Abstract
Study Objectives:
We investigated the moderation of caloric intake on the association between race/ethnicity and habitual sleep in adolescents.
Methods:
We analyzed the data obtained from 324 adolescents who completed the follow-up examination of the Penn State Child Cohort study. We collected actigraphy-measured sleep duration on 7 consecutive nights and computed their mean and standard deviation as habitual sleep duration (HSD) and habitual sleep variability (HSV), respectively. We also measured participants’ daily intakes of total calorie, total fat, carbohydrates, and protein, through the Youth/Adolescent Food Frequency Questionnaire. Adjusted mean HSD and HSV among non-Hispanic whites and racial/ethnic minorities were compared by using analysis of covariance (ANCOVA), while controlling for age, sex, BMI percentile, total caloric intake, and socioeconomic status. The significance of the interaction between race/ethnicity and caloric intake was further tested in ANCOVA models.
Results:
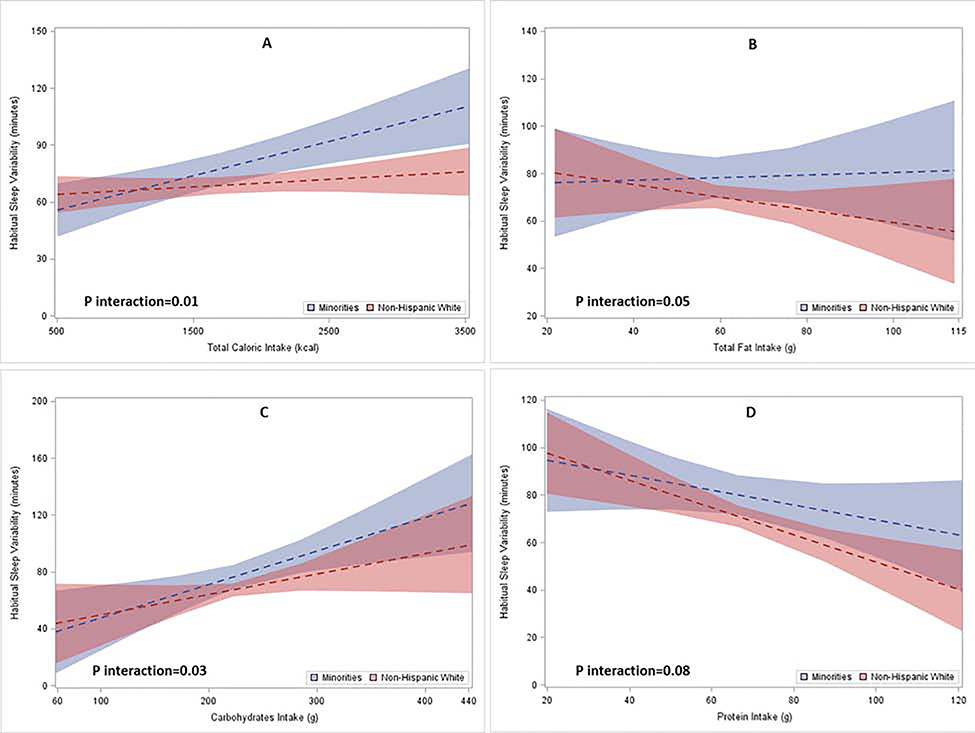
The study sample consisted of 79.3% non-Hispanic whites, 13.0% African American, 4.6% Hispanics, 2.2% Asian, and 0.9% American Indian. Adolescents who are racial/ethnic minorities showed shorter HSD (mean (SE): 6.80 (0.10) vs. 7.07 (0.05) hours/night, p=0.02) and higher HSV (mean (SE): 1.31 (0.07) vs. 1.15 (0.04) hours/night, p=0.04) than non-Hispanic whites. Racial/ethnic differences in HSV were significantly more pronounced among adolescents with high caloric intake (p interaction=0.01), especially from carbohydrates (p interaction=0.03) and fat (p interaction=0.05).
Conclusion:
Adolescents who are racial/ethnic minorities slept objectively shorter and with greater night-to-night variability than non-Hispanic whites. The racial/ethnic disparity in habitual sleep variability was more pronounced among adolescents with high caloric intake, particularly from carbohydrates and fat.
Keywords: racial disparity, habitual sleep, caloric intake, epidemiology
1. INTRODUCTION
Despite extensive knowledge on racial disparities on cardiovascular health has been gathered in the last few decades1–6, little reduction in the racial disparity in cardiometabolic risks has been noted. Concurrently, racial disparity in sleep has been documented7–10. Specifically, racial/ethnic minorities are at a higher risk of short sleep and worse sleep quality. After reviewing current scientific evidence, the American Heart Association (AHA) concluded that short sleep duration and low sleep quality are related to worsening cardiometabolic health11. Thus, it is plausible that racial/ethnic differences in sleep contribute to the disproportionate risk of cardiovascular disease.
While sleep duration and sleep quality are correlated, each of them may be independently associated with cardiometabolic outcomes12–14. For example, Dominguez et al. recently reported that actigraphy-measured short sleep duration and sleep fragmentation were independently associated with higher burden of subclinical atherosclerosis among middle-aged adults12. In contrast, Clark and colleagues found that self-reported sleep duration was not related to cardiovascular disease risk factors, while sleep disturbances were associated with risks of hypertension and dyslipidemia in Finnish residents13. The National Longitudinal Study of Adolescent Health suggested that short sleep duration during adolescence was associated with higher odds of hypercholesterolemia in young adulthood among U.S. females14. The independent, yet inconsistent, associations between sleep duration and sleep quality with cardiometabolic outcomes indicate that sleep duration and quality represents distinctive aspects of sleep physiology and induce differential impacts on cardiovascular health among various populations. Therefore, a better understanding of the contributing factors to the racial/ethnic disparity in sleep, from both quantity and quality perspectives, may reduce the disparity in cardiovascular health.
