Abstract
Nanobottles refer to colloidal particles with a hollow interior and a single opening in the wall. These unique features make them ideal carriers for the loading, encapsulation, release, and delivery of various types of theranostic agents in an array of biomedical applications. The hollow interior gives them a high loading capacity while the opening enables quick loading and controlled release of the payload(s). More significantly, on-demand release can be readily achieved by adding a stimuli-responsive material as the inner matrix or cork stopper. In this Progress Report, we begin with an introduction to the general structures and properties of nanobottles, followed by a brief discussion on the methods developed for their fabrication. We then showcase the use of nanobottles for loading different types of payloads, including small-molecule drugs, biomacromolecules, imaging contrast agents, and functional nanoparticles. We also highlight the strategies explored for controlling the release by varying the size of the opening and/or integrating with a stimuli-responsive material. We conclude with some perspectives on future directions for this class of nanomaterials in terms of fabrication, functionalization, and application.
Keywords: nanobottle, controlled release, drug delivery, encapsulation, phase-change material
1. Introduction
Bottle-like containers have existed for at least 10,000 years, with the earliest example related to pottery. Nowadays, bottles with very diverse sizes, shapes, and compositions are ubiquitous in our everyday life. They are widely used for the storage and transport of both liquids and solids, including water, milk, beer, oils, soft drinks, medicines, and chemicals, among others. The unique design of a bottle offers immediate advantages in terms of convenience for packaging, storage, and transportation. For example, the empty interior of a bottle ensures a high loading capacity while minimizing the amount and thus the cost of the material used for constructing the bottle. In addition, the well-defined opening on the top of a bottle offers the ease for loading and releasing both liquid and solid materials. More significantly, with the help of a simple cork or cap, the bottle can be utilized to hold the loaded content for a long period of time by protecting it from evaporation, decomposition, contamination, or deterioration.
Considering their so many advantages, tremendous efforts have been made over the past 15 years to bring the concept of bottles to the nanotechnology arena by downsizing their dimensions to the nanoscale regime.[1-4] Such nanobottles hold great promise for applications in areas ranging from catalysis to energy storage, pollutant separation, controlled release, and drug delivery for the encapsulation of various types of functional materials.[4-8] Among the applications, the delivery of a theranostic agent to the site of interest and under controlled release has attracted the most attention.[7-10] In such an application, the high loading capacity of nanobottles makes it possible to minimize the carrier material and thereby reduce its potential toxicity in vivo.[11-13] The opening allows for easy loading and release of essentially all types of theranostic agents regardless of their sizes and properties.[7,10] In addition, the opening can be designed with a smart feature to enable the on-demand release of payload(s),[14,15] for augmenting the therapeutic efficacy while reducing the off-target toxicity.[16,17] Similar to other types of carriers, the size and surface properties of nanobottles can also be engineered to improve their circulation half‐life, targeting ability, and clearance from the body.[17]
In this Progress Report, we summarize the recent developments in the design and fabrication of nanobottles, with a focus on their applications in controlled release and drug delivery. We begin with an introduction to the general design of a nanobottle, together with the unique properties for controlled release and related applications. We then discuss three general methods developed for the fabrication of colloidal particles with a bottle-like structure. We pay close attention to the strategies for ensuring the creation of only one opening in the wall of a hollow particle. We then showcase the biomedical applications of nanobottles in the context of encapsulation and controlled release of various types of theranostic agents, including small-molecule drugs, macromolecules, imaging contrast agents, and functional nanoparticles. We also highlight the methods capable of releasing the payload(s) in a controllable manner. At the end, we offer some perspectives on the challenges and opportunities with respect to both the synthesis and application of nanobottles.
2. Nanobottles
2.1. General Concept
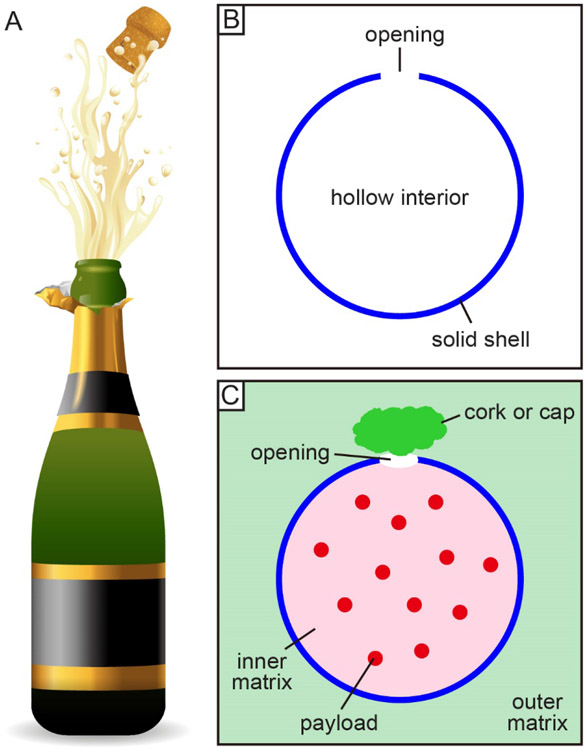
Figure 1A illustrates the situation one would observe when opening a champagne bottle made of glass. Typically, such a bottle has a hollow cylinder at the bottom, a narrow neck at the top, and an opening at the tip of the neck. The opening can be sealed with a cork or cap to protect the loaded liquid. The specific shape of a champagne bottle is designed for the convenience of holding and transportation in our everyday life. As for nanobottles, their major applications are in drug delivery so they can be simply fabricated with a spherical shape (Figure 1B) and typically used as a colloidal dispersion. In a sense, it is not necessary for them to take a cylindrical shape similar to that of a champagne bottle as long as they share the same general feature: a hollow body connected to the surrounding environment through a single opening. In general, a nanobottle can be characterized using three major parameters: overall dimension, size of the opening, and composition of the wall. In this Progress Report, we mainly focus on nanobottles made of biocompatible materials, together with an overall dimension below 1000 nm and an opening of greater than 10 nm.
Figure 1.
Nanobottles as a new class of carriers for controlled release and drug delivery. (A) A glass bottle used with a cork for the storage of champagne in our everyday life. (B) Schematic of a nanobottle that has one opening on its hollow body. (C) Schematic showing the encapsulation of a payload in the nanobottle and its release can be controlled by varying the size of the opening, choosing a proper inner/outer matrix, and adding a cork or cap as the stopper.
2.2. Unique Features for Controlled Release
The unique features of nanobottles make them ideal candidates as carriers for the controlled release and delivery of various theranostic agents for biomedical applications. As discussed above, the high loading capacity resulting from the completely empty interior can help minimize the use of carrier material and thereby reduce the level of toxicity in vivo.[11] In the meantime, the solid wall of a nanobottle can effectively protect most of the payloads from reacting with the outside environment during circulation. The opening also allows for easy loading and release of various types of payloads regardless of their size and hydrophilicity/hydrophobicity.[7,10] More importantly, the bottle-like structure offers a simple handle to realize the on-demand release of therapeutics, significantly improving their therapeutic efficacy while reducing the off-target toxicity.[16-17] Figure 1C shows a schematic illustration of the possible means for controlling the release profile of a payload from a nanobottle. In one approach, the size of the opening can be tuned to regulate the release kinetics. Alternatively, the payload can be co-loaded with a responsive material to enable on-demand release in the presence of a specific stimulus. Similarly, the nanobottle can be dispersed in an appropriate outer matrix to regulate the diffusion rate of the payload from the interior to the exterior. In a third approach, a stimulus-responsive cork or cap can be used to seal the opening, serving as an “on or off” gate to control the release. Of course, one can also combine any two or even three of these means to achieve a tight control over the release profile.
