, P = .025). Group II had significantly more stage III and IV lung cancer patients than group I (81.4% vs 57.1%, P = .023). There was no significant difference in mean time interval between first and second malignancy (46 vs 54 months, P = .36).
Table 1.
Patient Characteristic.
| Characteristic | Total (n = 69) | Group I (n = 42) | Group II (n = 27) | P value |
| Gender, Male | 51 (73.9%) | 29 (69.0%) | 22 (81.5%) | .251 |
| Age (lung cancer), yr-old | 70.2 ± 11.2 | 68.5 ± 11.6 | 72.8 ± 10.2 | .081 |
| Smoke (pack per yr) | 29.2 ± 32.5 | 26.0 ± 30.1 | 34.2 ± 36.1 | .389 |
| Lung cancer pathology | .094 | |||
| Squamous cell carcinoma | 19 | 9 | 11 | |
| Adenocarcinoma | 41 | 31 | 13 | |
| Small cell carcinoma | 5 | 2 | 3 | |
| Lung tumor dimension, cm | 3.9 ± 2.2 | 3.5 ± 2.1 | 4.7 ± 2.2 | .025∗ |
| Dimension range, cm | 0.8–10.2 | 0.8–9.0 | 1.3–10.2 | |
| Lung cancer stage | .023∗ | |||
| I | 16 (23.2%) | 15 (35.7%) | 1 (3.7%) | |
| II | 4 (5.8%) | 2 (4.8%) | 2 (7.4%) | |
| III | 15 (21.7%) | 9 (21.4%) | 6 (22.2%) | |
| IV | 31 (44.9%) | 15 (35.7%) | 16 (59.2%) | |
| Unknown | 3 (4.3%) | 1 (2.4%) | 2 (7.4%) | |
| Primary hepato-GI cancer stage | .019∗ | |||
| I + II | 25 (4.3%) | 15 (35.7%) | 10 (37.0%) | |
| III + IV | 27 (4.3%) | 21 (50%) | 6 (22.2%) | |
| Unknown | 17 (4.3%) | 6 (14.2%) | 11 (40.7%) | |
| Primary hepato-GI tumor treatment | .575 | |||
| Surgery | 55 | 33 | 22 | |
| TACE/RFA | 10 | 6 | 4 | |
| Chemotherapy | 14 | 11 | 3 | |
| Others | 3 | 2 | 2 | |
| Secondary lung cancer treatment | .009∗ | |||
| Surgery | 24 | 19 | 5 | |
| Chemotherapy | 22 | 14 | 8 | |
| Radiation therapy | 10 | 7 | 3 | |
| Target therapy | 11 | 7 | 4 | |
| Best supportive | 9 | 1 | 8 | |
| Chest image follow up in OPD | 38 (55.1%) | 28 (66.7%) | 10 (37.0%) | .025∗ |
| Interval between primary and secondary tumors (mo) | 51.2 ± 36.3 | 54.4 ± 30.3 | 46.2 ± 29.1 | .535 |
GI = gastrointestinal, OPD = outpatient department, RFA = radiofrequency ablation, TACE = trans-arterial cutaneous embolization.
P value less than .05;
The Kaplan–Meier curves showed group I had a significantly better survival than group II after the diagnosis secondary lung cancer (P = .014, median survival: 27 vs 10 months) (Fig. 3). The Kaplan–Meier curves showed CXR group had better survival than N-CXR (P = .042) (Fig. 4).
Figure 3.
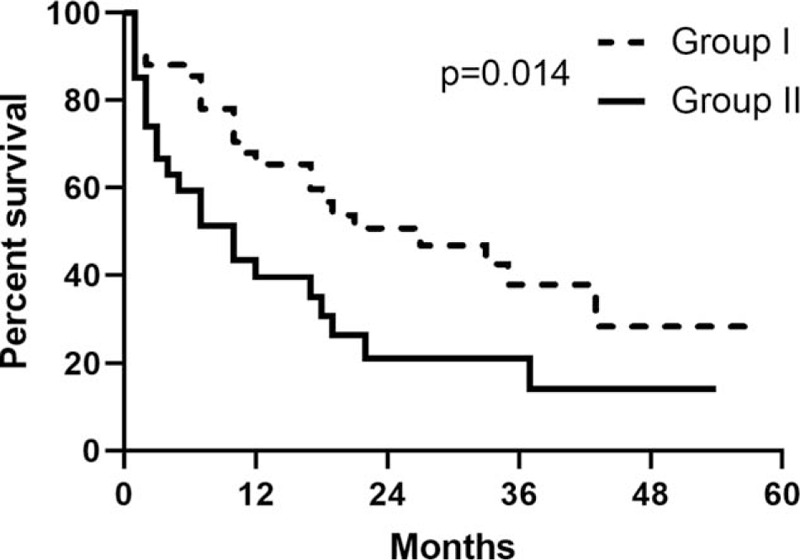
Five-year survival curves of group I and group II.
