Abstract
Regulatory T cells (Tregs) regulate immune responses and maintain host immune homeostasis. Tregs contribute to the disease progression of several chronic infections by oversuppressing immune responses via the secretion of immunosuppressive cytokines, such as transforming growth factor (TGF)-β and interleukin-10. In the present study, we examined the association of Tregs with Mycoplasma bovis infection, in which immunosuppression is frequently observed. Compared with uninfected cattle, the percentage of Tregs, CD4+CD25highFoxp3+ T cells, was increased in M. bovis-infected cattle. Additionally, the plasma of M. bovis-infected cattle contained the high concentrations of TGF-β1, and M. bovis infection induced TGF-β1 production from bovine immune cells in in vitro cultures. Finally, we analyzed the immunosuppressive effects of TGF-β1 on bovine immune cells. Treatment with TGF-β1 significantly decreased the expression of CD69, an activation marker, in T cells, and Th1 cytokine production in vitro. These results suggest that the increase in Tregs and TGF-β1 secretion could be one of the immunosuppressive mechanisms and that lead to increased susceptibility to other infections in terms of exacerbation of disease during M. bovis infection.
Keywords: TGF-β1, Mycoplasma bovis, regulatory T cell, immunosuppression, cattle
Introduction
Bovine mycoplasmosis caused by Mycoplasma bovis is prevalent in many countries, including Japan (1–4), and is characterized by chronic pneumonia, otitis, arthritis, and therapy-resistant mastitis (5–8). M. bovis has been well-documented as a causative agent of chronic pneumonia, and the exacerbation of disease is caused by co-infections with other agents (6, 7). However, the detailed mechanisms underlying the exacerbation of disease by co-infections during bovine mycoplasmosis have not been fully elucidated. The suppression of the immune response is frequently observed during M. bovis infection, leading to chronic progression. Several studies have demonstrated that M. bovis suppresses lymphocyte activities such as Th1 cytokine production and induces lymphocyte apoptosis in vitro (9, 10). In addition, our previous studies showed the association of immunosuppression by M. bovis with immunoinhibitory molecules, programmed death (PD)-1, PD-ligand 1 (PD-L1), and prostaglandin (PG) E2 (11, 12). PD-1/PD-L1 expression and PGE2 concentrations are increased in immune cells and the plasma of M. bovis-infected cattle, respectively (11, 12). The PD-1/PD-L1 pathway and PGE2 exert suppressive effects on Th1 cytokine production, such as interferon (IFN)-γ and tumor necrosis factor (TNF)-α, from bovine immune cells (13, 14). Therefore, the inhibition of the PD-1/PD-L1 pathway and PGE2 production in vitro activates M. bovis-specific Th1 responses (11, 12), which suggests that these inhibitory molecules might be involved in the immunosuppression during bovine mycoplasmosis. However, the detailed mechanisms of the immune suppression in this infection have not been fully elucidated.
Regulatory T cells (Tregs) constitute a subset of CD4+ T cells and are characterized by the expression of CD25, which is an interleukin (IL)-2 receptor α-chain, and forkhead box P3 (Foxp3), which is a transcription factor that is required for the development of Tregs (15). Tregs regulate the immune response by producing inhibitory cytokines, such as transforming growth factor (TGF)-β and IL-10, and maintain host immune homeostasis (16). Although Tregs are essential for host immune homeostasis, previous studies have reported the association of Tregs with the progression of chronic diseases by suppressing the immune response (17, 18). Tregs play an immunomodulatory role in several chronic infections, such as human immunodeficiency virus infection, hepatitis B virus infection, and hepatitis C virus infection (19–21). Several studies in the field of veterinary medicine have demonstrated that the immunomodulatory effects by Tregs are involved in the progression of chronic infections in cattle, such as Johne's disease and bovine leukemia virus infection (22–24). However, there are no studies demonstrating the association of Tregs with bovine mycoplasmosis.
The cytokine TGF-β exists in five isoforms, three of which (TGF-β1, TGF-β2, and TGF-β3) are expressed in mammals (25). TGF-β is a pleiotropic cytokine that is involved in both suppressive and inflammatory immune responses (26). Previous reports have shown that TGF-β–especially TGF-β1—plays an important role in immune modulation by regulating the activities of immune cells, including natural killer (NK) cells and T cells (27, 28). TGF-β controls innate immune responses such as NK cell cytotoxicity (29, 30). TGF-β also controls adaptive immunity by directly promoting the expansion of Treg cells and by inhibiting the generation and function of effector T cells and antigen presenting cells (31). In our previous study in cattle, we revealed that treatment with TGF-β reduces the expression of Th1 cytokines in T cells in vitro (23). However, the detailed immunosuppressive effects of TGF-β on bovine immune cells remain unclear.
In the present study, we examined the proportion of CD4+CD25highFoxp3+ cells and the concentration of plasma TGF-β1 in M. bovis-infected cattle by flow cytometry and enzyme-linked immunosorbent assay (ELISA), respectively. In addition, we analyzed the immunosuppressive effects of TGF-β1 on bovine peripheral blood mononuclear cells (PBMCs) in vitro.