3. How to Create Only One Opening in the Wall
3.1. Swelling and Freeze-Drying
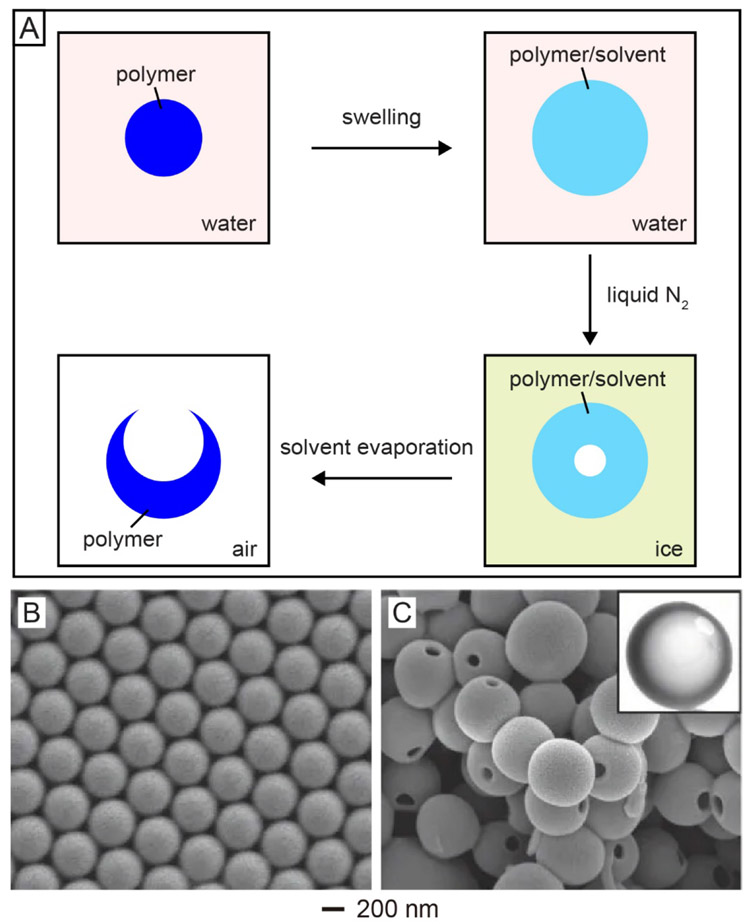
Over the last two decades, numerous hollow micro/nanoparticles with diverse sizes, shapes, and compositions have been reported.[18,19] However, how to create just one single opening in the surface of a hollow particle has been a major challenge. In one study, this was achieved by swelling polymer spheres with an organic solvent, followed by a freeze-drying process.[7] The major steps involved in this approach are shown in Figure 2A, with a polystyrene (PS) colloidal sphere serving as the starting point. In the first step, PS sphere was suspended in water and swollen by a solvent (e.g., styrene or toluene) good for the polymer but immiscible with water. The swollen sphere was then frozen with liquid nitrogen, during which both the solvent and water were solidified. With the solidification of the solvent, the sphere would experience volume shrinkage, pushing both the solvent molecules and polymer chains from the interior of the sphere to the outer surface and eventually leaving behind a void in the center. When the sample was kept in a freeze-dryer at a temperature above the melting point of the solvent but below that of water to remove the solvent, the flux of solvent associated with evaporation would lead to the formation of a single hole in the wall of the resultant hollow particle for the generation of a PS nanobottle. In this process, the ice around each swollen particle helped maintain the spherical shape by eliminating possible deformation and aggregation.
Figure 2.
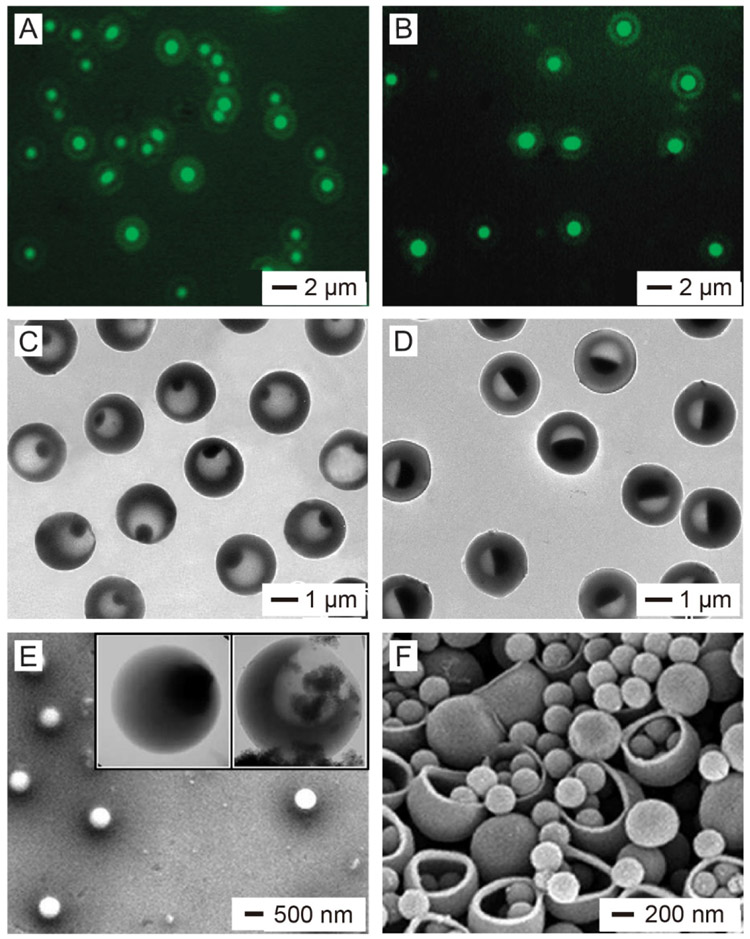
Synthesis of polymer nanobottles through swelling and subsequently freeze-drying. (A) Schematic illustration of the major steps involved in the synthesis of polymer nanobottles from a solid sphere made of the same polymer. (B) SEM image of PS solid spheres. (C) SEM and TEM (inset) images of PS nanobottles obtained by swelling the PS solid spheres with 5% (v/v) styrene-in-water emulsion, followed by freeze-drying. Reproduced with permission.[7] Copyright 2005 Springer Nature.
Figure 2B shows a scanning electron microscopy (SEM) image of the PS solid spheres with an average diameter of 400 nm. Figure 2C shows SEM and transmission electron microscopy (TEM) images of the resultant nanobottles obtained by swelling the PS solid spheres with a 5% (v/v) styrene-in-water emulsion for 30 min, followed by a freeze-drying process.[7] The as-obtained PS nanobottles had an average diameter of 580 nm, with an opening size of 100 nm in diameter. The increase in overall dimension relative to the original PS spheres confirmed the swelling by styrene. The size of the opening could be easily tuned by varying the type and/or amount of the solvent added into the aqueous suspension and thus the extent of swelling.[14] For example, when a 1% (v/v) toluene-in-water emulsion was used to swell PS spheres with an average diameter of 960 nm, the opening size of the resultant PS nanobottles was about 50 nm in diameter. In comparison, the opening was increased to 350 nm when the same batch of PS spheres were swollen in an aqueous emulsion containing 5% (v/v) toluene. This strategy has also been successfully applied to the fabrication of nanobottles made of biocompatible and biodegradable polymers such as poly(caprolactone) (PCL) and poly(L-lactide) (PLLA) that have been approved by the Food and Drug Administration for clinical use.[20] Notably, after filled with a payload, the opening on these nanobottles made of a polymer could be easily closed through a thermal or solvent treatment.[7]
3.2. Solvent Extraction or Extrusion
In the above approach, solvent evaporation is the major driven force to create a single opening. Along the same line, extraction or extrusion of the solvent encapsulated in the interior of hollow particles can also be used to generate a single hole in the surface.[21-24] As described in a recent report, PCL hollow particles with a single opening were fabricated by co-axially electrospraying poly(ethylene glycol) (PEG) and PCL solutions into ethanol.[24] In this process, a PEG solution in chloroform was applied as the inner fluid while a PCL solution in chloroform served as the outer fluid. When these two solutions were pumped out through a coaxial spinneret connected to a high voltage power supply, core-shell particles comprised of a PEG solution as the core and a PCL solution as the shell were obtained. Before reaching the collection medium based on an ethanol solution, a solid shell made of PCL would be formed as a result of the evaporation of chloroform. Once the particles reached the surface of ethanol solution, the chloroform in the core would be extracted due to its miscibility with ethanol. Since the extraction of chloroform would only occur at the site where the core-shell particle contacted the ethanol, only one opening would be formed on the final PCL hollow particles, generating a bottle-like structure.[24] Although the as-obtained PCL bottles had an overall dimension around 10 μm, the same strategy involving the extraction of a solvent has been used to prepare polymer bottles with sizes down to the nanoscale regime.[3,21,25] To this end, it can be potentially extended for the fabrication of biocompatible and biodegradable polymer-based nanobottles.
