Abstract
Infections are a common complication in patients with many hematologic malignancies, however whether patients with myeloproliferative neoplasms (MPN) also are at an increased risk of infections is largely unknown. To assess the risk of serious infections, we performed a large population-based matched cohort study in Sweden including 8 363 MPN patients and 32 405 controls using high-quality registers between the years 1992–2013 with follow-up until 2015. The hazard ratio (HR) of any infection was 2.0 (95% confidence interval 1.9–2.0), of bacterial infections 1.9 (1.8–2.0), and of viral infections 2.1 (1.9–2.3). One of the largest risk increases was that of sepsis, HR 2.6 (2.4–2.9). The HR of any infection was highest in primary myelofibrosis 3.7 (3.2–4.1), and significantly elevated in all MPN subtypes; 1.7 (1.6–1.8) in polycythemia vera and 1.7 (1.5–1.8) in essential thrombocythemia. There was no significant difference in risk of infections between untreated patients and patients treated with hydroxyurea or interferon-α during the years 2006–2013. These novel findings of an overall increased risk of infections in MPN patients, irrespective of common cytoreductive treatments, suggest the increased risk of infection is inherent to the MPN.
Keywords: Myeloproliferative neoplasms, Polycythemia Vera, Primary Myelofibrosis, Essential Thrombocythemia, Infections
Introduction
Infections are an important cause of morbidity and mortality in patients with hematologic malignancies such as acute myeloid leukemia, myelodysplastic syndromes, multiple myeloma, and malignant lymphoma.(1–3) Myeloproliferative neoplasms (MPN) are a group of chronic hematologic malignancies characterized by excess proliferation of myeloid cell lineages, and the classical Philadelphia negative MPNs are polycythemia vera (PV), essential thrombocythemia (ET), primary myelofibrosis (PMF), and MPN unclassifiable (MPN-U).(4, 5) MPNs are associated with an increased risk of disease and treatment complications including venous thrombosis and transformation to acute myeloid leukemia.(6–9) In recent years, an increased risk of infections in patients with PMF under treatment with the JAK1/2 inhibitor ruxolitinib has been observed, caused by both common and more opportunistic infectious agents like reactivation of tuberculosis and cryptococci infections.(10–15) A previous study on causes of death by our group showed an increased risk of death from infection (HR 2.7, 95% CI 2.4–3.1) in patients with MPN compared to population controls.(16) We were therefore motivated to determine the risk of any serious infection in patients with MPN compared to the general population, and elucidate the most common types of infections, in relation to time since diagnosis and cytoreductive treatment. We conducted a cohort study using nationwide registers, and included all patients diagnosed with MPN in Sweden 1992–2013, with matched population controls for comparison.
Patients and Methods
Population and central registers
Sweden is a country with a population of 10 million, where health care is publicly provided and tax financed for all residents. Each resident is given a personal registration number at birth or immigration, which is unique and life-long, and used in all contacts with health care and in all health care registers, allowing efficient cross linking of information.(17)
Reporting of all new malignant diagnoses to the Swedish Cancer Register has been mandatory by law since 1958, and since 1984 both clinicians and pathologists are obliged to report incident cases. This double-reporting routine has ensured a well-validated register with high degree of coverage and accuracy.(18–20) The Polycythemia Vera Study Group diagnostic criteria were used for MPN diagnosis initially during the study period and was after 2001 gradually replaced by the WHO classification system.(21–23) All dates and causes of death are registered in the nationwide Cause of Death Register.(24, 25) All hospital admissions along with information on discharge diagnoses are captured in the Inpatient Register, established in 1964 and with complete national coverage from 1987.(26) The Swedish Prescribed Drug Register has since 2005 collected information on all drugs prescribed and dispensed at pharmacies in Sweden.(27)
Study participants
We identified all MPN patients in the Swedish Cancer Register who were diagnosed between January 1st 1992 and December 31st 2013, aged 18 or older at diagnosis. For each patient, four controls, matched by birth year and sex, were identified from the Register of Total Population.(28) Patients and controls could not have had a previous hematologic malignancy at the time of MPN diagnosis or matching date. The controls had to be free of MPN, living in Sweden, and alive at the date of their corresponding patients’ MPN diagnosis.
Information on outcomes; hospitalization or death due to infection, was obtained from the Inpatient Register and the Cause of Death Register. All diagnoses, both main and contributing, during admission or from death certificates were included. Patients and controls were followed until time of event, or censored at time of emigration, death, or end of follow up, December 31st 2015. Since we aimed to assess the association between MPN and risk of infections, leukemic transformation was also considered a censoring event, defined as a diagnosis of acute leukemia in either the Cancer Register or the Inpatient Register.
This study was approved by the regional ethics committee in Stockholm, informed consent was waived since we had no contact with the study subjects.
Statistical methods
The main outcome was any infection requiring hospital admission. Separate analyses were performed for different types of infection; for example pneumonia and urinary tract infections, and for specific infectious agents; for example staphylococci and tuberculosis. Combined outcomes were also calculated for bacterial, viral, and fungal infections, respectively. Patients and controls were followed from the date of MPN diagnosis/matching date until the date of first hospital admission due to the first infection within each category. Recurrent events of the same category were not included in the analysis, and infections in other categories were ignored in the analyses.
Hazard ratios (HR), comparing the rate of infections among MPN patients to the rate among population controls, with 95 % confidence intervals (CI) were estimated using flexible parametric models assuming proportional hazards with 5 degrees of freedom for the baseline, adjusting for age category at diagnosis (18–49, 50–59, 60–69, 70–79, and 80 years or older), sex and calendar period (1992–1998, 1999–2005, and 2006–2013). HRs were also estimated using a flexible parametric model without assuming proportional hazards for the comparison of MPN patients to population controls to construct graphs over time-varying HRs. The non-proportional hazards model was adjusted for the same covariates, also had 5 degrees of freedom for modeling the baseline, and 3 degrees of freedom for the time-varying effect. Based on the model for any infection with non-proportional hazards, cumulative incidence (1 minus the survival function) was estimated with 95 % CIs. Deaths due to other reasons than infection were handled by censoring, thus the results are interpreted in the absence of competing risk of death. Separate analyses were performed for MPN combined as well as separately for each MPN subtype. Analyses were also performed by age category, sex, and calendar period.
