Abstract
Physical touch in the form of holding a loved one’s hand attenuates the neural response to threat (Coan, Schaefer, & Davidson, 2006). Speculation regarding the neural mediation of this effect points to the ventromedial prefrontal cortex (vmPFC), which is known to have inhibitory connections with threat responsive brain regions such as the amygdala. Despite the attractiveness of this hypothesis, a link between the vmPFC and diminished threat during handholding has been difficult to demonstrate empirically. Here we report that in a sample of 110 participants no evidence for vmPFC mediation of the handholding effect was obtained. Indeed, results indicated that connectivity patterns between threat responsive salience network structures and the vmPFC were in the opposite direction one would predict if the vmPFC mediated reductions in neural threat-responding caused by partner handholding. Our findings suggest that the vmPFC does not mediate the regulating effect of physical contact on neural threat responses.
Keywords: Emotion Regulation, Handholding, Close Relationships, vmPFC, Threat, Social Baseline
Social contact and social support are well-documented factors in health and well-being (Holt-Lunstad, Smith, & Layton, 2010). John Cacioppo (e.g., Cacioppo & Cacioppo, 2018) has long argued that loneliness is a major health issue. He spent much of his career exploring the importance of social connection for human health and well being To John, loneliness was an urgent public health issue, one requiring a detailed understanding of the social, behavioral, physiological, and neural mechanisms through which social connection realizes its effects..
Relationships may change health trajectories due to the impact of social connection on effort, stress, and emotion. Like, John, we have argued that access to reliable social resources constitutes a default or baseline assumption of the properly functioning human brain. Violations of this assumption compel increased investments in time, labor, and energy proportional to the increase in environmental demands brought about by relative isolation (Beckes and Coan; 2011, see also, Coan, Brown, & Beckes, 2014). Increased demand on personal resources is why isolation is so stressful, and why chronic isolation leads to both chronic stress (Gunnar & Quevedo, 2007) and impaired health (cf., Brown, Beckes, Allen, & Coan, 2017).
But we still do not know much about how the brain transforms social proximity into less threat-related activity and lower stress. What are the circuits or systems that cause other circuits or systems to be less active in the face of threat when social resources are available? Potentially the same mechanisms and systems that mediate other forms of emotion regulation. A prevailing hypothesis claims that links between relationships and health are mediated through inhibitory systems originating in the ventromedial prefrontal cortex (vmPFC). The vmPFC inhibits amygdala activity directly, and indirectly inhibits periaqueductal grey (PAG), dorsal anterior cingulate cortex (dACC) and anterior insula (Eisenberger, 2013; Morriss, Bell, Johnstone, van Reekum, & Hill, 2018). Indeed, the vmPFC is critical for the self-regulation of emotional responses, and the inhibitory connectivity between the vmPFC and affective excitatory systems (e.g., amygdala) strengthen during the extinction of conditioned fear (Ochsner & Gross, 2005). Thus, one possibility is that proximity to social resources increases inhibitory vmPFC activity, resulting in diminished activity in circuits mediating the salience of potential threats.
Previous studies have reported evidence consistent with the vmPFC hypothesis (Eisenberger et al., 2011; Morriss et al., 2018). For example, Eisenberger et al. (2011) found that during a pain task pictures of attachment figures activated a region of the ventromedial prefrontal cortex (vmPFC), and that activity in this vmPFC region was in turn negatively correlated with pain-related activity in the dACC. Similarly, Morriss et al. (2018) reported that during threat of shock, being told that an attachment figure was present in the scanning facility increased activation of portions of the vmPFC. At the same time, they observed decreased activation of amygdala, though they did not report any functional connectivity between the two systems.
It is interesting that in both of these studies, recruitment of vmPFC appears to follow or rely upon representations of a relational partner, as opposed to the partner’s actual, physical presence (either via direct observation or through physical touch such as handholding). Beckes and Coan (2011) have suggested that the presence of trusted relational partners is likely to regulate threat-related neural activations by acting directly on the perception of environmental demands, precluding the need for any intra-individual regulatory activity via vmPFC. Indeed, we (Coan et al., 2017; Coan, Beckes, & Allen, 2013; Coan, Schaefer, & Davidson, 2006; Johnson et al., 2013) have consistently found evidence that holding hands with a partner is associated with reduced neural responses to threat of shock, including prefrontal regions associated with emotion regulation, when compared to holding a stranger’s hand or being alone. In none of these studies have we observed threat-related increases during supportive handholding in the prefrontal cortex or elsewhere. This implies differences in how representations of relational partners and the actual presence of relational partners is mediated.
To further examine whether touch related reductions in threat activity are due to vmPFC regulation of the neural response to threat, data from our handholding studies could be subjected to a functional connectivity analysis like that used by Eisenberger and colleagues (2011). If the vmPFC is mediatingthe effects of partner touch, we should see greater negative correlations between threat responsive regions (e.g., dACC, anterior insula) and the vmPFC in the partner handholding condition. If no such evidence obtains, we might tentatively conclude that the mechanisms supporting representational social support (e.g., via digital images or imaginative procedures) and social support through physical contact (e.g., observable presence or physical touch) are distinct. For this study, we attempt to replicate Eisenberger and colleagues’ functional connectivity findings using the handholding data first reported in Coan and colleagues (2017).
Methods
Participants.
Participants (n=110) and an opposite gendered partner were brought into the lab for fMRI scans and data collection. (Data from this sample are also presented in Coan et al., 2017). Of the one hundred and ten dyads, 27 identified as friends, 29 were dating, 27 were cohabitating, and 27 were married. Scanned participants ranged in age from 23–26 and were 54% male and 46% female. Participants identified as 69% white, 26% African-American, 4% Hispanic, and 2% Asian. Participants were recruited from the VIDA longitudinal sample, (n = 86; Allen, Porter, McFarland, McElhaney, & Marsh, 2007; Coan et al., 2013; McElhaney, Antonishak, & Allen, 2008). Due to the lack of married couples in the VIDA sample, married couples were recruited from the general community (n = 24) and demographically matched to the VIDA sample. Pregnancy or risk of danger from the intense magnetic field of the MRI were used as exclusion criteria. We obtained informed consent from both partners and gave each $160.00 in compensation for their participation. This research was conducted under the approval of the University of Virginia’s Institutional Review Board.
Procedure.
Procedures are identical to Coan et al. (2017). Participants were screened for eligibility and brought in for questionnaires and scanning. Two AG-AgCL shock electrodes were applied to the participant’s ankle (left or right counterbalanced across participants). High resolution anatomical scans were then conducted on all participants.
