Abstract
Background.
In October 2018, a new heart allocation policy was implemented with intent of prioritizing the sickest patients and decreasing waitlist time. We examined the effects of the new policy on transplant practices and outcomes 1 year before and 1 year after the change.
Methods.
Transplant recipients from October 2017 to September 2019 at our institution were identified and divided into 2 cohorts, a preallocation and postallocation criteria change. Patient demographics, clinical data, and bridging strategy were assessed. Early outcomes including ischemic time, severe primary graft dysfunction, need for renal replacement therapy, and duration of hospital stay were investigated.
Results.
In the 12 months before the change, 38 patients were transplanted as compared to 33 patients in the 12 months after the change. The average wait-time to transplant decreased after the allocation change (49 versus 313 d, P = 0.02). Patients were more likely to be bridged with an intra-aortic balloon pump (45% versus 3%) and less likely to be supported with a durable left ventricular assist device (LVAD) after the change (24% versus 82%). There was an increase in total ischemic time after the change (177 versus 117 min, P ≤ 0.01). There were no significant differences in other early posttransplant outcomes.
Conclusions.
Implementation of the new allocation system for heart transplantation resulted in dramatic changes in the bridging strategy utilized at our institution. Temporary mechanical support usage increased following the change and the number of recipients supported with durable LVADs decreased. Early posttransplant outcomes appear similar.
INTRODUCTION
In October 2018, the United Network for Organ Sharing (UNOS) approved a new heart allocation criteria to prioritize the sickest patients with the goal of reducing waitlist mortality.1 Changes were made to address the increasing number of patients on the transplant waiting list, to better account for the severity of illness, and to reflect for an increasing population of patients being supported with a left ventricular assist device (LVAD).2,3 Notably, the survival rate of patients supported with LVAD without complications have been improving over the past decade,4 and the established guidelines at the time were not reflective of these improved outcomes.5
In the new allocation system, the prior 3-tiered system (status 1A, 1B, and 2) was changed to a 6-tiered system (Status 1–6). The current allocation criteria, as outlined in the Organ Procurement and Transplantation Network (OPTN) policy, are described in Table 1. Status 1A was divided into 3 separate categories (status 1, 2, and 3), while status 4 was created to correspond to the previous status 1B. As an example, under the previous criteria, a patient on venoarterial extracorporeal membrane oxygenation (VA-ECMO) and a stable patient on LVAD support who is using his or her discretional 30 days of Elective 1A time would have the same listing status. However, under the new criteria, those patients would instead be listed as status 1 and status 3, respectively. This stratification better reflects the severity of their illness and prioritizes the patient with the greatest chance of death while awaiting transplant. Stable patients with durable LVAD support are now listed as status 4 under the new allocation criteria instead of status 1B.6,7 In addition to changes in listing status, a geographical range of 500 nautical miles was instituted from the site of the donor hospital in an effort to prioritize available organs based on illness severity rather than geography.6
TABLE 1.
Change in heart allocation criteria resulting in a new 6-tiered system
| Old allocation system | New allocation system | Listing criteria |
|---|---|---|
| Status 1 | • VA-ECMO | |
| • Nondischargeable, surgically implanted, nonendovascular biventricular support device | ||
| • MCSD with life-treatening ventricular arrhythmia | ||
| Status 1A | Status 2 | • IABP |
| • Nondischargeable, surgically implanted, nonendovascular LVAD | ||
| • VT or VF without mechanical support | ||
| • MCSD with device malfunction or failure | ||
| • TAH, BiVAD, RVAD, or VAD for single ventricle patients | ||
| • Percutaneous endovascular MCSD | ||
| Status 3 | • Dischargeable LVAD for discretionary 30 d | |
| • Multiple inotropes or single high-dose inotrope with continuous hemodynamic monitoring | ||
| • Single inotrope with continuous monitoring | ||
| • VA-ECMO after 7 d; IABP or percutaneous endovascular circulatory support device after 14 d | ||
| • Nondischargeable, surgically implanted, nonendovascular LVAD after 14 d | ||
| • Mechanical support device with complication | ||
| Status 1B | Status 4 | • Dischargeable LVAD without discretionary 30 d |
| • Inotropes without hemodynamic monitoring | ||
| • Retransplant | ||
| • Diagnosis of CHD, ischemic heart disease with intractable angina, hypertrophic CM, restrictive CM, amyloidosis | ||
| Status 2 | Status 5 | • On waitlist for at least one other organ at the same hospital |
| Status 6 | • All other active candidates |
The new adult heart allocation criteria and its corresponding status from the previous criteria for medical urgency status is adopted from the OPTN website and policies, of which full details can be found at https://optn.transplant.hrsa.gov/media/1200/optn_policies.pdf.