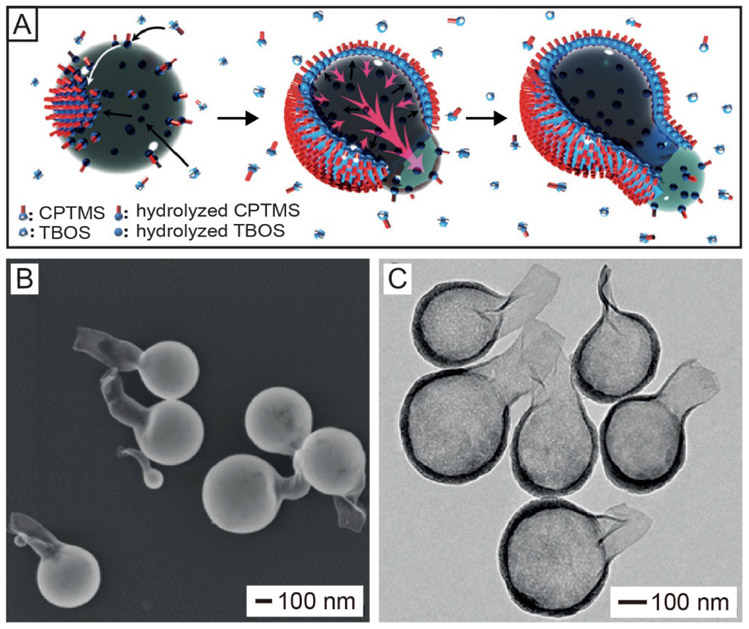
Besides solvent extraction, gradually extruding out the liquid template during deposition can also be used to create a single opening in the surface of a hollow nanoparticle. In one demonstration, silica nanobottles were synthesized using a water-in-n-pentanol emulsion system, in which the water droplets served as the template while (3-chloropropyl)trimethoxysilane (CPTMS) and tetrabutyl orthosilicate (TBOS) were employed as the precursors to silica.[6] Figure 3A shows a proposed mechanism to account for this synthesis. When the precursors were added into the emulsion, the relatively hydrophobic CPTMS molecules would hydrolyze at the water/n-pentanol interface when they met the alkaline water nanodroplets, forming a layer of hydrolyzed CPTMS molecules on the surface of the water droplets. In the meantime, the hydrophilic TBOS molecules would hydrolyze from the water phase and deposit at the interface, leading to the formation of a hybrid silica shell. With continuous co-condensation of hydrolyzed CPTMS and TBOS, the formed silica shell would extrude the water out, leading to the formation of a new water/oil interface. The co-condensation of silica at the newly formed water/oil interface eventually resulted in the formation of a flask bottle-like structure with just one opening in the surface. Figure 3, B and C, shows the SEM and TEM images of the as-obtained silica nanobottles, respectively. The nanobottles had an overall dimension of 350 nm and an opening size of 100 nm in diameter, which was connected to the nanobottle body through a neck. The thickness of the silica wall was about 10 nm, together with a gradual decrease in thickness from the body to the neck, indicating that the aqueous droplets were gradually extruded out through the neck during the deposition of silica. Currently, this method is mainly used to produce silica-based nanobottles.[6,26]
Figure 3.
Synthesis of silica-based nanobottles by extruding out a solvent from hollow particles. (A) Schematic illustration of the proposed mechanism involved in the synthesis of a silica-based nanobottle. Firstly, in the water-in-n-pentanol emulsion, the relatively hydrophobic CPTMS molecules hydrolyze upon meeting the alkaline water droplet and then assemble at the water/oil interface while the relatively hydrophilic TBOS molecules hydrolyze and evolve into silica at the interface from the water phase, leading to the formation of hybrid silica at the interface. Then, the continuous deposition of TBOS inside the water droplet extrudes water out, generating a new water/oil interface. Finally, the CPTMS and TBOS continue to hydrolyze and create hybrid silica at the newly formed water/oil interface, generating a neck with an opening on the silica-based nanobottle. (B) SEM and (C) TEM images of the as-obtained silica nanobottles. Reproduced with permission.[6] Copyright 2016 Wiley-VCH.
3.3. Site-Selected Protection and Growth
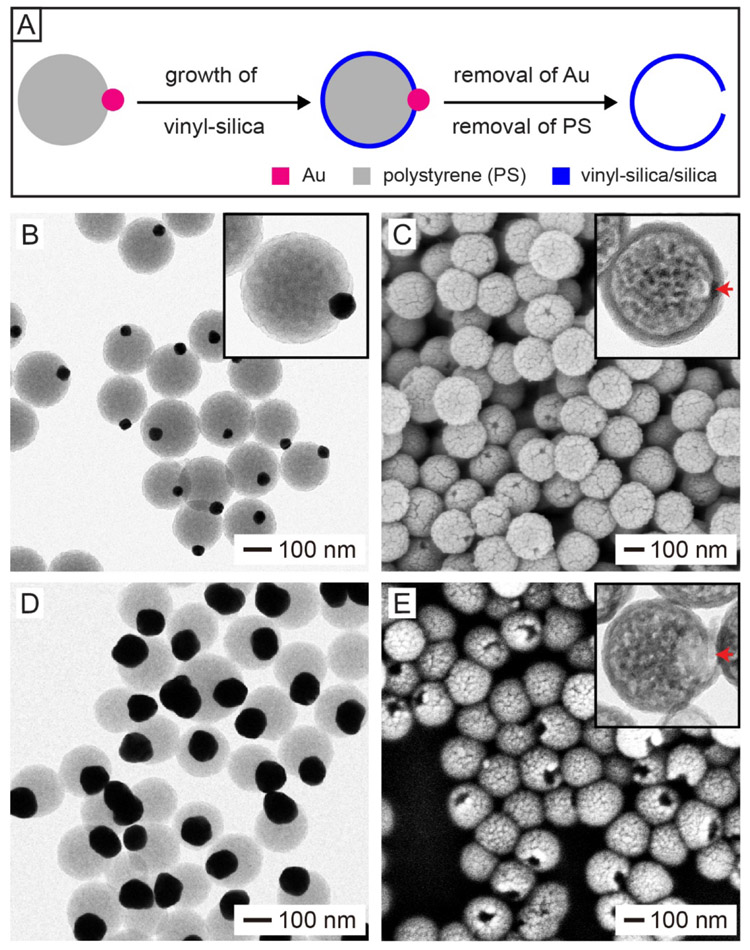
Site-selected protection and growth is another general method that can ensure the creation of only one hole in the surface of a hollow particle. In this approach, it is critical to deposit a thin layer of a solid material on the entire surface of a template particle except for a small portion.[27,28] To enable such site-selected protection and growth, one can use particles with a Janus structure as the template or locate the template particles at an interface prior to coating.[12,29-31] In a recent study, we reported a feasible approach to the preparation of silica nanobottles with a well-defined opening by employing Janus particles as the template.[15] Figure 4A shows the major steps involved in this approach, with Au−PS Janus particles serving as the template. The Au−PS Janus particles were produced by asymmetric growth of PS on citrated-covered Au nanoparticles through polymerization.[32,33] A thin shell made of vinyl-silica was then deposited on the hydrophobic PS portion while exempting the hydrophilic Au portion via the hydrolysis of vinyltrimethoxysilane (VTMS), a relatively hydrophobic precursor to silica. After sequential removal of the Au nanoparticles through etching and then the PS template by calcination, silica nanobottles with a well-defined hole in the surface were obtained. Alternatively, the PS template could be removed through dissolution with an organic solvent such as tetrahydrofuran or toluene.