During fMRI procedures, participants viewed stimuli projected onto a screen behind the magnet’s bore using a mirror placed on the head coil and were able to respond to stimuli using a button box. Trials were composed of a 1-second safety or threat cue, followed by 4–10 seconds of anticipation period indicated by a fixation cross. Then, a small dot appeared during which shocks were randomly delivered in threat trials only. The inter-trial interval was 4 to 10 seconds. Threat cues consisted of a red ‘X’ on a black background and indicated a 17% chance of electric shock while safety cues consisted of a blue ‘O’ on a black background, indicating no chance of shock. Participants viewed 10 threat cues with no shock, 2 with shock, and 12 safety cues in variable order within each of three counterbalanced blocks. During one block, the participant held the hand of their partner. During another they held the hand of an unseen confederate of the opposite sex and in another the participant was alone in the scanner. Shocks were generated by an isolated physiological stimulator (Coulbourn Instruments, Allentown, PA, USA) and lasted for 1 second at 4 mA. After each block, participants rated their subjective assessment of their current arousal and valence using the 9-point pictorial Self-Assessment Manakin (SAM) scales (Bradley & Lang, 1994).
Image acquisition and data preparation.
Functional images were acquired using a Simens 3.0 Tesla MAGNETOM Trio high-speed magnetic imaging device at University of Virginia’s Fontaine Research Park, with a circularly polarized transmit/receive head coil with integrated mirror. A total of 216 function T2*-weighted echo planar images (EPIs) sensitive to blood-oxygen-level-dependent contrasts were collected per block, in volumes of 28 3.5-mm transversal echo-planar slices (1-mm slice gap) covering the whole brain (1-mm slice gap, repetition time (TR) = 2000 ms, echo time (TE) = 40 ms, flip angle = 90 degrees, field of view (FOV)= 192, matrix = 64 X 64, voxel size = 3 X 3 X 3.5 mm). Before collection of functional images, 176 high-resolution T1-magnetization-prepared rapid-acquisition gradient echo images were acquired to determine the localization of function (1-mm slices, TR = 1900 ms, TE = 2.53 ms, flip angle = 9 degrees, FOV =250 mm, voxel size =1 X 1 X 1 mm).
Data were preprocessed and analyzed using FMRIB’s Software Library (FSL) software (Version 5.98; www.fmrib.ox.ac.uk/fsl, Worsley, 2001). Motion correction involved FMRIB’s Linear Image Registration Tool, and intra-modal correction algorithm tool (MCFLIRTl; Jenkinson, Bannister, Brady, & Smith, 2002), with slice scan time correction and a high-pass filtering cutoff point of 100 s, removing irrelevant signals. We used BET (Smith, Mulder, & Hill, 2001)brain extraction, eliminating non-brain material voxels in the fMRI data, and a 5-mm full width at half minimum Gaussian kernel for smoothing. Images were registered to the Montreal Neurological Institute (MNI) space by FLIRT (Jenkinson et al., 2002).
fMRI Data Analysis.
First level analysis of the functional data began with determination of functional regions of interests (ROI) for each subject using FEAT (FMRI Expert Analysis Tool) Version 5.98, part of FSL (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl) and time-series analysis by FILM (Worsley, 2001). Trials in which participants received shocks were modelled, but not analyzed in higher level analysis due to the potential of movement artifacts and our primary interest in anticipatory threat. Main effects of handholding using threat minus safe and safe minus threat contrasts were then individually explored using a second level analysis, collapsing across the handholding conditions and modelling all possible contrasts of handholding conditions. During a third level analysis, a group level analysis was performed using a whole brain corrected cluster-wise analysis. Details of the analytical methods and results from this analysis are available in Coan et al., (2017).
Psychophysiological Interaction (PPI) Data Analysis.
For the purposes of understanding whether the vmPFC is involved in reductions in the neural response to threat that occur during partner handholding we conducted PPI analyses using a 3-voxel3 ROI (3 X 3 X 3) centered on the vmPFC region used in Eisenberger et al.’s PPI analyses (2011; MNI coordinates: −6, 52, 16, modified from 1mm to a 2mm voxel size) as a seed region. Masks of this region were created for each subject in individual space. The mean time series in this ROI for the alone, stranger, and partner conditions was extracted for each participant using the fslmeants function. This time series was then used in a first level FEAT analysis for each subject as a physiological regressor. All other regressors used in the initial analysis were included as well. An additional interaction regressor (the PPI) modeling the interaction between the vmPFC and activity to the threat cues was included in the model. We also report the results of similar analyses on three alternative vmPFC ROIs identified in the previous literature in the supplementary materials. The first two ROIs were bilateral 3-voxel3 ROI coordinates from Urry et al. (2006; MNI coordinates: right 6, 42, −14 and left −22, 48, −10). The third was a 3-voxel3 ROI from Phelps et al. (2004; MNI coordinates: −4, 34, −8). First level analyses for these three ROIs were identical to the Eisenberg et al. seed region above.
At the second level we modeled the impact of handholding condition by collapsing lower level PPI effects across both alone and partner handholding conditions in a first analysis, and across both stranger and partner conditions in a second. We set up each analysis to model the PPI in each condition separately, and in the contrasts: alone minus partner and partner minus alone. The same procedure was followed to allow comparison between the partner and stranger conditions in a second analysis (stranger minus partner and partner minus stranger). This allowed us to detect simple correlations between the vmPFC and threat activity in other brain regions in each handholding condition and the relative difference in those correlations between the partner condition and each of the other two conditions. Second level results were collapsed across all participants for third level group analyses using FSL ordinary least squares.
A mask including only portions of the brain more active to threat than safety cues in the alone condition of the original analysis was used to constrain the PPI analysis to only those regions in which threat activity likely occurred. Third level results were corrected using a cluster-wise correction with a voxel level threshold of z > 1.96 and cluster extent threshold of p < .05 using random field theory. We then used an uncorrected threshold of voxel p < .05 in a second analysis to ensure that we were not missing any possible evidence for vmPFC mediation of the handholding effect and avoid any possible false negatives. We purposefully used liberal thresholds in an attempt to find any evidence for the hypothesis that the handholding effects are mediated by vmPFC activity.
