Abstract
Improving understanding of the prognostic factors associated with death resulting from sepsis in obstetric patients is essential to allow management to be optimized. This retrospective cohort study aimed to determine the risk factors for death in patients with sepsis admitted to the obstetric intensive care unit of a tertiary teaching hospital in northeastern Brazil between April 2012 and April 2016.
The clinical, obstetric, and laboratory data of the sepsis patients, as well as data on their final outcome, were collected. A significance level of 5% was adopted. Risk factors for death in patients with sepsis were evaluated in a multivariate analysis.
During the period analyzed, 155 patients with sepsis were identified and included in the study, representing 5.2% of all obstetric intensive care unit (ICU) admissions. Of these, 14.2% (n = 22) died. The risk factors for death were septic shock at the time of hospitalization (relative risk [RR] = 3.45; 95% confidence interval [CI]: 1.64–7.25), need for vasopressors during hospitalization (RR = 17.32; 95% CI: 4.20–71.36), lactate levels >2 mmol/L at the time of diagnosis (RR = 4.60; 95% CI: 1.05–20.07), and sequential organ failure assessment score >2 at the time of diagnosis (RR = 5.97; 95% CI: 1.82–19.94). Following multiple logistic regression analysis, only the need for vasopressors during hospitalization remained as a risk factor associated with death (odds ratio [OR] = 26.38; 95% CI: 5.87–118.51).
The need for vasopressors during hospitalization is associated with death in obstetric patients with sepsis.
Keywords: mortality, pregnancy, sepsis
1. Introduction
Sepsis, defined as life-threatening organ dysfunction caused by deregulated host response to infection, is currently considered one of the major causes of morbidity and mortality in the world.[1] According to the World Health Organization, it is the third leading cause of direct maternal death worldwide. In Latin America and the Caribbean, it corresponds to 8.3% of all deaths.[2]
In the United Kingdom, the incidence of severe sepsis in 2011 to 2012 was 0.47 per 10,000 live births. Of these, 20% went on to develop septic shock.[3] A study conducted in 2015 in the United States of America (USA) reported an incidence of maternal sepsis of 29.4 per 100,000 births, with a mortality rate of 4.4 per 100 live births.[4]
Infection is the third leading cause of direct maternal death in Brazil, corresponding to 5.2% of cases in 2016 (provisional data up to May 2017).[5] A Brazilian study, carried out in the city of Recife in 2006, reported that infection accounted for 5% of the admissions to an obstetric intensive care unit (ICU).[6]
Several studies have identified risk factors associated with a diagnosis of sepsis in the obstetric population; however, few have addressed how these patients should be treated and the factors associated with death.[7,8] A population-based cohort study conducted in the USA showed that the risk of maternal death was higher in patients with human immunodeficiency virus (HIV) infection. The risk of mortality was also high in patients with a history of cancer, chronic liver disease, and drug use, those without health insurance, patients with multiple organ failure, and those requiring mechanical ventilation or hemodialysis.[9] Another study conducted in the United Kingdom identified the presence of comorbidities, multiparity, absence of antibiotic use, and unemployment as being the principal factors associated with death.[7,9]
Despite its importance, few studies have been conducted on obstetric patients with sepsis, with most reports originating from the USA and Europe. In view of the aforementioned factors, it is essential to gain greater understanding regarding the factors associated with death resulting from sepsis in obstetric patients in order to identify prognostic factors and optimize management.
Given the scarcity of studies conducted for this purpose and in view of the high rate of sepsis and mortality in this geographical region, the present study was carried out to determine the factors related to death in patients with sepsis admitted to an obstetric ICU.
2. Methods
This study was conducted in the maternity unit of the Instituto de Medicina Integral Prof. Fernando Figueira (IMIP), in the city of Recife in northeastern Brazil. IMIP is a tertiary hospital, equipped with the only obstetric ICU in the state. An average of 6000 deliveries take place in the hospital annually, with the majority being high-risk patients. All patients receive care within Brazil's public health system.