Materials and Methods
Bacterial Strain
M. bovis strain PG45 (ATCC25523) was cultured in NK broth (Miyarisan Pharmaceutical, Tokyo, Japan) at 37°C for 72 h, collected by centrifugation, and washed with phosphate-buffered saline (PBS). Colony-forming units were counted using an NK agar plate (Miyarisan Pharmaceutical), followed by the resuspension of the bacteria in RPMI 1640 medium (Sigma-Aldrich, St. Louis, MO, USA) containing 10% heat-inactivated fetal bovine serum (Thermo Fisher Scientific, Waltham, MA, USA), 100 U/mL penicillin (Thermo Fisher Scientific), 100 μg/mL streptomycin (Thermo Fisher Scientific), and 2 mM L-glutamine (Thermo Fisher Scientific), and stored at −80°C until use.
Bovine Samples
Blood samples derived from Holstein cattle were collected at several farms in Hokkaido, Japan. M. bovis infection was diagnosed clinically and microbiologically at Rakuno Gakuen University, Ebetsu, Japan, and Hokkaido University, Sapporo, Japan, as described previously (12). The blood collections of uninfected cattle, which had no history of M. bovis infection, were conducted at a M. bovis free farm, the Field Science Center for Northern Biosphere, Hokkaido University. The number of lymphocytes in the peripheral blood of uninfected and M. bovis-infected cattle was counted using a Celltac α MEK-6450 automatic hematology analyzer (Nihon Kohden, Tokyo, Japan). All experimental procedures using bovine samples were conducted following approval from the local committee for animal studies at Hokkaido University (approval No. 17-0024). Informed consent was obtained from all owners of cattle sampled in the present study.
PBMC Culture
Buffy coat fraction was collected from blood samples by centrifugation (1,700 × g, 15 min, 25°C, without break). PBMCs were purified from collected buffy coat fraction by density gradient centrifugation (1,200 × g, 20 min, 25°C, without break) on 60% Percoll (GE Healthcare, Little Chalfont, UK). Then, collected PBMCs were washed 3 times with PBS by centrifugation (770 × g, 10 min, 25°C) and filtered through a 40-μm cell strainer (BD Biosciences, San Jose, CA, USA). PBMCs were stained with 0.4% Trypan Blue Stain (Thermo Fisher Scientific) and the number of the viable cells was counted using Countess II FL Automated Cell Counter (Thermo Fisher Scientific). In the PBMC cultures, PBMCs were cultured in RPMI 1640 medium as described above using 96-well plates (Corning, Corning, NY, USA) at 37°C under 5% CO2 atmosphere. PBMCs were incubated with live M. bovis at a multiplicity of infection (MOI) of 10:1. Culture supernatants were collected after 24 h, and TGF-β1 concentrations were measured by ELISA. To examine whether TGF-β1 suppresses Th1 responses in cattle, PBMCs were cultured with 10 ng/mL of recombinant human TGF-β1 (R&D Systems, Minneapolis, MN, USA) in the presence of 1 μg/mL of concanavalin A (Con A, Sigma-Aldrich). In accordance with manufacturer's protocols, recombinant human TGF-β1 was reconstituted in sterile 4 mM HCl (Kanto Chemical, Tokyo, Japan), and 4 mM HCl was used as a vehicle control in the experiments. Cells were harvested after 5 h, and CD69 expression was measured by flow cytometry. After 24h, culture supernatants were collected and IL-10 concentrations were determined by ELISA. After 72 h, culture supernatants were collected and the concentrations of IFN-γ and TNF-α were determined by ELISA.
ELISA
PGE2 concentrations in plasmas were measured by Prostaglandin E2 Express ELISA Kit (Cayman Chemical, Ann Arbor, MI, USA), following the manufacturer's instructions. IFN-γ, TNF-α, and TGF-β1 concentrations in culture supernatants were measured by Bovine IFN-γ ELISA Development Kit (Mabtech, Nacka Strand, Sweden), Bovine TNF alpha Do-It Yourself ELISA (Kingfisher Biotech, St. Paul, MN, USA), and Human TGF-β1 DuoSet ELISA (R&D Systems) respectively, according to the manufacturers' protocols. As described in a previous paper with slight modifications (13), sandwich ELISA of IL-10 was performed using two antibodies; anti-bovine IL-10 (CC318; Bio-Rad, Hercules, CA, USA) as a capture antibody and biotinylated anti-bovine IL-10 (CC320; Bio-Rad) as a detective antibody. Briefly, a 96-well Maxisorp Nunc-Immuno Plate (Thermo Fisher Scientific) was coated overnight with CC318 diluted with carbonate-bicarbonate buffer (Sigma-Aldrich). After washing with PBS, blocking was performed by PBS-T (PBS containing 0.05% Tween 20) containing 0.1% bovine serum albumin (Sigma-Aldrich) for 1 h. After washing with PBS-T, the samples were incubated in the wells for 2 h. Following washing with PBS-T, diluted detective antibodies (CC320) were added to the wells and incubated for 1 h. After further washing with PBS-T, Neutra-Avidin-HRP (Thermo Fisher Scientific) was added and incubated for 1 h. Finally, the plate was washed with PBS-T and incubated with TMB One Component Substrate (Bethyl Laboratories, Montgomery, TX, USA), and absorbance was measured using MTP-900 (Corona Electric, Ibaraki, Japan). The results were calculated based on a standard curve (from 78 to 5,000 pg/mL) constructed using recombinant bovine IL-10 (Kingfisher Biotech).
