Abstract
Background:
Randomized clinical trials have demonstrated that catheter ablation for atrial fibrillation (AF) in heart failure patients with reduced ejection fraction (HFrEF) may improve survival and other cardiovascular outcomes.
Methods:
We constructed a decision-analytic Markov model to estimate the costs and benefits of catheter ablation and medical management in patients with symptomatic HFrEF (LVEF ≤35%) and AF over a lifetime horizon. Evidence from the published literature informed the model inputs, including clinical effectiveness data from meta-analyses. Probabilistic and deterministic sensitivity analyses were performed. A 3% discount rate was applied to both future costs and benefits. The primary outcome was the incremental cost-effectiveness ratio (ICER) assessed from the US healthcare sector perspective.
Results:
Catheter ablation was associated with 6.47 (95% confidence interval (CI) 5.89 to 6.93) quality adjusted life-years (QALYs) and a total cost of $105,657 (95% CI $55,311 to $191,934) (2018 US dollars), compared to 5.30 (95% CI 5.20 to 5.39) QALYs and $63,040 (95% CI $37,624 to $102,260) for medical management. The ICER for catheter ablation compared to medical management was $38,496 (95% CI $5,583 to $117,510) per QALY gained. Model inputs with the greatest variation on ICER estimates were the cost of ablation and the effect of catheter ablation on mortality reduction. When assuming a more conservative estimate of the treatment effect of catheter ablation on mortality (hazard ratio of 0.86), the estimated ICER was $74,403 per QALY gained. At a willingness-to-pay threshold of $100,000 per QALY gained, AF ablation was found to be economically favorable compared to medical management in 95% of simulations.
Conclusions:
Catheter ablation in HFrEF patients with AF may be considered economically attractive at current benchmarks for societal willingness to pay in the United States.
Several small studies over the past decade suggest that patients with HF with reduced left ventricular ejection fraction (HFrEF) and concomitant atrial fibrillation (AF) have improved cardiovascular outcomes after catheter ablation when compared to medical therapy (rate or rhythm control) with regard to health-related quality of life, functional status, and left ventricular ejection fraction (LVEF).1–6 More recent clinical trials have also shown a benefit to catheter ablation in the form of reductions in both unplanned HF hospitalization and mortality.4, 6 Specifically, the CASTLE AF (Catheter Ablation versus Standard Conventional Therapy in Patients with Left Ventricular Dysfunction and Atrial Fibrillation) trial found a 44% risk reduction of worsening HF admissions and 47% risk reduction of all-cause mortality among patients randomized to catheter ablation compared to medical therapy alone.6
To date, data on the economics of catheter ablation for AF among HF patients in the United States (US) are quite limited. We conducted a model-based economic evaluation to assess the cost per quality-adjusted life year (QALY) gained for catheter ablation, compared to medical management alone, in patients with concomitant AF and HFrEF. Since the clinical effectiveness of catheter ablation in the HFrEF largely consists of a single randomized control trial, a major secondary objective was to identify the key factors upon which the cost-effectiveness of catheter ablation in this context rests.
METHODS
The study protocol and report were prepared in accordance with the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) Statement.7 The authors declare that all supporting data are available within the article.
Model Design and Structure
We constructed a decision-analytic Markov model to project the benefit and costs of two therapeutic strategies, catheter ablation and medical management, in a simulated cohort based on the baseline characteristics of CASTLE AF: 64-year old patients with paroxysmal or persistent atrial fibrillation (AF), heart failure with reduced ejection fraction (i.e. left ventricular ejection fraction (LVEF) of 35% or less) and New York Heart Association functional class II-III symptoms.
A two-state Markov model was constructed in TreeAge Pro 2020 (Williamstown, MA) and used to project costs, life-years (LYs) and quality adjusted life-years (QALYs) per-annual cycle over a lifetime horizon (Figure 1). Given the uncertainty in survival and the clinical effectiveness of AF ablation beyond the trial follow up, we adopted a conservative approach for model extrapolation by assuming an attenuated benefit of AF ablation on mortality, HF hospitalization and quality of life beyond the five year follow up of CASTLE AF. That is, the hazard ratios comparing the survival conferred by AF ablation and medical management arms were set to 1 beyond five years of follow up. To examine the effects of alternate approaches to model extrapolation, we modelled two other scenarios: 1) we assumed a sustained clinical benefit with AF ablation beyond the trial follow up over a lifetime horizon, without amplification or attenuation, to estimate lifetime benefits and costs; and 2) we assumed no additional benefits accrued beyond the follow up of CASTLE AF (i.e. no accrued QALYs beyond five years). A 3% discount rate was applied to all future costs and benefits.8
Figure 1.
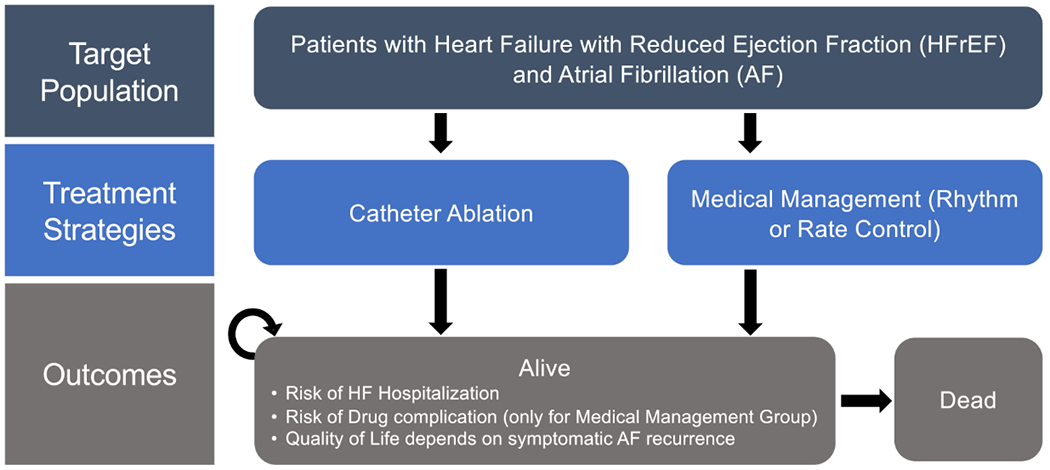
Schematic of Markov model structure. Abbreviations: AF, atrial fibrillation; HF, heart failure; HFrEF, heart failure with reduced ejection fraction.