This was a retrospective cohort study that included all patients admitted to the obstetric ICU between August 2012 and April 2016 whose discharge diagnosis was recorded as “sepsis” in the ICU's discharge database. The records were reviewed to confirm diagnosis according to the criteria established at the 2001 Sepsis Definitions Conference (Sepsis-2).[10] Cases in which the diagnosis of sepsis could not be confirmed following the review of the medical records were excluded from the study, including cases for which the relevant records had been misplaced and records with insufficient data to permit the analysis to be performed.
After the first 50 patients had been selected, sample size was calculated using OpenEpi, version 3.01. (www.OpenEpi.com) Considering a mortality risk of 36% for patients admitted with septic shock compared with 14% for those admitted without septic shock, a total of 122 patients would be required to demonstrate statistically significant differences between the 2 groups, for a power of 80% and a 95% confidence level. The sample was increased by 20% to compensate for any possible losses or exclusions, resulting in a total of 147 patients. Indeed, 166 patients with a diagnosis of sepsis were found in the obstetric ICU database, hence this was the number of medical records considered for inclusion.
Clinical, epidemiological, and laboratory data, as well as data regarding outcome, were extracted retrospectively from charts and collected using a specific standardized form. The dependent variable was maternal death. The independent variables consisted of: maternal age, parity, immediate origin (home or transfer from another hospital), origin (metropolitan region or another region within the state), type of delivery, comorbidities (hypertension, diabetes, chronic kidney disease, chronic liver disease, heart disease, lupus erythematosus, HIV infection, sickle cell disease, and others), obstetric complications (gestational diabetes, preterm labor, premature rupture of membranes, hemorrhage, and others), lifestyle (smoking, alcoholism, and use of illicit drugs), sepsis at admission (according to the criteria established at the 2001 International Sepsis Definitions Conference),[10] need for vasopressors at hospital admission, site of infection, sequential organ failure assessment (SOFA) score at the time of sepsis diagnosis, obtained by evaluating clinical and laboratory criteria that enable organ systems to be monitored,[1] serum lactate at the time of sepsis diagnosis (verified from an arterial blood gas analysis and considered high if >2 mmol/L, which is indicative of tissue hypoxia, in accordance with the new criteria of sepsis implemented in 2016 and known as “Sepsis-3”),[1] the time of initiation of antibiotic therapy (administration of antibiotics within an hour of diagnosis of sepsis, which included those patients who were already receiving antibiotics prior to diagnosis, or over an hour after diagnosis), adequate venous hydration (either achieved by fluid replacement with crystalloids, at least 30 mL/kg in 3 hours, when signs of hypoperfusion were present, or when the patient had maintained normal hydration, thus requiring no intervention),[11] and need for vasopressors during hospitalization (for all patients at admission and/or during hospitalization for whom the use of vasopressors was indicated).
Statistical analysis was performed using Epi Info, version 3.5.1 (Atlanta, GA). Frequency distribution measures with 95% confidence intervals (95% CI) were calculated for the categorical variables. Quantitative values were presented as means and standard deviations (SD). Contingency tables were then prepared to determine the association between the independent variables (predictive factors) and death, and relative risk (RR) and 95% CI were calculated. A significance level of 5% was adopted. All P-values were 2-tailed. A standard risk of 1.0 was assigned to the reference category.
Subsequently, a hierarchical logistic regression analysis was performed. Briefly, the variables were divided into blocks according to their proximity to the outcome. In each block, variables that remained associated with the result at a significance level of 20% were selected and reanalyzed down to the variables that remained associated with the result at a significance level of 5%. The resulting variables from each block were then evaluated in a new regression analysis where the variables that remained significantly associated with the result at a 5% significance level were selected to calculate the adjusted mortality risk.
IMIP's internal review board approved the study protocol (CAAE 49815615.9.0000.5201). The need for informed consent was waived based on the retrospective nature of the study.
