Abstract
Background
Coronavirus disease 2019 (COVID-19) has now brought major challenges to public health and the economy globally since December 2019, which requires effective treatment and prevention strategies to adapt to the impact of the pandemic. We, therefore, explored the prognostic factors for patients with COVID-19 and the contribution of immunomodulatory therapy on COVID-19 outcome.
Methods
From 1 February to 16 March 2020, consecutive cases with COVID-19 were analyzed in the West Campus of Wuhan Union Hospital, a tertiary care center that is designated to care for patients with COVID-19 in Wuhan, China. The observation was based on follow-up until in-hospital death or discharge. Logistic regressions were performed for prognostic factors associated with in-hospital death. Furthermore, a propensity score-matched analysis was done using a multivariable logistic regression model to analyze the contributions of multiple treatments on COVID-19 death.
Results
Three hundred and seventeen patients with COVID-19 were enrolled, of whom 269 were discharged and 48 died in hospital. After propensity score matching based on age, gender, symptoms and comorbidities, multivariable logistic regression was performed with the adjustment of other variables that were significant risk factors in the univariate regression. Treatments with glucocorticoids, immunoglobulin, thymosin, and ammonium glycyrrhizinate were significantly associated with a higher rate of COVID-19 death.
Conclusions
For in-hospital patients with COVID-19 of all severity levels, a high risk for fatal outcome was observed in those treated with glucocorticoids, immunoglobulin, thymosin, and ammonium glycyrrhizinate. The results of this study do not support immunomodulatory therapy in patients admitted to the hospital with COVID-19. Further prospective studies are essential to clarify our findings, especially for non-critically ill patients.
Keywords: COVID-19, immunomodulatory, mortality, critical medicine, prognostic factors
Introduction
The outbreak of coronavirus disease 2019 (COVID-19) occurred in Wuhan, China, in late December 20191. It is infected by novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is highly contagious and spreads by person-to-person transmission around the world. The World Health Organization (WHO) defined COVID-19 as a pandemic since March, 20202. As of 31 October 2020, COVID-19 has spread rapidly to over 236 countries and territories around the world and responsible for 45,518,009 confirmed cases of COVID-19, including 1,186,480 deaths, reported to WHO3. The impact on human health, the normal functioning of the global economy and society is unprecedented.
Despite the high number of cases reported globally, estimates of severity and fatality rate of the disease still remain very uncertain. Based on early statistics in mainland China, approximately 5% of COVID-19 patients become critically ill and 2.3% be dead (72,314 cases, updated through 11 February 2020)4. Most severe COVID-19 cases presented with acute respiratory distress syndrome (ARDS), multi-organ dysfunction and even death. Among the critical cases, the case-fatality rate is almost half [49.0% (1023 of 2087)] 4. Therefore, early predictors are crucial for the prediction and control of poor outcomes, and treatments are urgently needed to prevent deaths from COVID-19.
Since no effective vaccine or antiviral medication are available, though most patients are mild or moderate, there are still many severe and dead patients during the clinical course. Although some epidemiological studies have described the characteristics, treatment and clinical course of COVID-19 patients, the risk and protective factors for adverse outcomes are limited and inconsistent, remains to be elucidated. Growing evidence supports that systemic corticosteroids may decrease the mortality in critically ill patients with COVID-195. To our knowledge, there are rare previous studies exploring the association of multiple immunomodulatory treatments with COVID-19 outcomes among patients with different disease severity, different characteristics, past medical history, laboratory investigations, and complications.
Herein, to address this gap in the research, we conducted a retrospective propensity score-matched study to (1) investigate the predictive value of characteristics, past medical history, laboratory investigations, and complications for COVID-19 death, and (2) estimate the efficacy of multiple immunomodulatory treatments including glucocorticoids, immunoglobulin, thymosin, and ammonium glycyrrhizinate for COVID-19.
Methods
Study design and participants
This retrospective and observational, single-center study was conducted at the West Campus of Union Hospital, which was one of the main designated hospitals for treatments of patients with COVID-19 in Wuhan, China. We recruited 317 patients admitted from 1 February to 16 March 2020 with laboratory-confirmed SARS-CoV-2 infection. All included patients were diagnosed with COVID-19 according to WHO interim guidance6, and observed for follow-up until in-hospital death and discharge.