Flow Cytometry
Blood samples were treated with ACK buffer containing 8.26 mg/mL of NH4Cl, 1.19 mg/mL of NaHCO3, and 37.8 mg/mL of 2Na-EDTA (pH 7.3), and then washed twice with PBS. The staining of Tregs was then performed as described previously (23). Briefly, the cells were stained using the following reagents: FITC-conjugated anti-bovine CD4 antibody (CC8; Bio-Rad), Alexa Fluor 647-labeled anti-bovine CD25 antibody (IL-A111; Bio-Rad), FOXP3 Fix/Perm Buffer (BioLegend, San Diego, CA, USA), and PerCP/Cy5.5-conjugated anti-bovine Foxp3 antibody (FJK-16s; eBioscience, San Diego, CA, USA). PerCP/Cy5.5-conjugated rat IgG2a isotype control (eBR2a; eBioscience) was used as a negative control. After staining, the cells were analyzed by FACS Verse (BD Biosciences).
CD69 staining was performed as described in a previous paper (32). Briefly, collected cells were stained using the following antibodies: PerCP/Cy5.5-conjugated anti-bovine CD3 antibody (MM1A; Washington State University Monoclonal Antibody Center, Pullman, WA, USA), FITC-conjugated anti-bovine CD4 antibody (CC8), PE-conjugated anti-bovine CD8 antibody (CC63; Bio-Rad), and Alexa Fluor 647-labeled anti-bovine CD69 antibody (KTSN7A; Kingfisher Biotech). After staining, the cells were analyzed by FACS Verse.
Statistics
Differences were assessed using the Mann-Whitney U test and the Wilcoxon signed-rank test. Correlations were analyzed using the Spearman correlation. A p value of <0.05 was considered to indicate statistical significance.
Results
Increase in CD4+CD25highFoxp3+ T Cells in M. bovis-Infected Cattle
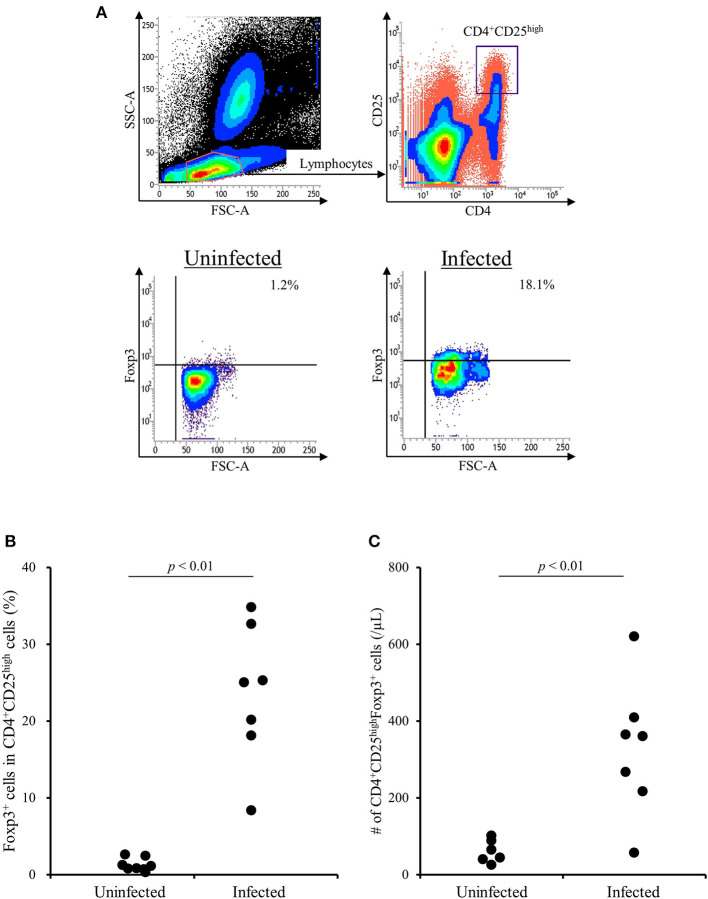
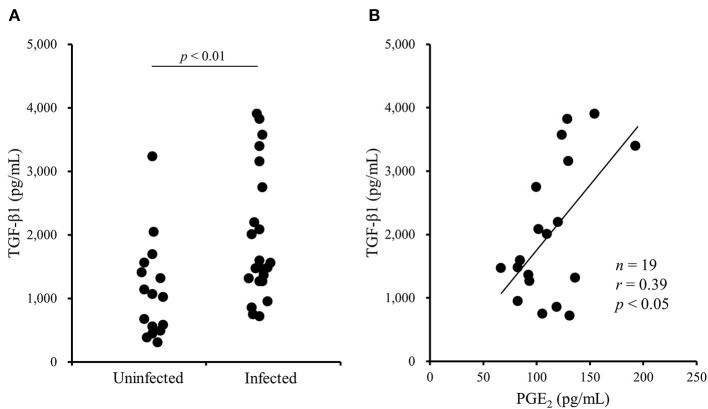
A previous study has shown that the TGF-β1 secretion from Tregs reduces antiviral cytokine activities and the cytotoxicity of NK cells in cattle infected with bovine leukemia virus (23). However, the association of Tregs with other bovine chronic infections was still unclear. In the present study, we examined the percentage of Tregs in peripheral blood samples from cattle infected with M. bovis. Flow cytometric analysis revealed that the proportion of Foxp3+ cells in CD4+CD25high cells was increased in M. bovis-infected cattle (Figures 1A,B, Table 1). The number of CD4+CD25highFoxp3+ cells in the peripheral blood was also increased in M. bovis-infected cattle (Figure 1C, Table 1). In addition, TGF-β1 concentrations in the plasma of M. bovis-infected cattle were significantly higher than those of cattle not infected with M. bovis (Figure 2A). Interestingly, TGF-β1 concentrations were positively correlated with PGE2 concentrations in the plasma of M. bovis-infected cattle (Figure 2B). Collectively, these results suggest the association of Tregs with M. bovis-infected cattle.