3. Results
Of all the patients admitted to the obstetric ICU between April 2012 and April 2016 (n = 3004), 166 were diagnosed with sepsis at some time during hospitalization. Of these, 3 were excluded because a review of the data failed to confirm their diagnosis of sepsis. Three incomplete medical records were excluded so as not to hamper analysis and in 5 cases the relevant medical records were missing. The remaining 155 patients, representing 5.1% of all admissions to the ICU, were included in the analysis.
Twenty-two of the patients who developed sepsis died (14.2%) (Fig. 1). The mean age of the patients who died was 27.1 years (SD = 6.8), significantly higher than the mean age of those who survived (23.6 years, SD = 6.6) (P = .02). In the bivariate analysis, however, when the extremes of age (<20 years or ≥35 years) were analyzed, no association was found between age and a risk of death. Furthermore, the distal variables parity, schooling, and origin were not associated with a risk of death (Table 1). Of the intermediate variables, in the bivariate analysis only urinary infection was identified as a protective factor against death in patients with sepsis (RR = 0.26, 95% CI = 0.08–0.85, P = .009) (Table 2). Of the patients who died, 45.5% had at least one obstetric complication (Table 2). At least one comorbidity was observed in 59.3% of all patients and in 27.3% of those who died (Table 2).
Figure 1.
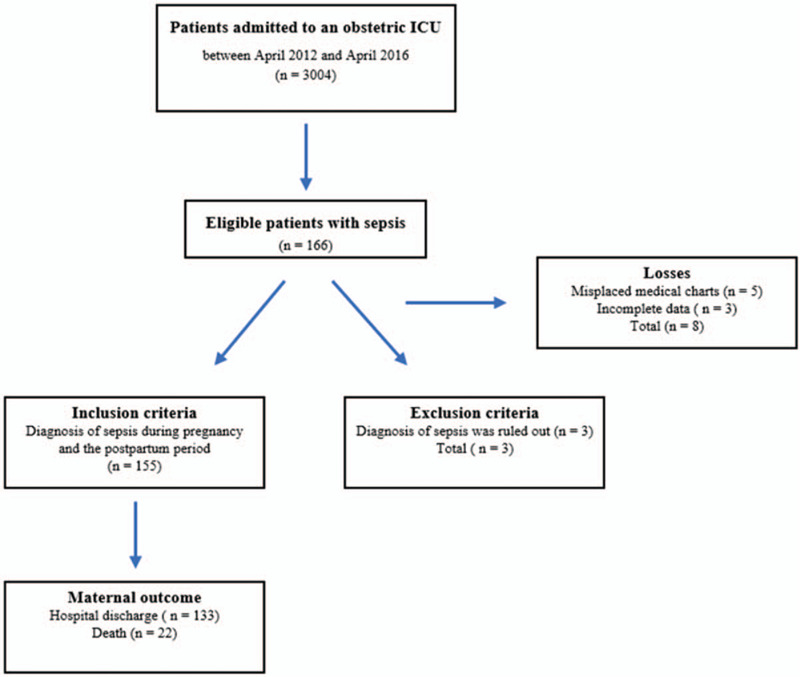
Flowchart showing the recruitment of patients.
Table 1.
Distal factors associated with death in obstetric patients with sepsis.
| Death | |||||||
| Characteristics | Yes | No | RR | 95% CI | P-value | ||
| Age (Mean/SD) | 27.1 | 6.8 | 23.6 | 6.6 | – | – | .02 |
| Age <20 years (n/%) | 3 | 13.6 | 40 | 30.1 | 0.41 | 0.12–1.31 | .08∗ |
| Age >35 years (n/%) | 2 | 9.1 | 9 | 6.8 | 1.30 | 0.35–4.89 | .48∗ |
| Number of pregnancies (Median/IQR) | 2 | 1–3 | 2 | 1–3 | – | – | .49 |
| Parity (Median/IQR) | 1 | 1–2 | 0 | 1–2 | – | – | .12 |
| Nulliparous (n/%) | 5 | 23.8 | 51 | 39.8 | 0.51 | 0.20–1.33 | .16 |
| Multiparous (n/%) | 5 | 23.8 | 17 | 13.3 | 1.80 | 0.73–4.42 | .20 |
| Schooling <8 years (n/%) | 2 | 9.1 | 32 | 24.1 | 0.35 | 0.08–1.44 | .09∗ |
| Resident of a city in Pernambuco other than the state capital or resident of another Brazilian state (n/%) | 10 | 45.5 | 53 | 39.8 | 1.21 | 0.56–2.64 | .62 |
Recife, Pernambuco, Brazil, 2018.