Model Inputs
The clinical effectiveness inputs were based on a recent meta-analysis of six randomized control trials that compared catheter ablation to medical therapy (i.e. rate or rhythm control) (Table 1).9 The meta-analysis reported a pooled relative risk for all-cause mortality of 0.52 (95% confidence interval (CI) 0.33 to 0.81) and 0.60 (95% CI 0.39 to 0.93) for heart failure hospitalization, with no observable heterogeneity in either pooled estimate (I2 = 0). The risk ratios, comparing catheter ablation to medical therapy, were applied to the cycle-specific all-cause death and HF hospitalization rates obtained from the Kaplan-Meier estimates for the medical therapy arm in the CASTLE AF trial to estimate the outcomes associated with catheter ablation. Mortality beyond the trial follow up period was modelled using a Weibull distribution, which was selected according to the ranked Akeike Information Criterion goodness-of-fit statistic.19, 20 Parameterization of survival was performed using R v3.6.1. In our sensitivity analyses, our model used alternate sources for the clinical effectiveness data reported in 1) the CASTLE AF trial (i.e. all-cause mortality HR 0.53 and HF hospitalization HR 0.56), and 2) a recent large observational study using US administrative data to evaluate AF catheter ablation in a real-world patient cohort with AF and HFrEF (i.e. all-cause mortality HR 0.67 and HF hospitalization HR 1.02) to further explore the uncertainty in catheter ablation benefit.6, 21
Table 1.
Base case clinical and costing inputs
| Variable | Base Case | Range | Distribution | Reference |
|---|---|---|---|---|
| Clinical Inputs | ||||
| All-cause death at 1 year (Medical Management) | 0.045 | n/a | Weibull | 6 |
| Relative risk of HF Hospitalization (Ablation vs. Medical Management) | 0.60 | 0.39 to 0.93 | Log-normal | 9 |
| Relative risk of death (Ablation vs. Medical Management) | 0.52 | 0.33 to 0.81 | Log-normal | 9 |
| Annual Rate of HF Hospitalization | 0.124 | 0.062 to 0.187 | Beta | 6 |
| Probability of Sinus Rhythm at 1 year with Medical Therapy | 0.275 | 0.2 to 0.35 | Beta | 4, 6, 10 |
| Relative Risk of AF Recurrence (Ablation vs. Medical Management) | 0.39 | 0.27 to 0.57 | Log-normal | 11 |
| Risk of Ablation Complication within 1 year | 0.029 | 0.026 to 0.032 | Beta | 12 |
| Annual risk of redo ablation procedure | 0.0546 | ±50% | Beta | 6 |
| Annual risk of antiarrhythmic drug toxicity | 0.0534 | ±50% | Beta | 4 |
| Utilities | ||||
| Alive in Sinus Rhythm | 0.779 | 0.770 to 0.788 | Beta | 13 |
| Disutility AF Recurrence | −0.080 | −0.099 to −0.062 | Beta | 13 |
| Disutility HF Hospitalization | −0.0066 | −0.0135 to 0 | Beta | 14, 15 |
| Costs | ||||
| Cost of AF Catheter Ablation | $36,475 | $16,408 to 48,679 | Gamma | 16 |
| Heart Failure Hospitalization Cost | $15,874 | ±50% | Gamma | 17 |
| Annual Outpatient Costs of AF | $3,843 | ±50% | Gamma | 18 |
For patients undergoing a medical management strategy, 31% of patients were on anti-arrhythmic drug (AAD) therapy in the CASTLE AF trial. For patients on AADs, patients could be modeled as starting on dofetilide or amiodarone. In the event of toxicity or therapeutic failure, it was assumed that patients on dofetilide would switch to amiodarone, and those on amiodarone would abandon a rhythm control strategy and convert to a rate control approach. Based on baseline AAD use in the ORBIT AF (Outcomes Registry for Better Informed Treatment of Atrial Fibrillation) study, we assumed that 8% of AF patients on AAD would start dofetilide as a first-line AAD with the remaining patients starting on amiodarone.22 That is, 3% of the total ORBIT AF cohort were on dofetilide at baseline. Among the 37% of the total cohort on baseline AAD therapy, dofetilide was the AAD therapeutic choice in 8%. Given the less favourable long-term side effect profile of amiodarone compared to dofetilide, we also conducted a sensitivity analysis that assumed AAD initiation would consist of 50% dofetilide and 50% amiodarone.
Utility inputs were obtained from the Euro heart study, which collected EQ-5D survey data from 5,050 cardiac patients (with AF, HF and concomitant AF and HF), and used a time-trade off method for utility weighting.13 Based on this data, the utility decrement from AF recurrence was set at −0.080, which is similar to the utility difference reported in a prior cost-effectiveness study by Reynolds et al. that derived utility scores through indirect comparison of several AF cohorts.23 We also applied a temporary utility decrement of −0.0066 for each HF hospitalization event.14, 15
Costing inputs for catheter ablation and annual outpatient AF management were obtained from recent analyses of commercial and Medicare claims in the United States.16, 18 Aside from the initial cost of catheter ablation, the annual cost of AF management per patient included medications, outpatient physician visits and other patient services, laboratory services, and emergency care for cardiovascular-related medical conditions. We assumed similar outpatient follow up costs in both the catheter ablation and medical management strategies. Based on data from the CASTLE AF trial, we assumed that 25% of patients in the catheter ablation arm would require a redo procedure incurring associated catheter ablation costs over the first 5 years of follow up. We made the conservative assumption that patients in the medical management arm could not accrue costs associated with crossover to catheter ablation. For HF re-hospitalization, we obtained the average cost per patient of a HF hospitalization with a median length of stay of 5 days from an analysis of the national 5% sample of Medicare beneficiaries.17 It was assumed that peri-procedural complications from AF ablation, antiarrhythmic drug toxicity or a change in antiarrhythmic drug therapy would incur costs associated with a typical cardiovascular hospitalization. While clinical studies allow for a “blanking period” (typically three months) of early AF recurrence, our economic model accrued all costs related to post-ablation clinical events and outpatient management regardless of the blanking period. Costs were valued in 2018 USD, and adjusted using the US Medical Care Consumer Price Index, where appropriate.24
Variability and Uncertainty
In order to understand how each individual factor influenced the estimated cost-effectiveness, one-way sensitivity analyses were performed in which we varied a single input parameter at a time using 95% CI bounds and recorded the change in incremental cost per QALY. Variables for which CIs were not provided were modelled with using a range of ± 50%. We also conducted a probabilistic sensitivity analysis, where a Monte Carlo simulation comprised of 10,000 iterations was used to propagate the uncertainty in individual model parameters to generate a distribution of expected costs and QALYs. We applied log-normal distributions for all hazard ratios, β-distributions to all probabilities and utilities, and γ-distributions to all costs (Supplemental Table I).
