Abstract
Background:
ECOG3805 is a randomized trial of testosterone suppression with or without docetaxel for metastatic hormone-sensitive prostate cancer (mHSPC). Deeper prostate-specific antigen (PSA) suppression is prognostic for outcome. However, the concordance of PSA rise and radiographic progression has not been examined previously in mHSPC, whereas this has been reported in metastatic castration-resistant prostate cancer.
Objective:
To determine the patterns of progression by PSA and radiographic parameters in patients in ECOG3805.
Design, setting, and participants:
We conducted a retrospective analysis of all patients in ECOG3805. Patients were classified according to the PSA level at progression (whether PSA level was below 2.0 ng/ml or not) and the type of progression event in the study (either PSA progression as defined by the study with or without clinical progression, or clinical progression alone). Baseline demographics, clinical outcomes, and patterns of progression were compared between the groups.
Results and limitations:
One in eight patients had clinical progression below a PSA level of 2 ng/ml, and approximately 25% developed clinical progression in the absence of confirmed PSA progression. Overall survival from randomization was shorter in patients with clinical progression without confirmed PSA progression than in patients with PSA progression alone as the first progression. Patient demographics at study entry were not predictive of the pattern of progression. Study limitations include its retrospective and post hoc nature.
Conclusions:
Clinical progression prior to PSA rise or at low PSA levels is a relatively frequent phenomenon in mHSPC and is associated with poorer overall survival. Further biological and clinical studies of these patients are warranted.
Patient summary:
Reliance on prostate-specific antigen (PSA) alone is an inadequate strategy to monitor patients undergoing treatment for metastatic hormone-sensitive prostate cancer. Prostate cancer can get worse on scans even with low PSA and/or no or small changes in PSA. Imaging should be added to PSA testing to monitor patients with metastatic prostate cancer.
Keywords: Chemotherapy, Clinical trial, Hormone therapy, Prostate cancer, Prostate-specific antigen
1. Introduction
Since 2010, many new life-prolonging therapies have emerged for metastatic castration-resistant prostate cancer (mCRPC). In 2014, ECOG3805 was the first study to report a major improvement in overall survival (OS) when an agent active in mCRPC, docetaxel, was added at the time of commencing testosterone suppression for metastatic hormone-sensitive prostate cancer (mHSPC) [1]. This finding was confirmed in 2016, and a closer analysis of long-term follow-up data showed that the OS benefit was most clearly seen in patients with high-volume disease [2,3], with a 17-mo improvement in median OS in high-volume patients. Subsequently, further clinical trials have shown improvements in OS with the addition of abiraterone, enzalutamide, and apalutamide in mHSPC [4-7].
There is no level 1 evidence to guide how to monitor patients with mHSPC for the emergence of castration-resistant prostate cancer (CRPC). Fortunately, a rising prostate-specific antigen (PSA) level often indicates emergent CRPC prior to radiographic progression. Given the limitations of imaging and PSA for prostate cancer management, the initial Prostate Cancer Working Group (PCWG) [8] guidelines were released in 1999 to provide some uniform guidelines for defining CRPC and eligibility criteria for trial enrollment. The guidelines are now in their third iteration as PCWG3 [9].
The adage that one should not order a test unless it is going to change management applies when following patients with mHSPC on testosterone suppression alone. There is wide variability in the potential benefit of testosterone suppression alone, with some patients developing early progression in the first few months, while others respond for many years [10]. To complicate matters more, some patients may progress with radiographic worsening before or concurrent with a modest PSA rise, as has been demonstrated with enzalutamide in the mCRPC setting [11].
Despite the application of working group criteria in a clinical trial setting, the approach to monitoring and clinical practice remains highly varied. The 2017 Advanced Prostate Cancer Consensus Conference [12] included a section on the topic of surveillance where 60 international prostate cancer experts discussed and voted on consensus guidelines for mHSPC. The results were highly variable, with 51% voting for baseline and follow-up imaging at PSA nadir or at the completion of six cycles of docetaxel. Surveillance imaging every 3–6 mo after baseline imaging was supported by 31% of the panelists. Baseline imaging followed by surveillance by PSA alone was supported by 18% [12].