CHD, congenital heart disease; CM, cardiomyopathy; ECMO, venoarterial extracorporeal membrane oxygenation; IABP, intra-aortic balloon pump; (R/L) VAD, (right/left) ventricular assist device; TAH, total artificial heart; VA-MCSD, mechanical circulatory support device; VF, ventricular fibrillation; VT, ventricular tachycardia.
Preliminary data presented in a 4-month report in April 2019 published by the OPTN Thoracic Committee demonstrated no major impact on the number of registrations made to the waiting list or the number of transplants performed, although it did report a significant increase in ischemic time.8 Newer data from the UNOS and OPTN registries have emerged regarding the effects of the new allocation criteria, revealing a shift in bridging strategies and a focus on temporary mechanical support. The percentage of patients supported by LVAD at the time of transplant has decreased significantly, whereas those supported by VA-ECMO and intra-aortic balloon pump (IABP) have increased.9-11 While an early analysis of the UNOS registry suggested worsening posttransplantation outcomes,9 a study of the OPTN registry at the 1-year mark did not show significant change observed in either waiting list mortality nor posttransplant survival after the policy change was instituted.11 However, the effect of the new allocation system on the types of temporary mechanical support being utilized, trends in clinical care at the institutional level, and early outcomes other than mortality is less well understood. Therefore, we sought to investigate how the new allocation criteria impacted patients transplanted at a single institution 1 year before and 1 year after the allocation change.
MATERIALS AND METHODS
Study Population
This was a retrospective cohort study that examined all adult patients who underwent heart transplantation at Barnes-Jewish Hospital/Washington University in St. Louis from October 2017 to September 2019. Patients were divided into two groups based on if they were transplanted before or after October 18, 2018, the date of the allocation change.
Data were gathered through review of electronic medical record, including baseline demographics, laboratory values, bridging strategy (none, IV inotropes, temporary mechanical devices such as IABP or LVAD), patient- and donor-specific factors, and early outcomes including the presence of severe primary graft dysfunction (PGD), need for renal replacement therapy (RRT) or tracheostomy after transplantation, duration of intensive care unit (ICU) stay, and total length of hospital stay. Study data were collected and managed using REDCap (Research Electronic Data Capture) tools hosted at Washington University in St. Louis. REDCap is a secure, web-based software platform designed to support data capture for research studies.12,13
Statistical Analysis
Continuous variables were analyzed using a 2-tailed t-test, while categorical variables were analyzed using Chi-square or Fisher exact test. A P value <0.05 was considered statistically significant. Data analysis was performed using Stata Statistical Software (Release 16, College Station, TX). This study was reviewed and approved by the Washington University in St. Louis Institutional Review Board.
RESULTS
Demographics and Baseline Clinical Data
In total, 71 patients were identified, with 38 patients transplanted the year before the change compared to 33 patients the year after the change. Baseline characteristics for each group are listed in Table 2. Compared with the year prior, patients that were transplanted after the allocation change had lower serum sodium and hemoglobin and higher total bilirubin and hemoglobin A1c. The most common reported etiology of heart failure was nonischemic cardiomyopathy in both cohorts, 68% (26/38) the year before versus 73% (24/33) the year after (P = 0.69). Single organ transplantation was performed most often, with 95% (36/38) the year before versus 97% (32/33) the year after (P = 0.64). While the average donor age did not change significantly, the amount of organ donations accepted from local organ procurement organizations decreased significantly from 97% (37/38) the year before to 33% (11/33) the year after (P < 0.01).