A separate model that estimated the effect of MPN treatment on risk of any infection was fitted within the MPN cohort during the most recent calendar period 2006–2013, with follow up until the end of 2015. The treatment categories analyzed were hydroxyurea, interferon-α, anagrelide, ruxolitinib, other MPN-related drugs (busulphan, danazol, erythropoietin, thalidomide, lenalidomide) and combined therapy, including medications from more than 1 category. Patients were defined as untreated if no prescription was dispensed for a period of 18 months, and if prescriptions of more than one drug were dispensed within a period of 6 months patients were considered to be receiving combination therapy. Patients could thus contribute time in different treatment categories during the disease course. Person-time without any cytoreductive or other MPN-related treatment were used as reference.
A sensitivity analysis was also performed, starting follow-up one year after the MPN diagnosis, where all patients with a diagnosis of infection within the first year of the MPN diagnosis were excluded, in order to assess potential detection bias at the time of MPN diagnosis.
Stata Statistical Software release 15 (Stata Corp 2017, Texas, United States) was used for all statistical analysis.
Results
A total of 8 363 patients and 32 405 controls were identified between the years 1992–2013. Median age was 71 years (range 18–98 years) at MPN diagnosis. The cohort consisted of 53% women and 47% men, the median age of the women was slightly higher than that of the men, 72 and 69 years, respectively. In the MPN cohort there were 2 868 patients diagnosed with PV (34%), 2 928 with ET (35%), 837 with PMF (10%), and with 1 730 MPN-U (21%) (Table 1).
Table 1.
Descriptive statistics on MPN patients and population controls.
| Number of patients, MPN | Number of population controls | |
|---|---|---|
| Women | 4 440 (53%) | 17 136 (53%) |
| Men | 3 923 (47%) | 15 269 (47%) |
| Age 18–49 | 915 (11%) | 3.528 (11%) |
| Age 50–59 | 1 124 (13%) | 4.432 (14%) |
| Age 60–69 | 1 897 (23%) | 7 355 (23%) |
| Age 70–79 | 2 619 (31%) | 10 092 (31%) |
| Age 80+ | 1 808 (22%) | 6 998 (22%) |
| Median age | 71 years | 70 years |
| Period 1992–1998 | 2 095 (25%) | 7 335 (23%) |
| Period 1999–2005 | 2 603 (31%) | 10 441 (32%) |
| Period 2006–2013 | 3 665 (43%) | 14 659 (45%) |
| PV | 2 868 (34%) | - |
| ET | 2 928 (35%) | - |
| PMF | 837 (10%) | - |
| MPN-U | 1 730 (21%) | - |
| Total | 8 363 | 32 405 |
MPN myeloproliferative neoplasms, PV polycythemia vera, ET essential thrombocythemia and PMF primary myelofibrosis, MPN-U myeloproliferative neoplasm unclassifiable
The HR of any infection in MPN patients compared to matched controls was 2.0 (1.9–2.0), with 3 095 events in MPN patients and 8 615 events in population controls (Table 2). The cumulative incidence of any infection in MPN and controls are shown in Figure 1. The mean follow-up time ranged between 7.2 and 8.1 years in different outcome categories. There was a trend towards a higher HR in the younger age categories than in the older, for any infection the HR was 2.8 (2.3–3.4) in the age group 18–50 years, 2.9 (2.4–3.9) in ages 50–59, 2.4 (2.2–2.6) in 60–69, 2.0 (1.8–2.1) in 70–79 and 1.5 (1.4–1.6) in ages 80 or above, but with fewer events in younger patients and controls. The HR of any infection was similar among men 2.0 (1.9–2.1) and women 1.9 (1.8–2.1) compared to their corresponding controls. There were no significant differences in HRs of infection between the calendar periods. For any infection, the HR was similar in PV, HR 1.7 (1.6–1.8), and in ET, HR 1.7 (1.5–1.8), while it was higher in PMF, HR 3.7 (3.2–4.1), with MPN-U representing an intermediate level, HR 2.4 (2.2– 2.6) (Table 3).
Table 2.
Hazard Ratio (HR) with 95 % Confidence Intervals (CI) in MPN and number of cases in each outcome. Estimated from flexible parametric models, adjusting for age group (18–49, 50–59, 60–69, 70–79, and 80 years and older), sex and calendar period (1992–1998, 1999–2005, and 2006–2013).
| Outcome | HR all MPN (95%CI) | Number of events, MPN | Number of events, controls |
|---|---|---|---|
| Combined outcomes | |||
| Any infections | 2.0 (1.9–2.0) | 3 095 | 8 615 |
| Bacterial infections | 1.9 (1.8–2.0) | 2 772 | 7 895 |
| Viral infections | 2.1 (1.9–2.3) | 488 | 1 212 |
| Fungal infections | 2.9 (2.5–3.5) | 206 | 374 |
| Infection type | |||
| Sepsis | 2.6 (2.4–2.9) | 634 | 1 250 |
| Pneumonia | 2.0 (1.9–2.1) | 1 416 | 3 868 |
| Urinary tract infections | 1.6 (1.5–1.7) | 1 124 | 3 815 |
| Meningitis and encephalitis | 2.8 (1.8–4.4) | 31 | 52 |
| Skin and soft tissue infections | 2.1 (1.8–2.4) | 287 | 715 |
| Gastrointestinal infections | 2.3 (2.1–2.6) | 447 | 1 010 |
| Osteomyelitis and spondylitis | 2.7 (1.9–3.9) | 46 | 89 |
| Endocarditis | 1.8 (1.3–2.6) | 39 | 116 |
| Infectious agent | |||
| Pneumococci | 1.7 (1.0–2.9) | 19 | 57 |
| Streptococci excl. pneumococci | 2.3 (1.7–3.1) | 70 | 150 |
| Staphylococci | 2.4 (2.0–2.8) | 231 | 511 |
| Escherichia coli | 1.9 (1.6–2.2) | 181 | 529 |
| Tuberculosis | 1.7 (0.8–3.3) | 11 | 31 |
| Hemophilus influenza | 2.3 (1.4–3.6) | 25 | 61 |
| Pneumocystis jiroveci | 8.6 (3.6–20.6) | 14 | 8 |
| Hepatitis B | 3.9 (1.7–8.9) | 11 | 12 |
| Varicella zoster | 2.0 (1.4–2.8) | 46 | 122 |
| Influenza | 1.9 (1.4–2.5) | 61 | 162 |
HR Hazard Ratio, CI Confidence Interval MPN myeloproliferative neoplasms
Figure 1.