The potential shortcomings of PSA as a marker of disease status are well known. These include cases of PSA decline in the absence of any therapy, the frequency of low or no PSA secretion by prostate cancer cells as the potential to evolve to more aggressive phenotypes including androgen receptor–negative anaplastic disease and small cell [13,14], and the potential for PSA to remain stable even as the patient develops clinical or radiographic progression. Examples of the first phenomenon were seen in the ALSYMPCA study where the rate of PSA decline on placebo therapy was 14% [15] and in the PREVAIL study with a PSA 50% decline in 4% of placebo patients [16]. The lack of PSA rise at the time of radiographic progression was first systematically described in patients treated with enzalutamide in a PREVAIL study [11], where 24.5% of first-line mCRPC patients had a lack of clear PSA progression at the time of radiographic progression on enzalutamide.
The definition of PSA progression as it relates to study conduct, and an understanding of the impact of these definitions and their validity are of critical importance. For ECOG3805, PSA progression was defined as a 50% increase over nadir, or a rise to at least 4.0 or 2.0 ng/ml if the nadir is less than these levels (Supplementary Table 1). For current studies, PCWG3 defines PSA progression as a 25% increase over nadir with a minimum absolute level of 1 ng/ml. Herein, we report an analysis of the patterns of progression in the ECOG3805 study with regard to clinical or radiographic progression prior to PSA progression, as defined by the study criteria. To our knowledge, this is the first analysis of clinical progression without PSA progression in an mHSPC population.
2. Patients and methods
A total of 790 men were accrued from July 28, 2006 to November 21, 2012, and randomized to androgen deprivation therapy (ADT) alone or ADT plus docetaxel at 75 mg/m2 every 3 wk for six cycles within 4 mo of commencing testosterone suppression (and possibly a weak antiandrogen at investigator discretion). Eligible patients had a pathological diagnosis of prostate cancer or a clinical scenario consistent with prostate cancer with an elevated PSA level, radiological evidence of metastatic disease, and an Eastern Cooperative Oncology Group (ECOG) performance status score of 0, 1, or 2. Prior adjuvant ADT was allowed if the duration of therapy was ≤24 mo and progression had occurred >12 mo after the completion of therapy. Patients who were receiving ADT for metastatic disease were eligible if there was no evidence of progression and treatment had commenced within 120 d before randomization. Patients were prospectively stratified into high-versus low-volume disease [1]. Clinical progression was defined as increasing symptomatic bone metastases, radiographic progression according to the RECIST criteria, or clinical deterioration due to cancer as per the investigator’s opinion. PSA progression was defined as an increase in PSA of ≥50% above the on-treatment nadir, with two consecutive increases at least 2 wk apart. The absolute PSA level to declare progression had to be at least 4.0 or 2.0 ng/ml if the nadir level was less than these levels (Supplementary Table 1). Concurrent PSA progression and clinical progression is defined as both events occurring with 1 mo.
PSA was assessed at baseline, and then at every cycle while on docetaxel and every 3 mo while on luteinizing hormone-releasing hormone therapy alone, and every 3 mo in the group not randomized to docetaxel. Imaging was obtained at baseline, and then only at the time of castration-resistant disease as determined by rising PSA or at the investigator’s discretion if the patient was deemed to have clinical deterioration. For this post hoc analysis, patients were defined as having had (1) PSA progression prior to clinical progression, (2) concurrent PSA and clinical progression, (3) PSA progression alone, or (4) clinical progression without PSA progression. Clinical progression is defined as either radiographic progression or symptoms indicative of disease progression based on the investigator’s discretion. In prostate cancer patients, the population of symptomatic progressors primarily comprises patients with increasing bone pain without radiographic progression as per the PCWG criteria. For this post hoc analysis for determining PSA status at the time of clinical progression, we required a PSA measurement within 30 d prior to determining clinical progression. This assessment was not done for all patients as it was not mandated by the protocol. For the purposes of our analysis, groups 1, 2, and 3 were all classified to have PSA progressive patients, where group 4 consists of our comparator cohort.
Time point–based analyses of PSA at 6 and 12 mo included only those patients who were progression free at these time points. Quality of life (QOL) assessment was administered at baseline and at 3, 6, 9, and 12 mo; only patients with baseline QOL assessment available and follow-up QOL assessment administered within 4 mo prior to first disease progression were included in this analysis. QOL change is defined as a change in FACT-P total score from baseline to the follow-up visit prior to disease progression. For example, a patient with disease progression at 8 mo has QOL change calculated as follows: FACT-P total score at 6 mo – FACT-P total score at baseline. Time to clinical progression is defined as the time from randomization to clinical progression. Patients without clinical progression were censored at the date of last disease assessment.