RESULTS
Model Validation
To assess model calibration, we compared the modelled survival probabilities of the medical management cohort to the reported survival in the CASTLE AF trial. Among the cohort treated with medical management alone, the modelled survival probabilities (94.6% (95% CI 91.3 - 97.8) at 1 year, 83.2% (95% CI 77.7 - 88.6) at 3 years and 62.0% (95% CI 54.9 - 69.9) at 5 years) were similar to the Kaplan Meier estimates reported in CASTLE AF (96.1% at 1 year, 82.8% at 3 years, and 62.5% at 5 years).6 Additionally, the model estimated a 10-year survival of 34.2% (95% CI 27.4 - 41.0), which is comparable to the 10-year survival of 35.4% (95% CI 24.3 - 36.6) reported for the subgroup of patients aged 65 to 74 years in a large contemporary cohort of primary care HF patients in the United Kingdom.25 Lastly, the model survival probabilities of the catheter ablation cohort (97.6% (95% CI 96.4 - 98.5) at 1 year, 89.8% (95% CI 84.6 - 93.4) at 3 years, 80.5% (95% CI 71.4 - 87.2 at 5 years) were also comparable to the CASTLE AF Kaplan Meier estimates (95.3% at 1 year, 84.9% at 3 years, 81% at 5 years).
Base Case Analysis
Over a lifetime horizon, patients with concomitant HFrEF and AF who underwent catheter ablation accrued an average of 6.47 (95% CI 5.89 - 6.93) QALYs or 8.43 (95% CI 7.66 - 9.02) LYs and had a total cost of $105,657 (95% CI 55,311 - 191,934). Patients who received medical management alone accrued an average of 5.30 (95% CI 5.20 - 5.39) QALYs or 7.21 (95% CI 6.93 - 7.48) LYs, and had a total cost of $63,040 (95% CI 37,624 - 102,260). The incremental cost for ablation was $42,617 and the incremental benefits were 1.17 QALYs and 1.22 LYs. The resulting incremental cost effectiveness ratios (ICERs) for catheter ablation compared to medical management alone were $38,496 (95% CI 5,583 - 117,510) per QALY gained and $35,335 (95% CI 5,413 - 125,472) per LY gained (Table 2).
Table 2.
Base Case and Lifetime Probabilistic Models
| Base Case: Probabilistic Model | Base Case: Deterministic Model | |||||
|---|---|---|---|---|---|---|
| Strategy | Total Costs (95% CI) | Total LYs (95% CI) | Total QALYs (95% CI) | Total Costs | Total LYs | Total QALYs |
| Medical Management | 63,040 (37,624-102,260) | 7.21 (6.93-7.48) | 5.30 (5.20-5.39) | 63,883 | 7.21 | 5.30 |
| Catheter Ablation | 105,657 (55,311-191,934) | 8.43 (7.66-9.02) | 6.47 (5.89-6.93) | 106,425 | 8.45 | 6.49 |
| ICER | $38,496 per QALY gained (95% CI 5,583 - 117,510) | $35,600 per QALY gained | ||||
Sensitivity Analyses
The inputs with the greatest variation effect on the model (Figure 2) were the clinical effectiveness of AF ablation on mortality and the cost of catheter ablation. When the catheter ablation cost was varied from $16,408 to $48,679, the range of the incremental cost per QALY gained was between $17,889 and $46,371.
Figure 2.
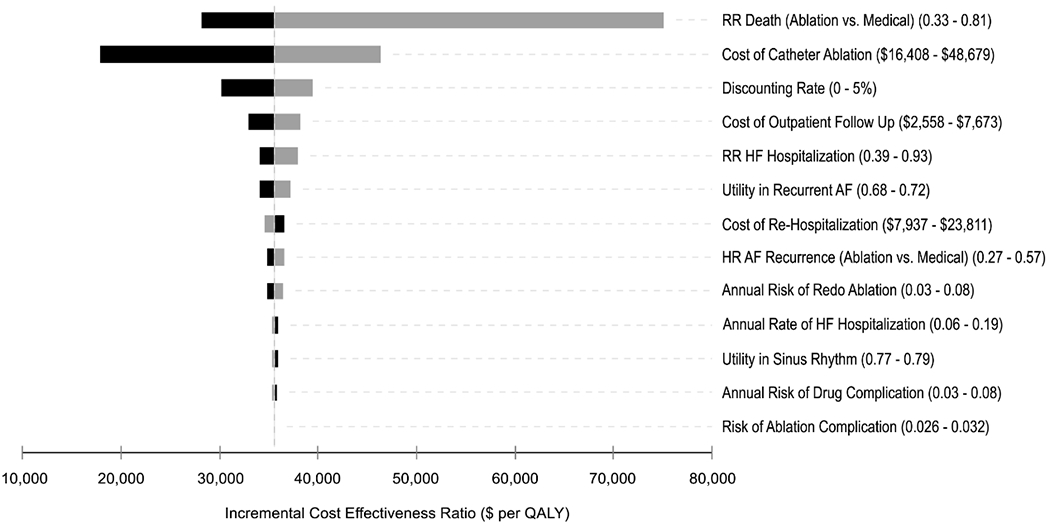
Tornado diagram summarizing one-way sensitivity analyses on incremental cost-effectiveness ratio (cost per quality-adjusted life years gained). Grey and black bars denote the effects of the upper and lower bounds of each variable input, respectively. That is, the upper bound may have differential effects on the incremental cost-effectiveness ratio depending on the variable input. Abbreviations: AF, atrial fibrillation; HF, heart failure; QALY, quality-adjusted life year; RR, relative risk.