After the identifications of the clinical outcome, we classified the patients into the survival group and dead group. The associations between multiple potential prognostic factors and the fatal outcomes were explored in univariable logistic analyses, including demographic information, chronic medical histories, clinical symptoms, complications, laboratory information, chest computed tomographic (CT) scans imaging features, and treatment information. Then propensity score-matching was done based on demographic information, chronic medical histories, and clinical symptoms. Multivariable logistic regression models were used to analyze the impact of multiple treatments on in-hospital death, with the adjustment for other variables that were statistically significant in the univariable logistic analyses.
The Ethics Committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology approved this study. Written informed consent was waived by the same committee, as only aggregated non-identifiable data for patients’ personal information were used.
Data collection
We extracted information of electronic clinical medical records, nursing records, laboratory information, and radiological examinations for all patients with COVID-19, using a modified version of case record form from the International Severe Acute Respiratory and Emerging Infection Consortium7. All data were collected and checked by two physicians (XY and YW). Any disagreements or uncertain records were resolved by direct contact with a third researcher (WX) or involved health-care providers.
Prognostic factors and outcomes
The extracted data were then tested to determine whether they were potential factors associated with COVID-19 death. The factors including (1) demographic information: age (≥65 vs <65 years), gender (male vs female); (2) chronic medical histories: chronic pulmonary disease, coronary heart disease, hypertensive heart disease, stroke, and diabetes; (3) symptoms from onset to hospital admission: fever, coughing and sputum, shortness of breath, and gastrointestinal symptoms; (4) complications: acute cardiac injury, acute kidney injury, acute liver injury. Acute cardiac injury was defined as the hypersensitive cardiac troponin I (hsTNI) above the upper limit of the reference range (>28 pg/mL), or new abnormalities were shown in electrocardiography and echocardiography according to the previous study8,9. Acute kidney injury was diagnosed on the basis of serum creatinine according to the KDIGO clinical practice guidelines10. Acute liver injury was identified by the serum levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT) and AST/ALT (ALT > 40 U/L; AST > 40 U/L; AST/ALT > 1); (5) laboratory information: white blood cell count, neutrophil count/lymphocyte count ratio (NLR), platelet count, d-dimer, high-sensitivity cardiac troponin I, lactate dehydrogenase (LDH), alanine aminotransferase (ALT), Aspartate aminotransferase (AST), serum creatinine, blood urea nitrogen; (6) CT scans image features: unilateral lung infiltration, bilateral pulmonary infiltration, multiple mottling and ground-glass opacity, and (7) treatment information: antivirals, glucocorticoids, immunoglobulin, thymosin, ammonium glycyrrhizinate, atomization inhalation, respiratory support like nasal cannula or mask, noninvasive mechanical ventilation (NIMV), and invasive mechanical ventilation (IMV).
The primary outcome was in-hospital mortality. Compared with the dead group, the cases in the survival group were the patients who were discharged till the end date of follow-up. The duration from hospital admission to release from hospital and death were also recorded.
Statistical analysis
Continuous data are shown as median [percentile 25 to percentile 75 (P25–P75)], and categorical data were presented as and n (%), respectively. We used the Mann–Whitney U test for continuous variables, χ2 test, or Fisher’s exact test for categorical variables to compare differences between survivors and non-survivors where appropriate.
Univariate logistic regression models were used to explore the variables associated with in-hospital death and calculated the odds ratio (OR). For propensity score-matched analysis, the matching variables would be demographic information, symptoms, and chronic medical histories if their between-group differences were statistically significant. Pairs of patients in the survival group and the dead group were derived using 5:1 greedy nearest neighbor matching within one-quarter of the standard deviation of the estimated propensity. Aimed to avoid overfitting in the multivariate model, we chose four variables to adjust the OR of each treatment in the multivariable analysis in light of the number of deaths (less than 50) in our study11. All tests were two-sided with significance set at α less than 0·05. The SAS 9.4 software (SAS Institute, Cary, NC) was applied for all analyses.