Figure 1.
The increase of CD4+CD25highFoxp3+ T cells in M. bovis-infected cattle. (A) The gating strategy and representative plots for Treg staining. (B) The percentage of Foxp3+ cells in CD4+CD25high T cells in M. bovis-infected (n = 7) and -uninfected (n = 8) cattle. (C) The number of CD4+CD25highFoxp3+ cells in M. bovis-infected (n = 7) and -uninfected (n = 6) cattle. (B,C) Statistical significance was determined by the Mann-Whitney U test. Uninfected, M. bovis-uninfected cattle; Infected, M. bovis-infected cattle.
Table 1.
The percentage and number of Tregs in M. bovis-infected and -uninfected cattle (raw data).
| Cattle | Foxp3+ /CD4+CD25high (%) | CD4+CD25highFoxp3+/lymphocyte (%) | # of lymphocytes (/μL) | # of Tregs (/μL) | |
|---|---|---|---|---|---|
| Uninfected | U-1 | 1.23 | 0.0089 | 2,800 | 24.92 |
| U-2 | 0.84 | 0.0141 | 2,800 | 39.48 | |
| U-3 | 0.78 | 0.0133 | 3,300 | 43.89 | |
| U-4 | 2.61 | 0.0362 | 2,800 | 101.36 | |
| U-5 | 0.71 | 0.0200 | 3,200 | 64.00 | |
| U-6 | 2.46 | 0.0419 | 2,100 | 87.99 | |
| U-7 | 0.32 | 0.0067 | NA | NA | |
| U-8 | 1.14 | 0.0182 | NA | NA | |
| M. bovis- infected | M-1 | 34.79 | 0.0677 | 3,200 | 216.64 |
| M-2 | 24.99 | 0.1384 | 2,600 | 359.84 | |
| M-3 | 25.27 | 0.0827 | 4,400 | 363.88 | |
| M-4 | 8.33 | 0.0087 | 6,500 | 56.55 | |
| M-5 | 32.61 | 0.0607 | 4,400 | 267.08 | |
| M-6 | 18.09 | 0.1016 | 6,100 | 619.76 | |
| M-7 | 20.13 | 0.1317 | 3,100 | 408.27 | |
NA, not available.
Figure 2.
The increase of TGF-β1 concentrations in blood plasma of M. bovis-infected cattle. (A) TGF-β1 concentrations in the plasma from M. bovis-infected (n = 22) and -uninfected (n = 16) cattle were determined by ELISA. Statistical significance was determined by the Mann-Whitney U test. (B) The correlation between TGF-β1 and PGE2 concentrations in the plasma of M. bovis-infected cattle (n = 19). Correlation statistic was analyzed using the Spearman correlation.
Induction of TGF- β1 Production by M. bovis Infection
To examine whether M. bovis directly induces TGF-β1 production during in vitro infection, PBMCs from uninfected cattle were cultivated with or without live M. bovis and TGF-β1 concentrations in culture supernatants were measured by ELISA. As shown in Figure 3, TGF-β1 concentrations were significantly increased in the cultures with live M. bovis when compared with that in the cultures without live M. bovis (Figure 3), suggesting that M. bovis can induce TGF-β1 production during its infection.
Figure 3.
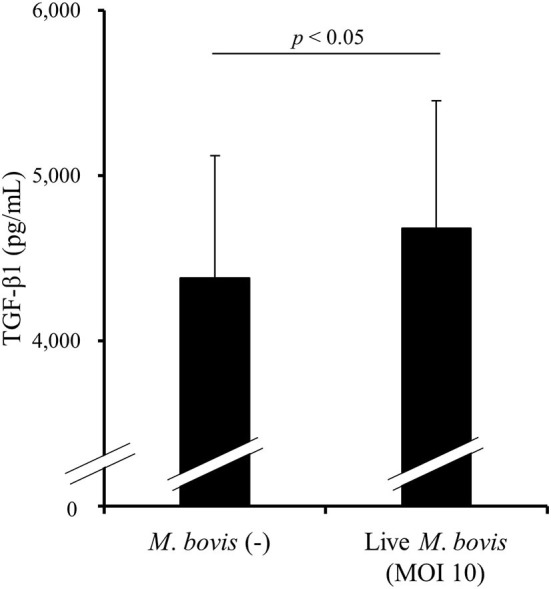
The induction of TGF-β1 production by M. bovis. PBMCs from uninfected cattle were cultured with live M. bovis, and TGF-β1 concentrations in culture supernatants were measured by ELISA (n = 8). Data are presented as means, and the error bars indicate standard errors. Statistical significance was determined by the Wilcoxon signed-rank test. MOI, multiplicity of infection.