CI = confidence interval, IQR = interquartile range, RR = relative risk, SD = standard deviation.
Fisher exact test.
Table 2.
Intermediate factors associated with death in obstetric patients with sepsis.
| Death (n/%) | |||||||
| Characteristics | Yes (n = 22) | No (n = 133) | RR | 95% CI | P-value | ||
| Transferred from another hospital | 16 | 72.4 | 81 | 60.9 | 1.59 | 0.66–3.84 | .28 |
| Pregnant at the time of sepsis | 11 | 50.0 | 92 | 69.2 | 0.50 | 0.23–1.08 | .07 |
| Caesarean section∗ | 6 | 30.0 | 41 | 54.7 | 0.43 | 0.18–1.04 | .050 |
| Obstetric complications | 10 | 45.5 | 50 | 37.6 | 1.31 | 0.60–2.86 | .48 |
| Comorbidities | 6 | 27.3 | 32 | 24.1 | 1.15 | 0.48–2.73 | .75 |
| Lifestyle factors (smoking + use of illicit drugs) | 1 | 4.5 | 3 | 2.3 | 1.79 | 0.31–10.27 | .46∗∗ |
| Respiratory infection | 7 | 31.8 | 32 | 24.1 | 1.38 | 0.61–3.15 | .43 |
| Uterine infection | 8 | 36.4 | 40 | 30.1 | 1.27 | 0.57–2.83 | .55 |
| Urinary infection | 3 | 13.6 | 55 | 41.4 | 0.26 | 0.08–0.85 | .009∗∗ |
| Other infections | 4 | 18.2 | 14 | 10.5 | 1.69 | 0.64–4.44 | .23∗∗ |
Recife, Pernambuco, Brazil, 2018. CI = confidence interval, RR = relative risk.
Analysis performed for the 95 patients who had the pregnancy interrupted shortly before admission or during hospitalization for sepsis. Forty-five women were discharged from hospital still pregnant, following an episode of sepsis.
Fisher exact test.
Antibiotic therapy was initiated within an hour of the diagnosis of sepsis in 45.6% of the cases, although 48% were already receiving antibiotics even before the diagnosis of sepsis (data not shown in table). Of the patients who died, there was a delay in the initiation of antibiotic therapy (begun over an hour after diagnosis) in 59.1% of cases (Table 3). The mean lactate level at diagnosis in the patients who survived was 2.5 mmol/L (SD = 2.1) compared with 6.2 mmol/L (SD = 5.2) in those who died, a difference that was statistically significant (P = .05) (Table 3). The median SOFA score at the time of diagnosis was 6 (interquartile range [IQR] 4–9.5) in the patients who died and 2 (IQR 0–5) in those who survived, a difference that was also statistically significant (P < .01). Volume resuscitation was considered adequate in 79.7% of cases; however, there was no difference between the groups in relation to this variable (Table 3). Of the proximal variables, septic shock at admission (RR = 3.45, 95% CI = 1.64–7.25), the need for vasopressor drugs during hospitalization (RR = 17.32, 95% CI = 4.20–71.36), lactate levels >2 at the time of diagnosis (RR = 4.60, 95% CI = 1.05–20.07), and SOFA score >2 at diagnosis (RR = 5.97, 95% CI = 1.82–19.94), were all associated with a greater risk of death (Table 3). After multiple logistic regression, only the need for vasopressors during hospitalization remained a risk factor associated with death (odds ratio [OR] = 26.38; 95% CI = 5.87–118.51).
Table 3.