Descriptive statistics were used to describe the baseline characteristics, disease progression pattern, and QOL data. Differences in the distributions of continuous and categorical variables between groups were evaluated using Wilcoxon rank-sum test and Fisher's exact test, respectively. The method of Kaplan and Meier was used to characterize event-time distributions. All analyses were performed using SAS software, version 9.4.
3. Results
As of the final data cutoff, disease progression was identified in 403 of 513 men with high-volume disease and 156 of 277 men with low-volume disease. A total of 435 patients qualified for the three populations of patients with clinical progression and any PSA status. Of these patients, a total of 274 had a PSA level measured within 30 d of the progression event (Table 1). A significant percentage of patients had a rise in PSA despite PSA remaining below 2 ng/ml, which was the defined cutoff for PSA progression. Without breaking down by treatment arm, this occurred in 11.3% (36/318) of high-volume patients and 12.8% of low-volume patients. PSA discordant progression, for example, clinical progression without PSA progression, occurred in 25% of evaluable patients in ECOG3805 (Table 2). It was seen in patients with both high- and low-volume disease, and in those treated with ADT alone as well as ADT and docetaxel. The most common pattern was the expected pattern of an initial PSA rise followed by clinical progression occurring >30 d after the PSA rise. This occurred in approximately 40% of patients. PSA progression without clinical PD at the time of the analysis had occurred in 22.1% of all patients.
Table 1 –
PSA status at clinical progression a
| PSA at clinical PD | High volume | Low volume | ||
|---|---|---|---|---|
| ADT + D | ADT alone | ADT + D | ADT alone | |
| N (number of pts with clinical PD) | 142 | 176 | 52 | 65 |
| Number of pts with PSA measurement within 30 d prior to clinical PD | 87 | 122 | 28 | 37 |
| Patients with PSA b ≤1 | 11.3% (16/142) |
5.1% (9/176) | 7.7% (4/52) | 10.8% (7/65) |
| Patients with PSA b ≤2 | 15.5% (22/142) |
8.0% (14/176) | 13.5% (7/52) | 12.3% (8/65) |
ADT = androgen deprivation therapy; D = docetaxel; PD = progressive disease; PSA = prostate-specific antigen; pts = patients.
Only patients with clinical PD with or without PSA PD were included in this analysis.
The latest PSA measurement observed within 30 d prior to clinical progression (including measurement observed on the date of clinical progression).
Table 2 –
Disease progression pattern by treatment arm and disease volume
| Disease progression pattern | High volume | Low volume | ||
|---|---|---|---|---|
| ADT + D | ADT alone | ADT + D | ADT alone | |
| Concurrent PSA PD and clinical PD a, n (%) |
18 (9.5) | 28 (13.2) | 8 (11.9) | 3 (3.4) |
| PSA PD first and then clinical PD b, n (%) | 81 (42.6) | 96 (45.1) | 23 (34.3) | 38 (42.7) |
| PSA PD only c, n (%) | 48 (25.3) | 37 (17.4) | 15 (22.4) | 24 (27.0) |
| Clinical PD only d, n (%) | 43 (22.6) | 52 (24.4) | 21 (31.3) | 24 (27.0) |
| Total | 190 | 213 | 67 | 89 |
ADT = androgen deprivation therapy; D = docetaxel; PD = progressive disease; PSA = prostate-specific antigen.
PSA PD and clinical PD were observed within a month (including 32 patients with onset of PSA PD observed within 1 mo of clinical PD but subsequent PSA to confirm that progression was not available).
PSA PD was observed at least 1 mo prior to clinical progression (including 21 patients with onset of PSA PD observed at least 1 mo prior to clinical progression but subsequent PSA to confirm that progression was not available).
Patients experienced PSA PD, but clinical PD has not been observed yet.
Patients experienced clinical PD without PSA PD.
Clinical progression was predominantly radiographic progression rather than symptomatic progression across all categories of patients (Supplementary Table 2). The proportion of patients with radiographic progression as the qualifying event for clinical progression ranged from 88% to 98% across low- and high-volume disease patients and patients with chemohormonal therapy versus hormonal therapy alone. Similarly, the proportions of radiographic versus symptomatic progression events within the clinical progression cohort did not vary according to the disease progression pattern (Supplementary Table 3). The proportions of radiographic progression were 91.2% when PSA progression preceded clinical progression, 94.7% when PSA progression and clinical progression were concurrent, and 92.9% when clinical progression occurred without PSA progression.