TABLE 2.
Baseline characteristics and clinical data
| Before allocation change (N = 38) | After allocation change (N = 33) | P | |
|---|---|---|---|
| Age (y) | 53 | 54 | 0.75 |
| Male sex | 71% | 70% | 0.90 |
| Caucasian race | 76% | 67% | 0.37 |
| Body mass index (kg/m2) | 29.3 | 27.5 | 0.12 |
| Sodium (mEq/L) | 139 | 135 | <0.01 |
| Creatinine (mg/dL) | 1.4 | 1.4 | 0.99 |
| Total bilirubin (mg/dL) | 0.7 | 1.0 | 0.05 |
| AST (units/L) | 37 | 39 | 0.72 |
| ALT (units/L) | 28 | 40 | 0.16 |
| Albumin (g/dL) | 4.1 | 4.0 | 0.26 |
| Hemoglobin (g/dL) | 12.0 | 11.0 | 0.02 |
| NT-proBNP (pg/mL) | 5734 | 5645 | 0.98 |
| Hemoglobin A1c | 5.8% | 6.4% | <0.01 |
| Blood type | |||
| A | 19 | 13 | 0.37 |
| B | 2 | 4 | 0.41 |
| O | 16 | 12 | 0.62 |
| AB | 1 | 4 | 0.17 |
| HF etiology | |||
| Ischemic | 10 | 7 | 0.62 |
| NICM | 26 | 24 | 0.69 |
| CAVa | 2 | 2 | 1 |
| Organ transplanted | |||
| Heart | 36 | 32 | 0.64 |
| Heart/kidney | 1 | 1 | |
| Heart/liver | 1 | 0 | |
| Donor age (y) | 27 | 27 | 0.80 |
| Local donor | 97% | 33% | <0.01 |
aTwo patients each in the year before the change as well as the year after had coronary allograft vasculopathy, requiring redo orthotopic heart transplantation.
CAV, coronary allograft vasculopathy; NICM, nonischemic cardiomyopathy.
Listing Information
The most common listing status before the allocation change was status 1B [28/38 (74%)]. Of the 10 patients listed as status 1A, 3 were inpatient before the change. One required inotropic support, one required IABP support, and the third was listed as a 1A exception for ventricular tachycardia. The other 7 patients were called in as an outpatient and were listed as status 1A exception due to pump malfunction, hemorrhagic stroke, hemolysis, aortic insufficiency, or refractory GI bleeding. The most common listing status after the allocation change was status 2 [19/33 (60%); Figure 1]. Of the 19 patients listed as status 2, 15 were supported with IABP, while the remaining four patients were listed as status 2 due to LVAD malfunction. All status 1 patients were supported with ECMO. After the change, there was a statistically significant decrease in days spent on the transplant waitlist from 314 to 49 days (P = 0.02; Table 3).
FIGURE 1.
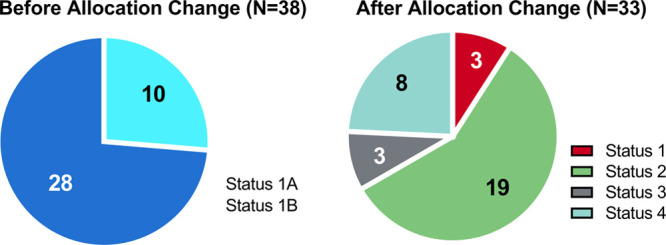
Listing status of heart transplants preallocation and postallocation criteria change.
TABLE 3.