Figure 4.
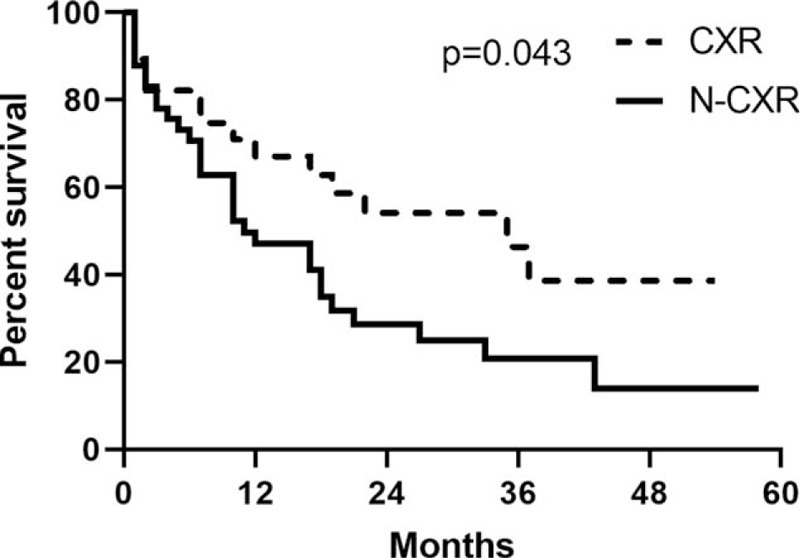
Five-year survival curves of group chest radiography and group N- chest radiography.
4. Discussion
In the study, approximately 7% of the CGMH cohort had another primary tumor before lung cancer and hepato-GI cancer was the most common primary malignancy. Patients had better survival outcome when secondary primary lung cancer was diagnosed in original primary cancer clinic. When second primary lung cancer was diagnosed, Group II had larger tumor size, more late stage lung cancer and shorter overall survival. Annual chest image follow-up contributed to better survival in secondary primary lung cancer. The study supported that increased surveillance after a first primary cancer leads to better outcome of second primary cancers.
The estimated prevalence for another primary malignancy varied between 0.5% and 10% among the various series.[4,11–13] In US National Cancer Institute's Surveillance, Epidemiology, and End Results program, multiple primary cancers account for about 17% of all incident cancers annually.[4] Patients with non-Hodgkin lymphoma, urology cancer, and colorectal cancer have a higher risk of developing a second primary malignancy.[4] Lung cancer is the most commonly diagnosed second primary cancer in cancer survivors in the US population-based study.[4] The risk factors to develop second primary cancers include genetics, smoke, radiotherapy, and chemotherapy of the first primary cancer.[2,3] In a study of breast cancer, smoking, and radiotherapy markedly enhanced risks to develop second primary lung cancer.[2] Geographic differences may exist in the distribution of primary tumors in lung cancer patients. In our cohort, 28.4% of the first primary tumors were hepato-GI cancer, followed by head and neck cancer and urology cancer, and the distribution of first primary malignancies was similar to previously report in Taiwan.[14]
Second primary cancers constitute a major threat to survival of cancer patients. The mortality rate of patients with second primary malignancies is higher than those who have only 1 single cancer.[4] Lung cancer is the most common and lethal second malignancy in a large, population-based cohort, in which 12% of patients died of second primary lung cancer.[4] Lu et al has reported the median survival was 12 months for second primary lung cancer.[14] In this study, the median survival was 27 months in patients whose lung cancer was diagnosed in primary hepato-GI clinic, better than the previously reported second primary lung cancer.[14] We also found that those with diagnosis of second primary lung cancer not in primary hepato-GI clinic had worse clinical outcome (median survival: 10 months) and included more stage III and IV lung cancer. Therefore, as patients survive first cancer, surveillance in clinic may reduce the mortality from second primary malignancies owing to early detection.