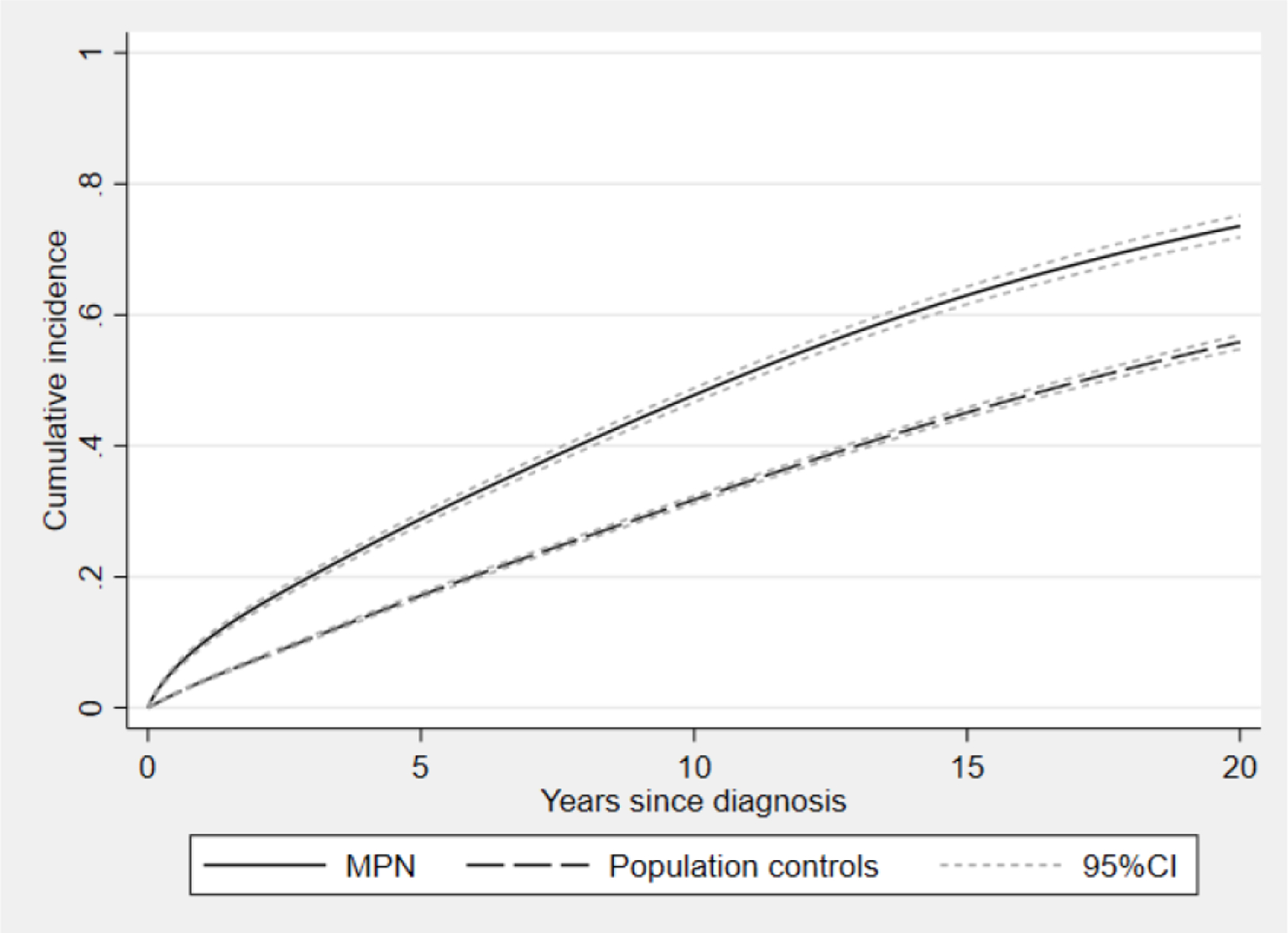
Cumulative incidence, based on a flexible parametric model, of any infection in patients with myeloproliferative neoplasm (MPN) and population controls with 95 % confidence intervals.
Table 3.
Hazard Ratio (HR) with 95 % Confidence Intervals (CI) in all MPN and subdivided by MPN subtype. Estimated from flexible parametric models, adjusting for age group (18–49, 50–59, 60–69, 70–79, and 80 years and older), sex and calendar period (1992–1998, 1999–2005, and 2006–2013).