Figure 4.
Synthesis of silica-based nanobottles through site-selected protection and deposition. (A) Schematic illustration showing the synthesis of a silica/vinyl-silica nanobottle by templating with a Au–PS Janus particle. (B, D) TEM images of Au–PS Janus particles derived from (B) 50- and (D) 100-nm Au nanoparticles. (C, E) SEM and TEM (insets) images of silica nanobottles obtained by templating with the Au–PS Janus particles in (B, D), respectively. Reproduced with permission.[15] Copyright 2019 Wiley-VCH.
Figure 4B shows a TEM image of the Au−PS Janus particles produced through asymmetric growth of PS from 50-nm Au nanoparticles. Figure 4C shows SEM and TEM (inset) images of the silica nanobottles obtained by templating with the Janus particles shown in Figure 4B. The nanobottles had an overall dimension of 190 nm, a wall of 16 nm in thickness, and an opening size of 24 nm in diameter. The size of the opening can be readily tuned by changing the diameter of the Au nanoparticles used for the synthesis of Au−PS Janus particles. For example, when 100-nm Au nanoparticles were employed as seeds to produce Au−PS Janus particles (Figure 4D), the opening of the resultant silica nanobottles was increased to 62 nm in diameter (Figure 4E). It is worth mentioning that the method by leveraging the concept of site-selected protection and growth is generally used to fabricate nanobottles made of inorganic materials, including biocompatible silica and Au.[12,28, 31]
4. Loading and Encapsulation of Functional Materials
4.1. Small-Molecule Drugs
Small-molecule drugs are widely used for the clinical treatment of various diseases.[34] For example, many small-molecule drugs are used in chemotherapy, one of the most commonly used procedures for cancer treatment.[35] Nanobottles have been explored as the carrier to deliver various types of small-molecule drugs. In one study, coumarin-6, a fluorescent small molecule, was used as a model compound of small-molecule drugs to demonstrate the encapsulation capability of PS nanobottles.[7] In a typical process, the PS nanobottles were immersed in an aqueous solution of coumarin-6, followed by annealing the system at 95 °C, a temperature slightly above the glass-transition temperature of PS, to close the opening and thus seal the coumarin-6 molecules inside the bottles. Figure 5A shows a fluorescence image of the resultant PS nanobottles, demonstrating the successful encapsulation of coumarin-6 molecules. As a major advantage of nanobottles, they offer temporal and spatial controls over the released drug, in addition to the dosage. In a recent study, doxorubicin (DOX), a commercial anticancer drug, was mixed with a eutectic mixture of lauric and stearic acids and then loaded into 190-nm silica nanobottles with an opening size of 24 nm in diameter for controlled release.[15] Upon heating, the mixture of fatty acids was melted to undergo a solid-to-liquid phase transition, triggering the release of the loaded drug for killing cancer cells. To this end, the drugs in the silica nanobottles could be released in a controllable manner in response to the changes in temperature (see Section 5 for a more detailed discussion).
Figure 5.
Loading of different types of payloads into nanobottles. (A, B) Fluorescence images of PS nanobottles loaded with (A) coumarin-6 and (B) DNP-BSA (C, D) TEM images of PS nnoabottles loaded with aqueous ioversol solutions at concentrations of (C) 25% and (D) 51%. (E) SEM and TEM (insets) images of PS nanobottles loaded with iron oxide nanoparticles. (F) SEM image of PS bottles loaded with PS solid nanoparticles. For the PS bottles in (A-D) and the left inset in (E), their openings were closed by heating the system at 95 °C for 30 min. (A, B, E, F) Reproduced with permission.[7] Copyright 2005 Springer Nature. (C, D) Reproduced with permission.[45] Copyright 2012 Wiley-VCH.
4.2. Biomacromolecules
Biomacromolecules including DNA, RNA, and proteins play essential roles in regulating the proliferation, division, and apoptosis of cells. They have been extensively used as therapeutic agents for cancer treatment and regenerative medicine.[36-39] The physical dimensions of these macromolecules can be as large as several nanometers and even close to tens of nanometers, making it difficult for them to pass through the solid wall of a carrier containing no sufficiently large pores in the surface.[7,10] As an immediate advantage of the relatively large opening (typically, >10 nm), nanobottles are strong candidates for the encapsulation and targeted delivery of biomacromolecules. In an early study, it was demonstrated that a fluorescence-labeled protein, dinitrophenol-conjugated bovine serum albumin (DNP-BSA), could be encapsulated in the PS nanobottles (Figure 5B).[7] The loaded biomacromolecules could also be released in a controllable manner by rationally modifying the nanobottles. For example, in one study, BSA was loaded into 220-nm silica nanobottles with an opening size of 22 nm in diameter and the bottles were then sealed with a layer of 1-tetradecanol (with a melting point at 38 °C).[40] The encapsulated BSA could be triggered to release by heating the system to a temperature above the melting point of 1-tetradecanol. It is worth mentioning that the bottle structure can protect the susceptible biomacromolecules from pre-degradation or denaturation by effectively separating them from the outside physiological environment.[10]
4.3. Imaging Contrast Agents
Imaging is indispensable for both diagnosis and therapy. The development of various imaging modalities such as magnetic resonance imaging (MRI), X-ray computed tomography (CT), thermoacoustic tomography (TAT), and ultrasound imaging has enabled rapid diagnosis of diseases through visualization and quantitative assessment.[41-43] For some clinical applications, contrast agents need to be delivered to specific lesions or organs to provide precise imaging results.[44] In the meantime, it is of great importance to improve the stability of a contrast agent in the biological medium and reduce the toxicity in vivo. To this end, nanobottles offer an ideal platform for encapsulating contrast agents and thus isolating them from the biological environment. Their outer surface could be further modified with appropriate ligands, enabling targeted delivery and molecular imaging. In one demonstration, ioversol, an iodinated contrast compound for CT imaging, was loaded into PS nanobottles in the form of an aqueous solution.[45] Afterwards, the opening could be sealed through a thermal annealing process, effectively retaining ioversol molecules inside the bottle. Figure 5, C and D shows TEM images of the PS nanobottles after encapsulation with aqueous solutions of ioversol at concentrations of 25% and 51%, respectively, demonstrating the simplicity and versatility of this platform. Other types of contrast agents, including perfluorooctane for MRI and NaCl for TAT, have also been successfully encapsulated in the PS nanobottles using a similar protocol.[45]