Due to sample, scanning, and individual brain differences, it is possible that the vmPFC ROI focused PPI analyses conducted missed the portion of the vmPFC involved in the social regulation of emotion. To mitigate this concern, we conducted additional PPIs with seed masks based on ROIs in which threat activity was significantly reduced in the partner condition (see, Coan et al., 2017). We used the same procedures as we did for the vmPFC ROIs and used a cluster-wise threshold of voxel z > 1.96 and cluster p < .05. These analyses were conducted twice, once using whole brain correction to find any connectivity differences across the entire brain, and once using a mask covering the vmPFC. This analysis of dlPFC and dACC connectivity employed small volume correction using a mask of all voxels included in the Harvard-Oxford Cortical Structural Atlas indicating a non-zero probability of location in the medial frontal cortex.
These PPI analyses focused on seed regions in the dorsolateral prefrontal cortex (dlPFC; 32, 30 ,22), a primary hub in the executive network, and the dACC (0, 6, 46), a primary hub in the salience network. We chose these ROIS as seed regions because they are representative of the networks that are most activated by threat and consistently emerge as moderated by handholding (see Coan et al., 2006; 2017; Johnson et al., 2013; These ROIs were functionally defined and reported in Coan et al. (2017).
Results
PPI Results.
Analyses were constructed to detect significant differences in vmPFC connectivity to threat regions as a function of handholding condition.
Alone minus Partner Contrast.
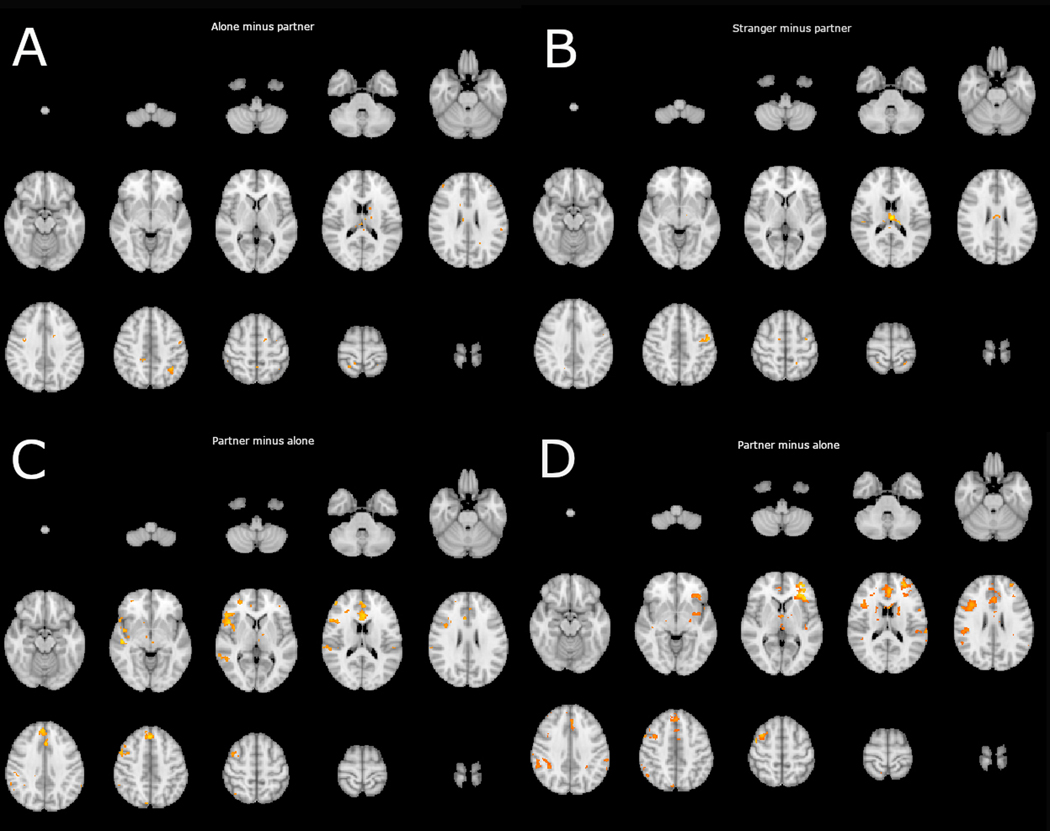
There were no regions in which the correlation between the vmPFC and threat activity was significantly more positive in the alone condition than in the partner condition. This is inconsistent with the theoretical stance that social contact down-regulates negative affect through vmPFC mediated inhibitory connections. If social contact were to work through these connections, providing a “safety signal” via the vmPFC, then this contrast should have revealed a pattern of increased negative connectivity between the vmPFC and threat responsive regions. Moreover, we found little evidence for this pattern of results in the uncorrected analysis. Small clusters of voxels hinted at it, but they were widely dispersed and minimal (see Figure 1 for a lightbox image of the uncorrected analysis).
Figure 1.
depicts significant (p<.05) voxels, uncorrected for multiple comparisons, detected in PPI analysis using a vmPFC (−6,52,16) seed region. Each panel shows a lightbox image of a comparison of interest. Panel A indicates voxels with which the vmPFC displayed more positive connectivity in the alone condition than in the partner condition. Panel B indicates voxels with which the vmPFC displayed more positive connectivity in the stranger condition than in the partner condition. Panel C indicates voxels with which the vmPFC displayed more positive connectivity in the partner condition than in the alone condition. Panel D indicates voxels with which the vmPFC displayed more positive connectivity in the partner condition than in the stranger condition.
Partner minus Alone Contrast.
Similarly, no significant clusters emerged in the partner minus alone contrast indicating no significant difference in the correlation between vmPFC activity and threat activity in the partner or the alone conditions. However, more voxels were found significant (voxel threshold p <.05) in the uncorrected analysis. Most critically, clusters of significant voxels uncorrected for multiple comparisons were detected in the dACC and portions of the anterior insula and its junction with the inferior frontal gyrus and orbitofrontal cortex (see Figure 1 for a lightbox image of this analysis). Similar findings emerged from analyses using different vmPFC regions of interest. These findings are reported in the supplementary material.
Stranger minus Partner Contrast.
There were no regions in which the correlation between the vmPFC and threat activity in the threat matrix was significantly more positive in the stranger condition than in the partner condition when corrected. This is also inconsistent with the vmPFC hypothesis and directly counter to Eisenberger and colleagues (2011) findings. Again, we found little evidence for this pattern of results in the uncorrected analysis. Small clusters of voxels indicated this pattern, but they were widely dispersed and minimal.
Partner minus Stranger Contrast.