Proximal factors associated with death in obstetric patients with sepsis.
| Death | |||||||
| Characteristics | Yes | No | RR | 95% CI | P-value | ||
| Septic shock at admission (n/%) | 8 | 36.4 | 14 | 10.5 | 3.45 | 1.64–7.25 | .001 |
| Need for vasopressors during hospitalization (n/%) | 20 | 90.9 | 36 | 27.5 | 17.32 | 4.20–71.36 | <.01∗ |
| Lactate levels at admission (Mean/SD) | 6.2 | 5.2 | 2.5 | 2.1 | – | – | <.01∗∗ |
| Lactate >2 at admission (n/%)a | 9 | 81.8 | 33 | 44.6 | 4.60 | 1.05–20.07 | .02∗ |
| SOFA at admission (Median/IQR) | 6 | 4–9.5 | 2 | 0–5 | – | – | <.01 |
| SOFA >2 at admission (n/%) | 17 | 85.0 | 57 | 43.2 | 5.97 | 1.82–19.94 | <.01∗ |
| Inadequate hydration (n/%) | 6 | 27.3 | 24 | 18.0 | 1.56 | 0.66–3.65 | .31 |
| Time until the initiation of antibiotics >1 h (n/%) | 13 | 59.1 | 67 | 50.4 | 1.35 | 0.61–2.98 | .44 |
Recife, Pernambuco, Brazil, 2018. CI = confidence interval, RR = relative risk, SD = standard deviation.
Data available for 85 patients only.
Fisher exact test.
Mann–Whitney test.
4. Discussion
Sepsis is a common cause of maternal admission to the ICU. In a previous study conducted in the same unit, sepsis accounted for 2.8% of admissions.[12] In the present study, in 3004 ICU admissions, 155 patients were diagnosed with sepsis (5.1%), a lower rate than that found in the United Kingdom, where severe sepsis accounted for 14.4% of admissions,[8] and in the Netherlands, where sepsis corresponded to 9.9% of maternal ICU admissions.[13] This lower rate can be explained by the fact that this is an exclusively obstetric ICU, with high rates of admission for hypertensive syndromes, a common complication of pregnancy and the major cause of maternal death in Brazil,[5] a situation that proportionately reduces the importance of sepsis as a cause of admission to ICU.
Twenty-two sepsis patients died (14.2%), resulting in a higher mortality rate compared with those reported from developed countries such as the United Kingdom (1.4%),[3] Ireland (no deaths between 2005 and 2012),[14] and the Netherlands (7.7%)[15];and similar to rates reported from African countries such as Malawi (16.4%)[16] and South Africa (9.5%).[17] The high death rate can be explained by the fact that the obstetric ICU at IMIP is the only obstetric ICU in the region, receiving patients referred from other units. Consequently, there is a natural tendency to receive cases of greater severity. In addition, differences in pre-hospital management and the characteristics of the patients may also explain this difference. However, the study design (a retrospective analysis of medical records) prevented a more in-depth analysis of these variables from being conducted.
Following logistic regression analysis, the only factor that remained associated with death was the need for vasopressors during hospitalization. Lactate level and the SOFA score, which in the bivariate analysis were factors associated with a risk of death, did not remain associated with death following logistic regression analysis. These factors, in addition to septic shock at admission, may have been confounded by the need for vasopressors and there is probably collinearity with the need for these drugs during hospitalization, thus resulting in the elimination of other associated factors.
Septic shock is a marker of severity in sepsis patients and is associated with high mortality.[1,18,19] Until recently, septic shock was defined as the need for vasopressors after adequate volume resuscitation.[10] More recently, lactate level was added to the diagnostic criteria, and septic shock is now defined as the need for vasopressors after adequate volume resuscitation and hyperlactatemia (>2 mmol/L).[1] This has also led to the definition of situations such as cryptic shock, which is a high-risk situation in which tissue hypoperfusion and organ dysfunction can lead to an increase in the SOFA score, without the initially expected hypotension; however, if not treated properly, it can also evolve to hypotension.[20–22]
These concepts may explain why the need for vasopressors remained associated with mortality in this study, while the other variables were excluded following logistic regression analysis. This may be the result of an overlap among these situations, since they frequently occur simultaneously. Patients who have organ dysfunction also have elevated lactate levels and ultimately need vasopressors.