Transplant listing data, including bridging strategy utilized before and after heart allocation change
| Before allocation change (N = 38) | After allocation change (N = 33) | P | |
|---|---|---|---|
| Days on transplant list | 314 | 49 | 0.02 |
| Called in as outpatienta (n, %) | 35 (92) | 10 (30) | <0.01 |
| Bridging strategy (n, %) | |||
| VAD | 31 (82) | 8 (24) | <0.01 |
| HM2 | 15 | 2 | |
| HM3 | 8 | 1 | |
| HVAD | 8 | 5 | |
| IABP | 1 (3) | 15 (45) | <0.01 |
| Inotropic support | 3 (8) | 5 (15) | 0.46 |
| VA-ECMO | 0 (0) | 3 (9) | 0.10 |
| Redo OHT | 2 (5) | 2 (6) | 1 |
| Noneb | 1 (3) | 0 (0) | 1 |
aPatients who were called in to the hospital while outpatient for admission to receive their heart transplant were listed as such, compared to patients that were already hospitalized for decompensated heart failure when they received their transplant.
bOne patient was admitted with multiple shocks from an implanted cardiac defibrillator and was listed as a status 1A exception for ventricular tachycardia. This patient went into PEA arrest after attempted defibrillation threshold testing and stayed in the hospital over 3 mo before transplant. The patient did not receive inotropic support or mechanical device support at any time during the hospitalization.
HM2, HeartMate 2; HM3, HeartMate 3; HVAD, HeartWare VAD; IABP, intra-aortic balloon pump; OHT, orthotopic heart transplant; VA-ECMO, venoarterial extracorporeal membrane oxygenation; VAD, ventricular assist device.
Shift in Bridging Strategy Utilized
There was a dramatic decrease in patients supported with LVAD at the time of transplant, from 82% (31/38) the year before the allocation change to 24% (8/33) the year after (P < 0.01; Table 3). In contrast, under the new allocation system, use of IABP increased from 3% (1/38) to 45% (15/33; P < 0.01). There was a suggestion of increased use of VA-ECMO (0% versus 9%, P = 0.1); however, other bridging strategies such as inotropic support and redo transplantation were not statistically different before and after the allocation change (Figure 2).
FIGURE 2.
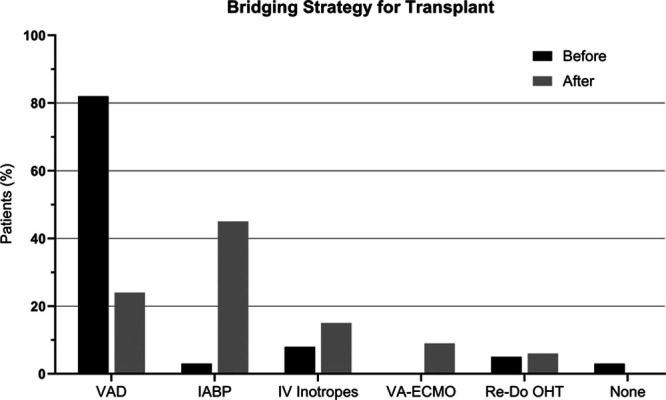
Bridging strategy before and after the allocation change. Patients transplanted after the heart allocation change were significantly more likely to be bridged with an IABP and less likely to be bridged with durable LVAD. IABP, intra-aortic balloon pump; LVAD, left ventricular assist device; OHT, orthotopic heart transplant.
Increasing Use of IABP
The increased use of IABP as a bridging strategy corresponded with increased insertion of IABP via the axillary approach after the allocation change (0% versus 68%; Table 4). Under the new allocation system, 19 patients in total had the use of either femoral or axillary IABP as a bridging strategy, with 15 of those able to be transplanted as status 2. Of the 4 other patients, 2 received heart transplants after utilization of a different bridging strategy. One required escalation to VA-ECMO and was eventually transplanted as status 1, and the other experienced bleeding complications necessitating IABP removal and was bridged to transplant on inotropes as a status 3. Further complications necessitating IABP exchange or removal are listed in Table 4. During the study period under the new allocation criteria, 2 other patients who were supported with IABP passed away before transplant due to refractory shock. In both cases, LVAD implantation was not an option due to severe biventricular dysfunction.
TABLE 4.