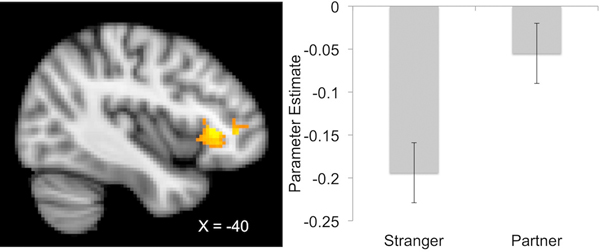
We detected a significant cluster corrected for multiple comparisons in the partner minus stranger contrast (754 voxels, peak MNI coordinates: −40, 28, 2, z-max = 3.86). This cluster was primarily located within the anterior insula, frontal operculum, inferior frontal gyrus, and orbitofrontal cortex (See Figure 2). More voxels were found significant in the uncorrected analysis. Clusters of significant voxels were detected in the dACC in addition to voxels bilaterally located in lateral prefrontal, insular, oppercular, and posterior parietal regions (see Figure 3 for a lightbox image of this analysis). Similar findings emerged from analyses using different vmPFC regions of interest. These findings are reported in the supplementary material.
Figure 2.
depicts a sagittal slice (x = −40) with a significant cluster detected in the junction between the anterior insula, frontal operculum, inferior frontal gyrus, and orbitofrontal cortex (−40, 28, 2) in a PPI analysis using a vmPFC (−6,52,16) seed region. The right portion of the figure depicts the mean parameter estimates of the correlation between the vmPFC and voxels within this significant cluster (corrected for multiple comparisons).
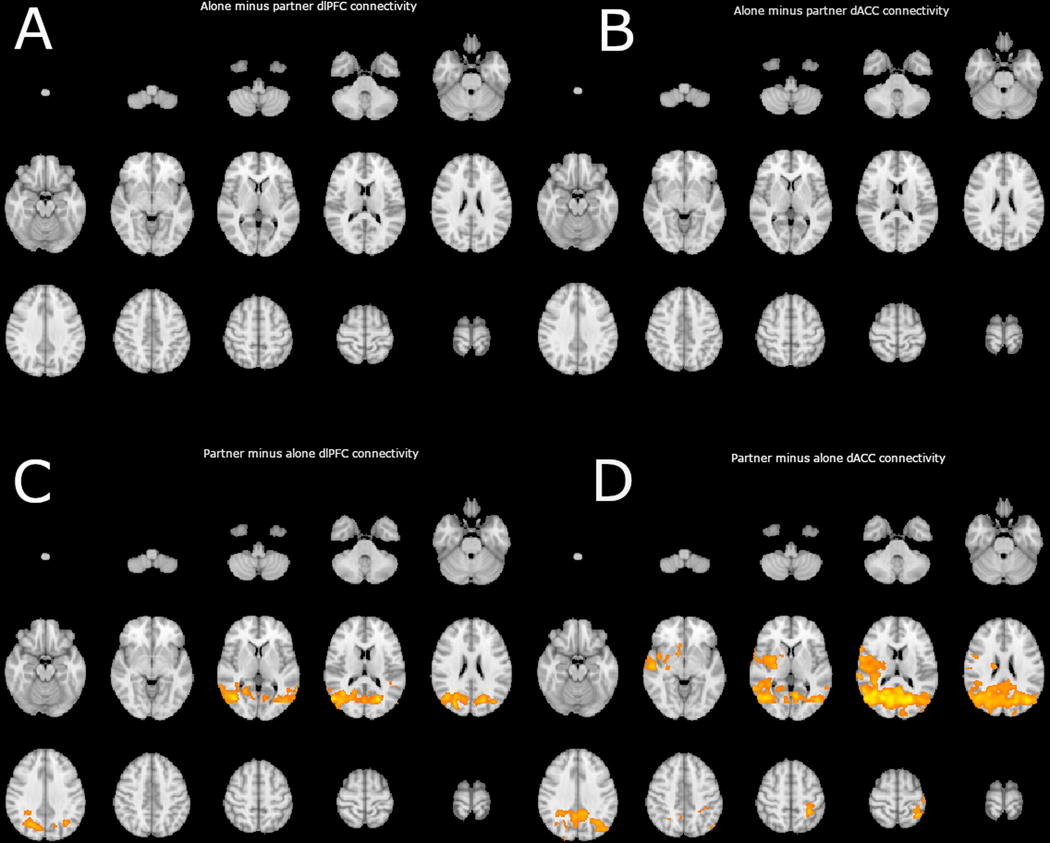
Figure 3.
depicts significant clusters of connectivity corrected for multiple comparisons, detected in PPI analysis using a dlPFC (32,30,22) seed region (depicted on the left) and a dACC (0, 6, 46) seed region (depicted on the right). Each panel shows a lightbox image of a comparison of interest between the partner and alone conditions. Panel A indicates clusters with which the dlPFC displayed more positive connectivity in the alone condition than in the partner condition, as indicated, no significant clusters emerged. Panel B indicates clusters with which the dACC displayed more positive connectivity in the alone condition than in the partner condition, as indicated, no significant clusters emerged. Panel C indicates clusters with which the dlPFC displayed more positive connectivity in the partner condition than in the alone condition. Panel D indicates clusters with which the dACC displayed more positive connectivity in the partner condition than in the alone condition.
Secondary PPI.
To further explore these findings, we conducted additional analyses using ROIs derived from Coan et al. (2017) in which threat activity was dampened during partner handholding. We report results from the dlPFC and dACC here as they are representative of the salience and executive networks and are reliably less active during partner handholding across studies. In an initial set of tests, we looked at differential connectivity between the partner handholding condition and each of the other two conditions across the entire brain. In a second set of tests, we looked for differential connectivity between the partner handholding condition and each of the other two conditions selectively in the medial prefrontal cortex. Analyses on additional regions of interest are available in the supplementary materials.
Alone minus Partner Contrasts.
No significant clusters emerge in the alone minus partner contrasts in either region of interest. This indicates that no region is significantly more negatively correlated with the dlPFC or the dACC during the partner handholding condition versus the alone condition (see Figure 3). An additional PPI analysis of dlPFC and dACC connectivity employed small volume correction using a mask of all voxels included in the Harvard-Oxford Cortical Structural Atlas indicating a non-zero probability of location in the medial frontal cortex. This analysis produced null effects and no indication of enhanced negative connectivity in the partner condition between either dlPFC or dACC.
Partner minus Alone Contrasts.