Of the patients who died, 36.4% had septic shock at admission and 90.9% received vasopressor drugs during hospitalization. Both situations were significantly associated with death in the bivariate analysis; however, only the need for vasopressors in hospital remained associated with death in the multivariate analysis. It is possible that there may be an overlap between these 2 groups, since patients admitted with septic shock and who needed vasopressors, but who were not treated with these drugs, are also included in the second group, in addition to those who developed septic shock after admission.
Even when analyzed together, the presence of comorbidities was not associated with death. This finding contradicts the results of some other studies that have reported an association, particularly in cases of immunosuppression, malignancy, and chronic liver disease.[7,9] In the present study, some comorbidities (such as HIV infection, lupus erythematosus, and chronic kidney disease) were rare or absent, and the small sample size may have been responsible for this finding.
The main source of infection was urinary tract infection (37.4%), unlike other studies in which genital and respiratory infections were those most commonly reported.[3,8,14,23] In spite of the high rate of infection, mortality in these cases was the lowest of all the different causes of sepsis (13.6%). This finding is consistent with results reported from a population-based study conducted in the USA in which urinary tract infection was considered a protective factor against death (adjusted OR = 0.3).[9] Although more frequent, this infection is possibly easier to treat, and the bacteria involved are less resistant, thus responding more quickly to treatment and being less likely to result in death.
When evaluating the approach adopted for treatment, results show that there was a delay of more than an hour in initiating antibiotic therapy in 49% of patients. In the group of patients in whom antibiotic therapy was initiated within an hour, around half (48%) were already using antibiotics even before the diagnosis of sepsis. Although no association was found between the initiation of antibiotic treatment and death, this is a cause for concern, since protocols for the treatment of sepsis stress the need to initiate antibiotic therapy within the first hour. Furthermore, the need for multidisciplinary team training is emphasized to ensure that medications can be administered promptly upon diagnosis of the disease.[11,24]
In a population-based study conducted in the United Kingdom, which evaluated factors associated with the progression from pregnancy-associated severe sepsis to death, in 19% of the patients who died, antibiotics were started at least 24 hours after diagnosis, with 14% of these patients never having received antibiotics (half of them died at home or upon arrival at the hospital). In the same study, antibiotics were administered in the first hour in only 33% of patients.[7] The results of that study cannot be compared with those of the present study, since the present study was conducted in a single center; however, it would appear that even in developed countries difficulties exist in the implementation of protocols.
Mean lactate level at admission was 2.5 mmol/L in the group of patients who survived and 6.2 mmol/L in the group of patients who died. Of those who died, lactate was >2 mmol/L in 81.8% of cases, compared with 72% of cases in a study conducted in the United Kingdom.[7] Hyperlactatemia is associated with tissue hypoperfusion, β-adrenergic stimulation, or other causes, and it is usually associated with unfavorable outcomes.[11,25] A mean lactate level on admission of around 6 mmol/L in the group of women who died suggests a much higher severity on arrival at the ICU, increasing the risk of death in these cases.