Results
Of 317 COVID-19 patients, 269 patients were discharged and 48 died. 169 (53.3%) were male, and 148 (46.7%) were female. 94 (29.7%) patients were beyond 65-years-old. The median age (years) in the dead group was significantly older than that in the survival group (66, P25–P75: 61–71 vs. 57, P25–P75: 47–66 years, p < .001). Higher proportions of male patients (50.9%) and elders (52.1%) were in the dead group (p < .001). 46.4% of patients reported chronic medical histories and more dead patients had the chronic pulmonary disease (16.7% vs. 5.6%, p = .006). Fever was the most common onset of symptoms in both groups (beyond 80%). More patients in the dead group had coughing and sputum (79.2% vs. 62.5%, p = .025), and shortness of breath (62.5% vs. 43.1%, p = .013) compared with survivors. Detailed information for demographic and clinical characteristics of patients with COVID-19 was shown in Table 1.
Table 1.
The demographic and clinical characteristics of patients with COVID-19.
| Total (n = 317) | Survival (n = 269) | Dead (n = 48) | p-Value | |
|---|---|---|---|---|
| Demographic information | ||||
| Age, year | 59 (49–67) | 57 (47–66) | 66 (61–71) | <.001 |
| Age groups | <.001 | |||
| <65 | 223 (70.3) | 200 (74.3) | 23 (47.9) | |
| ≥65 | 94 (29.7) | 69 (25.7) | 25 (52.1) | |
| Sex | .044 | |||
| Female | 148 (46.7) | 16 (33.3) | 132 (49.1) | |
| Male | 169 (53.3) | 32 (66.7) | 137 (50.9) | |
| Chronic medical histories | 147 (46.4) | 121 (45.0) | 26 (54.2) | .24 |
| Chronic pulmonary disease | 23 (7.3) | 15 (5.6) | 8 (16.7) | .006 |
| Coronary heart disease | 24 (7.6) | 21 (7.8) | 3 (6.3) | .707 |
| Hypertensive heart disease | 85 (26.8) | 68 (25.3) | 17 (35.4) | .144 |
| Stroke | 4 (1.3) | 4 (1.5) | 0 (0) | .395 |
| Diabetes | 42 (13.2) | 35 (13.0) | 7 (14.6) | .767 |
| Symptoms | ||||
| Fever | 256 (80.8) | 216 (80.3) | 40 (83.3) | .623 |
| Coughing and sputum | 206 (65.0) | 168 (62.5) | 38 (79.2) | .025 |
| Shortness of breath | 146 (46.1) | 116 (43.1) | 30 (62.5) | .013 |
| Gastrointestinal symptoms | 186 (52.7) | 154 (57.2) | 32 (66.7) | .222 |
Note. Continuous variables were expressed as median (P25–P75); categorical variables were expressed as number (%).
Abbreviation. P25–P75, percentile 25 to percentile 75.
Statistically significant differences between groups are marked in bold.
Table 2 showed the laboratory and CT findings of patients with COVID-19 on admission to the hospital. Of all patients, there were many typically abnormal laboratory findings. The average white blood cell count, ×109/L in the dead group was significantly higher than that in the survivor group (7.3, P25–P75: 5.8–9.7 vs. 5.8, P25–P75: 4.8–7.3, p = .003), as well as NLR (9.0, P25–P75: 4.8–16.0 vs. 3.2, P25–P75: 1.8–6.9, p < .001). The dead group mostly was with d-dimer > 1.5 μg/mL (50.0 vs. 18.2%, p < .001). Significant differences were not found in CT imaging features between the two groups.
Table 2.
The laboratory and CT findings of patients with COVID-19 on admission to hospital.