However, the determinants of racial/ethnic disparity in sleep pattern is not well-understood. The majority of the previous studies investigated the racial differences in sleep patterns attributed the disparities to sociocultural and occupational determinants7–10. For instance, Fuller-Rowell et al. reported that neighborhood economic disadvantage contributed to the differences in sleep efficiency between African American and European American adults9. Jackson and colleagues reported that the racial/ethnic difference in sleep duration was widest among those who held professional/management occupations, while less pronounced among occupations with less professional/management responsibilities. Although racial/ethnic difference in sleep pattern may well be attributed to sociocultural factors, biological and behavioral determinants are also important to consider when examining the racial/ethnic disparity in sleep. However, very little data has been published on the role of behavioral factors in the racial/ethnic disparity in sleep. On another note, previous studies mainly focused on the racial/ethnic difference in sleep quantity, as measured by habitual sleep duration, while overlooked the sleep quality, which may be measured by night-to-night variability in sleep duration. In fact, our group, along with others, has found that major lifestyle behavioral factors, particularly nutrition intake and dietary pattern, is correlated with both sleep duration and sleep variability15–19. For instance, Kjeldsen et al.19 reported that sleep duration was negatively associated with unhealthy dietary pattern, while sleep variability was positively associated with consumption of sugar-sweetened beverages. In addition, Nicklas et al. revealed that white women had higher resting metabolic rate, fat oxidation rate, and maximum oxygen consumption than black women20. The difference in the metabolic rate may result in a higher risk of obesity and related to poor sleep in racial/ethnic minorities. Therefore, we hypothesized that racial differences in sleep may vary by the dietary intake.
To evaluate this hypothesis, we conducted this study to assess the potential interaction between caloric intake and race/ethnicity on both habitual sleep duration (HSD) and sleep quality, as measured by habitual sleep variability (HSV), in a population-based sample of adolescents in central Pennsylvania.
2. METHODS
2.1. Study Sample
Available data from the population-based sample of adolescents who completed the follow-up examination of the Penn State Child Cohort (PSCC) study was used for this study. Detailed descriptions of the PSCC baseline examination have been published elsewhere21–23. Briefly, 700 children aged 6–12 years, who were in 3 school districts within the Harrisburg metropolitan area, were recruited and participated in the baseline examination during 2002–2006. After an average of 7.6 years, 421 out of the 700 subjects returned and completed the follow-up examination in 2010–2013. No major differences in the baseline characteristics were observed between those participants who did and did not complete the follow-up examination23.
During the follow-up examination, all participants underwent a detailed physical examination and completed a battery of questionnaires, including their socio-demographic information and dietary habits. After the physical examination and a standardized overnight polysomnography recording, the participants were released with an actigraphy device (GT3X+; ActiGraph, Pensacola, FL) to proceed with their daily routine for 1 week. Further details of the follow-up examination were published previously14,23. The study protocol was approved by the Penn State University College of Medicine Institutional Review Board. Written informed consent was obtained from participants and their parents or legal guardians if younger than 18 years.
2.2. Habitual Sleep Pattern
Actigraphy devices were used to assess the habitual sleep pattern of the participants. The participants were instructed to wear the actigraphy on their non-dominant hand wrist during nocturnal sleep for 8 consecutive nights over the study period. As the participants were instructed to wear the actigraphy at waist area during daytime, napping episodes were not identified through actigraphy. After removing recording artifacts, the actigraphy data were analyzed by using ActLife 6 software (ActiGraph LLC., Pensacola, FL), in which Sadeh’s algorithm24 was applied for sleep-wake scoring. Since the first night of the data were measured under a 9-hour sleep protocol in the sleep laboratory, they were excluded from the statistical analyses. Based on the remaining 7 nights (i.e. night 2-night 8), HSD and HSV were computed to represent the habitual sleep pattern of the participants. HSD was calculated as the mean of the actigraphy-measured sleep duration across the 7 nights of sleep period, while the intra-subject standard deviation (SD) of the sleep duration was used to represent the HSV. To maintain the validity of HSD and HSV, we further excluded those participants with less than 5 nights (i.e. 70% of 7 nights) of sleep data from the analyses.
2.3. Race/Ethnicity
Participant self-reported race/ethnicity was obtained through a self-administered demographic questionnaire. Each participant was asked to choose 1 from the 6 mutually exclusive options, including “American Indian”, “Native Hawaiian/Asian”, “Black non-Hispanic”, “Hispanic”, “White non-Hispanic”, or “Other”. Participants who did not identify themselves as “White non-Hispanic” were combined to construct the “Racial/Ethnic Minorities” group. To control for the potential biological, behavioral, and social demographic dissimilarities among different subgroups within the “Racial/Ethnic Minorities”, American Indian and Native Hawaiian/Asian were further excluded from sensitivity analyses.