Suppressive Effects of TGF- β1 on Th1 Responses Stimulated by Con A
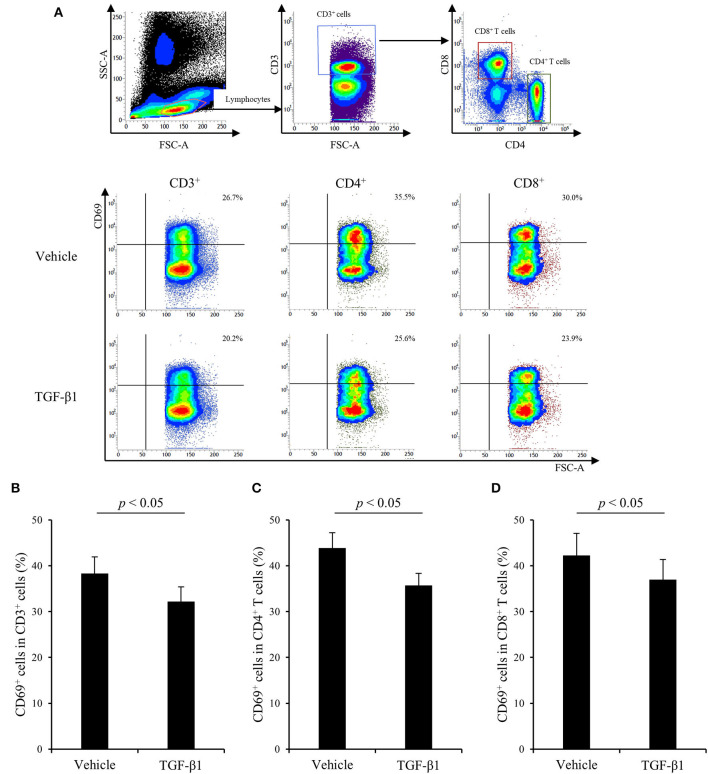
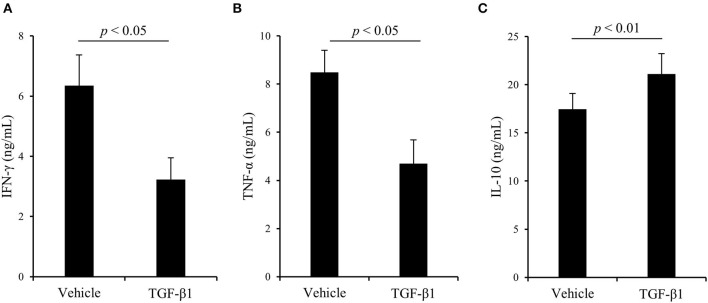
Finally, to examine the effects of TGF-β1 on bovine Th1 responses in detail, PBMCs from uninfected cattle were cultured with TGF-β1 in the presence of Con A stimulation, and T-cell activation and cytokine production were measured by flow cytometry and ELISA, respectively. As shown in Figure 4, treatment with TGF-β1 reduced the expression of CD69, an activation marker of lymphocytes, in CD3+, CD4+, and CD8+ T cells in vitro (Figures 4A–D). Additionally, treatment with TGF-β1 suppressed IFN-γ and TNF-α production from bovine PBMCs in vitro (Figures 5A,B). In contrast, treatment with TGF-β1 induced the production of IL-10, an immunosuppressive cytokine, from bovine PBMCs in vitro (Figure 5C). Collectively, these results suggest that TGF-β1 has the suppressive effects on immune responses, especially the Th1 response, in cattle.
Figure 4.
The effect of TGF-β1 on CD69 expression in cattle. (A–D) PBMCs from uninfected cattle (n = 7) were incubated with TGF-β1 in the presence of Con A. (A) The gating strategy and representative plots for CD69 expression. (B–D) CD69 expression in T cells (B), CD4+ T cells (C), and CD8+ T cells (D) were measured by flow cytometry. Data are presented as means, and the error bars indicate standard errors. Statistical significance was determined by the Wilcoxon signed-rank test.
Figure 5.
The effect of TGF-β1 on cytokine production in cattle. (A–C) PBMCs from uninfected cattle (n = 7) were incubated with TGF-β1 in the presence of Con A. After incubation, IFN-γ (A), TNF-α (B), and IL-10 (C) concentrations in culture supernatants were determined by ELISA. Data are presented as means, and the error bars indicate standard errors. Statistical significance was determined by the Wilcoxon signed-rank test.
Discussion
M. bovis has been shown to regulate bovine immune responses, including the induction of lymphocyte apoptosis and the suppression of Th1 cytokine production (9, 10). The effects of M. bovis on bovine immune response likely contribute to the chronic and nonresponsive progression of the disease. In the present study, we revealed the association of Tregs with M. bovis infection. We found that the percentage of CD4+CD25highFoxp3+ T cells was increased in cattle infected with M. bovis. Additionally, TGF-β1 concentrations in the plasma of M. bovis-infected animals were higher than in uninfected animals. Tregs are the suppressive subpopulation of CD4+ T cells and regulate immune responses by the secretion of inhibitory cytokines including TGF-β (16). Therefore, Tregs could be the source of TGF-β1 in the peripheral blood of M. bovis-infected cattle. These results suggest that the increase in Tregs could be one of the immunosuppressive mechanisms in M. bovis-infected cattle. Furthermore, the cultures using PBMCs of uninfected cattle revealed that treatment with TGF-β1 significantly downregulated Th1 responses, such as T-cell activation and Th1 cytokine production, in vitro. In vitro infection with M. bovis enhanced TGF-β1 production from bovine PBMCs. These data suggest that M. bovis promotes the secretion of TGF-β1 from host immune cells for its immune evasion, although the detailed mechanism remains unclear. Further experiments are required to elucidate the association of M. bovis-induced TGF-β1 with immunosuppression during M. bovis infection.