| Outcome | HR all MPN (95%CI) | PV HR (95%CI) | ET HR (95%CI) | PMF HR (95%CI) |
|---|---|---|---|---|
| Combined outcomes | ||||
| Any infections | 2.0 (1.9–2.0) | 1.7 (1.6–1.8) | 1.7 (1.5–1.8) | 3.7 (3.2–4.1) |
| Bacterial infections | 1.9 (1.8–2.0) | 1.7 (1.6–1.8) | 1.6 (1.5–1.8) | 3.4 (3.0–3.9) |
| Viral infections | 2.1 (1.9–2.3) | 1.8 (1.5–2.1) | 1.7 (1.4–2.1) | 5.2 (3.8–7.1) |
| Fungal infections | 2.9 (2.5–3.5) | 2.6 (1.9–3.4) | 2.2 (1.6–3.0) | 8.0 (4.9–13.3) |
| Infection type | ||||
| Sepsis | 2.6 (2.4–2.9) | 2.1 (1.8–2.5) | 2.0 (1.6–4.7) | 6.2 (4.8–8.0) |
| Pneumonia | 2.0 (1.9–2.1) | 1.8 (1.6–2.0) | 1.6 (1.4–1.8) | 3.8 (3.2–4.6) |
| Urinary tract infections | 1.6 (1.5–1.7) | 1.5 (1.4–1.7) | 1.5 (1.3–1.6) | 2.2 (1.8–2.8) |
| Skin and soft tissue infections | 2.1 (1.8–2.4) | 1.8 (1.4–2.3) | 1.6 (1.2–2.0) | 4.9 (3.2–7.4) |
| Gastrointestinal infections | 2.3 (2.1–2.6) | 2.3 (1.9–2.8) | 1.7 (1.3–2.0) | 4.8 (3.4–6.8) |
| Infectious agent | ||||
| Streptococci excl. pneumococci | 2.3 (1.7–3.1) | 2.4 (1.5–3.8) | 1.1 (0.6–2.0) | 8.7 (4.0–19.1) |
| Staphylococci | 2.4 (2.0–2.8) | 1.6 (1.2–2.1) | 1.8 (1.3–2.4) | 5.5 (3.6–8.6) |
| Escherichia coli | 1.9 (1.6–2.2) | 1.8 (1.4–2.4) | 1.7 (1.2–2.2) | 3.7 (2.1–6.5) |
| Varicella zoster | 2.0 (1.4–2.8) | 0.98 (0.5–2.0) | 2.5 (1.4–4.5) | 7.3 (2.9–18.3) |
HR Hazard Ratio, CI Confidence Interval MPN myeloproliferative neoplasms, PV polycythemia vera, ET essential thrombocythemia and PMF primary myelofibrosis
The rate of bacterial infections was 2-fold elevated in the MPN population, HR 1.9 (1.8–2.0), the rate of viral infections was similarly elevated, HR 2.1 (1.9–2.3), while the HR of fungal infections was higher, 2.9 (2.5–3.5). For bacterial and fungal infections, the HRs were similar in all calendar periods, but there was a tendency towards an increased rate of viral infections during the most recent calendar period, HR 2.5 (2.1–3.1) in 2006–2013 compared to HR 1.8 (1.5–2.2) in 1992–1998. The HRs were similar in patients with PV and ET but higher in those with PMF; the HR for bacterial infections was 1.7 (1.6–1.8) in PV, 1.6 (1.5–1.8) in ET, and 3.4 (3.0–3.9) in PMF. For viral infections, the HR was 1.8 (1.5–2.1) in PV, 1.7 (1.4–2.1) in ET, and 5.2 (3.8–7.1) in PMF (Table 3). This pattern was similar in all outcomes where analysis per MPN subtype was performed. The HRs in relation to time from MPN diagnosis for any infection, bacterial, and viral infections, the pattern was similar with a slightly higher risk shortly after diagnosis and then the HRs were stable over the disease duration. The HR of infection remained significantly elevated throughout the disease course (Figure 2a–c).
Figure 2.
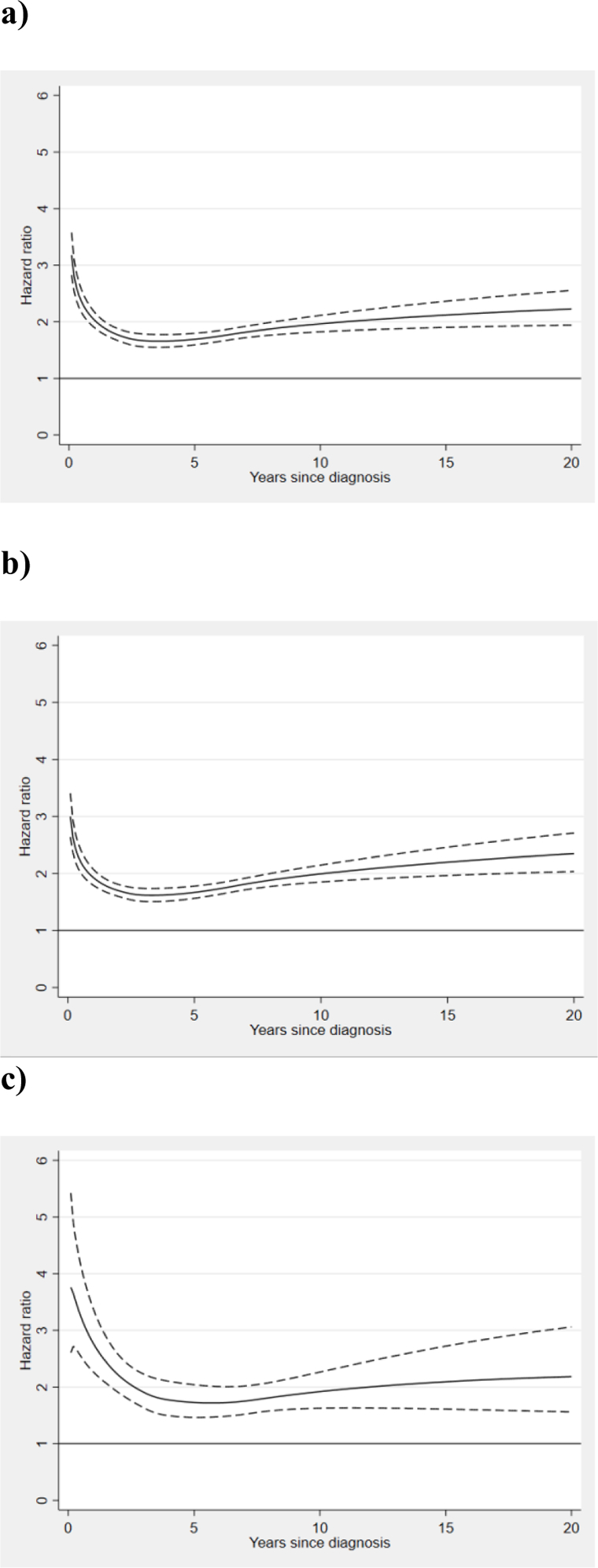
Non-proportional hazard ratios of infections among myeloproliferative neoplasm (MPN) patients in comparison to matched controls, with 95 % confidence intervals in relation to time after MPN diagnosis for a) any infection, b) bacterial infections c) viral infections.
The HR of sepsis was 2.6 (2.4–2.9) with 634 events in MPN patients and 1 250 in controls. The rate of sepsis was significantly higher in patients with PMF, HR 6.2 (4.8–8.0) than in PV 2.1 (1.8–2.5) and ET 2.0 (1.6–4.7). Other types of infections with high HR in MPN were common infections such as pneumonia, HR 2.0 (1.9–2.1), and skin and soft tissue, HR 2.1 (1.8–2.4), as well as less common infections such as meningitis/encephalitis, HR 2.8 (1.8–4.4) and osteomyelitis/spondylitis, HR 2.7 (1.9–3.9) (Table 2).