Significant clusters of voxels emerged in the partner minus alone contrasts in both the dlPFC and dACC. Extra-striate visual areas were more positively correlated with dlPFC during partner handholding than during the alone condition (see Table 1 and Figure 3). The same pattern of connectivity emerged with dACC connectivity indicating stronger positive connectivity between dACC and occipital cortex in the partner condition. This pattern of enhanced positive connectivity in the partner condition also extended in parietal and temporal association cortex, primary interoceptive cortex, and salience network (insula, operculum, and inferior frontal gyrus), dorsal and ventral striate, thalamus, and primary somatosensory and motor cortex.
Table 1.
Significant clusters of connectivity detected in comparisons between the partner condition and alone condition using a dlPFC (32,30,22) seed region (depicted on the top) and a dACC (0, 6, 46) seed region (depicted on the bottom).
| dlPFC Connectivity Partner - Alone | MNI Coordinates of Z-Max | |||||
|---|---|---|---|---|---|---|
| # Voxels | Peak Location | Z-Max | X | Y | Z | |
| Cluster 1 | 6136 | Occipital Cortex | 4.24 | 54 | −70 | 16 |
| dACC Connectivity Partner - Alone | MNI Coordinates of Z-Max | |||||
| # Voxels | Peak Location | Z-Max | X | Y | Z | |
| Cluster 1 | 18105 | Occipital Cortex* | 4.12 | 42 | −62 | 12 |
Extends into parietal and temporal association cortex, primary somatosensory and motor cortex, dorsal and ventral striate, salience network (insula, inferior frontal gyrus, opperculum), and thalamus
An additional PPI analysis of dlPFC and dACC connectivity employed small volume correction using a mask of all voxels included in the Harvard-Oxford Cortical Structural Atlas indicating a non-zero probability of location in the medial frontal cortex. This analysis produced null effects and no indication of enhanced positive connectivity in either condition between the vmPFC and either dlPFC or dACC.
Stranger minus Partner Contrasts.
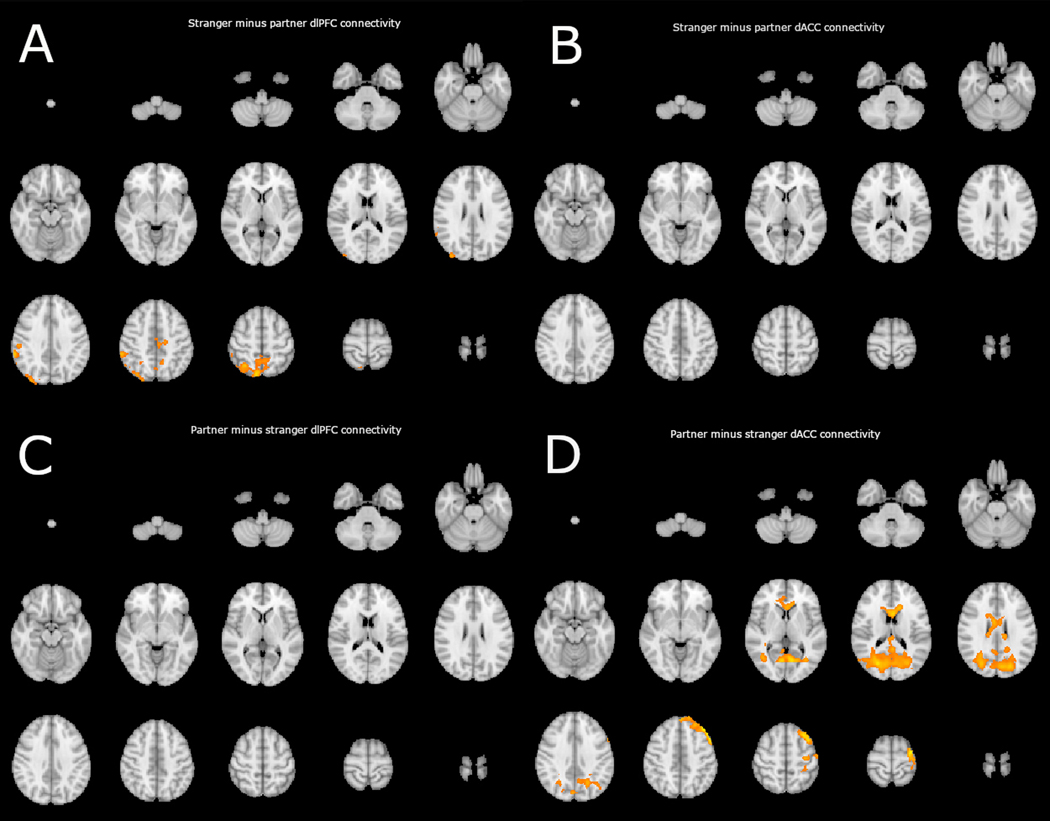
No significant clusters emerged in the stranger minus partner contrasts with the dACC seed region. This indicates that no region is significantly more negatively correlated with dACC during the partner condition versus the stranger condition (see Figure 4). Alternatively, one significant cluster was detected using the dlPFC seed region (see table 2). This cluster peaked in the ipsilateral posterior parietal lobe consistent with more positive connectivity in the right executive network during stranger handholding than during partner handholding.
Figure 4.
depicts significant clusters of connectivity corrected for multiple comparisons, detected in PPI analysis using a dlPFC (32,30,22) seed region (depicted on the left) and a dACC (0, 6, 46) seed region (depicted on the right). Each panel shows a lightbox image of a comparison of interest between the partner and stranger conditions. Panel A indicates clusters with which the dlPFC displayed more positive connectivity in the alone condition than in the partner condition. Panel B indicates clusters with which the dACC displayed more positive connectivity in the stranger condition than in the partner condition, as indicated, no significant clusters emerged. Panel C indicates clusters with which the dlPFC displayed more positive connectivity in the partner condition than in the stranger condition, as indicated, no significant clusters emerged. Panel D indicates clusters with which the dACC displayed more positive connectivity in the partner condition than in the stranger condition.
Table 2.