The cost-effectiveness acceptability curve displays the probability of each strategy accruing the best net health benefit at different willingness-to-pay thresholds (Figure 3). At a willingness-to-pay threshold of $50,000 per QALY gained, catheter ablation was cost-effective in 75% of simulations (Figure 4). Using a threshold of $100,000 per QALY gained benchmark, catheter ablation was cost-effective in 95% of simulations.
Figure 3.
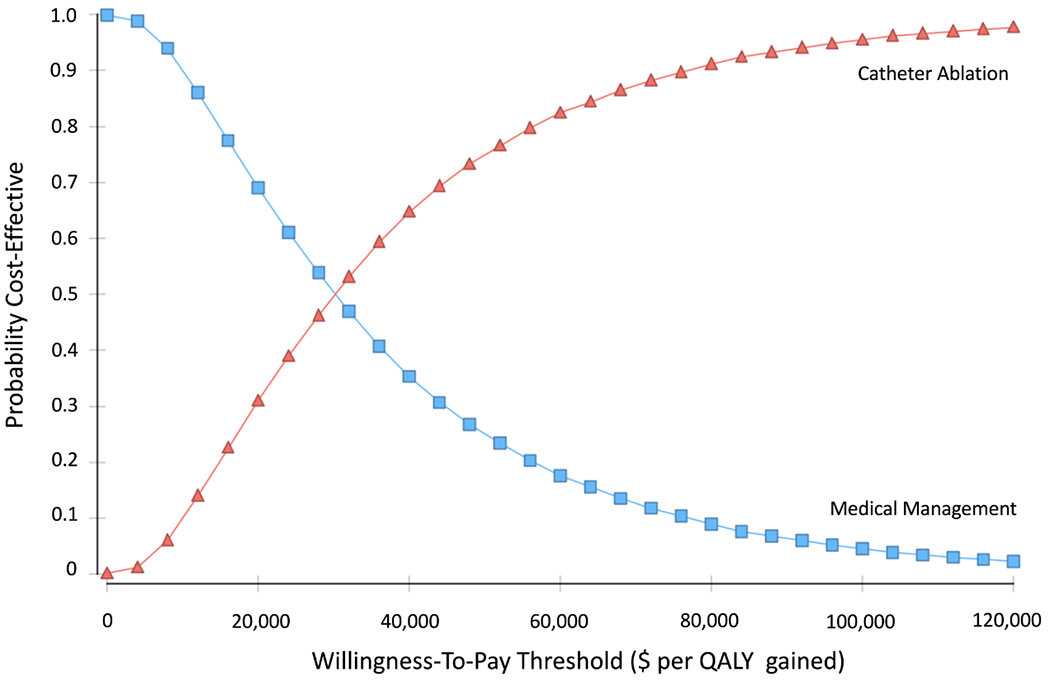
Cost-effectiveness acceptability curve showing the probability of a strategy being cost-effective over a range of willingness-to-pay (WTP) thresholds.
Figure 4.
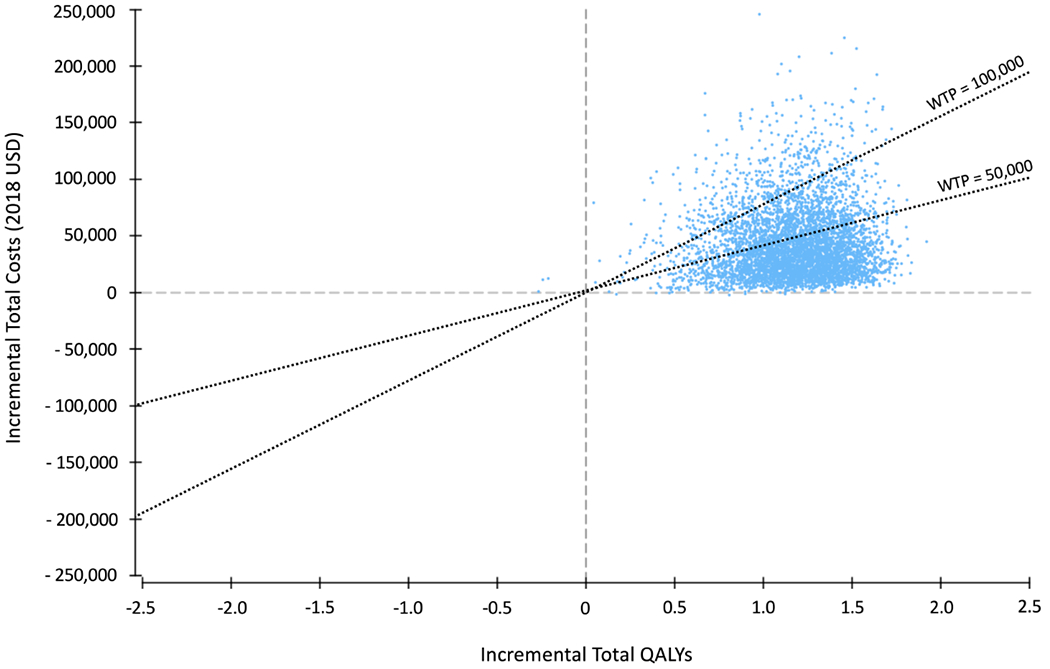
Incremental cost-effectiveness plane comparing medical management to ablation
In a secondary analysis using clinical effectiveness inputs from CASTLE AF rather than the meta-analysis, the value proposition was similar to the base case with an estimated ICER of $35,831 per QALY gained. Given the uncertainty in the generalizability of the CASTLE AF findings,21 we attenuated the estimated treatment benefit of AF ablation on all-cause mortality to assess the corresponding effect on ICER estimate. Assuming a more modest ablation effect on mortality compared to medical management (HR of 0.86, or the upper limit of the 95% CI of treatment effect), the estimated ICER was $74,403 per QALY gained.
We also conducted a sensitivity analysis using clinical effectiveness inputs derived from a large US administrative cohort of patients comparing the efficacy of catheter ablation versus medical management in patients with concomitant HFrEF and AF. In this study, ablation was associated with a lower risk of death (HR 0.67) but there was no difference in the risk of HF hospitalization (HR 1.02).21 The ICER using these more modest estimates of clinical effectiveness was $49,783 per QALY gained.