| Normal range | Total (n = 317) | Survival (n = 269) | Dead (n = 48) | p-Value | |
|---|---|---|---|---|---|
| Laboratory findings | |||||
| White blood cell count, ×109 /L | 3.5–9.5 | 5.9 (4.8–7.7) | 5.8 (4.8–7.3) | 7.3 (5.8–9.7) | .003 |
| <3.5 | 57 (18.0) | 49 (18.2) | 8 (16.7) | .040 | |
| 3.5–9.5 | 222 (70.0) | 193 (71.7) | 29 (60.4) | ||
| >9.5 | 38 (12.0) | 27 (10.0) | 11 (22.9) | ||
| Neutrophil count, ×109/L | 1.8–6.3 | 3.9 (2.8–5.8) | 3.7 (2.7–5.4) | 5.8 (4.0–8.0) | <.001 |
| Lymphocyte count, ×109/L | 1.1–3.2 | 1.2 (0.7–1.8) | 1.3 (0.9–1.9) | 0.6 (0.4–1.2) | <.001 |
| NLR | 3.2 (1.8–6.9) | 2.6 (1.7–5.3) | 9.0 (4.8–16.0) | <.001 | |
| Platelet count, ×109/L | 125–350 | 204 (144–256) | 209 (147–257) | 178 (128–256) | .119 |
| <100 | 32 (10.1) | 25 (9.3) | 7 (14.6) | .262 | |
| D-dimer, μg/mL | 0–0.55 | 0.63 (0.26–1.28) | 0.51 (0.25–1.14) | 1.24 (0.54–6.14) | .034 |
| >1.5 | 73 (23.0) | 49 (18.2) | 24 (50.0) | <.001 | |
| High-sensitivity cardiac troponin I, ng/mL | <26.2 | 7.0 (3.0–15.0) | 5.5 (2.4–12.0) | 29.0 (13.8–32.8) | <.001 |
| >28 | 44 (13.9) | 16 (5.9) | 28 (58.3) | <.001 | |
| Lactate dehydrogenase, U/L | 109–245 | 223 (171–394) | 205 (167–307) | 497 (287–585) | <.001 |
| Alanine aminotransferase, U/L | 5–40 | 29 (21–44) | 28 (21–42) | 39 (29–74) | <.001 |
| Aspartate aminotransferase, U/L | 8–40 | 34 (22–53) | 34 (21–53) | 37 (25–63) | .419 |
| Serum creatinine, μmol/L | 57–111 | 68 (56–81) | 68 (56–80) | 68 (54–89) | .954 |
| Blood urea nitrogen | 2.9–8.2 | 5.1 (3.9–6.6) | 5.0 (3.9–6.5) | 6.0 (4.0–9.3) | .419 |
| CT imaging features | |||||
| Unilateral lung infiltration | 29 (9.1) | 26 (9.7) | 3 (6.3) | .450 | |
| Bilateral pulmonary infiltration | 286 (90.2) | 241 (89.6) | 45 (93.8) | .372 | |
| Multiple mottling and ground-glass opacity | 205 (64.7) | 173 (64.3) | 32 (66.7) | .753 |
Note. Continuous variables were expressed as median (P25–P75); categorical variables were expressed as number (%).
Abbreviations. P25–P75, percentile 25 to percentile 75; NLR, neutrophil count/lymphocyte count ratio.
Statistically significant differences between groups are marked in bold.
Patients in the dead group were more likely to have organ injury including acute cardiac injury (58.3 vs. 5.9%, p < .001), and acute kidney injury (35.4 vs. 11.2%, p < .001). Most patients were treated with antiviral in both groups (beyond 90%). The proportions of patients receiving glucocorticoids, immunoglobulin, thymosin, and ammonium glycyrrhizinate in the dead group were higher than that in the survivor group (detailed information was shown in Table 3).
Table 3.
The treatment measures and complications of patients with COVID-19.
| Total (n = 317) | Survivor (n = 269) | Dead (n = 48) | p-Value | |
|---|---|---|---|---|
| Treatment measures | ||||
| Antivirals | 301 (95.0) | 253 (94.1) | 48 (100) | .083 |
| Glucocorticoids | 101 (31.9) | 61 (22.7) | 40 (83.3) | <.001 |
| Immunoglobulin | 118 (37.2) | 76 (28.3) | 42 (87.5) | <.001 |
| Thymosin | 68 (21.5) | 43 (16.0) | 25 (52.1) | <.001 |
| Ammonium glycyrrhizinate | 46 (14.5) | 32 (11.9) | 14 (29.2) | .003 |
| Atomization inhalation | 71 (22.4) | 62 (23.0) | 9 (18.8) | .511 |
| Respiratory support | ||||
| Nasal cannula or mask | 303 (95.6) | 255 (94.8) | 48 (100) | .106 |
| NIMV | 79 (24.9) | 31 (11.5) | 48 (100) | <.001 |
| IMV | 67 (21.1) | 19 (7.1) | 48 (100) | <.001 |
| Clinical outcomes | ||||
| Cardiac injury | 44 (13.9) | 16 (5.9) | 28 (58.3) | <.001 |
| Kidney injury | 47 (14.8) | 30 (11.2) | 17 (35.4) | <.001 |
| Liver injury | 144 (45.4) | 118 (43.9) | 26 (54.2) | .187 |
| Hospital stay | 22 (13–29) | |||
| Survival day | 19 (10–27) |
Note. Continuous variables were expressed as median (P25–P75); categorical variables were expressed as number (%).