Patients bridged with IABP before and after heart allocation change
| Before allocation change | After allocation change | |
|---|---|---|
| Patients bridged with IABP | 1 | 19 |
| Transplanted as status 2 (n, %) | 1 (100) | 15 (79) |
| Transplanted as different statusa | 0 | 2 (11) |
| Passed away before transplant | 0 | 2 (11) |
| Mean days with IABP | 6 | 13 |
| Axillary IABP placement (n, %) | 0 | 13 (68) |
| Complicationsb (n, %) | 0 | 5 (26) |
| Balloon rupture | 0 | 1 |
| Bacteremia | 0 | 1 |
| Kinking of catheter shaft | 0 | 1 |
| Bleeding | 0 | 1 |
| Limb ischemia | 0 | 1 |
aTwo patients initially started out with IABP bridging. One required escalation with VA-ECMO and was up-listed and eventually transplanted as status 1. The other had IABP removal due to bleeding complication and was transplanted as status 3.
bBalloon rupture and bacteremia required axillary IABP exchange, kinking of the catheter shaft required replacement from axillary to femoral positioning, bleeding resulted in IABP removal, limb ischemia necessitated thrombectomy and IABP removal.
IABP, intra-aortic balloon pump; VA-ECMO, venoarterial extracorporeal membrane oxygenation.
Trends in Clinical Care
After the allocation change, patients were less likely to be outpatient at the time of heart transplant. The year before the change, 92% (35/38) of patients were called in from home to be admitted to the hospital to receive their heart transplant, compared with 30% (10/33) the year after. This coincides with increased length of stay in the hospital before heart transplant after the system change (5 versus 14 d, P = 0.01; Table 5). To evaluate how our center adjusted to the new allocation system, we assessed transplant volume by quarter over this 2-years window. As seen in Figure 3, transplant volumes per quarter remained consistent until the allocation change, after which there was a considerable decrease in the first 2 calendar quarters. Over time, clinical practice was adjusted to align with the prioritization scheme of the new system, resulting in an increase in transplant volume over the final two quarters of the year.
TABLE 5.
Early outcomes after heart transplantation
| Before allocation change (N = 38) | After allocation change (N = 33) | P | |
|---|---|---|---|
| Ischemic time (min) | 117 | 177 | <0.01 |
| Days intubated | 3 | 3 | 0.82 |
| Days in ICU | 7 | 9 | 0.33 |
| Days in hospital before OHT | 5 | 14 | 0.01 |
| Days in hospital after OHT | 7 | 9 | 0.33 |
| Severe PGD (n, %) | 6 (16) | 5 (15) | 1 |
| PGD patients with LVAD | 6 (100) | 4 (80) | 0.12 |
| % LVAD patients with PGD | 19% | 50% | 0.19 |
| Days on vasopressors/inotropes | 8 | 7 | |
| vasopressors >7 d (n, %) | 16 (42) | 9 (27) | |
| Usage of RRT (n, %) | 12 (32) | 14 (42) | 0.34 |
| Tracheostomy (n, %) | 4 (11) | 6 (18) | 0.50 |
| Infection (n, %) | 8 (21) | 9 (27) | 0.54 |
| Treated rejection (n,%) | 8 (21) | 3 (9) | 0.16 |
| Expired (n, %) | 0 (0) | 3 (9) | 0.10 |
ICU, intensive care unit; OHT, orthotopic heart transplant; PGD, primary graft dysfunction; RRT, renal replacement therapy.
FIGURE 3.
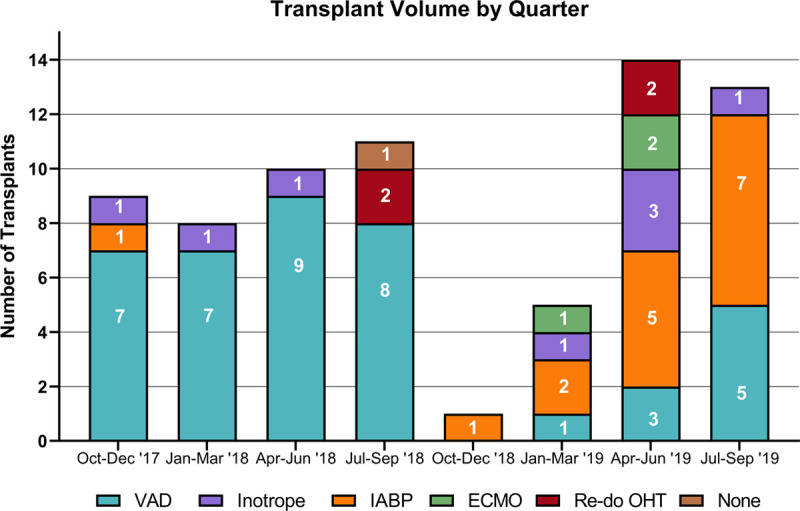
Transplant volume by calendar quarter. Transplant volume was lowest in the two quarters immediately following the allocation change on October 18, 2018. ECMO, extracorporeal membrane oxygenation; IABP, intra-aortic balloon pump; OHT, orthotopic heart transplant; VAD, ventricular assist device.