4.4. Functional Nanoparticles
Another advantage of the nanobottles is that they can be used to load functional nanoparticles as long as the particles are smaller enough to pass through the opening. The nanoparticles may offer specific functions for a variety of applications. For example, encapsulation of magnetic iron oxide nanoparticles into nanobottles can provide functions such as targeted delivery and MRI contrast enhancement.[29,46] The SEM and TEM (inset) images in Figure 5E shows the PS nanobottles after loading with iron oxide nanoparticles.[7] It can be clearly seen that some iron oxide nanoparticles had been loaded into the nanobottle through the opening. In another study, 10-nm Pt nanoparticles capable of catalyzing the decomposition of H2O2 were loaded into 350-nm silica nanobottles with an opening size of 100 nm in diameter, and the loaded nanobottles could then serve as nanomotors.[6] When dispersed in aqueous H2O2, the Pt nanoparticles would decompose H2O2 into H2O and O2 inside the nanobottle. The generated O2 bubbles would exit from the opening, propelling the nanobottle forward. Such fuel-driven nanomotors possess several advantages in drug delivery such as rapid drug transport and deep tissue penetration.[47] It is worth mentioning that one can easily tune the opening size of the nanobottles to accommodate nanoparticles with different sizes. For example, 260-nm PS nanoparticles could be easily loaded into 540-nm PS nanobottles when the opening was increased to about 450 nm in diameter (Figure 5F).[7]
5. How to Control the Release Profile
5.1. Varying the Size of the Opening
As discussed in Section 2, the release profile of a payload from a nanobottle can be tuned by varying the size of the opening, using an appropriate inner or outer matrix, or corking the opening with a stopper. Obviously, varying the size of the opening is the most straightforward and simplest method. For a nanobottle with an inner space of V and an opening of D in diameter, if we assume the payload molecules are evenly distributed inside the nanobottle during the whole release process and the released molecules are evenly distributed in an infinite outer space, the mass transfer of the payload can be described using the following equation:
| (1) |
where C is the concentration of the payload in the nanobottle, k is the mass transfer coefficient of the payload, and t is time. Equation (1) can be transformed into:
| (2) |
After integration, we obtain:
| (3) |
where C0 is the initial concentration of the payload in the nanobottle. The cumulative release profile of the payload can be described using equation (4):
| (4) |
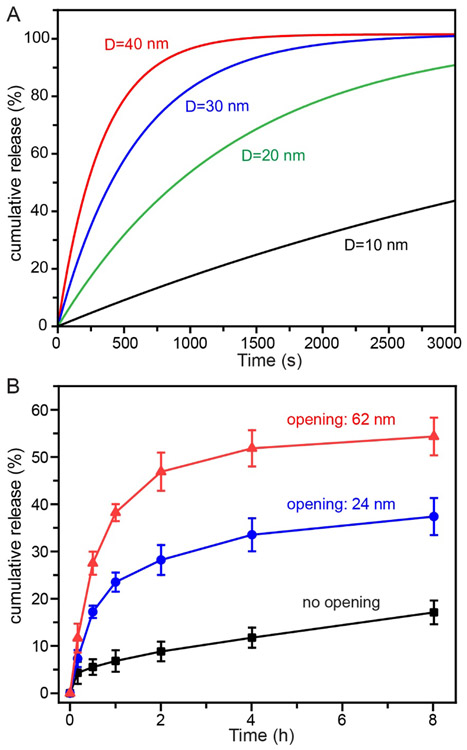
Clearly, the size of the opening has a major impact on the release rate, that is, a smaller opening size will lead to a slower release rate. The effect of opening size on the release kinetics can be more clearly observed from the calculated cumulative release profiles shown in Figure 6A, which were obtained from equation (4) when a payload with a mass transfer coefficient of 1×10−8 m/s was released from a nanobottle with an inner space of 200 nm in diameter while the diameter of the opening was decreased from 40 to 30, 20, or 10 nm, respectively. This phenomenon was also verified experimentally in a recent study, in which 190-nm silica nanobottles with opening diameters of 24 or 62 nm, respectively, were used for the loading and release of a commercial anticancer drug (DOX).[15] Figure 6B shows the release profiles of DOX from the nanobottles, demonstrating that a larger opening would support faster release kinetics. As expected, the silica hollow nanoparticles without opening in the wall showed the slowest release rate.
Figure 6.
Controlling the release of a payload by varying the size of the opening. (A) Calculated cumulative release profiles of a payload from a nanobottle when its opening diameter (D) is reduced from 40 to 30, 20, or 10 nm, respectively, where the mass transfer coefficient (k) of the payload is set to be 1×10−8 m/s and the inner space is set to be 200 nm in diameter. (B) Cumulative DOX release from 190-nm silica nanobottles with the size of opening controlled at 24 nm and 62 nm, respectively, with silica hollow nanoparticles with no opening in the surface serving as a control. (B) Reproduced with permission.[15] Copyright 2019 Wiley-VCH.
5.2. Incorporating a Stimuli-Responsive Filler
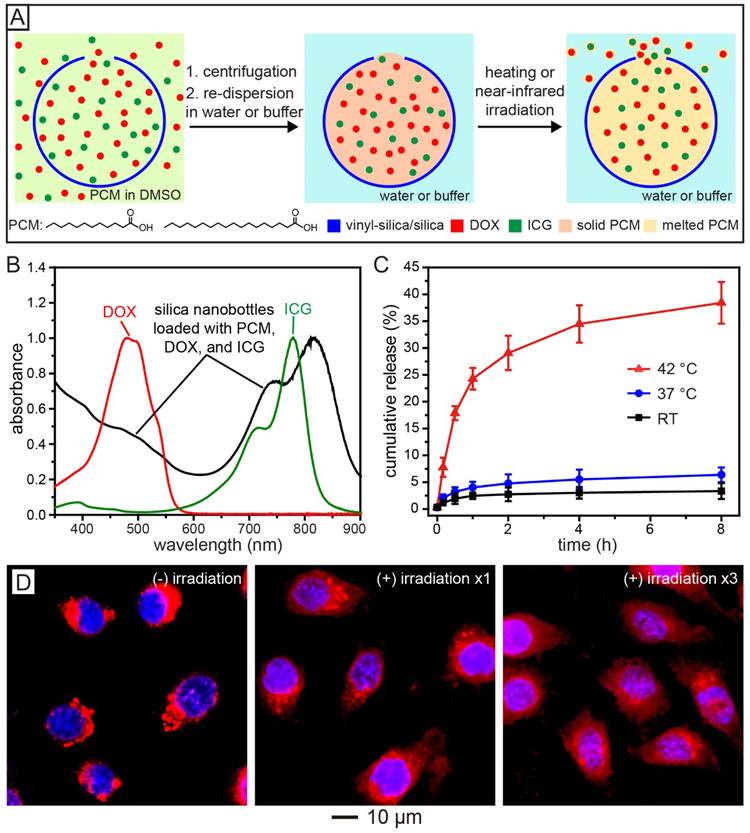
Another effective strategy for controlled release is to co-load the payload with a stimuli-responsive material into the interior of the nanobottles. For example, phase-change material (PCM),[48-50] a thermo-responsive material, can be used as a smart filler for the controlled release of anti-cancer drugs in response to the change in temperature.[15] Figure 7A shows a schematic illustration of the major steps involved in the loading of DOX, with indocyanine green (ICG, a near-infrared dye), into a silica nanobottle, together with a PCM serving as the smart matrix for temperature-regulated release. Specifically, ICG was added to enable remote and localized heating under near-infrared irradiation. In a typical loading process, the silica nanobottles were dispersed in a dimethyl sulfoxide (DMSO) solution containing a PCM (the eutectic mixture of lauric and stearic acids), DOX, and ICG, followed by removal of the unloaded materials via centrifugation and washing. Addition of water to the above system would induce the solidification of PCM because of the immiscibility between them, retaining DOX and ICG in the nanobottles. Upon direct heating or ICG-enabled photothermal heating, the PCM would be melted to undergo a solid-to-liquid phase transition, triggering the release of DOX. Notably, the PCM used in this study was based on natural fatty acids, which are biocompatible and biodegradable.[48,51,52]
Figure 7.