To better understand the theoretical upper limit of the treatment effect that would remain below a willingness-to-pay threshold of $100,000 per QALY gained, we performed a threshold analysis. In this analysis, the hazard ratio of death (AF ablation versus medical management) would need to be less than 0.91 assuming that the clinical benefit of catheter ablation was primarily obtained through a reduction in the risk of death.
Scenario Analyses
Our base case model provided a conservative ICER estimate by assuming attenuated benefit beyond 5 years. With a model that allowed clinical benefits of catheter ablation to be sustained over a lifetime horizon without amplification or attenuation, catheter ablation was associated with 7.93 QALYs and $115,109, and medical management was associated with 5.07 QALYs and $63,883. The resulting ICER was $17,899 per QALY gained (Supplemental Table II).
The most conservative estimate is derived when assuming that no additional costs or benefits accrue beyond the follow up of CASTLE AF (i.e. no accrued QALYs beyond five years). In this scenario, catheter ablation was associated with 3.23 QALYs and $68,247, and medical management was associated with 2.78 QALYs and $33,413. The estimated ICER was $76,826 per QALY gained.
Finally, we assessed the influence of initial AAD therapy on the estimated ICER. Among the 31% of patients in the medical management arm who were started on AAD therapy, 8% were started on dofetilide for initial management of AF, based on practice patterns described in the ORBIT AF registry, and the remaining patients were started on amiodarone.22 When the proportion of dofetilide was increased to 50%, the estimated ICER was $33,878 per QALY gained comparing catheter ablation to medical management. In this scenario, the improved value proposition of catheter ablation compared to medical management alone was due to the added upfront costs in the medical management arm associated with hospitalization for dofetilide initiation.
DISCUSSION
The main finding of our study is that catheter ablation appears to be an economically attractive strategy compared to medical management alone for patients with AF and HFrEF under the conservative assumptions that clinical benefits only persisted for 5 years or that the size of the mortality benefit was substantially smaller than that reported in the CASTLE-AF Trial. Our base case incremental cost effectiveness ratio was $38,496 which meets conventional criteria for cost effectiveness in the United States.26 When varied over their reported ranges, the model inputs that resulted in the greatest estimated ICER variations were the initial cost of catheter ablation, the clinical effectiveness of catheter ablation on mortality reduction, and the health utility with recurrent symptomatic AF. However, despite the uncertainty in these input parameters, 95% of our simulations found that catheter ablation had an incremental cost effectiveness ratio less than $100,000 per QALY gained.
Our results are consistent with a recent cost-utility analysis comparing catheter ablation and medical management among HFrEF patients with AF.27 Conducted from the perspective of the Australian health system, Gao et al. estimated an ICER of $55,942 (Australian Dollars) per QALY gained for patients treated with catheter ablation compared to medical management. In contrast to our study, Gao et al. assumed a similar base utility among the catheter ablation and medical management cohorts. That is, the model assumed that catheter ablation did not confer an improvement in health-related quality of life, which may underestimate the benefit of catheter ablation and the subsequent ICER. Additionally, this assumption is not consistent with the published literature; catheter ablation is associated with significant improvements in quality of life in both generic and disease-specific quality of life measures.28, 29
Other prior economic models over the past decade have not specifically assessed the cost-effectiveness of AF catheter ablation in patients with concomitant HFrEF.23, 30–33 These prior studies assessed the cost-effectiveness of catheter ablation in the overall symptomatic AF population, and relied on model assumptions that predated the availability of adequate clinical effectiveness data.
The present analysis is motivated in part by the intriguing and unexpected finding of a large mortality benefit from catheter ablation in the CASTLE-AF Trial, which reported improved survival among those randomized to catheter ablation as well as a reduced the risk of HF hospitalization compared to medical therapy alone.6 The coexistence of AF and HFrEF may represent a distinct and more clinically severe patient subgroup that derives differential benefit from rhythm control with catheter ablation.34 It is worth noting that CASTLE AF trial has been subjected to several criticisms including the relatively high proportion of participants lost to follow up, the non-standardized approach to optimization of HF therapy, and slow enrollment with a highly selective inclusion criteria.35 These limitations have led to skepticism in the generalizability of the trial results.
Additionally, the recent AMICA (Atrial Fibrillation Management in Congestive Heart Failure with Ablation) trial showed similar LVEF changes in the ablation and medical management groups, raising the question of whether ablation produces important amounts of remodeling in the heart failure population with reduced left ventricular function as suggested by CASTLE-AF.36 However, AMICA results are best viewed as inconclusive due to an insufficient sample size (with premature study termination due to enrolment futility) and due to the exclusion of 12% of randomized participants from primary outcome analysis due to technically inadequate follow-up transthoracic echocardiogram studies.
Contrary to the results of the AMICA study, the clinical benefit of AF catheter ablation in heart failure with regard to improvement in LVEF and clinical outcomes has been described in prior trials.3, 4 In a recent meta-analysis of six randomized trials, catheter ablation was found to reduce the risk of all-cause mortality (RR 0.52; 95% CI 0.33 to 0.81) and HF hospitalization (RR 0.60; 95% CI 0.39 to 0.93) compared to medical management among patients with concomitant HFrEF and AF.9 Additionally, the prespecified subgroup analysis of HF patients in CABANA (Catheter Ablation vs Antiarrhythmic Drug Therapy for Atrial Fibrillation) provides potential support to the treatment benefit associated with catheter ablation in the HFrEF population.37 Among patients with NYHA class II or greater symptoms, there was a trend towards a decreased risk of the primary composite endpoint of death, disabling stroke, serious bleeding, or cardiac arrest (HR 0.68, 95% CI 0.44 to 1.05; p=0.15). Although the classification of HF is not directly comparable between CABANA and CASTLE AF (i.e. HF with NYHA Class > II and HF with reduced EF, respectively), the similar direction of treatment effect among the catheter ablation arm compared to medical management provides encouragement that patients with HF and concomitant AF may derive benefit.
Even assuming a more conservative estimate of the treatment effect on mortality (i.e. HR 0.86, the upper limit of the 95% CI of catheter ablation versus medical management in the CASTLE AF trial), the estimated ICER was $74,403 per QALY gained. Under this scenario, the cost-effectiveness of ablation relative to medical management could improve with decreased ablation costs, increased symptomatic benefit, or a lower rate of redo procedures in the setting of improved catheter ablation technology. Nevertheless, the findings of the current economic evaluation should be taken with caution until the clinical benefit observed in CASTLE AF is corroborated in additional, larger studies.