Significant clusters of connectivity detected in comparisons between the partner condition and stranger condition using a dlPFC (32,30,22) seed region (depicted on the top) and a dACC (0, 6, 46) seed region (depicted on the bottom).
| dlPFC Connectivity Stranger - Partner | MNI Coordinates of Z-Max | |||||
|---|---|---|---|---|---|---|
| # Voxels | Peak Location | Z-Max | X | Y | Z | |
| Cluster 1 | 6136 | Posterior Parietal Cortex | 4.24 | 54 | −70 | 16 |
| dACC Connectivity Partner - Stranger | MNI Coordinates of Z-Max | |||||
| # Voxels | Peak Location | Z-Max | X | Y | Z | |
| Cluster 1 | 7281 | Occipital Cortex* | 3.64 | 24 | −74 | 16 |
| Cluster 2 | 1690 | dlPFC** | 4.1 | −40 | 32 | 46 |
| Cluster 3 | 1263 | Corpus Callosum | 3.99 | −4 | 12 | 18 |
Extends into parietal and temporal association cortex
Extends into primary motor and somatosensory cortex
An additional PPI analysis of dlPFC and dACC connectivity employed small volume correction using a mask of all voxels included in the Harvard-Oxford Cortical Structural Atlas indicating a non-zero probability of location in the medial frontal cortex. This analysis produced null effects and no indication of enhanced negative connectivity in the partner condition between either dlPFC or dACC.
Partner minus Stranger Contrasts.
Significant clusters of voxels emerged in the partner minus stranger contrasts with the dACC seed region. DACC was more positively correlated with extrastriate visual cortex, corpus callosum, and left dlPFC during partner handholding than during the stranger condition (see Table 2 and Figure 4).
An additional PPI analysis of dlPFC and dACC connectivity employed small volume correction using a mask of all voxels included in the Harvard-Oxford Cortical Structural Atlas indicating a non-zero probability of location in the medial frontal cortex. This analysis produced null effects and no indication of enhanced positive connectivity in the partner condition between the vmPFC and either dlPFC or dACC.
Discussion
Competing Hypotheses.
This analysis pitted two competing hypotheses against each other regarding the mediating role of the vmPFC in the social regulation of threat response. On the one hand Beckes and Coan (2011) argue that the decreased threat response of individuals holding hands with a partner is not mediated through the vmPFC, and on the other Eisenberger (2013) argues that the vmPFC mediates this threat reduction. The results here support the former hypothesis, that the vmPFC is not mediating these effects. If the vmPFC were mediating the effects of handholding, then one would expect increased vmPFC activity during the handholding condition relative to the alone condition (which is not the case, see Coan et al., 2017, 2006, and the supplementary materials). Barring an increase in vmPFC activity, it is possible that the vmPFC mediates this effect through increased inhibitory or negative connectivity with threat responsive neural structures during the partner condition. The PPI analysis in this study, however, indicates that the main effect of partner handholding is not mediated through the vmPFC as the pattern of results is more consistently opposite that predicted in Eisenberger’s (2013) model. Whereas we find the typical negative association between vmPFC activity and threat activity in the alone and stranger conditions, this pattern is either diminished or absent in the partner condition.
Alternative Explanations.
This brings up the concern discussed in the introduction regarding why Eisenberger et al. (2011) and others have found vmPFC mediation of social contact on the neural threat and pain responses, whereas we have not. We posit that images or thoughts of a loved one act on different mechanisms than does real, physical social contact (and possibly presence – cf, Connor et al., 2012). To date all of the studies indicating vmPFC mediation of the decreased threat or pain response have relied on either a pictorial or mental representation of the partner, rather than contact with the partner per se. There are at least three possible explanations for these discrepancies.
The first explanation assumes that mental representations are evoked by pictures and that mental representations of the partner activate the vmPFC as part of the social mentalizing network (see Frith & Frith, 2006 for a review). This is a plausible explanation for vmPFC activity in the aforementioned studies, but it does not necessarily explain either the apparent mediating role of the vmPFC in previous studies of social emotion regulation, nor does it provide any insight into the potential mechanisms of the handholding studies.
The second possible explanation is that handholding and pictures/mental representations engage two distinct types of social reward. If the social regulation of threat and pain responding is mediated through a reward or safety signal (Eisenberger, 2013), then it is plausible, and consistent with current neurobiology, that the type of reward engaged in touch manipulations is fundamentally different than that engaged in pictorial or mentalizing manipulations. Behaviorists have long recognized a distinction between primary and secondary reward, and recent neuroimaging studies support the hypothesis that primary rewards are not prefrontally mediated, whereas secondary rewards may be (Beck, Locke, Savine, Jimura, & Braver, 2010). Touch acts as a primary reward for a variety of social species (Dunbar, 2010). This discovery was made famous in a series of classic studies by Harry Harlowe (1958) on infant monkeys, in which he demonstrated the monkey’s preferred the soft feel of cloth surrogate mothers to wire surrogates who provided milk.
Secondary reward emerges from repeatedly associating a stimulus with primary reward until it adopts the reward signal of the primary reward. Pictures and mental representations are likely secondary reinforcers, although they may contain some features that act as primary reinforcers (Beckes, Simpson, & Erickson, 2010; c.f., Hofer, 2006). A variety of neural systems are likely involved in secondary reward, but in this context the regulating properties of pictures or mental representations depend on the vmPFC. The vmPFC is known to represent the reward value of stimuli in a variety of situations (Winecoff et al., 2013), and possesses inhibitory pathways to the amygdala typically involved in fear extinction (Phelps, Nearing, Delgado, & LeDoux, 2004) indicating its role in modulation of lower level learning circuits.
A third explanation is similar to that of the primary versus secondary reward hypothesis, but does not depend on reward per se. This alternative may work independently of or in parallel with the social reward explanation. From this view, the presence of a predictable, reliable source of social support changes the individual’s estimation of environmental demands and personal resources through shifts in early perception (Gibson, 1979; Beckes, Ijzerman, & Tops, 2015;c.f., Beckes & Coan, 2011). A reliable partner can do many things for us, such as care for us when ill or injured, help share effort in achieving goals, or protect us from threats. Indeed, the partner may become intrinsically incorporated into the individual’s mental model of the self (Beckes, Coan, & Hasselmo, 2013) and modify the perception of the threat directly, making the world look, sound or feel less dangerous.
Such a shift in perception might obviate the need for self-regulation, diminishing reliance on prefrontal mechanisms to manage responses to threat. The hypothesis that the partner changes the perception of threat is bolstered by the consistent findings of reduced activity in the dlPFC and dACC during partner handholding, as if the partner’s literal presence (and thus their immediately accessible resources) obviate the need for costly top-down control (including emotion regulation) in responding to the threat. This would be consistent with the finding that dACC is more positively connected with extra-striate visual cortex, primary somatosensory, motor, and interoceptive cortex, left dlPFC, and other portions of the salience network in the partner condition. The same connectivity pattern between salience network and primary somatosensory cortex is also consistent with findings indicating a mediating role for such connectivity in the social regulation of pain (López-Solà, Geuter, Koban, Coan, & Wager, 2019). Alternatively, in the stranger condition, the dlPFC is more positively correlated primarily with other portions of the executive network. This may indicate more perseveration on the threat in the stranger condition, and the top-down coordination of executive networks to try to “solve the problem.”