2.4. Caloric Intake
A self-administered Youth/Adolescents food frequency Questionnaire (YAQ) was used to assess the quantity for major nutrients intake of each participant. Briefly, each participant was asked to report the frequency of consumption on 152 food items during the one-year period prior to the study. The frequencies were analyzed and converted to a series of nutrients indices representing the daily nutrition intake. The reproducibility and validity of the YAQ have been reported previously25,26. For this report, we included daily total calorie, total fat, protein, and carbohydrate intakes to represent the participants’ dietary pattern. Subjects with a daily total caloric intake <500 kcal or > 5000 kcal were excluded from the analyses due to implausible responses.
2.5. Other Covariables
Participants’ other demographic information, including age and sex, were also collected through the demographic questionnaire. We measured participants’ height and weight during the physical exam and calculated their body mass index (BMI). Age- and gender-adjusted BMI percentile was then calculated based on the 2000 Centers for Disease Control and Prevention growth charts27. Zip-code level socioeconomic status (SES), including 5-year average percentage (year 2009–2013) of high school graduates and median household income, were extracted from American Community Survey 28.
2.6. Statistical Analysis
Due to insufficient sleep data (N=94) and/or implausible nutrition intake (N=4), 97 of the 421 participants were excluded from the analyses, which resulted in an effective sample size of 324. Major demographic characteristics of the entire study sample, as well as stratified by racial/ethnic groups, were presented as mean (SD) or proportions. To compare these characteristics between non-Hispanic whites and racial/ethnic minorities, t-tests or chi-square tests were used. To assess the overall racial/ethnic differences in HSD and HSV, analysis of covariance (ANCOVA) models were used to estimate the multivariable-adjusted mean and standard error (SE) of the HSD and HSV for non-Hispanic whites and racial/ethnic minorities, respectively. In these models, age, sex, BMI percentile, Zip-code level SES, and daily total caloric intake were included as covariates. These covariables were selected based on the existing knowledge regarding the inter-relationship among HSD, HSV, nutrition intake, and obesity14,21, as well as the observed empirical associations between these variables with demographic characteristics in our study sample. As HSD and HSV were correlated, HSD was controlled for when estimating the adjusted mean of HSV, and vice versa.
To further evaluate whether caloric intake modified the racial/ethnic differences in HSD and HSV, interaction terms between race/ethnicity and total calorie, total fat, carbohydrates, and protein intakes were created. To assess the significance of the interactions, these interaction terms, with their respective lower ordered terms, were included in the ANCOVA models. Since the caloric and nutritional intake variables were modeled as continuous variables, the multivariable-adjusted mean (SE) at the three levels (25th percentile, median, and 75th percentile) of each nutrient intake were computed and compared to enhance the interpretability of the effect sizes. It is worthwhile noting that total caloric intake was further adjusted for, when testing the interaction between race/ethnicity and other macro-nutrients. The linear trend in the HSD and HSV across different nutrient intake levels were assessed using multivariable adjusted linear regression models. Given the heterogeneity of biological and social characteristics in the racial/ethnic minority groups, American Indian and Hawaiian/Asian were further excluded from the sensitivity analyses.
All analyses in this report were performed by using Statistical Analysis Software (SAS 9.4; SAS Institute, Cary, NC, USA). A two-sided p-value of ≤ 0.5 was used to determine statistical significance.
3. RESULTS
3.1. Sample Characteristics
Table 1 summarizes the demographic characteristics, dietary intakes, and habitual sleep pattern of the study sample. As expected from a representative sample of central Pennsylvania population, over 60% of the racial/ethnic minorities in our study sample were African American. The univariate comparisons suggested that non-Hispanic white adolescents slept significantly longer (p<0.01) and with less variability (p=0.03) during the 1-week study period than racial/ethnic minorities. Compared to non-Hispanic white adolescents, racial/ethnic minorities consumed significantly less fat (p=0.05), but had higher BMI percentile (p<0.01). No significant racial/ethnic difference in age, sex, intakes of total calorie, carbohydrates, or protein was observed.
Table 1.
Demographic characteristics of the study sample1
| Characteristics | Overall (N=324) | Non-Hispanic White (N=257) | Racial/Ethnic Minority (N=67) | P |
|---|---|---|---|---|
| Age (years) | 16.7 (2.3) | 16.7 (2.4) | 16.9 (1.9) | 0.38 |
| Male (%) | 51.9 | 53.7 | 44.8 | 0.19 |
| Race/Ethnicity (%) | ||||
| Non-Hispanic White | 79.3 | 100.0 | 0.0 | |
| African American | 13.0 | 0.0 | 62.7 | |
| Hispanics | 4.6 | 0.0 | 22.4 | N/A |
| Hawaiian/Asian | 2.2 | 0.0 | 10.4 | |
| American Indian | 0.9 | 0.0 | 4.5 | |
| BMI percentile | 65.9 (28.2) | 63.7 (29.2) | 73.9 (22.7) | <0.01 |
| HSD (hours) | 7.00 (0.83) | 7.07 (0.80) | 6.72 (0.91) | <0.01 |
| HSV (hours) | 1.18 (0.60) | 1.14 (0.58) | 1.32 (0.64) | 0.03 |
| Total caloric intake (kcal/day) | 1765.4 (658.4) | 1797.3 (652.6) | 1643.0 (671.2) | 0.09 |
| Total fat intake (g/day) | 63.7 (26.3) | 65.1 (26.2) | 58.1 (25.8) | 0.05 |
| Carbohydrate intake (g/day) | 232.4 (88.8) | 236.1 (87.3) | 218.5 (93.5) | 0.15 |
| Protein intake (g/day) | 70.4 (27.7) | 71.7 (27.8) | 65.4 (27.2) | 0.10 |
| % High School graduate or above | 91.9 (3.0) | 92.2 (2.7) | 90.9 (3.6) | <0.01 |
| Household median income ($) | 64806 (11132) | 66005 (10583) | 60532 (12021) | <0.01 |
The results were expressed as mean (SD) and percentage for continuous and categorical variables, respectively. HSD: Habitual Sleep Duration. HSV: Habitual Sleep Variability.