The prevention or early detection of second primary cancers is important for cancer survivors. However, to the best of our knowledge, less is known regarding the surveillance strategies to reduce the risk of second primary malignancies. CXR is a noninvasive and available tool for lung cancer screen. Although it has lower sensitivity than chest CT for stage Ia lung cancer, its detection rate was comparable in stage Ib-IV in National Lung Screening Trial.[17] In this study, patients receiving at least once CXR annually had better outcome than those who did not. It is likely that a significant number of cancer survivors may need lung cancer screen based on smoking history and other risk factors. CXR should also be applied to surveillance strategy to reduce mortality attributed to second primary cancers.
Delay diagnosis in cancer multiplicity was associated with a worse prognosis. As a consequence, screening practices could be done after a first primary cancer is diagnosed for early detection of recurrence or second primary malignancy. For hepato-GI cancer, post-treatment surveillance, including radiological image screen, endoscopic evaluation or tumor markers, has been suggested to detect recurrence.[15,16] CT screen has been used to detect primary lung cancer at earlier stage. CT screening for lung cancer was proved to be effective in early diagnosis with a 20% reduction in lung cancer mortality and a 7% reduction in all-cause mortality.[17] However, these trials of CT screen were not designed to screen second primary lung cancer, and patients with a history of malignancy were excluded. Although CT may play an important role in screen of cancer, in this study, the patients with annual CT follow-up did not show significant survival benefit. Because a large proportion of these patients received only abdomen CT (not including chest field), second primary lung cancer may not be detected in early stages in this study. For patients with hepato-GI cancer, the benefits of routine chest CT screen to reduce mortality due to second primary lung cancer need to be investigated in future studies.
Our study has several limitations. First, although this study was based on a large lung cancer cohort, the number of patients with primary hepato-GI cancer was relatively small and may limit the applicability of the results. Second, we could not clarify the reasons of patient's loss of follow up in primary hepato-GI cancer clinic because this was a real-world retrospective study. Third, future large-scale prospective studies would be required to identify the features of high-risk cancer survivors and to determine whether screening strategy for second primary lung cancer in cancer survivors could reduce mortality.
5. Conclusion
In the cohort, 7% of lung cancer patients was found to have a previous malignancy, with hepato-GI cancer as the most. Due to the increasing number of cancer survivors and the high mortality risk of second primary cancers, effective screen, and treatment strategies should be investigated. This study provided evidence that surveillance after primary hepato-GI cancer leads to detection of earlier stages of second primary lung cancer and better survival. Further trials will be needed to develop the appropriate long-term surveillance strategies of cancer survivors.
Acknowledgments
The authors thank all the members of the Cancer Center, Chang Gung Memorial Hospital, for their invaluable help in cancer registry data.
Author contributions
Analyzed the data: Min-Wei Lu, Mei-Chi Chen, Hsiu-Mei Chang
Conceived and designed the study: Hung-Yu Huang, Fu-Tsai Chung
Conceptualization: Hung-Yu Huang, Fu-Tsai Chung.
Data curation: Min-Wei Lu, Mei-Chi Chen, Hsiu-Mei Chang.
Methodology: Chih-Hsi Kuo, Shu-Min Lin.
Supervision: Hsiu-Mei Chang, Chun-Hua Wang.
Writing – original draft: Min-Wei Lu, Mei-Chi Chen.
Writing – review & editing: Hung-Yu Huang, Fu-Tsai Chung.
Footnotes
Abbreviations: CGMH = Chang Gung Memorial Hospital, CT= computed tomography, CXR= chest radiography, GI = gastrointestinal.
How to cite this article: Huang H-Y, Lu M-W, Chen M-C, Chang H-M, Kuo C-H, Lin S-M, Wang C-H, Chung F-T. Clinic image surveillance reduces mortality in patients with primary hepato-gastrointestinal cancer who develop second primary lung cancer: a STROBE-compliant retrospective study. Medicine. 2020;99:50(e23440).