Future studies should investigate whether neural substrates involved in reward, such as endogenous opioids, are necessary for the threat reducing impact of social contact. If so, such a finding would support the distinct reward pathways hypothesis for the effects of handholding. If not, then the case for a feed-forward process in which perception is directly changed by partner presence would seem more plausible given the fewer number of plausible alternative mechanisms.
Notably this study has limitations. First, handholding and picture viewing were not manipulated in the same experiment. This limits direct comparisons between the two and allows only hypotheses to be generated regarding the relationship between actual physical contact with loved ones and graphic or mental representations of loved ones. Second, self-regulation is not explicitly manipulated in this study. In most neuroscience studies of emotion regulation, the participant is instructed to manipulate their emotional state by reappraisal, suppression, or another self-regulation strategy, which helps disentangle important intra-psychic processes involved in emotion regulation. Future studies should be conducted to correct for both of these problems. Finally, we found only a few significant effects after correction for multiple comparisons with relatively liberal thresholds. Despite this limitation, given our hypothesis, the trend of data in the opposite direction of the hypothesis that vmPFC mediates the regulatory effects of handholding on the neural response to threat is reasonable evidence for our inferences. Moreover, we found no evidence in support of the vmPFC hypothesis despite using rather weak thresholds.
Conclusion
Social emotion regulation is not a simple singular process. Both representation of loved ones and their physical presence/touch are associated with decreased neural and affective responses to threat and pain (Coan et al., 2017; Eisenberger et al., 2011; O’Conner, Siegle, McFarland, Silk, Ladouceur, Dahl, Coan, & Ryan, 2012). The use of internal representations of loved ones appears to rely on prefrontal feedback mechanisms, whereas physical touch does not. This suggests multiple pathways through which interpersonal relationships impact an individual’s ability to cope with the stressors of everyday life. Social baseline theory (Beckes & Coan, 2011) argues that actual contact and interaction are superior to representational processes in part because they are less resource intensive, and more automatic. Despite these potential benefits, both types of social emotion regulation are likely critical for the benefits of positive relationships and social connection on human health and well-being. As John Cacioppo (e.g., Cacioppo & Patrick, 2009) has long argued, humans need social connection to live well. Positive thoughts and images of our social resources certainly do contribute to our health and well being, though perhaps not to the same extant or in the same way as the perceived physical presence and immediate availability of those social resources. This paper was also inspired by John’s insistence that in these and other questions the matter of primary concern is what the data have to say—and, to that end, that we be willing to publish and discuss apparently “null” results. In the end, we dedicate this paper to his memory, not only because of the subject matter, but because of the way he inspired us to critically examine our data as we continue the long and sometimes vexing search for the neural and physiological mechanisms of the social and psychological phenomena that matter to us.
Supplementary Material
Acknowledgements:
We thank Joseph P. Allen, Casey Brown, Karen Hasselmo, Erin Maresh, Marlen Gonzalez, Zoe Englander, Alexander Tatum, and Cat Thrasher for their assistance in preparing and conducting this study.
Disclosure Statement: This research was supported by a National Institute of Mental Health grant, Award Number R01MH080725, to James A. Coan.
Footnotes
The authors declare no conflicts of interest.
References
- Allen JP, Porter M, McFarland C, McElhaney KB, & Marsh P. (2007). The Relation of Attachment Security to Adolescents’ Paternal and Peer Relationships, Depression, and Externalizing Behavior. Child Development, 78(4), 1222–1239. 10.1016/j.pestbp.2011.02.012.Investigations [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck SM, Locke HS, Savine AC, Jimura K, & Braver TS (2010). Primary and secondary rewards differentially modulate neural activity dynamics during working memory. PLoS ONE, 5(2). 10.1371/journal.pone.0009251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckes L. & Coan JA (2011). Social baseline theory: The role of social proximity in emotion and economy of action. Social and Personality Psychology Compass, 5, 976–988. [Google Scholar]
- Beckes L, Coan JA, & Hasselmo K. (2013). Familiarity promotes the blurring of self and other in the neural representation of threat. Social Cognitive and Affective Neuroscience, 8, 670–677. 10.1093/scan/nss046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckes L, Ijzerman H. & Tops M. (2015). Toward a radically embodied neuroscience of attachment and relationships. Frontiers in Human Neuroscience. 10.3389/fnhum.2015.00266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckes L, Simpson JA, & Erickson A. (2010). Stimulus Pairings Of Snakes and Succor : Learning Secure Attachment Associations With Novel Faces via Negative. Psychological Science, 21(5), 721–728. 10.1177/0956797610368061 [DOI] [PubMed] [Google Scholar]
- Bradley M, & Lang PJ (1994). Measuring Emotion: The Self-Assessment Semantic Differential Manikin and the. Journal of Behavior Therapy and Experimental Psychiatry, 25(I), 49–59. 10.1016/0005-7916(94)90063-9 [DOI] [PubMed] [Google Scholar]
- Brown CL, Beckes L, Allen JP, & Coan JA (2017). Subjective General Health and the Social Regulation of Hypothalamic Activity. Psychosomatic Medicine, 79(6), 670–673. 10.1097/PSY.0000000000000468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, & Patrick W. (2009). Loneliness: Human nature and the need for social connection. New York: W. W. Norton & Company. [Google Scholar]