Regarding infections with specific infectious agents; the elevated rate among MPN patients was consistent over many common pathogens, for example staphylococci 2.4 (2.0–2.8) and streptococci (excluding pneumococci) 2.3 (1.7–3.1), pneumococci 1.7 (1.0–2.9), varicella zoster 2.0 (1.4–2.8), with a more marked increase in PMF, where the HR of varicella zoster was 7.3 (2.9–18.3). The rate of tuberculosis was not significantly increased, HR 1.7 (0.8–3.3), with only 11 cases in the MPN cohort (Table 2). We also investigated more rare opportunistic infections such as pneumocystis jiroveci where the HR was 8.6 (3.6–20.6). For, cryptococci, and progressive multifocal leukoencephalopathy, there were too few events for any comparative statistical analysis, however there was a higher number of cases in the MPN cohort than in the population controls, 6 to 1 and 3 to 0, respectively.
In the most recent calendar period, 2006–2013, there was 3 695 patients with 15 211 years at risk and 957 events, available for analysis on the effect of cytoreductive treatment on risk of any infection. No treatment or hydroxyurea were the most common therapeutic strategies. There were no significant differences in infection rates during follow-up when patients were untreated (reference, 1.0) compared to patients who were treated with hydroxyurea 1.0 (0.9–1.2), interferon-α 1,1 (0.7–1.8), or anagrelide 0.9 (0.5–1.7). The rates of any infection were increased during follow-up in patients who were treated with other MPN-related drugs, combinations of drugs, or ruxolitinib (Table 4).
Table 4.
Effect of treatment on risk of any infection in the MPN cohort during the last calendar period, patients diagnosed 2006–2013 with follow up until 2015. Analyses are adjusted for age category, sex and year of MPN diagnosis. The category assigned other MPN-related drugs include busulphan, danazol, erythropoietin, thalidomide, lenalidomide. No treatment is used as reference.
| Treatment | MPN (all) HR (95%CI) | Number of patients, MPN | Time at risk, years, MPN | PV HR (95%CI) | ET HR (95%CI) | PMF HR (95%CI) |
|---|---|---|---|---|---|---|
| No cytoreductive treatment | 1.0 reference | 3 501 | 4 994 | 1.0 reference | 1.0 reference | 1.0 reference |
| Hydroxyurea | 1.0 (0.9–1.2) | 2 432 | 8 276 | 1.1 (0.8–1.4) | 1.0 (0.7–1.4) | 1.1 (0.7–1.7) |
| Interferon | 1.1 (0.7–1.8) | 301 | 778 | 1.0 (0.4–2.4) | 0.6 (0.1–2.5) | 1.1 (0.4–3.1) |
| Anagrelid | 0.9 (0.5–1.7) | 148 | 337 | 1.3 (0.4–2.0) | 0.6 (0.2–1.6) | 2.2 (0.5–9.4) |
| Ruxolitinib | 4.1 (1.3–13.0) | 35 | 23 | NA | NA | 4.6 (1.4–15.8) |
| Other MPN related drugs | 2.8 (2.2–3.6) | 302 | 436 | 0.9 (0.3–3.0) | 2.2 (1.1–4.3) | 2.6 (1.7–3.9) |
| Combinations of any of the above | 1.9 (1.3–2.7) | 492 | 368 | 1.4 (0.5–3.4) | 1.1 (0.5–2.6) | 1.8 (1.0–3.3) |
| Total number of patients, MPN | 3 659 | 1 182 | 1 369 | 461 | ||
| Total time at risk, years | 15 211 | 5 140 | 6 174 | 1 378 |
HR Hazard Ratio, CI Confidence Interval MPN myeloproliferative neoplasms, PV polycythemia vera, ET essential thrombocythemia and PMF primary myelofibrosis
The sensitivity analysis did not convey any major differences compared to our main analysis, the HR with all events during the first year of MPN diagnosis excluded, was 1.8 (1.8–1.9).
Discussion
In this large population-based cohort study we observed a 2-fold increased risk of serious infection in patients with MPN compared to matched controls. The risk was particularly increased in patients with PMF, and significantly elevated in all MPN subtypes. The risk increase was distributed over a wide range of infection types and infectious agents, both bacterial and viral. Interestingly, no significant difference was observed among untreated patients and patients treated with cytoreductive treatment such as hydroxyurea or interferon-α.
The information on risk of infections in MPN is limited, making comparison to earlier literature difficult. In a previous report by our group, an increased rate (HR 2.7 (2.4–3.1)) of death from infection were observed in patients with MPN compared to controls. In that study only infection as cause of death was analyzed,(16) as opposed to the present report where all infections requiring hospitalization are included.
There are several possible mechanisms behind the increased risk of infections. In MPNs, there is a state of chronic inflammation and a dysregulation of the immune system, with increased levels of pro-inflammatory cytokines.(29, 30) Furthermore, patients with MPN and PMF in particular, have been reported to have lower levels of B lymphocytes, with an inverse correlation to JAK2-V617F allele burden.(31) JAK2V617F, CALR, and MPL mutations may in some patients occur in early lympho-myeloid precursors and thus affect the lymphoid lineages as well as the myeloid.(32–34) In PMF, abnormalities in the immune system may differ between patients with different driver mutation; JAK2-mutated cases have been reported to have more alterations in T-regulatory cell populations while CALR-mutated cases had dysregulations of the interferon-γ-axis.(35) In addition, the MPN population is also at an increased risk of other comorbidities, such as cardiovascular and thromboembolic disease,(36) autoimmune disease, (37) and solid malignancies,(9) and they may therefore have an increased risk of infections related to the treatment of comorbidities, hospitalization of other reasons, or a generally increased frailty. Taken together, several underlying factors may coexist and lead to the increased risk of infections observed in MPNs.