Early Outcomes
A statistically significant increase in cold ischemic time was seen under the new system with an average of 177 minutes compared with 117 minutes before the change (P < 0.01; Table 5). Otherwise, there were no statistically significant differences in any other measured outcomes, including the overall number of days intubated, presence of severe PGD, days on vasopressor and inotropic support after transplant, use of RRT, need for tracheostomy, postoperative infection, treated rejection at 6 months, overall ICU length of stay, or length of stay in the hospital after heart transplantation. Of note, nearly all cases of PGD occurred in patients who went into transplant on LVAD support (Table 5). There was a nonstatistically significant increase in posttransplant mortality after the allocation change with 3 patients occurring the year after compared with zero patients the year prior. All 3 deaths occurred in patients supported with a durable LVAD.
DISCUSSION
Our study illustrates the profound impact of the new UNOS allocation system on transplant practices at a large tertiary academic center. Using the 12 months before the implementation of the system as a control, our data revealed a significant decrease in the number of patients transplanted who were bridged with a durable LVAD and a simultaneous increase in patients who were supported with an IABP. This was associated with a significant reduction in time on the waitlist before transplant. After the change, patients were more likely to be hospitalized for several weeks before transplant, and ischemic time was increased. However, posttransplant outcomes were similar between the cohorts.
Our experience has been compatible with other recent analyses demonstrating the shift in bridging strategy following the allocation change. An early investigation of the UNOS registry reported that among patients receiving a heart transplant those bridged with LVADs decreased from 42% to 23%, while those bridged with temporary mechanical support increased from 10% to 41% after the allocation change.9 Another recent study by Varshney et al14 demonstrated a significant increase in temporary mechanical circulatory support (MCS) among patients with acute decompensated heart failure in the US transplant centers. At our institution, the use of IABP as a bridging strategy increased significantly from 3% to 45% after the allocation change. Furthermore, 68% of patients who received IABP had the device placed in the axillary position, which has been shown to represent a well-tolerated bridging strategy.15 IABP complications necessitating removal or exchange occurred in 26% of cases at our center. These results are comparable to recent study by Bhimaraj et al,16 who reported that in a large series of patients with axillary IABP insertion that 15.8% required at least one IABP replacement. Two other reviews have suggested vascular complication rates of 8%–18%17 and 0.94%–31.1%.18 In our series, 79% of IABP bridged patients successfully made it to transplant, further supporting the concept that this is an acceptable strategy to bridge patients to transplant. In comparison, other studies have shown rates of ~70% success in bridging patients with axillary IABP support to transplant or LVAD.16 Similar findings have also been reported using the Impella 5.0 as a bridge to durable MCS or heart transplant.19
Another striking observation was that the lower priority assigned to those on LVAD support (status 4) has resulted in fewer of these patients receiving a heart transplant, a finding echoed in recent analyses of the UNOS9 and OPTN10,11 registries. At our institution, only 3 patients (9%) were transplanted from a status 4 listing under the new allocation criteria. The other 5 patients on durable LVAD support were up-listed due to complications related to their LVAD. The combination of a lower listing status along with an increase in geographic range for available donors has led to decreased offers for status 4 patients when compared with status 1B patients the year prior. Thus, the new system has increased wait times for those who are doing well on LVAD support. This has created a system of practice where durable MCS and transplant are effectively parallel pathways rather than events in series. Although there may be some advantages to this approach moving forward, it has disadvantaged many LVAD patients who were implanted as a bridge to transplant before the allocation change. In addition, the practice of only transplanting LVAD patients with complications has the potential to worsen both short-term and long-term transplant outcomes.