Abbreviations. P25–P75, percentile 25 to percentile 75; NIMV, noninvasive mechanical ventilation; IMV, invasive mechanical ventilation.
Statistically significant differences between groups are marked in bold.
Among the included 317 patients, univariable analyses showed 12 variables were significantly related to death, including age (≥65 vs <65 years) (OR 3.2, 95% CI 1.7–5.9), male sex (OR 1.9, 95% CI 1.0–3.7), coughing and sputum (OR 2.3, 95% CI 1.1–4.8), shortness of breath (OR 2.2, 95% CI 1.2–4.1), chronic pulmonary disease (OR 3.4, 95% CI 1.3–8.5), NLR (OR 1.1, 95% CI 1.1–1.2), d-dimer (>1.5 vs ≤1.5 μg/mL) (OR 4.5, 95% CI 2.4–8.6), acute cardiac injury (OR 22.1, 95% CI 10.3–47.6), acute kidney injury (OR 4.4, 95% CI 2.2–8.8), glucocorticoids (OR 17.0, 95% CI 7.6–38.4), immunoglobulin (OR 17.8, 95% CI 7.3–43.5), thymosin (OR 5.7, 95% CI 2.9–11.7), and ammonium glycyrrhizinate (OR 3.0, 95% CI 1.5–6.3). The detailed information was shown in Table 4.
Table 4.
Univariate logistic regression for prognostic factors of death among COVID-19 patients.
| Factors | OR | Lower 95% CI | Upper 95% CI | p-Value |
|---|---|---|---|---|
| Age, year (>65 vs <65) | 3.2 | 1.7 | 5.9 | <.001 |
| Sex (Female vs Male) | 0.5 | 0.3 | 1.0 | .047 |
| Coughing and sputum | 2.3 | 1.1 | 4.8 | .028 |
| Shortness of breath | 2.2 | 1.2 | 4.1 | .015 |
| Chronic medical histories | 1.4 | 0.8 | 2.7 | .241 |
| Chronic pulmonary disease | 3.4 | 1.3 | 8.5 | .009 |
| Coronary heart disease | 0.8 | 0.2 | 2.7 | .708 |
| Hypertensive heart disease | 1.6 | 0.8 | 3.1 | .147 |
| Diabetes | 1.1 | 0.5 | 2.7 | .767 |
| WBC (nomal vs abnomal) | 1.7 | 0.9 | 3.1 | .117 |
| NLR | 1.1 | 1.1 | 1.2 | <.001 |
| D-dimer (>1.5 vs < 1.5) | 4.5 | 2.4 | 8.6 | <.001 |
| Platelet count (<100 vs > 100) | 1.7 | 0.7 | 4.1 | .267 |
| Unilateral lung infiltration | 0.6 | 0.2 | 2.1 | .453 |
| Bilateral pulmonary infiltration | 1.7 | 0.5 | 6.0 | .377 |
| Multiple mottling and ground-glass opacity | 1.1 | 0.6 | 2.1 | .753 |
| Atomization inhalation | 0.8 | 0.4 | 1.7 | .511 |
| Glucocorticoids | 17.0 | 7.6 | 38.4 | <.001 |
| Immunoglobulin | 17.8 | 7.3 | 43.5 | <.001 |
| Thymosin | 5.7 | 3.0 | 11.0 | <.001 |
| Ammonium glycyrrhizinate | 3.0 | 1.5 | 6.3 | .003 |
| Cardiac injury | 22.1 | 10.3 | 47.6 | <.001 |
| Kidney injury | 4.4 | 2.2 | 8.8 | <.001 |
| Liver injury | 1.5 | 0.8 | 2.8 | .189 |
Note. p-Values are from logistic regression model.