Limitations
The results of the study need to be interpreted in the context of several limitations. First, the clinical effectiveness inputs were primarily based on a single randomized control trial.6 To date, CASTLE AF remains the largest trial of AF catheter ablation in the HFrEF population and these findings will need to be corroborated by additional studies. Several ongoing trials will help address this area of uncertainty: (a) The Ablation of Atrial Fibrillation in Heart Failure Patients (CONTRA-HF) trial is investigating a rhythm control strategy using cryoablation in patients with HF and implanted cardiac devices (ClinicalTrials.gov NCT03062241); and (b) Randomised Ablation-based Atrial Fibrillation Rhythm Control Trial in Patients with Heart Failure and High Burden Atrial Fibrillation (RAFT AF) is assessing the impact of a catheter ablation-based rhythm control strategy versus rate control in patients with AF stratified by HFrEF and HF with preserved EF (ClinicalTrials.gov NCT01420393). In our sensitivity analyses, catheter ablation continued to represent a cost-effective strategy compared to medical management as long as there was a mortality benefit large enough to be demonstrable in a plausibly sized clinical trial.
Second, the utility inputs from CASTLE AF and other large randomized trials of AF in HFrEF were not available. Similar to prior cost-effectiveness analyses,23 we assigned a higher utility value to patients who maintained sinus rhythm compared to those with recurrent AF. Our model assumes that AF is the primary driver of quality of life and the weighting of AF takes precedence over the contributions of HF to utilities. This assumption may overestimate the magnitude of QALY benefit. Nevertheless, in the scenario where utility weights were not considered, our model estimated an ICER of $35,335 per LY gained, which is still within the conventional threshold for value within in US.
Third, we may have underestimated the cost offsets conferred by the catheter ablation strategy. That is, while CASTLE AF reported a reduction in the rate of HF hospitalization following AF ablation, additional health resource use was not reported by treatment group. It is possible that a strategy of AF catheter ablation in HFrEF also reduces the frequency of arrhythmia-related hospitalization or emergency department visits, which would result in fewer long-term costs associated with catheter ablation and a more favourable ICER.
Last, costs were not directly measured in the CASTLE AF trial by gold standard micro-costing methods. The current study relied on published costs for AF ablation derived using the MarketScan, which is a large administrative claims database comprising of individuals covered by employer insurance, and Medicare beneficiaries who possess supplemental insurance paid by their employers. Since this database contains a convenience sample of claims, our results may not be fully generalizable across the United States among patients covered by Medicare or Medicaid. It is worth noting that the median age of our modelled cohort was 64 years old, where the costing inputs obtained from commercial insurance may be more relevant.
However, when extrapolating our findings to the Medicare population, our base case cost input for initial AF ablation may underestimate the value proposition of catheter ablation for AF in HFrEF. The estimated ICER would be more favorable when using lower ablation costs, such as Medicare reimbursement ($19,800 in 2018 USD including the National Medicare Outpatient Hospital Rate for Level 3 Electrophysiology procedures and physician fees)38, 39 or mean ablation costs derived from administrative databases primarily comprised of Medicare or managed care insurance plans ($21,563 in 2014 USD).40 Nevertheless, when using a conservative AF ablation cost in our base case model, catheter ablation of AF was still considered economic attractive compared to medical management alone.
CONCLUSIONS
Based upon the available randomized evidence to date, catheter ablation may be an economically attractive strategy for treatment of AF in HFrEF patients, but our results are dependent on the assumption that unambiguous mortality reduction can be corroborated in future trials in this patient population.
Supplementary Material
Supplemental Methods.
Supplemental Table I. Parameters for Probabilistic Sensitivity Analysis
Supplemental Table II. Base Case and Lifetime Deterministic Models
WHAT IS KNOWN
In heart failure patients with reduced ejection fraction (HFrEF), randomized clinical trials have shown than catheter ablation for atrial fibrillation (AF) reduces the risk of hospitalization and mortality, and improves health-related quality of life compared to medical therapy alone.
Few studies have evaluated the value proposition of catheter ablation in heart failure patients, and the lifetime costs and benefits from the US healthcare sector perspective have not been estimated.
WHAT THE STUDY ADDS
Catheter ablation in HFrEF patients with AF may be considered economically attractive compared to medical therapy at current benchmarks for societal willingness to pay in the United States with an incremental cost effectiveness ratio of $38,496 (95% CI $5,583 to $117,510) per QALY gained.
The key factors influencing the value proposition of catheter ablation included the initial cost of catheter ablation and the clinical effectiveness of catheter ablation on mortality reduction. Despite the uncertainty in these input parameters, 95% of our simulations found that catheter ablation had an incremental cost effectiveness ratio less than $100,000 per QALY gained.
Acknowledgments
SOURCES OF FUNDING
DSC is supported by a Canadian Institutes of Health Research Banting Fellowship and an Arthur JE Child Cardiology Fellowship. ZL and AL are supported by National Health Institute T32 training grant (#5T32HL069749).
DISCLOSURES
JPP receives grants for clinical research from Abbott, American Heart Association, Association for the Advancement of Medical Instrumentation, Bayer, Boston Scientific, NHLBI, and Philips and serves as a consultant to Abbott, Allergan, ARCA Biopharma, Biotronik, Boston Scientific, LivaNova, Medtronic, Milestone, Sanofi, Philips, and Up-to-Date. ZL receives grant support from Boston Scientific. MF serves as a consultant to AxonTherpaies, Galvani, Daxor. BDA receives grants for clinical research from Abbott and Boston Scientific and serves as a consultant to Abbott, Biotronik, Boston Scientific, Medtronic, and Seimens. DVE receives research grants from Abbott, Boston Scientific, Medtronic and GE Healthcare outside of the submitted work. ADD reports research funding from the American Heart Association, Amgen, AstraZeneca, Bayer, Intra-Cellular Therapies, Luitpold Pharmaceuticals, Merck, the NHLBI, Novartis and PCORI. He also provides consulting services for Amgen, AstraZeneca, Bayer, InnaMed, LivaNova, Mardil Medical, Novartis, Procyrion, scPharmaceuticals, and Zoll. The remaining authors have no conflicts of interest to disclose. The remaining authors report no additional disclosures.