Controlling the release of a payload by incorporating a stimuli-responsive inner matrix. (A) Schematic illustration of the encapsulation of a therapeutic drug (DOX) and a near-infrared dye (ICG) into a silica nanobottle for controlled release, with PCM serving as a smart matrix. (B) UV-vis spectrum of the silica nanobottles after loading with a mixture of PCM, DOX, and ICG. (C) Cumulative DOX release from the silica nanobottles at room temperature (RT), 37 °C, and 42 °C, respectively. (D) Fluorescence images of HeLa cells after incubation with the silica nanobottles loaded with a mixture of PCM, DOX, and ICG: (left) without laser irradiation, (middle) after laser irradiation for 8 min, and (right) after three rounds of 8-min laser irradiation. The red fluorescence came from DOX while the blue fluorescence indicated nuclei. The laser had an output peaked at 808 nm, together with an irradiance of 0.4 W/cm2. Reproduced with permission.[15] Copyright 2019 Wiley-VCH.
The successful loading of DOX and ICG was verified by the UV-vis spectra shown in Figure 7B.[15] The loading capacity of DOX was measured to be 84 mg per gram of the nanobottles. The controlled release of DOX was demonstrated by incubating the loaded nanobottles at different temperatures (Figure 7C). At room temperature and 37 °C, the amount of the released drug was essentially negligible, indicating that the drug was retained by the solid PCM. In contrast, when the sample was heated to 42 °C, a temperature slightly higher than the melting point (39 °C) of the PCM, about 37% of the loaded DOX was released within 8 h, confirming that the release of the payload could be readily tuned by triggering the solid-to-liquid phase transition of the PCM matrix. The controlled release was further conducted inside cancer cells through near-infrared irradiation. As shown in Figure 7D, after incubation with HeLa cells, the red fluorescence from DOX was mainly distributed outside the nuclei before irradiation, while it gradually emerged inside the nuclei upon irradiation, indicating the release of DOX from the nanobottles under near-infrared-induced heating. The cancer cells could be effectively killed as a result of both the release of DOX and the slight rise in local temperature.
5.3. Corking the Opening with a Stopper
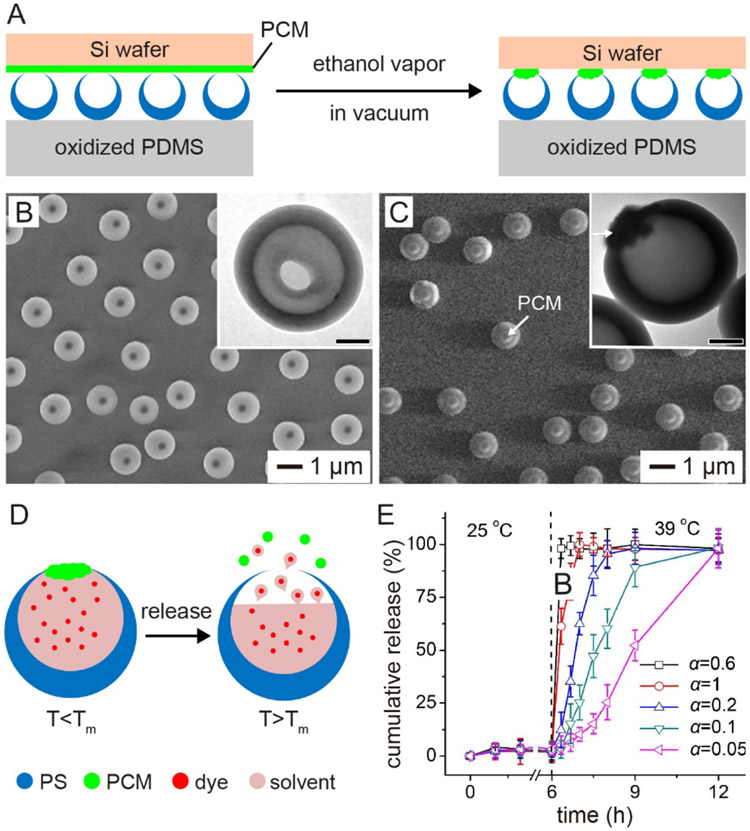
In our everyday life, corks or caps are used to seal the openings of bottles, helping store the loaded liquids or solids and control their release. To this end, it is rational to believe that putting a stopper made of a stimuli-responsive material at the opening of a nanobottle can immediately bring a tight control to the release process. However, considering the extremely small dimensions of nanobottles, it would be a grand challenge to achieve such a capability. To address this challenge, we developed an approach to cork the opening of PS nanobottles with a PCM for controlled release (Figure 8A).[14] One of the most critical steps is to prepare well-oriented PS nanobottles on a polydimethylsiloxane (PDMS) substrate. In this case, PS solid spheres were partially embedded in a poly(4-vinylpyridine) (P4VP) film coated on a PDMS substrate, followed by swelling the PS beads in a 5% (v/v) toluene-in-water emulsion. After freeze-drying and then removal of P4VP film, well-oriented PS nanobottles with an average opening of 350 nm in diameter were obtained (Figure 8B). Because the bottom of the PS beads was protected by P4VP, the swelling of PS and the evaporation of toluene would prefer to occur at the top of each PS sphere, leading to the formation of an opening on the top of each nanobottle (Figure 8B).
Figure 8.
Controlling the release of a payload by corking the opening with a stimuli-responsive stopper. (A) Schematic showing how to cork the openings of PS bottles with PCM-based stoppers. (B, C) SEM and TEM (insets) images of PS bottles (B) before and (C) after the opening had been corked with a PCM. Scale bars in the insets are 300 nm. (D) Schematic showing how to control the release of a payload from a PCM-capped PS bottle by heating. Tm stands for the melting point of the PCM. (E) Cumulative release of a payload from the PCM-capped PS bottles at the indicated temperatures. The opening of the bottles was corked with a PCM made of a mixture of 1-tetradecanol and lauric acid at different volume ratios (α). The melting point of the PCM was dependent on the ratio. Adapted with permission.[14] Copyright 2013, Wiley-VCH.
The openings on the arrayed nanobottles were corked with PCM by bringing a PCM film coated on a silicon wafer into contact with the well-oriented nanobottles. Afterwards, the system was exposed to ethanol vapor under vacuum, which would soften the PCM, leaving behind a cork on the opening of each nanobottle. Figure 8C shows SEM and TEM (inset) images of the PS nanobottles after their openings had been corked with a PCM. The thermo-responsive cork can then serve as a smart, “on or off” gate to control the release of payload inside the bottle (Figure 8D). Significantly, the release profile of the payload could be tuned by varying the composition of the cork (Figure 8E).[14] For example, when the PCM cork was based on a mixture of 1-tetradecanol (with a melting point at 38 °C) and lauric acid (with a melting point at 44 °C) at a ratio of 0.6, almost all the pre-loaded dye in the nanobottles was released within 30 min at 39 °C. In comparison, when the cork was based on a mixture of 1-tetradecanol and lauric acid at a ratio of 0.05, it needed nearly12 h for the dye to be released.
6. Perspectives
In this Progress Report, we have discussed the concept of nanobottles and reviewed the recent developments on their syntheses, as well as applications in controlled release and drug delivery. The most attractive feature of a nanobottle is that there is only one opening on the hollow body. To this end, we have focused on experimental approaches capable of creating only one hole in the surface of a hollow nanoparticle. When explored as a carrier for drug delivery, this unique feature offers some immediate advantages, including a high loading capacity, quick loading and release process, and full protection of the payload(s). It has been demonstrated that many different types of payloads, including small molecules, biomacromolecules, imaging contrast agents, and functional nanoparticles, can all be easily and conveniently loaded into a nanobottle through the relatively large opening. The release profile of the payload(s) can be conveniently tuned by controlling the size of the opening, co-loading a stimuli-responsive material into the hollow interior, and/or corking the opening with a stopper. Despite the major successes achieved in the fabrication and utilization of nanobottles, a number of challenges remain to be addressed in the future research.