3.2. Racial Disparity in Habitual Sleep
The multivariable-adjusted mean (SE) of HSD and HSV for non-Hispanic whites and racial/ethnic minorities are shown in Table 2. As summarized in the table, the HSD among racial/ethnic minorities was 0.27 hours shorter (p=0.02) than the non-Hispanic white adolescents. Adolescents from racial/ethnic minorities also had a significantly greater HSV (p=0.04). The racial/ethnic differences in the HSD and HSV were approximately equivalent to 30% of their respective SDs (HSD: 0.27/0.83=32.5%; HSV: 0.16/0.59=27.1%). The differences remained significant after excluding American Indian and Hawaiian/Asian from the analyses.
Table 2.
Multivariable adjusted mean (SE) of habitual sleep duration and variability by race/ethnicity.
| Overall | Non-Hispanic White | Racial/ethnic Minority | P value | |
|---|---|---|---|---|
| HSD (hour) | 7.00 (0.05) | 7.07 (0.05) | 6.80 (0.10) | 0.02 |
| HSV (hour) | 1.18 (0.03) | 1.15 (0.04) | 1.31 (0.08) | 0.04 |
HSD: Habitual Sleep Duration. HSV: Habitual Sleep Variability.
Adjusted for age, sex, BMI percentile, total caloric intake, ZIP-code level socioeconomic status, and HSD or HSV.
3.3. Interaction between Race/Ethnicity and Nutrients Intake
There was no significant interaction between race/ethnicity and dietary intake with regard to the racial/ethnic difference in HSD. In contrast, the interactions between race/ethnicity and dietary intake variables, except protein intake, were statistically significant when assessing the relationship between race/ethnicity and HSV. To graphically illustrate the interaction between race/ethnicity and caloric intake on HSV, we plotted the multivariable-adjusted mean and the corresponding 95% confidence interval (CI) of HSV according to dietary intake levels by race/ethnicity in Figure 1. As shown in Figure 1, p for interaction between race/ethnicity and total calorie (Panel A), total fat (Panel B), carbohydrates (Panel C), and protein (Panel D) were 0.01, 0.05, 0.03, and 0.08, respectively. To enhance the interpretability of the results, we further present the HSD and HSV among non-Hispanic whites and racial/ethnic minorities according to different levels of nutrients intake in Table 3. The multivariable-adjusted mean (SE) of HSV, accompanied by the significant interactions, suggests the racial/ethnic difference in HSV was more pronounced at higher intake levels. For example, while HSV was positively associated with total caloric intake among both non-Hispanic whites and racial/ethnic minorities, HSV increased significantly faster among racial/ethnic minorities (panel A of Figure 1). As shown in Table 3, the difference in HSV between non-Hispanic whites and racial/ethnic minorities was 0.07 hours at the 25th percentile of total caloric intake, while the difference substantially increased to 0.28 hours when their caloric intake were at the 75th percentile of the study sample. Similarly, the difference in HSV between non-Hispanic whites and minorities increased with higher carbohydrate intake (panel C of Figure 1). Specifically, the racial/ethnic difference in HSV were 0.07 hours and 0.23 hours as their carbohydrate intake increased from the 25th and 75th percentiles of the entire sample, respectively. In the same vein, racial/ethnic difference in HSV increased with increased total fat intake (panel B of Figure 1). The significance of the interaction terms did not change in the sensitivity analyses, in which American Indian and Native Hawaiian/Asian were excluded.
Figure 1. Multivariable adjusted mean (SE) HSV among non-Hispanic white and racial/ethnic minority adolescents across nutrients intake levels.
P for interaction between race and total caloric, total fat, carbohydrates, and protein intakes were 0.01, 0.05, 0.03, and 0.08 respectively.
Table 3.
Multivariable adjusted mean (SE) of habitual sleep duration and variability by race/ethnicity across nutrients intake levels.