REFERENCES
- 1.MacDonald MR, Connelly DT, Hawkins NM, Steedman T, Payne J, Shaw M, Denvir M, Bhagra S, Small S, Martin W et al. Radiofrequency ablation for persistent atrial fibrillation in patients with advanced heart failure and severe left ventricular systolic dysfunction: a randomised controlled trial. Heart (British Cardiac Society). 2011;97:740–7. [DOI] [PubMed] [Google Scholar]
- 2.Jones DG, Haldar SK, Hussain W, Sharma R, Francis DP, Rahman-Haley SL, McDonagh TA, Underwood SR, Markides V and Wong T. A randomized trial to assess catheter ablation versus rate control in the management of persistent atrial fibrillation in heart failure. J Am Coll Cardiol. 2013;61:1894–903. [DOI] [PubMed] [Google Scholar]
- 3.Hunter RJ, Berriman TJ, Diab I, Kamdar R, Richmond L, Baker V, Goromonzi F, Sawhney V, Duncan E, Page SP et al. A randomized controlled trial of catheter ablation versus medical treatment of atrial fibrillation in heart failure (the CAMTAF trial). Circ Arrhythm Electrophysiol. 2014;7:31–8. [DOI] [PubMed] [Google Scholar]
- 4.Di Biase L, Mohanty P, Mohanty S, Santangeli P, Trivedi C, Lakkireddy D, Reddy M, Jais P, Themistoclakis S, Dello Russo A et al. Ablation Versus Amiodarone for Treatment of Persistent Atrial Fibrillation in Patients With Congestive Heart Failure and an Implanted Device: Results From the AATAC Multicenter Randomized Trial. Circulation. 2016;133:1637–44. [DOI] [PubMed] [Google Scholar]
- 5.Prabhu S, Taylor AJ, Costello BT, Kaye DM, McLellan AJA, Voskoboinik A, Sugumar H, Lockwood SM, Stokes MB, Pathik B et al. Catheter Ablation Versus Medical Rate Control in Atrial Fibrillation and Systolic Dysfunction: The CAMERA-MRI Study. J Am Coll Cardiol. 2017;70:1949–1961. [DOI] [PubMed] [Google Scholar]
- 6.Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, Merkely B, Pokushalov E, Sanders P, Proff J et al. Catheter Ablation for Atrial Fibrillation with Heart Failure. The New England journal of medicine. 2018;378:417–427. [DOI] [PubMed] [Google Scholar]
- 7.Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, Augustovski F, Briggs AH, Mauskopf J, Loder E et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ. 2013;346:f1049. [DOI] [PubMed] [Google Scholar]
- 8.Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, Kuntz KM, Meltzer DO, Owens DK, Prosser LA et al. Recommendations for Conduct, Methodological Practices, and Reporting of Cost-effectiveness Analyses: Second Panel on Cost-Effectiveness in Health and Medicine. Jama. 2016;316:1093–103. [DOI] [PubMed] [Google Scholar]
- 9.Turagam MK, Garg J, Whang W, Sartori S, Koruth JS, Miller MA, Langan N, Sofi A, Gomes A, Choudry S et al. Catheter Ablation of Atrial Fibrillation in Patients With Heart Failure: A Meta-analysis of Randomized Controlled Trials. Ann Intern Med. 2019;170:41–50. [DOI] [PubMed] [Google Scholar]
- 10.Greene SJ, Fonarow GC, DeVore AD, Sharma PP, Vaduganathan M, Albert NM, Duffy CI, Hill CL, McCague K, Patterson JH et al. Titration of Medical Therapy for Heart Failure With Reduced Ejection Fraction. J Am Coll Cardiol. 2019;73:2365–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asad ZUA, Yousif A, Khan MS, Al-Khatib SM and Stavrakis S. Catheter Ablation Versus Medical Therapy for Atrial Fibrillation. Circulation Arrhythmia and electrophysiology. 2019;12:e007414. [DOI] [PubMed] [Google Scholar]
- 12.Gupta A, Perera T, Ganesan A, Sullivan T, Lau DH, Roberts-Thomson KC, Brooks AG and Sanders P. Complications of catheter ablation of atrial fibrillation: a systematic review. Circulation Arrhythmia and electrophysiology. 2013;6:1082–8. [DOI] [PubMed] [Google Scholar]
- 13.Berg J, Lindgren P, Nieuwlaat R, Bouin O and Crijns H. Factors determining utility measured with the EQ-5D in patients with atrial fibrillation. Qual Life Res. 2010;19:381–90. [DOI] [PubMed] [Google Scholar]
- 14.Jaagosild P, Dawson NV, Thomas C, Wenger NS, Tsevat J, Knaus WA, Califf RM, Goldman L, Vidaillet H and Connors AF Jr. Outcomes of acute exacerbation of severe congestive heart failure: quality of life, resource use, and survival. SUPPORT Investigators. The Study to Understand Prognosis and Preferences for Outcomes and Risks of Treatments. Arch Intern Med. 1998;158:1081–9. [DOI] [PubMed] [Google Scholar]
- 15.Sandhu AT, Ollendorf DA, Chapman RH, Pearson SD and Heidenreich PA. Cost-Effectiveness of Sacubitril-Valsartan in Patients Who Have Heart Failure With Reduced Ejection Fraction. Ann Intern Med. 2017;166:607–608. [DOI] [PubMed] [Google Scholar]
- 16.Perino AC, Fan J, Schmitt SK, Kaiser DW, Heidenreich PA, Narayan SM, Wang PJ, Chang AY and Turakhia MP. Patient and facility variation in costs of catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2018;29:1081–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kilgore M, Patel HK, Kielhorn A, Maya JF and Sharma P. Economic burden of hospitalizations of Medicare beneficiaries with heart failure. Risk Manag Healthc Policy. 2017;10:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim MH, Johnston SS, Chu BC, Dalal MR and Schulman KL. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011;4:313–20. [DOI] [PubMed] [Google Scholar]
- 19.Royston P and Parmar MK. Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat Med. 2002;21:2175–97. [DOI] [PubMed] [Google Scholar]
- 20.Soikkeli F, Hashim M, Ouwens M, Postma M and Heeg B. Extrapolating Survival Data Using Historical Trial-Based a Priori Distributions. Value Health. 2019;22:1012–1017. [DOI] [PubMed] [Google Scholar]