Diversity in terms of composition and size.
Even though a variety of nanobottles have been reported, their diversity in terms of composition and size still needs to be expanded. For most biomedical applications, it is preferable for the carriers to be biocompatible and biodegradable.[17] To this end, nanobottles made of biocompatible and biodegradable polymers such as PCL, poly(lactic-co-glycolic acid) (PLGA), or natural biopolymers will offer an advantage, especially when moving to in vivo applications. Size is another important parameter of the carrier, which plays a critical role in determining the biodistribution, toxicity, and targeting ability.[17,53] For example, a smaller size typically leads to a longer circulation time and higher uptake efficiency by the malignant cells.[17] However, most of the nanobottles reported in literature have an overall dimension larger than 100 nm. To this end, development of nanobottles with size less than 100 nm, or even less than 50 nm, will allow us to greatly improve the performance of nanobottles in drug delivery and related applications.
Surface modification.
The surface properties of nanoparticles is essential to their circulation half‐life, targeting capability, and clearance from the body.[17,54] For example, coating the surface of a carrier with a layer of PEG can protect it from the adsorption of plasma proteins and thus avoid being uptaken by macrophages.[55] In addition, a targeting ligand often needs to be added to the surface of a carrier to enable site-specific drug delivery.[56,57] To this end, modifying the outer surface of nanobottles with an appropriate ligand will enhance their performance in drug delivery and related applications. In the ideal situation, the solid wall on the nanobottles should provide well-defined functional groups for surface modification.
Integration with stimuli-responsive materials.
Incorporation of a stimuli-responsive material into the design of nanobottles offers the ability to precisely control the release of a payload. The on-demand release can be leveraged to improve the therapeutic efficacy while reducing the off-target toxicity. To this end, a thermo-responsive material such as PCM has been used as a smart filler or a smart cork to trigger and control the release by varying the temperature. The release is controlled by the change in molecular mobility of PCM during its solid-to-liquid phase transition. Materials responsive to other types of stimuli (e.g., pH, ultrasound, light, mechanical stress, or reactive biomolecules) can also be used to achieve on-demand release for nanobottles.[58-60]
Other applications involving encapsulation.
Here we have mainly focus on the biomedical applications of nanobottles in terms of controlled release and drug delivery. It is worth pointing out that nanobottles can also be used to encapsulate many other types of payloads, including reactive chemical agents, catalytic materials, cosmetics, inks, and pigments that are widely used in applications ranging from energy storage to chemical conversion, as well as stabilization of colloidal particles and removal of pollutants.[5-8,10] Furthermore, the payloads can be encapsulated in many different forms, including solutions, colloidal suspensions, nanoparticles, and mixtures of various components. As an example, silica nanobottles loaded with Au nanoparticles have been explored as nanoreactors for the catalytic reduction of 4-nitrophenol.[6]
We hope the readers will enjoy the concept of nanobottles, as well as their synthetic methods and biomedical applications, discussed in this Progress Report and perhaps find some inspiration to move this unique class of nanomaterials to the next level of success.
Acknowledgments
This work was supported in part by startup funds from the Georgia Institute of Technology, a grant from the National Institutes of Health (NIH, R01 CA138527), and an NIH Director’s Pioneer Award (DP1 OD000798). We are grateful to our coworkers and collaborators for their invaluable contributions to this project.
Biography

Jichuan Qiu received his Ph.D. in materials chemistry and physics from Shandong University in 2018 with Prof. Hong Liu. He joined the Xia group at the Georgia Institute of Technology as a visiting graduate student in 2016 and then continued as a postdoctoral fellow since 2018. His current research interest includes the design and rational synthesis of nanostructured materials for biomedical applications.

Jianchang Xu received his B.S. degree in materials engineering from China University of Petroleum (East China) in 2017. He is pursuing his Ph.D. degree with Prof. Lijuan Zhang at the South China University of Technology. As a visiting student, he joined the Xia group at the Georgia Institute of Technology in 2019 and is working on the synthesis of nanostructured materials for biomedical applications.

Younan Xia studied at the University of Science and Technology of China (B.S., 1987) and University of Pennsylvania (M.S., 1993) before receiving his Ph.D. from Harvard University in 1996 (with George M. Whitesides). He started as an assistant professor of chemistry at the University of Washington (Seattle) in 1997 and was promoted to associate professor and professor in 2002 and 2004, respectively. He joined the Department of Biomedical Engineering at Washington University in St. Louis in 2007 as the James M. McKelvey Professor. Since 2012, he holds the position of Brock Family Chair and GRA Eminent Scholar in Nanomedicine at the Georgia Institute of Technology.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Contributor Information
Jichuan Qiu, The Wallace H. Coulter Department of Biomedical Engineering, Georgia Institute of Technology and Emory University, Atlanta, GA 30332, USA.
Jianchang Xu, The Wallace H. Coulter Department of Biomedical Engineering, Georgia Institute of Technology and Emory University, Atlanta, GA 30332, USA.
Younan Xia, The Wallace H. Coulter Department of Biomedical Engineering, Georgia Institute of Technology and Emory University, Atlanta, GA 30332, USA; School of Chemistry and Biochemistry, Georgia Institute of Technology, Atlanta, GA 30332, USA.