| Non-Hispanic White |
Racial/ethnic Minorities |
|||||||
|---|---|---|---|---|---|---|---|---|
| 25th percentile | Median | 75th percentile | P for trend | 25th percentile | Median | 75th percentile | P for trend | |
| Total calorie | ||||||||
| HSD (hour) | 7.01 (0.06) | 7.06 (0.05) | 7.11 (0.06) | 0.14 | 6.71 (0.11) | 6.75 (0.10) | 6.79 (0.11) | 0.38 |
| HSV (hour) | 1.11 (0.05) | 1.14 (0.04) | 1.17 (0.04) | 0.22 | 1.18 (0.08) | 1.31 (0.07) | 1.45 (0.08) | <0.01 |
| Total Fat | ||||||||
| HSD (hour) | 7.15 (0.10) | 7.09 (0.06) | 7.00 (0.08) | 0.32 | 6.82 (0.17) | 6.77 (0.10) | 6.70 (0.14) | 0.50 |
| HSV (hour) | 1.22 (0.07) | 1.17 (0.04) | 1.09 (0.06) | 0.23 | 1.31 (0.10) | 1.32 (0.07) | 1.34 (0.10) | 0.79 |
| Carbohydrates | ||||||||
| HSD (hour) | 6.96 (0.14) | 7.05 (0.05) | 7.16 (0.11) | 0.36 | 6.69 (0.15) | 6.74 (0.10) | 6.80 (0.14) | 0.59 |
| HSV (hour) | 1.00 (0.09) | 1.12 (0.04) | 1.27 (0.08) | 0.07 | 1.07 (0.11) | 1.27 (0.07) | 1.52 (0.10) | <0.01 |
| Protein | ||||||||
| HSD (hour) | 7.08 (0.13) | 7.07 (0.05) | 7.05 (0.08) | 0.86 | 6.75 (0.13) | 6.76 (0.10) | 6.77 (0.08) | 0.88 |
| HSV (hour) | 1.35 (0.07) | 1.18 (0.04) | 0.98 (0.06) | <0.01 | 1.42 (0.10) | 1.33 (0.07) | 1.22 (0.10) | 0.14 |
Adjusted for age, sex, BMI percentile, zip-code level socioeconomic status, and total caloric intake (when analyzing other macronutrients).
4. DISCUSSION
4.1. Racial Differences in Sleep Pattern
Racial/ethnic minority status has been identified as a predictor of both self-reported29–32 and objectively-measured 32,33 short sleep duration. For example, Lauderdale et al. showed that white participants slept approximately 1 hour longer than African American participants, based on 3 nights of actigraphy data33. We confirmed that the difference in objectively-measured HSD is already presented in adolescence. Specifically, racial/ethnic minority adolescents, on average, slept approximately 0.25 hours shorter per night compared to non-Hispanic whites. Moreover, not only there was a discrepancy in quantity of sleep, but also a significant racial difference in sleep quality31. We also observed that adolescents belong to racial/ethnic minorities showed a significantly larger HSV than non-Hispanic whites. While an inconsistent racial/ethnic difference in sleep variability has been documented in adults 34–37, this is the first time that a significant racial/ethnic disparity in HSV was observed in population-based sample of adolescents. The adjustment of HSD ensured that the observed difference in HSV was independent of average sleep duration.
4.2. Role of Dietary Intake
Due to the strong evidence of the causal effects of dietary habits on cardiovascular health, the AHA included healthy diet as one of the Life’s Simple 7 factors for cardiovascular health38. As sleep has been repeatedly correlated with dietary intake15–19, it is crucial to assess whether dietary intake play a role in the racial/ethnic differences in HSD and HSV. Our study revealed that racial/ethnic disparity in HSV varies by caloric intake. As the total caloric intake may be considered as the sum of energy intake from carbohydrates, fat, and protein, we further identified the sources contributing to the overall moderating effect by evaluating the interactions between each macronutrient and race/ethnicity. The significant interactions between carbohydrates and fat intakes with race/ethnicity further suggested the moderating effect of total caloric intake was mainly driven by intakes of carbohydrates and fat, but not protein. Moreover, the moderating effects of carbohydrates and fat intakes on racial/ethnic disparity in HSV showed different patterns. Specifically, while increased carbohydrates intake was associated with higher HSV in both groups, the association was significantly more pronounced among minorities. Total fat intake, on the other hand, was associated with a decreased HSV among non-Hispanic whites, but not among racial/ethnic minorities. The different patterns may suggest carbohydrates and fat intakes moderate the racial/ethnic disparity in sleep through different mechanisms.
To our knowledge, our study is the first to observe such an interaction between race/ethnicity and dietary intake on HSV. The findings suggest that unhealthy dietary intake may not only directly associate with cardiometabolic health39, but also indirectly relate to the disproportionate cardiovascular diseases risks via its correlation with the racial disparity in sleep, even in healthy adolescents. Thus, it is of great public health importance to promote and facilitate healthy diets and dietary habits among racial/ethnic minorities at the community level.
4.3. Potential Mechanisms
The potential mechanisms responsible for racial/ethnic disparities in sleep have been discussed previously7–10. Since short sleep may be a surrogate marker of stress40,41, the difference in sleep pattern has been, in part, attributed to exposure of life stressors that disproportionally affect racial/ethnic minorities. For example, it has already been documented that racial discrimination, and associated psychological distress42–44, is associated with sleep problems in adolescents. In addition, Jackson et al. showed that employment status and racial discrimination may result in shorter sleep duration among African Americans.10 Other forms of systemic racism, including less favorable neighborhood conditions, have also been found to be contributors to the racial/ethnic disparities in sleep9. As sleep is a complex physiological phenomenon, racial/ethnic difference in sleep pattern may be also determined by physiological factors, such as differences in endogenous circadian periods and likelihood of being affected by a misalignment between the internal circadian clock and time of sleep45. This likelihood may well be affected by the interplay of other psychosocial and environmental factors that contribute to circadian misalignment. Taken together, a combination of social, environmental, and physiological factors may results in a difference in the demand for and/or ability to sleep between racial/ethnic minorities and non-Hispanic white adolescents.