Abbreviations. OR, odds ratio; CI, confidence interval; NLR, neutrophil count/lymphocyte count ratio.
Estimates with statistical significance are marked in bold.
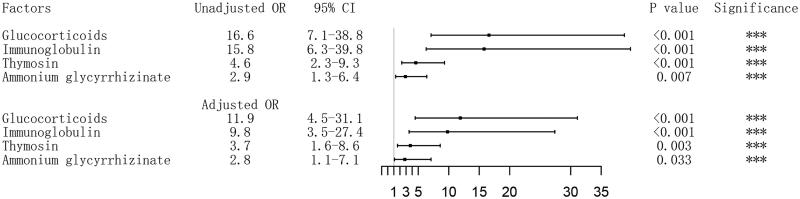
After propensity score matching based on age, sex, coughing and sputum, shortness of breath, chronic pulmonary disease, 46 dead COVID-19 patients and 162 survivors were included for the next logistic analyses. For univariable logistic analyses, the association between treatments and in-hospital death were also statistically significant, with the OR of 16.6 (95% CI 7.1−38.8) for glucocorticoids, 15.8 (95% CI 6.3–39.8) for immunoglobulin, 4.6 (95% CI 2.3–9.3) for thymosin, 2.9 (95% CI 1.3–6.4) for ammonium glycyrrhizinate. After the adjustment for acute cardiac injury, acute kidney injury, NLR and d-dimer in the multivariable logistic analyses, glucocorticoids (OR 11.9, 95% CI 4.5–31.1), immunoglobulin (OR 9.8, 95% CI 3.5–27.4), thymosin (OR 3.7, 95% CI 1.6–8.6), and ammonium glycyrrhizinate (OR 2.8, 95% CI 1.1–7.1) were also independent risk factors for in-hospital death (Figure 1).
Figure 1.
Association of treatment with in-hospital death in patients with coronavirus disease 2019 (COVID-19). Odds ratios (ORs) of each medication variable were obtained using separate multivariate logistic models after adjustment for NLR, D-dimer, any cardiac injury and kidney injury; The patients of survivors group were derived using 5:1 greedy nearest neighbor matching within one-quarter of the standard deviation of the estimated propensity, based the potential variables including age, gender, coughing and sputum, shortness of breath, and chronic pulmonary disease. Abbreriations. OR, odds ratio; CI, confidence interval.
Discussion
This study provided comprehensive data on the demographic, clinical, laboratory, and radiological characteristics as well as the complications, and treatment of discharged or dead hospitalized patients with COVID-19 in Wuhan. We observed several potential risk factors associated with the fatal outcome, furthermore, those treated with multiple treatments including glucocorticoids, immunoglobulin, thymosin, and ammonium glycyrrhizinate were also observed high risk for fatal outcome. The results of this study do not support immunotherapy in patients admitted to hospital with COVID-19 for in-hospital patients with COVID-19 of different severity.
The potential risk factors for fatal outcome identified in this study included age (>65 years), males, coughing and sputum, shortness of breath, chronic pulmonary disease, elevated NLR, elevated d-dimer (>1.5), acute cardiac injury, and acute kidney injury, and most were in line with those in several recent reports11–15. However, different from the findings of previous studies, chronic pulmonary disease was the only comorbidity associated with the death of COVID-1911,12. Few studies reported the role of initial symptoms on admission in prognosis predictions, and our studies showed the patients with coughing and sputum and shortness of breath may suffer a higher risk of COVID-19 death. For the predicted value of laboratory findings, we calculated the ratio of neutrophil-to-lymphocyte ratio (NLR) which are inflammation markers that reflect systemic inflammatory response and might be the most available in emergency circumstances13. The results in our study indicated that elevated NLR levels may reflect an enhanced inflammatory response may suggest a poor prognosis.
We also observed the COVID-19 patients treated with glucocorticoids, immunoglobulin, thymosin, and ammonium glycyrrhizinate may face more risk of death. Considering the total number of deaths (n = 48) in our study and to avoid overfitting in the regression model, only five variables could be included for multivariable analysis11. The adjusted ORs for the immunomodulatory treatments attenuated with adjustment of covariates while still statistically significant.