Since 2016, the SOFA score has been applied in cases of sepsis. Over the data collection period, this criterion was still not being used for diagnosis, but was included in the analysis as a means of assessing organ and system failure. The median SOFA score at admission was 2 (IQR 0–5) for the patients who survived and 6 (IQR 4–9.5) for those who died, showing that even if the new criteria were applied, there would probably be no significant change in the diagnosis. Of the patients who died, the SOFA score at admission was >2 in 85% of cases, confirming the presence of organ failure, and there was a statistically significant association with death. A similar result has been reported from other studies in which organ failure was directly associated with death.[9]
This study has some limitations, since the analysis was retrospective and referred to a single center. Because this study was based on the review of medical records, the fact that there were some missing data may raise doubts about the chronology of certain events, preventing a more in-depth analysis from being made. Due to time limitations and the uncommonness of the disease, only a limited number of patients could be included, thus reducing the statistical power of the study. Therefore, we cannot exclude the possibility that other potential factors associated with death could have been present but could not be detected with this study design. Nevertheless, the number of deaths that were observed over the time period of this study was comparable to those reported in large population studies. The fact that the survey was carried out in the only obstetric ICU in the region means that the relative proportion of severe cases admitted to the ICU was high. However, we believe that this sample accurately represents the general population, reflecting the local situation and ensuring good internal validation. Other strengths of the study include the point-to-point assessment of sepsis management and the population included in the study (pregnant women). Studies with this population and with this type of presentation are rare. The issues that remain to be clarified will require larger, preferably multicenter, prospective studies.
5. Conclusions
The results of the present study show that mortality due to sepsis in the obstetric population was high in this obstetric ICU in Brazil. The need for vasopressors during hospitalization was the main factor associated with death in obstetric patients with sepsis.
Acknowledgments
The authors are grateful to the Instituto de Medicina Integral Prof. Fernando Figueira for supporting the research.
Author contributions
Conceptualization: Ana Maria Coêlho Holanda, Melania Maria Ramos de Amorim, Leila Katz.
Formal analysis: Ana Maria Coêlho Holanda, Melania Maria Ramos de Amorim, Leila Katz.
Investigation: Sammyle Maria Barros Bezerra, Larissa Miranda Silva Aschoff.
Methodology: Ana Maria Coêlho Holanda, Melania Maria Ramos de Amorim, Leila Katz.
Writing – original draft: Ana Maria Coêlho Holanda, Melania Maria Ramos de Amorim, Leila Katz.
Writing – review & editing: Ana Maria Coêlho Holanda, Melania Maria Ramos de Amorim, Sammyle Maria Barros Bezerra, Larissa Miranda Silva Aschoff, Leila Katz.
Footnotes
Abbreviations: CI = confidence interval, HIV = human immunodeficiency virus, ICU = intensive care unit, IMIP = Instituto de Medicina Integral Prof. Fernando Figueira, IQR = interquartile range, OR = odds ratio, RR = relative risk, SD = standard deviation, SOFA score = sequential organ failure assessment score.
How to cite this article: Holanda AM, Amorim MM, Bezerra SM, Aschoff LM, Katz L. Risk factors for death in patients with sepsis admitted to an obstetric intensive care unit: a cohort study. Medicine. 2020;99:50(e23566).
Ethical aspects: The project was approved by IMIP's internal review board (CAAE 49815615.9.0000.5201). The need for informed consent was waived based on the retrospective nature of the study.
Funding: None.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Singer M, Deutschman CS, Seymour CW, et al. The Third International consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016;315:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health 2014;2:e323–33. [DOI] [PubMed] [Google Scholar]
- [3].Acosta CD, Kurinczuk JJ, Lucas DN, et al. Severe Maternal Sepsis in the UK, 2011–2012: A National Case-Control Study. PLoS Med 2014;11:e1001672. 10.1371/journal.pmed.1001672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Al-Ostad G, Kezouh A, Spence AR, et al. Incidence and risk factors of sepsis mortality in labor, delivery and after birth: population-based study in the USA. J Obstet Gynaecol Res 2015;41:1201–6. [DOI] [PubMed] [Google Scholar]