An interesting result in our study is that there was no significant difference in infectious risk among untreated patients and patients treated with common cytoreductive agents such as hydroxyurea or interferon-α. In patients with PV and ET, the Nordic guidelines issued by the Nordic MPN Study Group proposes hydroxyurea or pegylated interferon-α as first line treatment for patients above 60 years of age and pegylated interferon-α as first line in individuals below 60 years of age in need of cytoreduction.(38) Historically, hydroxyurea is by far the most common choice of cytoreductive agent in Sweden.(39) Anagrelide is proposed as an option for patients in need of only platelet reduction, while busulphan, radioactive phosphorus or phlebotomy alone are less common alternatives in selected cases, in particular in patients of advanced age. There is increasing evidence of an elevated risk of infections in MPN patients treated with ruxolitinib. (11, 40, 41) Possible mechanisms of immunodeficiency associated to ruxolitinib includes reduced number or activity of T-cells, in particular T-regulatory cells, NK-cells, and dendritic cells.(42–46) JAK 1/2-inhibitors are also increasingly used and investigated in inflammatory diseases such as rheumatoid arthritis and psoriasis for its broad immunosuppressing properties. (47, 48) Ruxolitinib is effective for treatment of graft-versus-host disease, with unaffected graft-versus-leukemia effect.(49, 50) Selectivity of JAK inhibition is of importance for its immunosuppressive effects, where inhibition of JAK1 seems to be of major importance for T-cell inhibition in vitro.(51) In 2019, the JAK2-selective inhibitor fedratinib was approved by FDA, and whether the selectivity leads to reduced risk of infections remains to be answered.(52) Ruxolitinib was approved by FDA in 2011 for PMF and 2014 for PV with resistance or intolerance to hydroxyurea, corresponding to a short part of the study period. It was thus expected that a limited number of patients in our study would be treated with ruxolitinib and the results should be interpreted with caution. Since the analysis on impact of cytoreductive treatments on risk of infection is based on observational data from registers, confounding by indication cannot be ruled out, although we have adjusted the analysis for important factors such as age. Even so, our results indicate that the increased risk of infection in patients with MPN is not solely caused by cytoreductive treatments such as hydroxyurea and interferon-α.
There is a general recommendation on immunization against pneumococci and influenza in Sweden in all individuals above 65 years of age and in certain risk groups, MPN not included, but it may differ how the counties provide and subsidize it for its inhabitants. Immunization against pneumococci is also included in the recommended program for infants since 2009. The degree of coverage of the influenza vaccine is reported to be 50 % (2014) in the older population.(53) Immunization against zoster reactivation is not generally recommended, but has been available at the individual’s own request in Sweden since 2013. What preventative or prophylactic measures are indicated in the MPN population cannot be fully based on observational results, however, certain immunizations could be considered, e.g. varicella zoster vaccine in certain high-risk individuals, in particular in patients with PMF.
The strengths of our study are that it relies on prospectively collected data from high-quality health registers, it includes over 40 000 individuals with a long follow-up time and minimal loss to follow-up, as well as the cohort design with matched population controls for comparison. The population-based setting ensures generalizability and minimizes the risk of selection bias regarding the patient population. In 2015, Sweden had 244 hospital beds per 100.000 inhabitants, which is in the lower range of European countries,(54) however the results are likely applicable to other countries with similar health care systems. Since MPN patients in Sweden are seen by a hospital-based hematologist, and most population controls are seen in the primary care setting, which is not captured in the Patient Register, we chose to only include infections requiring inpatient care to avoid ascertainment bias between patients and controls. The study does not include less serious infections managed in the outpatient setting and we can therefore only speculate whether the reported infections proportionally represent the more serious infections in both MPN patients in controls, or if patients with MPN have a similar number of infections, but are at higher risk of more serious outcomes. This may require further elucidation, however the serious infections are of highest concern. Other limitations are the lack of detailed patient information, e.g. mutational status, blood counts, spleen size, or disease stage in the registers as well as potential misclassification of early PMF patients as ET and underreporting of acute leukemia. Overall, given the robust results of our study, we do not believe that the limitations affect the validity of our findings.
In summary, we present clinically important findings that patients with MPN, particularly those with PMF, are at a significantly higher risk of severe infections compared to population controls. The lack of differences in risk of infections among untreated and treated patients in our study, suggests that the increased risk of infections is inherent to the MPN, and that risk of infection should not be a determining factor when considering cytoreductive treatments such as hydroxyurea or interferon-α when otherwise indicated. Our presented risk estimates can be considered a baseline risk of infections in MPN prior to JAK-inhibitor era. The increased risks of infections should be recognized in clinical management of these patients as well as when defining how medications, particularly JAK inhibitors, affect the susceptibility of infection in patients with MPN.
Research funding:
The regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet, Blodcancerfonden, the Cancer Research Foundations of Radiumhemmet, Åke Olsson Foundation for Hematologic Research, and the Memorial Sloan Kettering Core Grant (P30 CA008748).
Footnotes
Conflict of interest: TM Andersson, S Eloranta, KE Smedby, and M Björkholm are involved in an ongoing public-private real world evidence collaboration between Karolinska Institutet and Janssen Pharmaceuticals, however, the current project is not related to this collaboration. M Hultcrantz has received honoraria from Intellisphere, LLC, not related to this project. The remaining authors declare no conflict of interest.