There have been concerns in the transplant community that the changes in the new allocation policy designed to reduce death on the waitlist could worsen posttransplant outcomes. In particular, the combination of increased allograft ischemic time and more severe illness in recipients has been considered a recipe for early complications such as PGD. Although an early analysis of the UNOS registry reported by Cogswell et al9 found a significant increase in post-heart transplant death in the new system, analysis of the OPTN data by Goff et al11 did not show significant change in posttransplant survival at the 1-year mark. Similar to other studies,8,9,11 we observed a significant increase in ischemic time under the new allocation system. This was likely a result of a significant increase in accepted organs from outside our local organ procurement organization. However, despite the longer ischemic time and increased use of temporary support, we did not observe significant differences in the use of RRT, tracheostomy, length of stay in the ICU, length of stay in the hospital, or severe PGD after transplant. In fact, 91% of the severe PGD occurred in those with LVADs in both years of this study. This observation is in line with prior data that LVADs are a major risk factor for PGD.20 A concerning finding is that under the new system severe PGD occurred in 50% of patients who had an LVAD going into transplant. This may be a consequence of the new system favoring organ allocation to LVAD patients with complications. To this point, we recently demonstrated that patients with the right heart failure on LVAD support are at higher risk of PGD posttransplant compared with stable patients on LVAD support.21
Although our study was too small to address mortality concerns, there were 3 patients who expired the year after the change. All of these patients were supported with LVADs and the cause of death was attributable to complications of severe PGD. At the time of this reporting, there were no further deaths among patients transplanted in the year after the allocation change. Thus, we conclude that increased use of temporary support devices and longer ischemic times did not worsen early posttransplant outcomes at our institution. Rather, the mortality observed in our study was seen only in patients supported with LVADs. Further investigation of these relationships in larger patient series’ with longer follow-up will be necessary to delineate the implications of the allocation change on early and late posttransplant outcomes.
LIMITATIONS
First, this a single-center study with a limited sample size, and the results cannot necessarily be generalized to clinical practice at other institutions. However, over the study period, all heart transplants were evaluated by the same groups of advanced heart failure physicians and cardiac surgeons at our institution. Next, this study was performed at the 1-year mark of allocation change, and only short-term outcomes were available. Finally, this study was not large enough to address the increasing use of VA-ECMO. At our institution, 9% of patients were bridged with VA-ECMO successfully; however, this approach must be used carefully.22
CONCLUSION
Implementation of the new heart allocation system at our institution resulted in dramatic changes in utilization of the bridging strategy for transplantation. Patients were less likely to be supported with durable LVADs and were more likely to be supported by IABP after the allocation change, resulting in decreased days on the waitlist and increased inpatient hospital length of stay before the transplant. Early posttransplant outcomes appear similar. Further monitoring of posttransplant outcomes will be needed to evaluate the longer-term effects of the allocation criteria changes.
Footnotes
Published online 15 December, 2020.
All authors contributed to research design, data collection, and writing of the paper. J.L. performed the data analysis.
J.D.S. is a speaker for CareDx. The other authors declare no conflicts of interest.
REFERENCES
- 1.Stevenson LW, Kormos RL, Young JB, et al. Major advantages and critical challenge for the proposed United States heart allocation system. J Heart Lung Transplant. 2016; 35:547–549. [DOI] [PubMed] [Google Scholar]
- 2.Meyer DM, Rogers JG, Edwards LB, et al. The future direction of the adult heart allocation system in the United States. Am J Transplant. 2015; 15:44–54. [DOI] [PubMed] [Google Scholar]
- 3.Rao P, Smith R, Khalpey Z. Potential impact of the proposed revised UNOS thoracic organ allocation system. Semin Thorac Cardiovasc Surg. 2018; 30:129–133. [DOI] [PubMed] [Google Scholar]
- 4.Kirklin JK, Pagani FD, Kormos RL, et al. Eighth annual INTERMACS report: special focus on framing the impact of adverse events. J Heart Lung Transplant. 2017; 36:1080–1086. [DOI] [PubMed] [Google Scholar]
- 5.Wever-Pinzon O, Drakos SG, Kfoury AG, et al. Morbidity and mortality in heart transplant candidates supported with mechanical circulatory support: is reappraisal of the current United network for organ sharing thoracic organ allocation policy justified? Circulation. 2013; 127:452–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liz Robbins C. OPTN/UNOS proposal to modify the adult heart allocation system. 2016 Available at https://optn.transplant.hrsa.gov.pdf.