References
- [1].Xuan M, Mestre R, Gao C, Zhou C, He Q, Sánchez S, Angew. Chem. Int. Ed 2018, 57, 6838. [DOI] [PubMed] [Google Scholar]
- [2].Sun H, Liu D, Du J, Chem. Sci 2019, 10, 657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chang M-W, Stride E, Edirisinghe M, Langmuir 2010, 26, 5115. [DOI] [PubMed] [Google Scholar]
- [4].Han F, Wang R, Feng Y, Wang S, Liu L, Li X, Han Y, Chen H, Nat. Commun 2019, 10, 1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Guan G, Zhang Z, Wang Z, Liu B, Gao D, Xie C, Adv. Mater 2007, 19, 2370. [Google Scholar]
- [6].Yi D, Zhang Q, Liu Y, Song J, Tang Y, Caruso F, Wang Y, Angew. Chem. Int. Ed 2016, 55, 14733. [DOI] [PubMed] [Google Scholar]
- [7].Hyuk Im S, Jeong U, Xia Y, Nat. Mater 2005, 4, 671. [DOI] [PubMed] [Google Scholar]
- [8].Qiu J, Camargo PHC, Jeong U, Xia Y, Acc. Chem. Res 2019, 52, 3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Van Herk A, Forcada J, Pastorin G, Controlled Release Systems: Advances in Nanobottles and Active Nanoparticles, CRC Press, 2016. [Google Scholar]
- [10].Si Y, Chen M, Wu L, Chem. Soc. Rev 2016, 45, 690. [DOI] [PubMed] [Google Scholar]
- [11].An K, Hyeon T, Nano Today 2009, 4, 359. [Google Scholar]
- [12].Xia Y, Li W, Cobley CM, Chen J, Xia X, Zhang Q, Yang M, Cho EC, Brown PK, Acc. Chem. Res. 2011, 44, 914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang N, Cheng X, Li N, Wang H, Chen H, Adv. Healthcare Mater 2019, 8, 1801002. [DOI] [PubMed] [Google Scholar]
- [14].Hyun DC, Lu P, Choi S-I, Jeong U, Xia Y, Angew. Chem. Int. Ed 2013, 52, 10468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Qiu J, Huo D, Xue J, Zhu G, Liu H, Xia Y, Angew. Chem. Int. Ed 2019, 58, 10606. [DOI] [PubMed] [Google Scholar]
- [16].Li J, Mooney DJ, Nat. Rev. Mater 2016, 1, 16071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sun T, Zhang YS, Pang B, Hyun DC, Yang M, Xia Y, Angew. Chem. Int. Ed 2014, 53, 12320. [DOI] [PubMed] [Google Scholar]
- [18].Wang X, Feng J, Bai Y, Zhang Q, Yin Y, Chem. Rev 2016, 116, 10983. [DOI] [PubMed] [Google Scholar]
- [19].Yu L, Yu XY, Lou XW, Adv. Mater 2018, 30, 1800939. [Google Scholar]
- [20].Jeong U, Hyuk Im S, Camargo PHC, Kim JH, Xia Y, Langmuir 2007, 23, 10968. [DOI] [PubMed] [Google Scholar]
- [21].Li M, Xue J, Langmuir 2011, 27, 3229. [DOI] [PubMed] [Google Scholar]
- [22].Xu H, Liu X, Wang D, Chem. Mater 2011, 23, 5105. [Google Scholar]
- [23].Wang W, Zhang M-J, Xie R, Ju X-J, Yang C, Mou C-L, Weitz DA, Chu L-Y, Angew. Chem. Int. Ed 2013, 52, 8084. [DOI] [PubMed] [Google Scholar]
- [24].Zhou F-L, Chirazi A, Gough JE, Hubbard Cristinacce PL, Parker GJM, Langmuir 2017, 33, 13262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Han J, Song G, Guo R, Chem. Mater 2007, 19, 973. [Google Scholar]
- [26].Jiang S, Kaltbeitzel A, Hu M, Suraeva O, Crespy D, Landfester K, ACS Nano 2020, 14, 498. [DOI] [PubMed] [Google Scholar]
- [27].Qiu J, Xie M, Lyu Z, Gilroy KD, Liu H, Xia Y, Nano Lett. 2019, 19, 6703. [DOI] [PubMed] [Google Scholar]
- [28].Zhang H, Chen J, Li N, Jiang R, Zhu X-M, Wang J, ACS Appl. Mater. Interfaces 2019, 11, 5353. [DOI] [PubMed] [Google Scholar]
- [29].Mo AH, Landon PB, Gomez KS, Kang H, Lee J, Zhang C, Janetanakit W, Sant V, Lu T, Colburn DA, Akkiraju S, Dossou S, Cao Y, Lee K-F, Varghese S, Glinsky G, Lal R, Nanoscale 2016, 8, 11840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ye J, Lagae L, Maes G, Van Dorpe P, J. Mater. Chem 2011, 21, 14394. [Google Scholar]
- [31].Ridelman Y, Singh G, Popovitz-Biro R, Wolf SG, Das S, Klajn R, Small 2012, 8, 654. [DOI] [PubMed] [Google Scholar]
- [32].Ohnuma A, Cho EC, Camargo PHC, Au L, Ohtani B, Xia Y, J. Am. Chem. Soc 2009, 131, 1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ohnuma A, Cho EC, Jiang M, Ohtani B, Xia Y, Langmuir 2009, 25, 13880. [DOI] [PubMed] [Google Scholar]
- [34].Gallego J, Varani G, Acc. Chem. Res 2001, 34, 836. [DOI] [PubMed] [Google Scholar]
- [35].Schroeder A, Heller DA, Winslow MM, Dahlman JE, Pratt GW, Langer R, Jacks T, Anderson DG, Nat. Rev. Cancer 2012, 12, 39. [DOI] [PubMed] [Google Scholar]
- [36].Tayalia P, Mooney DJ, Adv. Mater 2009, 21, 3269. [DOI] [PubMed] [Google Scholar]
- [37].Rupaimoole R, Slack FJ, Nat. Rev. Drug Discov 2017, 16, 203. [DOI] [PubMed] [Google Scholar]
- [38].Subbiah R, Guldberg RE, Adv. Healthcare Mater 2019, 8, 1801000. [DOI] [PubMed] [Google Scholar]
- [39].Cheng L, Yang L, Meng F, Zhong Z, Adv. Healthcare Mater 2018, 7, 1800685. [DOI] [PubMed] [Google Scholar]
- [40].Li X, Zhou L, Wei Y, El-Toni AM, Zhang F, Zhao D, J. Am. Chem. Soc 2015, 137, 5903. [DOI] [PubMed] [Google Scholar]
- [41].Nie L, Xing D, Yang D, Zeng L, Zhou Q, Appl. Phys. Lett 2007, 90, 174109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kiessling F, Fokong S, Bzyl J, Lederle W, Palmowski M, Lammers T, Adv. Drug Deliv. Rev 2014, 72, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Banerjee R, Pavlides M, Tunnicliffe EM, Piechnik SK, Sarania N, Philips R, Collier JD, Booth JC, Schneider JE, Wang LM, Delaney DW, Fleming KA, Robson MD, Barnes E, Neubauer S, J. Hepatol 2014, 60, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Caldorera-Moore ME, Liechty WB, Peppas NA, Acc. Chem. Res 2011, 44, 1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Bai M-Y, Moran CH, Zhang L, Liu C, Zhang Y, Wang LV, Xia Y, Adv. Funct. Mater 2012, 22, 764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lee N, Yoo D, Ling D, Cho MH, Hyeon T, Cheon J, Chem. Rev 2015, 115, 10637. [DOI] [PubMed] [Google Scholar]
- [47].Peng F, Tu Y, Wilson DA, Chem. Soc. Rev 2017, 46, 5289. [DOI] [PubMed] [Google Scholar]
- [48].Qiu J, Huo D, Xia Y, Adv. Mater 2020, DOI: 10.1002/adma.202000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Hyun DC, Levinson NS, Jeong U, Xia Y, Angew. Chem. Int. Ed 2014, 53, 3780. [DOI] [PubMed] [Google Scholar]
- [50].Moon GD, Choi S-W, Cai X, Li W, Cho EC, Jeong U, Wang LV, Xia Y, J. Am. Chem. Soc 2011, 133, 4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Zhu C, Huo D, Chen Q, Xue J, Shen S, Xia Y, Adv. Mater 2017, 29, 1703702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Xue J, Wu T, Li J, Zhu C, Xia Y, Angew. Chem. Int. Ed 2019, 58, 3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kang H, Rho S, Stiles WR, Hu S, Baek Y, Hwang DW, Kashiwagi S, Kim MS, Choi HS, Adv. Healthcare Mater 2020, 9, 1901223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Qiu J, Xia Y, Nat. Nanotechnol 2020, 15, 252. [DOI] [PubMed] [Google Scholar]
- [55].Xia X, Yang M, Wang Y, Zheng Y, Li Q, Chen J, Xia Y, ACS Nano 2012, 6, 512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R, Nat. Nanotechnol 2007, 2, 751. [DOI] [PubMed] [Google Scholar]
- [57].Sun T, Wang Y, Wang Y, Xu J, Zhao X, Vangveravong S, Mach RH, Xia Y, Adv. Healthcare Mater 2014, 3, 1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Wang Y, Shim MS, Levinson NS, Sung H-W, Xia Y, Adv. Funct. Mater 2014, 24, 4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Mura S, Nicolas J, Couvreur P, Nat. Mater 2013, 12, 991. [DOI] [PubMed] [Google Scholar]
- [60].Yavuz MS, Cheng Y, Chen J, Cobley CM, Zhang Q, Rycenga M, Xie J, Kim C, Song KH, Schwartz AG, Wang LV, Xia Y, Nat. Mater 2009, 8, 935. [DOI] [PMC free article] [PubMed] [Google Scholar]