On the other hand, little is understood regarding the mechanisms through which nutrients intake modifies the racial/ethnic disparities in habitual sleep, in particular HSV. We postulate that the racial/ethnic differences in metabolic rates may be one of mechanisms. For example, African American postmenopausal women showed a significantly lower fat oxidation rate than white women20. Similar racial difference in lipolysis has also been observed in children46. Since leptin is a stimulant of lipolysis and fatty acid oxidation47,48, lower rates of fat metabolism commonly coexists with low leptin level and obesity. With the mounting evidence that shows low leptin level and obesity are related to reduced sleep duration49 and higher sleep variability21,50, the difference in fat metabolism may be a physiological mechanism that contribute to the different levels of racial/ethnic discrepancy in HSV. Specifically, due to the lower rates of metabolism, higher caloric intake among race/ethnic minorities may lead to abnormal fat storage and alternations in sleep.
4.4. Strength and Limitation
A major strength of our study is that we used 7-night actigraphy data to objectively measure HSD and HSV. This data collection approach allowed us to accurately measure habitual sleep patterns and prevented us from the potential biases and limitations of subjectively-reported sleep, 1-night polysomnography, or short-term actigraphy data. To further increase the validity of our data, we excluded the participants with < 5 nights (70% of 7 nights) of sleep data. A second strength is that we purposively controlled for HSD as a covariable when analyzing the racial/ethnic disparity in HSV, and vice versa. As a result, we were able to conclude that the racial/ethnic difference in HSV, as well as the interaction between race/ethnicity and nutrient intake, were not confounded by HSD. Lastly, we adjusted for total caloric intake when evaluating the potential interaction between race/ethnicity and other macronutrients, which enabled us to conclude that the interaction with carbohydrate intakes was independent of total caloric intake. The adjustment was equivalent to including the proportion of energy from various macronutrients in the model. Therefore, our results may be also interpreted as the racial/ethnic difference in sleep variability varies by the total caloric intake, as well as the proportion of calorie obtained through carbohydrates and fat.
The present study has some limitations. First, self-reported race/ethnicity was used to form a dichotomized race/ethnicity classification (i.e., non-Hispanic whites vs. racial/ethnic minorities). The heterogeneity among multiple race/ethnicity groups, genetic ancestries, and psychosocial exposures in the “racial/ethnic minorities” group may confound the findings. However, the racial/ethnic disparity in sleep and the interaction between race/ethnicity and caloric intakes remained significant after excluding Native American and Asian participants. In fact, the racial/ethnic differences in sleep patterns were more pronounced after the exclusion. It suggested greater disparities in sleep between non-Hispanic whites and African American or Hispanic adolescents, which is consistent with the underlying impact of social determinants of health, such as racism, discrimination, prejudice, neighborhood conditions, food security, or healthcare access in these specific groups in the United States. Second, the response rate of the follow-up examination was 60%. However, no significant difference in the demographic characteristics was found between adolescents who participated in the follow-up examination and those who did not. Third, zip-code level median household income and education level were used to represent the participants’ SES. While aggregated education and household income data may not be able to entirely represent the individual level SES, we expect that community-level SES is highly correlated with individual-level SES. Therefore, the adjustment would partially eliminated the confounding effect of SES. Additionally, daytime napping behavioral was not measured through actigraphy, which may confound our findings. Finally, the current study is a cross-sectional analysis of the PSCC follow-up examination data. Thus, causal inferences should not be made.
5. CONCLUSION
In conclusion, our study suggests significant racial/ethnic disparities in habitual sleep patterns. Specifically, adolescents who are racial/ethnic minorities slept objectively shorter but with greater night-to-night variability than non-Hispanic whites. More importantly, the racial/ethnic disparity in habitual sleep variability was more pronounced with increased caloric intake, particularly from carbohydrates and fat. This finding suggests that the racial/ethnic disparity in sleep variability is associated with food consumption and may indirectly relate to the known racial/ethnic disparities in cardiometabolic risk. In the meantime, longitudinal studies are necessary to confirm our findings and uncover the underlying mechanisms through which nutrients intake modifies the racial/ethnic disparity in habitual sleep.
Supplementary Material
Highlights:
There is a significant racial disparity in habitual sleep even in healthy adolescents.
Compared to Caucasian adolescents, adolescents who belong to racial/ethnic minority groups, habitually, slept shorter and with a larger night-to-night variability in sleep duration.
The racial disparity in habitual sleep was more pronounced among adolescents with high caloric intake, especially from carbohydrates and fat.
Unhealthy dietary intake may exacerbate the racial/ethnic disparity in habitual sleep among adolescents and through which contribute to the disproportionate cardiovascular disease risk among racial/ethnic minorities.
ACKNOWLEDGEMENTS
This work is supported by National Institutes of Health (NIH) grant: R01 HL097165, R01 HL63772, R21 HL087858 and the Penn State Clinical and Translational Science Institute (CTSI) grant: UL TR000127.