Recently, several studies have revealed the relationship between Tregs and other Mycoplasma infections in humans and mice (33, 34). Odeh and Simecka have demonstrated that CD4+CD25+ T-cell population is important to dampen inflammatory disease in Mycoplasma pulmonis infection of mice. However, the cell population does not contribute to persistence of infection (33). Guo and colleagues have shown that the association of an imbalance of circulating Tregs and Th17 cells with the deterioration of patients with Mycoplasma pneumoniae pneumonia. Although the Th17/Treg ratio is significantly higher in the patients with refractory M. pneumoniae pneumonia in comparison with healthy control, there is no significant difference with the frequencies of Tregs and the levels of TGF-β1 in the patients with refractory M. pneumoniae pneumonia (34). These studies suggest that Tregs might not be involved in the chronic progression of Mycoplasma infections. However, to the best of our knowledge, this is the first study to show the association of Tregs with cattle infected with M. bovis. Additionally, it is still unclear whether the increase in Tregs and Treg-derived cytokines truly leads to the progression of M. bovis infection. Therefore, future experiments are necessary to further elucidate roles of Tregs in the pathogenesis of Mycoplasma infections including M. bovis infection.
PGE2 is an inflammatory mediator derived from arachidonic acid by several enzymes, such as cyclooxygenase (COX)-1 and COX-2 (35). PGE2 inhibits the activity of immune cells, such as T cells, dendritic cells, and NK cells (36). Our previous studies have revealed that the immunosuppressive effects of PGE2 contribute to the disease progression of several chronic infections in cattle, including M. bovis infection (12, 14, 37). Interestingly, in this study, TGF-β1 concentrations were positively correlated with PGE2 concentrations in the plasma of M. bovis-infected cattle. Previous studies on human research have described that treatment with TGF-β1 in vitro induces PGE2 production from several cell types including CD4+ T cells (38, 39). Our previous and present studies have shown that M. bovis upregulates PGE2 and TGF-β1 production from bovine immune cells (12). Hence, PGE2 upregulation in M. bovis-infected cattle might be caused via TGF-β1 production by M. bovis. Conversely, previous studies have shown that PGE2 induces Foxp3 gene expression and enhances the induction and differentiation of Foxp3+CD25+CD4+ Tregs (40–42). Our previous study has shown that treatment with PGE2 upregulates TGF-β1 and Foxp3 expression in bovine immune cells (37). Thus, the cross-interaction between M. bovis-induced TGF-β1 and PGE2 might have the potential for exacerbating immune suppression during M. bovis infection.
Here, we demonstrate the association of Tregs with M. bovis-infected cattle. Additionally, M. bovis promotes the secretion of TGF-β1, which has a suppressive effect on immune responses, especially Th1 immune responses, in cattle. These findings might contribute to the increase in susceptibility to other infections regarding the exacerbation of disease because co-infection with other bacteria and viruses has been frequently observed during M. bovis infection (6, 7). Further experiments are required to elucidate the influence of M. bovis-induced TGF-β1 in the exacerbation of disease by co-infections during the bovine mycoplasmosis. Presently, M. bovis infection is spreading globally (1–4), and there are no effective vaccines due to the immunosuppression caused by M. bovis. Therefore, a greater understanding of the immunosuppressive mechanism is necessary to develop a novel control strategy of bovine mycoplasmosis. Our results could contribute to the development of an effective control method against this infection.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The animal study was reviewed and approved by Local committee for animal studies at Hokkaido University (Approval No. 17-0024). Informed consent was obtained from all owners of cattle sampled in the present study. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author Contributions
SK, SM, and KOha: designed the work. YS, SGot, TO, HS, KOhi, and NM: performed the experiments. YS, SK, SGot, TO, and KOhi: acquired, analyzed, and interpreted the data. SGon, HH, MT, YH, and JK: provided blood samples and laboratory reagents. YS and SK: wrote the manuscript. SK, TO, NM, SM, and KOha: revised the manuscript. All authors reviewed and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to Dr. Hideyuki Takahashi, Dr. Yasuyuki Mori, and Dr. Tomio Ibayashi for valuable advice and discussions. We would like to thank Enago (www.enago.jp) for the English language review.
Footnotes
Funding. This work was supported by JSPS KAKENHI grant number 19KK0172 (to SK), grants from the Project of the NARO, Bio-oriented Technology Research Advancement Institution [Research Program on Development of Innovative Technology 26058 BC (to SK) and Special Scheme Project on Regional Developing Strategy, Grant 16817557 (to SK)].