- Cacioppo JT, & Cacioppo S. (2018). The growing problem of loneliness. The Lancet, 391, 426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan JA, Beckes L, & Allen JP (2013). Regulatory Impact of Social Relationships in Adulthood. International Journal of Psychophysiology, 88(3), 224–231. 10.1016/j.ijpsycho.2013.04.006.Childhood [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan JA, Beckes L, Gonzalez MZ, Maresh EL, Brown CL, & Hasselmo K. (2017). Relationship status and perceived support in the social regulation of neural responses to threat. Social Cognitive and Affective Neuroscience, 12(10), 1574–1583. 10.1093/scan/nsx091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan JA, Brown C, & Beckes L. (2014). Our social baseline: The role of social proximity in economy of action In Mikulincer M. & Shaver PR (Eds.) Nature and Formation of Social Connections: From Brain to Group (pp. 89–104). Washington DC, American Psychological Association Press. [Google Scholar]
- Coan JA, Schaefer HS, & Davidson RJ (2006). Lending a Hand: Social Regulation of the Neural Response to Threat. Psychological Science, 17(12). Retrieved from https://collab.itc.virginia.edu/access/content/group/c84012f3-0222-46b5-ae2d-2efd2fe5d96a/Coan%2CSchaefer_Davidson%2C2006.pdf [DOI] [PubMed] [Google Scholar]
- Conner OL, Siegle GJ, McFarland AM, Silk JS, Ladouceur CD, Dahl RE, … Ryan ND (2012). Mom—It Helps When You’re Right Here! Attenuation of Neural Stress Markers in Anxious Youths Whose Caregivers Are Present during fMRI. PLoS ONE, 7(12), e50680 10.1371/journal.pone.0050680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar RIM (2010). The social role of touch in humans and primates: Behavioural function and neurobiological mechanisms. Neuroscience & Biobehavioral Reviews, 34(2), 260–268. 10.1016/J.NEUBIOREV.2008.07.001 [DOI] [PubMed] [Google Scholar]
- Eisenberger NI (2013). Social ties and health: A social neuroscience perspective. Current Opinion Neurobiology, 23(3), 407–413. 10.1016/j.conb.2013.01.006.Social [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Master SL, Inagaki TI, Taylor SE, Shirinyan D, Lieberman MD, & Naliboff B. (2011). Attachment figures activate a safety signal-related neural region and reduce pain experience. Proceedings of the National Academy of Sciences, 1081, 11721–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CR, & Frith U. (2006). The neural basis of mentalizing. Neuron, 50, 531–534. [DOI] [PubMed] [Google Scholar]
- Gibson JJ (1979). The ecological approach to visual perception. Boston: Houghton-Mifflin [Google Scholar]
- Gunnar M, & Quevedo K. (2007). The Neurobiology of Stress and Development. Annual Review of Psychology, 58(1), 145–173. 10.1146/annurev.psych.58.110405.085605 [DOI] [PubMed] [Google Scholar]
- Harlow HF (1958). The nature of love. American psychologist, 13(12), 673. [DOI] [PubMed] [Google Scholar]
- Hofer MA (2006). Psychobiological Roots of Early Attachment. Current Directions in Psychological Science, 15(2), 84–88. 10.1111/j.0963-7214.2006.00412.x [DOI] [Google Scholar]
- Holt-Lunstad J, Smith TB, & Layton JB (2010). Social Relationships and Mortality Risk: A Meta-analytic Review. PLoS Medicine, 7(7). Retrieved from https://collab.itc.virginia.edu/access/content/group/c84012f3-0222-46b5-ae2d-2efd2fe5d96a/journal.pmed.1000316.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, & Smith S. (2002). Improved Optimization for the Robust and Accurate Linear Registration and Motion Correction of Brain Images. NeuroImage, 17, 825–841. 10.1006/nimg.2002.1132 [DOI] [PubMed] [Google Scholar]
- Johnson SM, Moser MB, Beckes L, Smith A, Dalgleish T, Halchuk R, … Coan JA (2013). Soothing the threatened brain: Leveraging contact comfort with emotionally focused therapy. PLoS ONE, 8(11). 10.1371/journal.pone.0079314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Solà M, Geuter S, Koban L, Coan JA & Wager TD (2019). Brain mechanisms of social touch-induced analgesia in females. Pain, 160, 2072–2085. [DOI] [PubMed] [Google Scholar]
- McElhaney KB, Antonishak J, & Allen JP (2008). “They Like Me, They Like Me Not”: Popularity and Adolescents’ Perceptions of Acceptance Predicting Social Functioning Over Time. Child Development, 79(3), 720–731. 10.1109/TMI.2012.2196707.Separate [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morriss J, Bell T, Johnstone T, van Reekum CM, & Hill J. (2019). Social domain based modulation of neural responses to threat: The different roles of romantic partners versus friends. Social Neuroscience, 14(4), 398–408. 10.1080/17470919.2018.1486735 [DOI] [PubMed] [Google Scholar]
- Ochsner KN, & Gross JJ (2005). The cognitive control of emotion. Trends in Cognitive Sciences, 9(5), 242–249. 10.1016/j.tics.2005.03.010 [DOI] [PubMed] [Google Scholar]
- O’Conner OL, Siegle GJ,. McFarland AM, Silk JS, Ladouceur CD Dahl RE, Coan JA, & Ryan ND (2012). Mom-it helps when you’re right here! Attenuation of neural stress markers in anxious youths whose caregivers are present during fMRI. PLoS One, 7, e50680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoda K, Okamoto Y, Nakashima K, Nittono H, Ura M, & Yamawaki S. (2009). Decreased ventral anterior cingulate cortex activity is associated with reduced social pain during emotional support. Social Neuroscience, 4(5), 443–454. 10.1080/17470910902955884 [DOI] [PubMed] [Google Scholar]
- Phelps EA, Nearing KI, Delgado MR, & LeDoux JE (2004). Extinction learning in humans: Role of the amygdala and vmPFC. Neuron, 43(6), 897–905. 10.1016/j.neuron.2004.08.042 [DOI] [PubMed] [Google Scholar]
- Smith EA, Mulder MB, & Hill J. (2001). Controversies in the evolutionary social sciences: a guide for the perplexed. TRENDS in Ecology & Evolution, 16(3). Retrieved from http://tree.trends.com [DOI] [PubMed] [Google Scholar]
- Uddin LQ (2017). Salience network of the human brain. Academic Press: London. [Google Scholar]
- Urry HL Van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL, & Davidson RJ (2006). Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the dirurnal pattern of cortisol excretion among older adults. Journal of Neuroscience, 26, 4415–4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winecoff A, Clithero JA, Carter RM, Bergman SR, Wang L, & Huettel SA (2013). Ventromedial prefrontal cortex encodes emotional value. The Journal of Neuroscience, 33(27), 11032–11039. 10.1523/JNEUROSCI.4317-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ (2001). Statistical Analysis of Activation Images In Functional MRI: An Introduction to Methods. Oxford University Press; Retrieved from https://pdfs.semanticscholar.org/0b27/ffcba6e006f86c7e52fce4cc853af9405329.pdf [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.