In the last few months, new evidence came to light strongly supporting that immunomodulatory therapy may result in lower mortality in patients with COVID-195. These studies mainly explored the effect of corticosteroids and glucocorticoids, included dexamethasone16, and hydrocortisone17,18 at low and high doses. While in our study, we observed more intricate and comprehensive immunotherapy therapy, including the use of glucocorticoids, immunoglobulin, thymosin, and ammonium glycyrrhizinate at any reasonable dose.
These randomized controlled trials (RCTs) indicated that the use of corticosteroids and glucocorticoids may decrease the mortality of critically ill patients with COVID-19. Nevertheless, it should be noted that the results of the above RCTs may be applicable to critically ill patients with COVID-19, but may not to all the patients with COVID-19 of different disease severity. For example, the RECOVERY trial16 only focused on patients who received IMV in the main analysis. Besides, they stated that the association of dexamethasone with reduced 28-day mortality was among patients receiving IMV, but not among patients not receiving respiratory support. The other two trials on the effect of hydrocortisone (NCT0424459117 and NCT0251748918) only included patients admitted to an intensive care unit (ICU). In our study, we enrolled all consecutive patients diagnosed with COVID-19, including critical cases of the infection. Of all the 317 patients, only 67 (21.1%) patients receiving IMV, this may suggest that more studies are needed in the future to explore the immunomodulatory effect on COVID-19 patients with different severity, especially for non-critically ill patients.
Immunomodulatory therapy did not improve the outcome of death in this study for in-hospital patients with COVID-19 of all severity. The possible causes are as follows. Firstly, patients in the death group may have a longer time from onset to admission and receive treatment later. From late January to early March, Wuhan experienced a high peak of the COVID-19 outbreak. A previous study reported that long wait for access to medical care was observed in severe cases compared with that in nonsevere cases, and more than half of the patients experienced at least two hospital visits.15 Rigorous monitoring and treatment may be delayed due to the long wait for access to medical care. These COVID-19 patients would likely develop multiple organ dysfunction and died of respiratory failure. Therefore, the time from onset to hospital admission may bias the effect of the immunomodulatory treatments.
Second, severe COVID-19 is related to a dysregulated host inflammatory response, suggesting that immunomodulator may be an effective treatment19. Treatments such as glucocorticoids and immunoglobulins are therefore often used in more critical patients, may be an indicator of disease severity rather than a predisposing factor. Randomized controlled trials of the effects of immunomodulators in patients with COVID-19 are urgently needed.
The pathophysiology of COVID-19 caused by SARS-CoV-2 infection is aggressive inflammatory responses strongly implicated in the resulting damage to the airways Therefore, poor prognosis is not only related to the viral infection but also the host response. Thymosins was a family of polypeptides originally derived and characterized from the bovine thymus, and have been studied for a long time to characterize their immune restorative and immune-modulating properties. Interestingly, some molecules (thymosin α1 and thymosin β4), have been identified that their immunomodulatory efficacy including both immune enhancement and regulation20,21. Immunoglobulins have also been used to prevent and treat illness, neutralize drugs and poisons, and accentuate or depress the immune system22. Our study suggested that the use of thymosins or immunoglobulins is associated with fatal outcomes of COVID-19. On the other hand, there were also other studies that suggested that glucocorticoids are more common in fatal cases and indeed associated with COVID-19 death outcomes15,23. These may be related to the risks of prolonged viremia, corticosteroid-induced diabetes, avascular necrosis and psychosis24. Ammonium glycyrrhizate can produce glucocorticoid like effects for immunomodulatory therapy.
Although there is no effective antiviral drug or vaccine for COVID-19, timely diagnosis and early respiratory support can alleviate severe cases and reduce mortality. The severities of the initial confirmed cases were mostly mild. Therefore, we propose that timely treatment and strict monitoring are essential, and immunomodulatory therapy should be used with caution to avoid aggravating complications.