References
- 1.Janardhan KS, Hays M, Dyer N, Oberst RD, Debey BM. Mycoplasma bovis outbreak in a herd of North American bison (Bison bison). J Vet Diagn Invest. (2010) 22:797–801. 10.1177/104063871002200528 [DOI] [PubMed] [Google Scholar]
- 2.Nicholas RA. Bovine mycoplasmosis: silent and deadly. Vet Rec. (2011) 168:459–62. 10.1136/vr.d2468 [DOI] [PubMed] [Google Scholar]
- 3.Higuchi H, Iwano H, Gondaira S, Kawai K, Nagahata H. Prevalence of Mycoplasma species in bulk tank milk in Japan. Vet Rec. (2011) 169:442. 10.1136/vr.d5331 [DOI] [PubMed] [Google Scholar]
- 4.Higuchi H, Gondaira S, Iwano H, Hirose K, Nakajima K, Kawai K, et al. Mycoplasma species isolated from intramammary infection of Japanese dairy cows. Vet Rec. (2013) 172:557. 10.1136/vr.101228 [DOI] [PubMed] [Google Scholar]
- 5.Gagea MI, Bateman KG, Shanahan RA, van Dreumel T, McEwen BJ, Carman S, et al. Naturally occurring Mycoplasma bovis-associated pneumonia and polyarthritis in feedlot beef calves. J Vet Diagn Invest. (2006) 18:29–40. 10.1177/104063870601800105 [DOI] [PubMed] [Google Scholar]
- 6.Caswell JL, Archambault M. Mycoplasma bovis pneumonia in cattle. Anim Health Res Rev. (2007) 8:161–86. 10.1017/S1466252307001351 [DOI] [PubMed] [Google Scholar]
- 7.Caswell JL, Bateman KG, Cai HY, Castillo-Alcala F. Mycoplasma bovis in respiratory disease of feedlot cattle. Vet Clin North Am Food Anim Pract. (2010) 26:365–79. 10.1016/j.cvfa.2010.03.003 [DOI] [PubMed] [Google Scholar]
- 8.Fox LK. Mycoplasma mastitis: causes, transmission, and control. Vet Clin North Am Food Anim Pract. (2012) 28:225–37. 10.1016/j.cvfa.2012.03.007 [DOI] [PubMed] [Google Scholar]
- 9.Vanden Bush TJ, Rosenbusch RF. Mycoplasma bovis induces apoptosis of bovine lymphocytes. FEMS Immunol Med Microbiol. (2002) 32:97–103. 10.1111/j.1574-695X.2002.tb00540.x [DOI] [PubMed] [Google Scholar]
- 10.Mulongo M, Prysliak T, Scruten E, Napper S, Perez-Casal J. In vitro infection of bovine monocytes with Mycoplasma bovis delays apoptosis and suppresses production of gamma interferon and tumor necrosis factor alpha but not interleukin-10. Infect Immun. (2014) 82:62–71. 10.1128/IAI.00961-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goto S, Konnai S, Okagawa T, Nishimori A, Maekawa N, Gondaira S, et al. Increase of cells expressing PD-1 and PD-L1 and enhancement of IFN-γ production via PD-1/PD-L1 blockade in bovine mycoplasmosis. Immun Inflamm Dis. (2017) 5:355–63. 10.1002/iid3.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goto S, Konnai S, Hirano Y, Kohara J, Okagawa T, Maekawa N, et al. Upregulation of PD-L1 expression by prostaglandin E2 and the enhancement of IFN-γ by anti-PD-L1 antibody combined with a COX-2 inhibitor in Mycoplasma bovis infection. Front Vet Sci. (2020) 7:12. 10.3389/fvets.2020.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ikebuchi R, Konnai S, Okagawa T, Yokoyama K, Nakajima C, Suzuki Y, et al. Blockade of bovine PD-1 increases T cell function and inhibits bovine leukemia virus expression in B cells in vitro. Vet Res. (2013) 44:59. 10.1186/1297-9716-44-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sajiki Y, Konnai K, Okagawa T, Nishimori A, Maekawa N, Goto S, et al. Prostaglandin E2 induction suppresses the Th1 immune responses in cattle with Johne's disease. Infect Immun. (2018) 86:e00910–7. 10.1128/IAI.00910-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. (2003) 299:1057–61. 10.1126/science.1079490 [DOI] [PubMed] [Google Scholar]
- 16.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. (2008) 133:775–87. 10.1016/j.cell.2008.05.009 [DOI] [PubMed] [Google Scholar]
- 17.Belkaid Y, Rouse B. Natural regulatory T cells in infectious disease. Nat Immunol. (2005) 6:353–60. 10.1038/ni1181 [DOI] [PubMed] [Google Scholar]
- 18.Belkaid Y. Regulatory T cells and infection: a dangerous necessity. Nat Rev Immunol. (2007) 7:875–88. 10.1038/nri2189 [DOI] [PubMed] [Google Scholar]
- 19.Cabrera R, Tu Z, Xu Y, Firpi RJ, Rosen HR, Liu C, et al. An immunomodulatory role for CD4+CD25+ regulatory T lymphocytes in hepatitis C virus infection. Hepatology. (2004) 40:1062–71. 10.1002/hep.20454 [DOI] [PubMed] [Google Scholar]
- 20.Eggena MP, Barugahare B, Jones N, Okello M, Mutalya S, Kityo C, et al. Depletion of regulatory T cells in HIV infection is associated with immune activation. J Immunol. (2005) 174:4407–14. 10.4049/jimmunol.174.7.4407 [DOI] [PubMed] [Google Scholar]
- 21.Stoop JN, van der Molen RG, Baan CC, van der Laan LJ, Kuipers EJ, Kusters JG, et al. Regulatory T cells contribute to the impaired immune response in patients with chronic hepatitis B virus infection. Hepatology. (2005) 41:771–8. 10.1002/hep.20649 [DOI] [PubMed] [Google Scholar]