ABBREVIATIONS
- PSCC
Penn State Child Cohort
- HSD
Habitual Sleep Duration
- HSV
Habitual Sleep Variability
- SD
Standard Deviation
- YAQ
Youth and adolescents food frequency questionnaire
- BMI
Body mass index
- SES
Socioeconomic status
- ANCOVA
Analysis of covariance
- SE
Standard error
Footnotes
Declaration of Interests: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Graham G Disparities in cardiovascular disease risk in the United States. Curr Cardiol Rev. 2015;11(3):238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lackland DT. Racial differences in hypertension: implications for high blood pressure management. Am J Med Sci. 2014;348(2):135–138. doi: 10.1097/MAJ.0000000000000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spanakis EK, Golden SH. Race/ethnic difference in diabetes and diabetic complications. Curr Diab Rep. 2013;13(6):814–823. doi: 10.1007/s11892-013-0421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leigh JA, Alvarez M, Rodriguez CJ. Ethnic Minorities and Coronary Heart Disease: an Update and Future Directions. Curr Atheroscler Rep. 2016;18(2):9. doi: 10.1007/s11883-016-0559-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howard G, Moy CS, Howard VJ, McClure LA, et al. Where to Focus Efforts to Reduce the Black-White Disparity in Stroke Mortality: Incidence Versus Case Fatality? Stroke. 2016;47(7):1893–1898. doi: 10.1161/STROKEAHA.115.012631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benjamin EJ, Virani SS, Callaway CW, et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation. 2018. 20;137(12):e67–e492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 7.Williams NJ, Grandne MA, Snipes A, Rogers A, Williams O, Airhihenbuwa C, Jean-Louis G. Racial/ethnic disparities in sleep health and health care: importance of the sociocultural context. Sleep Health. 2015;1(1):28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grandner MA, Williams NJ, Knutson KL, Roberts D, Jean-Louis G. Sleep disparity, race/ethnicity, and socioeconomic position. Sleep Med. 2016;18:7–18. doi: 10.1016/j.sleep.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuller-Rowell TE, Curtis DS, El-Sheikh M, Chae DH, Boylan JM, Ryff CD. Racial disparities in sleep: the role of neighborhood disadvantage. Sleep Med. 2016;27–28:1–8. doi: 10.1016/j.sleep.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson CL, Redline S, Kawachi I, Williams MA, Hu FB. Racial disparities in short sleep duration by occupation and industry. Am J Epidemiol. 2013;178(9):1442–1451. doi: 10.1093/aje/kwt159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.St-Onge MP, Grandner MA, Brown D, et al. Sleep Duration and Quality: Impact on Lifestyle Behaviors and Cardiometabolic Health: A Scientific Statement From the American Heart Association. Circulation. 2016;134(18):e367–e386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dominguez F, Fuster V, Fernandez-Alvira JM et al. Association of sleep duration and quality with subclinical atherosclerosis. J AM Coll Cardiol 2019; 73(2):134–144. doi: 10.1016/j.jacc.2018.10.060. [DOI] [PubMed] [Google Scholar]
- 13.Clark Aj, Salo P, Lange T, et al. Onset of impaired sleep and cardiovascular disease risk factors: a longitudinal study. Sleep. 2016;39(9):1709–1718. doi: 10.5665/sleep.6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gangwisch JE, Malaspina D, Babiss LA, et al. Short sleep duration as a risk factor for hypercholesterolemia: analyses of the National Longitudinal Study of Adolescent Health. Sleep. 2010;33(7):956–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He F, Bixler EO, Berg A, et al. Habitual sleep variability, not sleep duration, is associated with caloric intake in adolescents. Sleep Med. 2015;16(7):856–861. doi: 10.1016/j.sleep.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peuhkuri K, Sihvola N, Korpela R. Diet promotes sleep duration and quality. Nutr Res. 2012;32(5):309–319. doi: 10.1016/j.nutres.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Dashti HS, Scheer FA, Jacques PF, Lamon-Fava S, Ordovás JM. Short sleep duration and dietary intake: epidemiologic evidence, mechanisms, and health implications. Adv Nutr. 2015;6(6):648–659. doi: 10.3945/an.115.008623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grandner MA, Jackson N, Gerstner JR, Knutson KL. Sleep symptoms associated with intake of specific dietary nutrients. J Sleep Res. 2014;23(1):22–34. doi: 10.1111/jsr.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kjeldsen JS, Hjorth MF, Andersen R et al. Short sleep duration and large variability in sleep duration are independently associated with dietary risk factors for obesity in Danish school children. Int J Obes (Lond). 2014:38:32–39. doi: 10.1038/ijo.2013.147. [DOI] [PubMed] [Google Scholar]
- 20.Nicklas BJ, Berman DM, Davis DC, Dobrovolny CL, Dennis KE. Racial differences in metabolic predictors of obesity among postmenopausal women. Obes Res. 1999;7(5):463–468. [DOI] [PubMed] [Google Scholar]
- 21.Bixler EO, Vgontzas AN, Lin HM, et al. Blood pressure associated with sleep disordered-breathing in a population sample of children. Hypertension. 2008;52:841–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He F, Bixler EO, Liao J, et al. Habitual sleep variability, mediated by nutrition intake, is associated with abdominal obesity in adolescents. Sleep Med. 2015;16(12):1489–1494. doi: 10.1016/j.sleep.2015.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bixler EO, Fernandez-Mendoza J, Liao D et al. Natural history of sleep disordered breathing in prepubertal children transitioning to adolescence. Eur Respir J. 2016;47:1402–1409. doi: 10.1183/13993003.01771-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sadeh A, Sharkey KM, Carskadon MA. Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep. 1994;17:201–207. [DOI] [PubMed] [Google Scholar]