References
- 1.Blimark C, Holmberg E, Mellqvist UH, Landgren O, Bjorkholm M, Hultcrantz M, et al. Multiple myeloma and infections: a population-based study on 9253 multiple myeloma patients. Haematologica. 2015;100(1):107–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Safdar A, Armstrong D. Infections in patients with hematologic neoplasms and hematopoietic stem cell transplantation: neutropenia, humoral, and splenic defects. Clin Infect Dis. 2011;53(8):798–806. [DOI] [PubMed] [Google Scholar]
- 3.Goldberg SL, Chen E, Corral M, Guo A, Mody-Patel N, Pecora AL, et al. Incidence and clinical complications of myelodysplastic syndromes among United States Medicare beneficiaries. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(17):2847–52. [DOI] [PubMed] [Google Scholar]
- 4.Barbui T, Thiele J, Gisslinger H, Finazzi G, Vannucchi AM, Tefferi A. The 2016 revision of WHO classification of myeloproliferative neoplasms: Clinical and molecular advances. Blood Rev. 2016;30(6):453–9. [DOI] [PubMed] [Google Scholar]
- 5.Hultcrantz M, Ravn Landtblom A, Andreasson B, Samuelsson J, Dickman PW, Kristinsson SY, et al. Incidence of myeloproliferative neoplasms - trends by subgroup and age in a population-based study in Sweden. J Intern Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bjorkholm M, Hultcrantz M, Derolf AR. Leukemic transformation in myeloproliferative neoplasms: therapy-related or unrelated? Best Pract Res Clin Haematol. 2014;27(2):141–53. [DOI] [PubMed] [Google Scholar]
- 7.Hultcrantz M, Bjorkholm M, Landgren O, Kristinsson SY, Andersson TML. Risk for Arterial and Venous Thrombosis in Patients With Myeloproliferative Neoplasms. Ann Intern Med. 2018;169(4):268. [DOI] [PubMed] [Google Scholar]
- 8.Hultcrantz M, Kristinsson SY, Andersson TM, Landgren O, Eloranta S, Derolf AR, et al. Patterns of survival among patients with myeloproliferative neoplasms diagnosed in Sweden from 1973 to 2008: a population-based study. J Clin Oncol. 2012;30(24):2995–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Landtblom AR, Bower H, Andersson TM, Dickman PW, Samuelsson J, Bjorkholm M, et al. Second malignancies in patients with myeloproliferative neoplasms: a population-based cohort study of 9379 patients. Leukemia. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polverelli N, Palumbo GA, Binotto G, Abruzzese E, Benevolo G, Bergamaschi M, et al. Epidemiology, outcome, and risk factors for infectious complications in myelofibrosis patients receiving ruxolitinib: A multicenter study on 446 patients. Hematol Oncol. 2018. [DOI] [PubMed] [Google Scholar]
- 11.Polverelli N, Breccia M, Benevolo G, Martino B, Tieghi A, Latagliata R, et al. Risk factors for infections in myelofibrosis: role of disease status and treatment. A multicenter study of 507 patients. American journal of hematology. 2017;92(1):37–41. [DOI] [PubMed] [Google Scholar]
- 12.Manduzio P Ruxolitinib in myelofibrosis: to be or not to be an immune disruptor. Ther Clin Risk Manag. 2017;13:169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lescuyer S, Ledoux MP, Gravier S, Natarajan-Ame S, Duval C, Maloisel F, et al. Tuberculosis and atypical mycobacterial infections in ruxolitinib-treated patients with primary or secondary myelofibrosis or polycythemia vera. Int J Infect Dis. 2019;80:134–6. [DOI] [PubMed] [Google Scholar]
- 14.Hirano A, Yamasaki M, Saito N, Iwato K, Daido W, Funaishi K, et al. Pulmonary cryptococcosis in a ruxolitinib-treated patient with primary myelofibrosis. Respir Med Case Rep. 2017;22:87–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sylvine P, Thomas S, Pirayeh E, French Network of Regional Pharmacovigilance C. Infections associated with ruxolitinib: study in the French Pharmacovigilance database. Annals of hematology. 2018;97(5):913–4. [DOI] [PubMed] [Google Scholar]
- 16.Hultcrantz M, Wilkes SR, Kristinsson SY, Andersson TM, Derolf AR, Eloranta S, et al. Risk and Cause of Death in Patients Diagnosed With Myeloproliferative Neoplasms in Sweden Between 1973 and 2005: A Population-Based Study. J Clin Oncol. 2015;33(20):2288–95. [DOI] [PubMed] [Google Scholar]
- 17.Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. European journal of epidemiology. 2009;24(11):659–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turesson I, Linet MS, Bjorkholm M, Kristinsson SY, Goldin LR, Caporaso NE, et al. Ascertainment and diagnostic accuracy for hematopoietic lymphoproliferative malignancies in Sweden 1964–2003. Int J Cancer. 2007;121(10):2260–6. [DOI] [PubMed] [Google Scholar]
- 19.Barlow L, Westergren K, Holmberg L, Talback M. The completeness of the Swedish Cancer Register: a sample survey for year 1998. Acta oncologica. 2009;48(1):27–33. [DOI] [PubMed] [Google Scholar]
- 20.Socialstyrelsen. Cancer incidence in Sweden 2014. www.socialstyrelsen.se; 2015. 2014-12-26. [Google Scholar]
- 21.Berlin NI. Diagnosis and classification of the polycythemias. Semin Hematol. 1975;12(4):339–51. [PubMed] [Google Scholar]
- 22.Swerdlow SH, International Agency for Research on Cancer., World Health Organization. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon, France: International Agency for Research on Cancer; 2008. 439 p. p. [Google Scholar]
- 23.Jaffe ES, World Health Organization. Pathology and genetics of tumours of haematopoietic and lymphoid tissues. Lyon Oxford: IARC Press ; Oxford University Press (distributor); 2001. 351 p. p. [Google Scholar]
- 24.Socialstyrelsen. Dödsorsaksstatistik - Historik, produktionsmetoder och tillförlitlighet. In: Socialstyrelsen, editor. 2010. [Google Scholar]
- 25.Brooke HL, Talback M, Hornblad J, Johansson LA, Ludvigsson JF, Druid H, et al. The Swedish cause of death register. Eur J Epidemiol. 2017;32(9):765–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wettermark B, Hammar N, Fored CM, Leimanis A, Otterblad Olausson P, Bergman U, et al. The new Swedish Prescribed Drug Register--opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf. 2007;16(7):726–35. [DOI] [PubMed] [Google Scholar]
- 28.Ludvigsson JF, Almqvist C, Bonamy AK, Ljung R, Michaelsson K, Neovius M, et al. Registers of the Swedish total population and their use in medical research. European journal of epidemiology. 2016;31(2):125–36. [DOI] [PubMed] [Google Scholar]