- 22.Coussens PM, Sipkovsky S, Murphy B, Roussey J, Colvin CJ. Regulatory T cells in cattle and their potential role in bovine paratuberculosis. Comp Immunol Microbiol Infect Dis. (2012) 35:233–9. 10.1016/j.cimid.2012.01.004 [DOI] [PubMed] [Google Scholar]
- 23.Ohira K, Nakahara A, Konnai S, Okagawa T, Nishimori A, Maekawa N, et al. Bovine leukemia virus reduces anti-viral cytokine activities and NK cytotoxicity by inducing TGF-β secretion from regulatory T cells. Immun Inflamm Dis. (2016) 4:52–63. 10.1002/iid3.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roussey JA, Oliveira LJ, Langohr IM, Sledge DG, Coussens PM. Regulatory T cells and immune profiling in johne's disease lesions. Vet Immunol Immunopathol. (2016) 181:39–50. 10.1016/j.vetimm.2016.03.008 [DOI] [PubMed] [Google Scholar]
- 25.Khalil N. TGF-β: From latent to active. Microbes Infect. (1999) 1:1255–63. 10.1016/S1286-4579(99)00259-2 [DOI] [PubMed] [Google Scholar]
- 26.Sanjabi S, Oh SA, Li MO. Regulation of the immune response by TGF-β: from conception to autoimmunity and infection. Cold Spring Harb Perspect Biol. (2017) 9:a022236. 10.1101/cshperspect.a022236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang L, Pang Y, Moses HL. TGF-beta and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends Immunol. (2010) 31:220–7. 10.1016/j.it.2010.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haque S, Morris JC. Transforming growth factor-β: a therapeutic target for cancer. Hum Vaccin Immunother. (2017) 13:1741–50. 10.1080/21645515.2017.1327107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. (2003) 198:1875–86. 10.1084/jem.20030152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bellone G, Aste-Amezaga M, Trinchieri G, Rodeck U. Regulation of NK cell functions by TGF-b1. J Immunol. (1995) 155:1066–73. [PubMed] [Google Scholar]
- 31.Batlle E, Massagué J. Transforming growth factor-β signaling in immunity and cancer. Immunity. (2019) 50:924–40. 10.1016/j.immuni.2019.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sajiki Y, Konnai S, Okagawa T, Maekawa N, Nakamura H, Kato Y, et al. A TLR7 agonist activates bovine Th1 response and exerts antiviral activity against bovine leukemia virus. Dev Comp Immunol. (2021) 114:103847. 10.1016/j.dci.2020.103847 [DOI] [PubMed] [Google Scholar]
- 33.Guo H, He Z, Li M, Wang T, Zhang L. Imbalance of peripheral blood Th17 and treg responses in children with refractory Mycoplasma pneumoniae pneumonia. J Infect Chemother. (2016) 22:162–6. 10.1016/j.jiac.2015.12.006 [DOI] [PubMed] [Google Scholar]
- 34.Odeh AN, Simecka JW. Regulatory CD4+CD25+ T cells dampen inflammatory disease in murine mycoplasma pneumonia and promote IL-17 and IFN-γ responses. PLoS ONE. (2016) 11:e0155648. 10.1371/journal.pone.0155648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phipps RP, Stein SH, Roper RL. A new view of prostaglandin E regulation of the immune response. Immunol Today. (1991) 12:349–52. 10.1016/0167-5699(91)90064-Z [DOI] [PubMed] [Google Scholar]
- 36.Kalinski P. Regulation of immune responses by prostaglandin E2. J Immunol. (2012) 188:21–8. 10.4049/jimmunol.1101029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sajiki Y, Konnai S, Okagawa T, Nishimori A, Maekawa N, Goto S, et al. Prostaglandin E2-induced immune exhaustion and enhancement of antiviral effects by anti-PD-L1 antibody combined with COX-2 inhibitor in bovine leukemia virus infection. J Immunol. (2019) 203:1313–24. 10.4049/jimmunol.1900342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baratelli F, Lee JM, Hazra S, Lin Y, Walser TC, Schaue D, et al. PGE2 contributes to TGF-beta induced T regulatory cell function in human non-small cell lung cancer. Am J Transl Res. (2010) 2:356–67. [PMC free article] [PubMed] [Google Scholar]
- 39.Fang L, Chang HM, Cheng JC, Leung PC, Sun YP. TGF-β1 induces COX-2 expression and PGE2 production in human granulosa cells through Smad signaling pathways. J Clin Endocrinol Metab. (2014) 99:E1217–26. 10.1210/jc.2013-4100 [DOI] [PubMed] [Google Scholar]
- 40.Baratelli F, Lin Y, Zhu L, Yang SC, Heuzé-Vourc'h N, Zeng G, et al. Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. J Immunol. (2005) 175:1483–90. 10.4049/jimmunol.175.3.1483 [DOI] [PubMed] [Google Scholar]
- 41.Mahic M, Yaqub S, Johansson CC, Tasken K, Aandahl EM. FOXP3+CD4+CD25+ adaptive regulatory T cells express cyclooxygenase-2 and suppress effector T cells by a prostaglandin E2-dependent mechanism. J Immunol. (2006) 177:246–54. 10.4049/jimmunol.177.1.246 [DOI] [PubMed] [Google Scholar]
- 42.Sreeramkumar V, Fresno M, Cuesta N. Prostaglandin E2 and T cells: friends or foes? Immunol Cell Biol. (2012) 90:579–86. 10.1038/icb.2011.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.