- 7.Colvin-Adams M, Valapour M, Hertz M, et al. Lung and heart allocation in the United States. Am J Transplant. 2012; 12:3213–3234. [DOI] [PubMed] [Google Scholar]
- 8.Lindblad K, Lehman R. Four-month monitoring of heart allocation proposal to modify the heart allocation system. 2019 Available at https://optn.transplant.hrsa.gov/media/2924/post-implementation-heart-policy-report_20190417_ready-to-post.pdf. Accessed September 4, 2019.
- 9.Cogswell R, John R, Estep JD, et al. An early investigation of outcomes with the new 2018 donor heart allocation system in the United States. J Heart Lung Transpl. 2020; 39:1–4. [DOI] [PubMed] [Google Scholar]
- 10.Hanff TC, Harhay MO, Kimmel SE, et al. Trends in mechanical support use as a bridge to adult heart transplant under new allocation rules. JAMA Cardiol. 2020; 5:728–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goff RR, Uccellini K, Lindblad K, et al. A change of heart: preliminary results of the US 2018 adult heart allocation revision. Am J Transplant. 2020; 20:2781–2790. [DOI] [PubMed] [Google Scholar]
- 12.Harris PA, Taylor R, Minor BL, et al. ; REDCap Consortium. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019; 95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009; 42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varshney AS, Berg DD, Katz JN, et al. ; Critical Care Cardiology Trials Network Investigators. Use of temporary mechanical circulatory support for management of cardiogenic shock before and after the united network for organ sharing donor heart allocation system changes. JAMA Cardiol. 2020; 5:703–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Estep JD, Cordero-Reyes AM, Bhimaraj A, et al. Percutaneous placement of an intra-aortic balloon pump in the left axillary/subclavian position provides safe, ambulatory long-term support as bridge to heart transplantation. JACC Heart Fail. 2013; 1:382–388. [DOI] [PubMed] [Google Scholar]
- 16.Bhimaraj A, Agrawal T, Duran A, et al. Percutaneous left axillary artery placement of intra-aortic balloon pump in advanced heart failure patients. JACC Heart Fail. 2020; 8:313–323. [DOI] [PubMed] [Google Scholar]
- 17.Parissis H, Graham V, Lampridis S, et al. IABP: history-evolution-pathophysiology-indications: what we need to know. J Cardiothorac Surg. 2016; 11:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Jong MM, Lorusso R, Al Awami F, et al. Vascular complications following intra-aortic balloon pump implantation: an updated review. Perfusion. 2018; 33:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall SA, Uriel N, Carey SA, et al. Use of a percutaneous temporary circulatory support device as a bridge to decision during acute decompensation of advanced heart failure. J Heart Lung Transplant. 2018; 37:100–106. [DOI] [PubMed] [Google Scholar]
- 20.Truby LK, Takeda K, Topkara VK, et al. Risk of severe primary graft dysfunction in patients bridged to heart transplantation with continuous-flow left ventricular assist devices. J Heart Lung Transplant. 2018; 37:1433–1442. [DOI] [PubMed] [Google Scholar]
- 21.King PM, Raymer DS, Shuster J, et al. Right heart failure while on left ventricular assist device support is associated with primary graft dysfunction. Asaio J. 2020; 66:1137–1141. [DOI] [PubMed] [Google Scholar]
- 22.Habal MV, Truby L, Ando M, et al. VA-ECMO for cardiogenic shock in the contemporary era of heart transplantation: which patients should be urgently transplanted? Clin Transplant. 2018; 32:e13356. [DOI] [PubMed] [Google Scholar]