- 25.Rockett HR, Wolf AM, Colditz GA. Development and reproducibility of a food frequency questionnaire to assess diets of older children and adolescents. J Am Diet Assoc 1995;95:336–340. [DOI] [PubMed] [Google Scholar]
- 26.Rockett HR, Breitenbach M, Frazier AL, et al. Validation of a youth/adolescent food frequency questionnaire. Prev Med 1997;26:808–816. [DOI] [PubMed] [Google Scholar]
- 27.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development . Vital Health Stat 11 2002;(246):1–190. [PubMed] [Google Scholar]
- 28.U.S. Census Bureau, Understanding and Using American Community Survey Data: What All Data Users Need to Know, U.S. Government Printing Office, Washington, DC, 2018. [Google Scholar]
- 29.Whinnery J, Jackson N, Rattanaumpawan P, Grandner MA. Short and long sleep duration associated with race/ethnicity, sociodemographics, and socioeconomic position. Sleep. 2014. 1;37(3):601–611. doi: 10.5665/sleep.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hale L, Do DP. Racial differences in self-reports of sleep duration in a population-based study. Sleep. 2007;30(9):1096–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biggs SN, Lushington K, James Martin A, van den Heuvel C, Declan Kennedy J. Gender, socioeconomic, and ethnic differences in sleep patterns in school-aged children. Sleep ed. 2013;14(12):1304–1309. doi: 10.1016/j.sleep.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 32.Marczyk Organek KD, Taylor DJ, Petrie T, Martin S, Greenleaf C, Dietch JR, Ruiz JM. Adolescent sleep disparities: sex and racial/ethnic differences. Sleep Health. 2015;1(1):36–39. doi: 10.1016/j.sleh.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 33.Lauderdale DS, Knutson KL, Yan LL, Rathouz PJ, Hulley SB, Sidney S, Liu K. Objectively measured sleep characteristics among early-middle-aged adults: the CARDIA study. Am J Epidemiol. 2006;164(1):5–16. [DOI] [PubMed] [Google Scholar]
- 34.Matthews KA, Hall M, Dahl RE. Sleep in healthy black and white adolescents. Pediatrics. 2014;133(5):e1189–1196. doi: 10.1542/peds.2013-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lemola S, Ledermann T, Friedman EM. Variability of sleep duration is related to subjective sleep quality and subjective well-being: an actigraphy study. PLoS One. 2013;8(8):e71292. doi: 10.1371/journal.pone.0071292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bei B, Wiley JF, Trinder J, Manber R. Beyond the mean: A systematic review on the correlates of daily intraindividual variability of sleep/wake patterns. Sleep Med Rev. 2016;28:108–124. doi: 10.1016/j.smrv.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Mezick EJ, Matthews KA, Hall M, Kamarck TW, Buysse DJ, Owens JF, Reis SE. Intra-individual variability in sleep duration and fragmentation: associations with stress. Psychoneuroendocrinology. 2009;34(9):1346–1354. doi: 10.1016/j.psyneuen.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: The American Heart Association’s strategic impact goal through 2020 and beyond. Circulation. 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 39.Funtikova AN, Navarro E, Bawaked RA, Fíto M, Schröder H. Impact of diet on cardiometabolic health in children and adolescents. Nutr J. 2015;14:118. doi: 10.1186/s12937-015-0107-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knutson KL, Rathouz PJ, Yan LL, Liu K, Lauderdale DS. Intra-individual daily and yearly variability in actigraphically recorded sleep measures: the CARDIA study. Sleep. 2007;30(6):793–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vgontzas AN, Bixler EO. Short sleep and obesity: are poor sleep, chronic stress, and unhealthy behaviors the link? Sleep 2008;31:1203. [PMC free article] [PubMed] [Google Scholar]
- 42.Hall MH, Casement MD, Troxel WM, et al. Chronic stress is prospectively associated with sleep in midlife women: The SWAN sleep study. Sleep. 2015;38(10):1645–1654. doi: 10.5665/sleep.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lewis TT, Cogburn CD, Williams DR. Self-reported experiences of discrimination and health: scientific advances, ongoing controversies, and emerging issues. Annu Rev Clin Psychol. 2015;11:407–440. doi: 10.1146/annurev-clinpsy-032814-112728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miranda R, Polanco-Roman L, Tsypes A, Valderrama J. Perceived discrimination, ruminative subtypes, and risk for depressive symptoms in emerging adulthood. Cultur Divers Ethnic Minor Psychol. 2013;19(4):395–403. doi: 10.1037/a0033504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eastman CI, Tomaka VA, Crowley SJ. Circadian rhythms of European and African-Americans after a large delay of sleep as in jet lag and night work. Sci Rep. 2016;6:36716. doi: 10.1038/srep36716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Danadian K, Lewy V, Janosky JJ, Arslanian S. Lipolysis in African-American children: is it a metabolic risk factor predisposing to obesity? J Clin Endocrinol Metab. 2001;86(7):3022–3026. [DOI] [PubMed] [Google Scholar]
- 47.Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Müller C, Carling D, Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415(6869):339–343. [DOI] [PubMed] [Google Scholar]
- 48.Ruud J, Brüning JC. Metabolism: Light on leptin link to lipolysis. Nature. 2015. November 5;527(7576):43–44. doi: 10.1038/527043a. [DOI] [PubMed] [Google Scholar]
- 49.Chaput JP, Després JP, Bouchard C, Tremblay A. Short sleep duration is associated with reduced leptin levels and increased adiposity: Results from the Quebec family study. Obesity (Silver Spring). 2007;15(1):253–261. [DOI] [PubMed] [Google Scholar]
- 50.Patel SR, Hayes AL, Blackwell T, Evans DS, Ancoli-Israel S, Wing YK, Stone KL; Osteoporotic Fractures in Men (MrOS); Study of Osteoporotic Fractures (SOF) Research Groups. The association between sleep patterns and obesity in older adults. Int J Obes (Lond). 2014;38(9):1159–1164. doi: 10.1038/ijo.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.