Firstly, we comprehensively considered the potential factors related to prognosis, including characteristics, comorbidities, complications, and treatments. The estimation of predictive factors for fatal outcomes in our study is therefore robust. Additionally, our study is the first study to address the impact of multiple immunomodulatory therapies in COVID-19, using standardized and detailed data collection. The main limitation of this study is the retrospective observational design that it only accounts for some covariates, other factors potentially affecting both the indication for glucocorticoid treatment and mortality may not have been accounted for. Propensity score matching and multivariable logistic regression methods were used to calculate the adjusted ORs of the treatments, however, these adjustments may not be fully accounted for the small sample size of death outcome. Another limitation is that we fail to take into account the detailed information of treatment such as the beginning time and continuous dosage. More randomized controlled trials are needed to explore the effect of immunomodulatory therapy on the outcome of COVID-19.
Conclusion
In our study for in-hospital patients with COVID-19 of all severity levels, the use of corticosteroids, immunoglobulin, thymosin, ammonium glycyrrhizinate may be associated with a higher risk of mortality. Further prospective studies are essential to clarify our findings, especially for non-critically ill patients.
Acknowledgements
None reported.
Transparency
Declaration of funding
No funding to disclose.
Declaration of financial/other relationships
All authors declare that they have no relevant financial or other relationships to disclose. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published. Author contributions were as follows: design: YW, XY, WX, YL; data collection: XY, CH, YS, CY; analysis and manuscript writing: YW, XY. All authors reviewed and approved the final manuscript.
Data availability statement
All related data were displayed in the manuscript. Further information regarding the data can be obtained by contacting the corresponding authors.
References
- 1.Wang C, Horby PW, Hayden FG, et al. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coronavirus Disease 2019 (COVID-19) Situation Report-63 HIGHLIGHTS [Internet]. Geneva (Switzerland): World Health Organization; 2020. [cited 2020 March 24]. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200323-sitrep-63-covid-19.pdf?sfvrsn=b617302d_2
- 3.Coronavirus disease (COVID-19) Situation dashboard [Internet]. Geneva (Switzerland): World Health Organization; 2020. [cited 2020 October 31]. https://who.sprinklr.com/
- 4.Wu Z, McGoogan JM.. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. [DOI] [PubMed] [Google Scholar]
- 5.Sterne JAC, Murthy S, Diaz JV, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324(13):1330–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected [Internet]. Geneva (Switzerland): World Health Organization; 2020. [cited 2020 February 8]. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected
- 7.The International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC) [Internet]. Oxford (UK): ISARIC; 2020. [cited 2002 January 25]. https://isaric.tghn.org/
- 8.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. Lancet. 2020;395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–c184. [DOI] [PubMed] [Google Scholar]
- 11.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L, He W, Yu X, et al. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect. 2020;80(6):639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lagunas-Rangel FA. Neutrophil-to-lymphocyte ratio and lymphocyte-to-C-reactive protein ratio in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. J Med Virol. 2020;92(10):1733–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng Y, Luo R, Wang K, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146(1):110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. 2020. DOI: 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dequin P-F, Heming N, Meziani F, et al. Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with COVID-19: a randomized clinical trial. JAMA. 2020;324(13):1298–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Angus DC, Derde L, Al-Beidh F, et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA. 2020;324(13):1317–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tay MZ, Poh CM, Rénia L, et al. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.King R, Tuthill C.. Immune modulation with thymosin alpha 1 treatment. Vitam Horm. 2016;102:151–178. [DOI] [PubMed] [Google Scholar]
- 21.Marshall GJ. Thymosin-induced immunoregulation: clinical potentials for allergy and asthma endotypes. Expert Opin Biol Ther. 2018;18(1):95–97. [DOI] [PubMed] [Google Scholar]
- 22.Stiehm ER, Orange JS, Ballow M, et al. Therapeutic use of immunoglobulins. Adv Pediatr. 2010;57(1):185–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang JJY, Lee KS, Ang LW, et al. Risk factors of severe disease and efficacy of treatment in patients infected with COVID-19: a systematic review, meta-analysis and meta-regression analysis. Clin Infect Dis. 2020;71(16):2199–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arabi YM, Mandourah Y, Al-Hameed F, et al. Corticosteroid therapy for critically ill patients with middle east respiratory syndrome. Am J Respir Crit Care Med. 2018;197(6):757–767. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All related data were displayed in the manuscript. Further information regarding the data can be obtained by contacting the corresponding authors.