This study was supported by Chang Gung Memorial Hospital Research Project Grants (CMRPG3H0931, CMRPG3B0831, CMRPG3B0832, CMRPG3B0833) and Saint Paul's Hospital Research Project Grant (SPMRP-U1–3001).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
References
- [1].Watanabe S, Kodama T, Shimosato Y, et al. Multiple primary cancers in 5,456 autopsy cases in the national cancer center of Japan. J Natl Cancer Inst 1984;72:1021–7. [PubMed] [Google Scholar]
- [2].Ford MB, Sigurdson AJ, Petrulis ES, et al. Effects of smoking and radiotherapy on lung carcinoma in breast carcinoma survivors. Cancer 2003;98:1457–64. [DOI] [PubMed] [Google Scholar]
- [3].Sosnowski R, Bjurlin MA, Verze P, et al. Role of cigarette smoking in urological malignancies and clinical interventions for smoking cessation. Cent European J Urol 2016;69:366–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Donin N, Filson C, Drakaki A, et al. Risk of second primary malignancies among cancer survivors in the United States, 1992 through 2008. Cancer 2016;122:3075–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Teppo L, Salminen E, Pukkala E. Risk of a new primary cancer among patients with lung cancer of different histological types. Eur J Cancer 2001;37:613–9. [DOI] [PubMed] [Google Scholar]
- [6].Liu YY, Chen YM, Yen SH, et al. Multiple primary malignancies involving lung cancer-clinical characteristics and prognosis. Lung Cancer 2002;35:189–94. [DOI] [PubMed] [Google Scholar]
- [7].Duchateau CS, Stokkel MP. Second primary tumors involving non-small cell lung cancer: prevalence and its influence on survival. Chest 2005;127:1152–8. [DOI] [PubMed] [Google Scholar]
- [8].Chuang SC, Scelo G, Lee YC, et al. Risks of second primary cancer among patients with major histological types of lung cancers in both men and women. Br J Cancer 2010;102:1190–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Son C, Lee SK, Choi PJ, et al. Characteristics of additional primary malignancies in Korean patients with non-small cell lung cancer. J Thorac Dis 2013;5:737–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kim SW, Kong KA, Kim DY, et al. Multiple primary cancers involving lung cancer at a single tertiary hospital: clinical features and prognosis. Thorac Cancer 2015;6:159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rosso S, De Angelis R, Ciccolallo L, et al. Multiple tumours in survival estimates. Eur J Cancer 2009;45:1080–94. [DOI] [PubMed] [Google Scholar]
- [12].Amer MH. Multiple neoplasms, single primaries, and patient survival. Cancer Manag Res 2014;6:119–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Preyer O, Concin N, Obermair A, et al. The relative risk of second primary cancers in Austria's western states: a retrospective cohort study. BMC Cancer 2017;17:699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lu MS, Chen MF, Huang YK, et al. Clinical outcome in lung cancer with a second malignancy: the time sequence matters. Medicine (Baltimore) 2016;95:e5203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Baiocchi GL, Marrelli D, Verlato G, et al. Follow-up after gastrectomy for cancer: an appraisal of the Italian research group for gastric cancer. Ann Surg Oncol 2014;21:2005–11. [DOI] [PubMed] [Google Scholar]
- [16].Lepage C, Phelip JM, Cany L, et al. Effect of 5 years of imaging and CEA follow-up to detect recurrence of colorectal cancer: The FFCD PRODIGE 13 randomised phase III trial. Dig Liver Dis 2015;47:529–31. [DOI] [PubMed] [Google Scholar]
- [17].National Lung Screening Trial Research T, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Infante M, Cavuto S, Lutman FR, et al. A randomized study of lung cancer screening with spiral computed tomography: three-year results from the DANTE trial. Am J Respir Crit Care Med 2009;180:445–53. [DOI] [PubMed] [Google Scholar]
- [19].Pastorino U, Rossi M, Rosato V, et al. Annual or biennial CT screening versus observation in heavy smokers: 5-year results of the MILD trial. Eur J Cancer Prev 2012;21:308–15. [DOI] [PubMed] [Google Scholar]
- [20].Saghir Z, Dirksen A, Ashraf H, et al. CT screening for lung cancer brings forward early disease. The randomised Danish lung cancer screening trial: status after five annual screening rounds with low-dose CT. Thorax 2012;67:296–301. [DOI] [PubMed] [Google Scholar]
- [21].Groome PA, Bolejack V, Crowley JJ, et al. The IASLC lung cancer staging project: validation of the proposals for revision of the T, N, and M descriptors and consequent stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol 2007;2:694–705. [DOI] [PubMed] [Google Scholar]