- 21.Noseworthy PA, Van Houten HK, Gersh BJ, Packer DL, Friedman PA, Shah ND, Dunlay SM, Siontis KC, Piccini JP and Yao X. Generalizability of the CASTLE-AF trial: Catheter ablation for patients with atrial fibrillation and heart failure in routine practice. Heart Rhythm. 2020;17:1057–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pokorney SD, Holmes DN, Shrader P, Thomas L, Fonarow GC, Mahaffey KW, Gersh BJ, Kowey PR, Naccarelli GV, Freeman JV et al. Patterns of Amiodarone use and outcomes in clinical practice for atrial fibrillation. American Heart Journal. 2020;220:145–154 [DOI] [PubMed] [Google Scholar]
- 23.Reynolds MR, Zimetbaum P, Josephson ME, Ellis E, Danilov T and Cohen DJ. Cost-effectiveness of radiofrequency catheter ablation compared with antiarrhythmic drug therapy for paroxysmal atrial fibrillation. Circ Arrhythm Electrophysiol. 2009;2:362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.United States Bureau of Labor Statistics. Consumer Price Index for All Urban Consumers: Medical care, not seasonally adjusted (Series ID CUUR0000SAM). https://www.bls.gov/cpi/factsheets/medical-care.htm [access date: November 1, 2019]
- 25.Taylor CJ, Ordonez-Mena JM, Roalfe AK, Lay-Flurrie S, Jones NR, Marshall T and Hobbs FDR. Trends in survival after a diagnosis of heart failure in the United Kingdom 2000-2017: population based cohort study. BMJ. 2019;364:l223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neumann PJ, Cohen JT and Weinstein MC. Updating cost-effectiveness--the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371:796–7. [DOI] [PubMed] [Google Scholar]
- 27.Gao L and Moodie M. Modelling the lifetime cost-effectiveness of catheter ablation for atrial fibrillation with heart failure. BMJ Open. 2019;9:e031033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blomstrom-Lundqvist C, Gizurarson S, Schwieler J, Jensen SM, Bergfeldt L, Kenneback G, Rubulis A, Malmborg H, Raatikainen P, Lonnerholm S et al. Effect of Catheter Ablation vs Antiarrhythmic Medication on Quality of Life in Patients With Atrial Fibrillation: The CAPTAF Randomized Clinical Trial. Jama. 2019;321:1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mark DB, Anstrom KJ, Sheng S, Piccini JP, Baloch KN, Monahan KH, Daniels MR, Bahnson TD, Poole JE, Rosenberg Y et al. Effect of Catheter Ablation vs Medical Therapy on Quality of Life Among Patients With Atrial Fibrillation: The CABANA Randomized Clinical Trial. JAMA. 2019;321:1275–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aronsson M, Walfridsson H, Janzon M, Walfridsson U, Nielsen JC, Hansen PS, Johannessen A, Raatikainen P, Hindricks G, Kongstad O et al. The cost-effectiveness of radiofrequency catheter ablation as first-line treatment for paroxysmal atrial fibrillation: results from a MANTRA-PAF substudy. Europace. 2015;17:48–55. [DOI] [PubMed] [Google Scholar]
- 31.Blackhouse G, Assasi N, Xie F, Gaebel K, Campbell K, Healey JS, O’Reilly D and Goeree R. Cost-effectiveness of catheter ablation for rhythm control of atrial fibrillation. Int J Vasc Med. 2013;2013:262809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan PS, Vijan S, Morady F and Oral H. Cost-effectiveness of radiofrequency catheter ablation for atrial fibrillation. J Am Coll Cardiol. 2006;47:2513–20. [DOI] [PubMed] [Google Scholar]
- 33.Neyt M, Van Brabandt H and Devos C. The cost-utility of catheter ablation of atrial fibrillation: a systematic review and critical appraisal of economic evaluations. BMC Cardiovasc Disord. 2013;13:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Willems S, Meyer C, de Bono J, Brandes A, Eckardt L, Elvan A, van Gelder I, Goette A, Gulizia M, Haegeli L et al. Cabins, castles, and constant hearts: rhythm control therapy in patients with atrial fibrillation. Eur Heart J. 2019;40:3793–3799c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Packer M and Kowey PR. Building Castles in the Sky. Circulation. 2018;138:751–753. [DOI] [PubMed] [Google Scholar]
- 36.Kuck KH, Merkely B, Zahn R, Arentz T, Seidl K, Schluter M, Tilz RR, Piorkowski C, Geller L, Kleemann T et al. Catheter Ablation Versus Best Medical Therapy in Patients With Persistent Atrial Fibrillation and Congestive Heart Failure: The Randomized AMICA Trial. Circulation Arrhythmia and electrophysiology. 2019;12:e007731. [DOI] [PubMed] [Google Scholar]
- 37.Packer DL, Mark DB, Robb RA, Monahan KH, Bahnson TD, Poole JE, Noseworthy PA, Rosenberg YD, Jeffries N, Mitchell LB et al. Effect of Catheter Ablation vs Antiarrhythmic Drug Therapy on Mortality, Stroke, Bleeding, and Cardiac Arrest Among Patients With Atrial Fibrillation: The CABANA Randomized Clinical Trial. JAMA. 2019;321:1261–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Centers for Medicare and Medicaid. Hospital Outpatient Prospective Payment- Notice of Final Rulemaking (NFRM) with Comment Period CY2018 Payment Rates. CMS-1678-FC 2018. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/Hospital-Outpatient-Regulations-and-Notices-Items/CMS-1678-FC. Access date: December 9, 2019.
- 39.Centers for Medicare and Medicaid. Physician Fee Schedule Search. 2020. https://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx. Access date: August 8, 2020.
- 40.Hunter TD, Palli SR and Rizzo JA. Cost comparison of radiofrequency catheter ablation versus cryoablation for atrial fibrillation in hospitals using both technologies. J Med Econ. 2016;19:959–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Methods.
Supplemental Table I. Parameters for Probabilistic Sensitivity Analysis
Supplemental Table II. Base Case and Lifetime Deterministic Models