- [5].Brazilian mortality information system (SIM). Maternal mortality according to cause. [Sistema de informações sobre mortalidade (SIM). Mortalidade materna por causa]; 2017. Available at: https://mortalidade.inca.gov.br/MortalidadeWeb/. Accessed on October 15, 2017. [Google Scholar]
- [6].Amorim MMR, Katz L, Ávila MB, et al. [Admission profile in an obstetrics intensive care unit in a maternity hospital of Brazil]. Rev Bras Saude Mater Infant 2006;6: suppl: s55–62. [in Portuguese]. [Google Scholar]
- [7].Mohamed-Ahmed O, Nair M, Acosta C, et al. Progression from severe sepsis in pregnancy to death: a UK population-based case-control analysis. BJOG 2015;122:1506–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Acosta CD, Harrison DA, Rowan K, et al. Maternal morbidity and mortality from severe sepsis: a national cohort study. BMJ Open 2016;6:e012323.doi: 10.1136/bmjopen-2016-012323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Oud L, Watkins P. Evolving trends in the epidemiology, resource utilization, and outcomes of pregnancy-associated severe sepsis: a population-based cohort study. J Clin Med Res 2015;7:400–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Levy MM, Fink MP, Marshall JC, et al. International sepsis definitions conference. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med 2003;29:530–8. [DOI] [PubMed] [Google Scholar]
- [11].Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock: 2016. Crit Care Med 2017;45:486–552. [DOI] [PubMed] [Google Scholar]
- [12].Martins Filho ED, Santos AC, Rodrigues Junior RST, et al. [Epidemiological and clinical profile of patients admitted at an obstetrical ICU with diagnosis of puerperal pelvic sepsis in Northeastern Brazil]. Rev Bras Saude Matern Infant 2010;10:469–75. [in Portuguese]. [Google Scholar]
- [13].Zwart JJ, Dupuis JR, Richters A, et al. Obstetric intensive care unit admission: a 2-year nationwide population-based cohort study. Intensive Care Med 2010;36:256–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Knowles SJ, O'Sullivan NP, Meenan AM, et al. Maternal sepsis incidence, aetiology and outcome for mother and fetus: a prospective study. BJOG 2015;122:663–71. [DOI] [PubMed] [Google Scholar]
- [15].Kramer HM, Schutte JM, Zwart JJ, et al. Maternal mortality and severe morbidity from sepsis in the Netherlands. Acta Obstet Gynecol Scand 2009;88:647–53. [DOI] [PubMed] [Google Scholar]
- [16].van den Akker T, de Vroome S, Mwagomba B, et al. Peripartum infections and associated maternal mortality in rural Malawi. Obstet Gynecol 2011;118(2 pt 1):266–72. [DOI] [PubMed] [Google Scholar]
- [17].National Committee for the Confidential Enquiries into Maternal Deaths (NCCEMD). Saving Mothers: Fourth Report on Confidential Enquiries into Maternal Deaths in South Africa 2005-2007. Health (San Francisco). 2008. [Google Scholar]
- [18].Song JE, Kim MH, Jeong WY, et al. Mortality risk factors for patients with septic shock after implementation of the surviving sepsis campaign bundles. Infect Chemother 2016;48:199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med 2013;369:840–51. [DOI] [PubMed] [Google Scholar]
- [20].Arnold RC, Sherwin R, Shapiro NI, et al. Emergency Medicine Shock Research Network (EM Shock Net) Investigators. Multicenter observational study of the development of progressive organ dysfunction and therapeutic interventions in normotensive sepsis patients in the emergency department. Acad Emerg Med 2013;20:433–40. [DOI] [PubMed] [Google Scholar]
- [21].Javed A, Guirgis FW, Sterling SA, et al. Clinical predictors of early death from sepsis. J Crit Care 2017;42:30–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ranzani OT, Monteiro MB, Ferreira EM, et al. Reclassifying the spectrum of septic patients using lactate: severe sepsis, cryptic shock, vasoplegic shock and dysoxic shock. Rev Bras Ter Intensiva 2013;25:270–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bauer ME, Lorenz RP, Bauer ST, et al. Maternal deaths due to sepsis in the state of Michigan, 1999-2006. Obstet Gynecol 2015;126:747–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Seymour CW, Kahn JM, Martin-Gill C, et al. Delays from first medical contact to antibiotic administration for sepsis. Crit Care Med 2017;45:759–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Albright CM, Ali TN, Lopes V, et al. Lactic acid measurement to identify risk of morbidity from sepsis in pregnancy. Am J Perinatol 2015;32:481–6. [DOI] [PubMed] [Google Scholar]