- 29.Mendez Luque LF, Blackmon AL, Ramanathan G, Fleischman AG. Key Role of Inflammation in Myeloproliferative Neoplasms: Instigator of Disease Initiation, Progression. and Symptoms. Current hematologic malignancy reports. 2019;14(3):145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barosi G An immune dysregulation in MPN. Current hematologic malignancy reports. 2014;9(4):331–9. [DOI] [PubMed] [Google Scholar]
- 31.Sorensen AL, Bjorn ME, Riley CH, Holmstrom M, Andersen MH, Svane IM, et al. B-cell frequencies and immunoregulatory phenotypes in myeloproliferative neoplasms: Influence of ruxolitinib, interferon-alpha2, or combination treatment. European journal of haematology. 2019;103(4):351–61. [DOI] [PubMed] [Google Scholar]
- 32.Larsen TS, Christensen JH, Hasselbalch HC, Pallisgaard N. The JAK2 V617F mutation involves B- and T-lymphocyte lineages in a subgroup of patients with Philadelphia-chromosome negative chronic myeloproliferative disorders. British journal of haematology. 2007;136(5):745–51. [DOI] [PubMed] [Google Scholar]
- 33.Kjaer L, Holmstrom MO, Cordua S, Andersen MH, Svane IM, Thomassen M, et al. Sorted peripheral blood cells identify CALR mutations in B- and T-lymphocytes. Leukemia & lymphoma. 2018;59(4):973–7. [DOI] [PubMed] [Google Scholar]
- 34.Pardanani A, Lasho TL, Finke C, Mesa RA, Hogan WJ, Ketterling RP, et al. Extending Jak2V617F and MplW515 mutation analysis to single hematopoietic colonies and B and T lymphocytes. Stem Cells. 2007;25(9):2358–62. [DOI] [PubMed] [Google Scholar]
- 35.Romano M, Sollazzo D, Trabanelli S, Barone M, Polverelli N, Perricone M, et al. Mutations in JAK2 and Calreticulin genes are associated with specific alterations of the immune system in myelofibrosis. Oncoimmunology. 2017;6(10):e1345402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hultcrantz M, Bjorkholm M, Dickman PW, Landgren O, Derolf AR, Kristinsson SY, et al. Risk for Arterial and Venous Thrombosis in Patients With Myeloproliferative Neoplasms: A Population-Based Cohort Study. Ann Intern Med. 2018;168(5):317–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kristinsson SY, Landgren O, Samuelsson J, Bjorkholm M, Goldin LR. Autoimmunity and the risk of myeloproliferative neoplasms. Haematologica. 2010;95(7):1216–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nordic care program for patients with Essential Thrombocythemia, Polycythemia Vera and Primary Myelofibrosis http://nmpn.org/index.php/guidelines/17-nmpn-care-program-2017/file: Nordic MPN Study Group; 2017. [Available from: http://nmpn.org/index.php/guidelines/17-nmpn-care-program-2017/file.
- 39.Andreasson B, Lofvenberg E, Westin J. Management of patients with polycythaemia vera: results of a survey among Swedish haematologists. Eur J Haematol. 2005;74(6):489–95. [DOI] [PubMed] [Google Scholar]
- 40.Lussana F, Cattaneo M, Rambaldi A, Squizzato A. Ruxolitinib-associated infections: A systematic review and meta-analysis. American journal of hematology. 2018;93(3):339–47. [DOI] [PubMed] [Google Scholar]
- 41.Tremblay D, King A, Li L, Moshier E, Coltoff A, Koshy A, et al. Risk factors for infections and secondary malignancies in patients with a myeloproliferative neoplasm treated with ruxolitinib: a dual-center, propensity score-matched analysis. Leukemia & lymphoma. 2019:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heine A, Held SA, Daecke SN, Wallner S, Yajnanarayana SP, Kurts C, et al. The JAK-inhibitor ruxolitinib impairs dendritic cell function in vitro and in vivo. Blood. 2013;122(7):1192–202. [DOI] [PubMed] [Google Scholar]
- 43.Massa M, Rosti V, Campanelli R, Fois G, Barosi G. Rapid and long-lasting decrease of T-regulatory cells in patients with myelofibrosis treated with ruxolitinib. Leukemia. 2014;28(2):449–51. [DOI] [PubMed] [Google Scholar]
- 44.Keohane C, Kordasti S, Seidl T, Perez Abellan P, Thomas NS, Harrison CN, et al. JAK inhibition induces silencing of T Helper cytokine secretion and a profound reduction in T regulatory cells. British journal of haematology. 2015;171(1):60–73. [DOI] [PubMed] [Google Scholar]
- 45.Schonberg K, Rudolph J, Vonnahme M, Parampalli Yajnanarayana S, Cornez I, Hejazi M, et al. JAK Inhibition Impairs NK Cell Function in Myeloproliferative Neoplasms. Cancer research. 2015;75(11):2187–99. [DOI] [PubMed] [Google Scholar]
- 46.Parampalli Yajnanarayana S, Stubig T, Cornez I, Alchalby H, Schonberg K, Rudolph J, et al. JAK1/2 inhibition impairs T cell function in vitro and in patients with myeloproliferative neoplasms. British journal of haematology. 2015;169(6):824–33. [DOI] [PubMed] [Google Scholar]
- 47.Nakayamada S, Kubo S, Iwata S, Tanaka Y. Chemical JAK inhibitors for the treatment of rheumatoid arthritis. Expert Opin Pharmacother. 2016;17(16):2215–25. [DOI] [PubMed] [Google Scholar]
- 48.Shreberk-Hassidim R, Ramot Y, Zlotogorski A. Janus kinase inhibitors in dermatology: A systematic review. J Am Acad Dermatol. 2017;76(4):745–53 e19. [DOI] [PubMed] [Google Scholar]
- 49.Spoerl S, Mathew NR, Bscheider M, Schmitt-Graeff A, Chen S, Mueller T, et al. Activity of therapeutic JAK 1/2 blockade in graft-versus-host disease. Blood. 2014;123(24):3832–42. [DOI] [PubMed] [Google Scholar]
- 50.Choi J, Cooper ML, Alahmari B, Ritchey J, Collins L, Holt M, et al. Pharmacologic blockade of JAK1/JAK2 reduces GvHD and preserves the graft-versus-leukemia effect. PLoS One. 2014;9(10):e109799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perner F, Schnoder TM, Ranjan S, Wolleschak D, Ebert C, Pils MC, et al. Specificity of JAK-kinase inhibition determines impact on human and murine T-cell function. Leukemia. 2016;30(4):991–5. [DOI] [PubMed] [Google Scholar]
- 52.Mullally A, Hood J, Harrison C, Mesa R. Fedratinib in myelofibrosis. Blood Adv. 2020;4(8):1792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jorgensen P, Mereckiene J, Cotter S, Johansen K, Tsolova S, Brown C. How close are countries of the WHO European Region to achieving the goal of vaccinating 75% of key risk groups against influenza? Results from national surveys on seasonal influenza vaccination programmes, 2008/2009 to 2014/2015. Vaccine. 2018;36(4):442–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eurostat. Hospital beds. ec.europa.eu2020 [Google Scholar]