3.1. Patient characteristics and clinical course
The baseline clinical characteristics of patients who developed clinical progression without PSA rise versus any pattern of PSA progression with or without clinical progression are shown in Table 3. Most baseline features were similar between the populations. They were similar in terms of age, Gleason score at diagnosis, and history of prior local therapy. Patients with clinical progression first tended to have a lower baseline PSA. A PSA <0.2 response at 6 and 12 mo was maintained, respectively, in 32 of 99 (32.3%) and 16 of 56 (28.6%) patients in the clinical progression group versus 54 of 254 (17.5%) and 37 of 182 patients (20.3%) in the PSA progression groups. However, QOL change from baseline and OS were inferior in the group with clinical progression alone.
Table 3 –
Baseline characteristics, clinical course, and QOL by disease progression type
| Clinical PD first | Current PSA and clinical PD PSA PD then clinical PD PSA PD only |
|||
|---|---|---|---|---|
| N = 140 % | % | N = 419 % | % | |
| Age at randomization | ||||
| Median | 62.5 | 62 | ||
| Range | 36–91 | 39–88 | ||
| Gleason score | ||||
| <7 | 10 | 7.8 | 20 | 5.4 |
| 7 | 20 | 15.6 | 83 | 22.6 |
| 8–10 | 98 | 76.6 | 265 | 72.0 |
| Missing | 12 | 51 | ||
| Prior local therapy | ||||
| No | 104 | 74.3 | 336 | 80.2 |
| Yes | 36 | 25.7 | 83 | 19.8 |
| Baseline PSA | ||||
| Median | 20.4 | 93.8 | ||
| Range | 0.1–2960 | 0.4–8540.1 | ||
| PSA <0.2 at 6 mo a, n (%) | ||||
| No | 67 (67.7) | 254 (82.5) | ||
| Yes | 32 (32.3) | 54 (17.5) | ||
| PSA <0.2 at 12 mo b, n (%) | ||||
| No | 40 (71.4) | 145 (79.7) | ||
| Yes | 16(28.6) | 37 (20.3) | ||
| QOL change from baseline c,d | ||||
| N | 70 | 212 | ||
| Mean (standard deviation) | −5.6(16.4) | −1.1 (17.5) | ||
| Median (range) e | −4.7 (−55.3, 35.0) | −2.0 (−69.4, 64.0) | ||
| Time to clinical progression (both arms) f | ||||
| N | 140 | 419 | ||
| Number of events | 140 | 295 | ||
| Median (mo) | 9.1 | 20.5 | ||
| 95% CI | (7.8, 11.4) | (18.2, 22.9) | ||
| Time to clinical progression (docetaxel arm) | ||||
| N | 64 | 193 | ||
| Number of events | 64 | 130 | ||
| Median (mo) | 11.1 | 24.1 | ||
| 95% CI | (8.0, 13.5) | (20.7, 29.1) | ||
| Time to clinical progression (ADT-alone arm) | ||||
| N | 76 | 226 | ||
| Number of events | 76 | 165 | ||
| Median (mo) | 8.5 | 17.8 | ||
| 95% CI | (5.7, 11.3) | (14.2, 20.1) | ||
ADT = androgen deprivation therapy; CI = confidence interval; PD = progressive disease; PSA = prostate-specific antigen; QOL = quality of life.
Only patients with first disease progression observed at least 6 mo after randomization were included in the analysis.
Only patients with first disease progression observed at least 12 mo after randomization were included in the analysis.
As QOL assessment was administered at baseline, and at 3, 6, 9, and 12 mo, only patients with baseline QOL assessment available and follow-up QOL assessment administered within 4 mo prior to first disease progression were included in this analysis. There are 70 and 212 patients meeting the criterion in the “clinical PD first” and “other” categories, respectively.
QOL change is defined as change in the FACT-P total score from baseline to the follow-up visit prior to disease progression. For example, a patient with disease progression at 8 mo has QOL change calculated as follows: FACT-P total score at 6 mo – FACT-P total score at baseline.
p = 0.14 by Wilcoxon rank-sum test.
Time to clinical progression is defined as the time from randomization to clinical progression. Patients without clinical progression were censored at the date of last disease assessment.
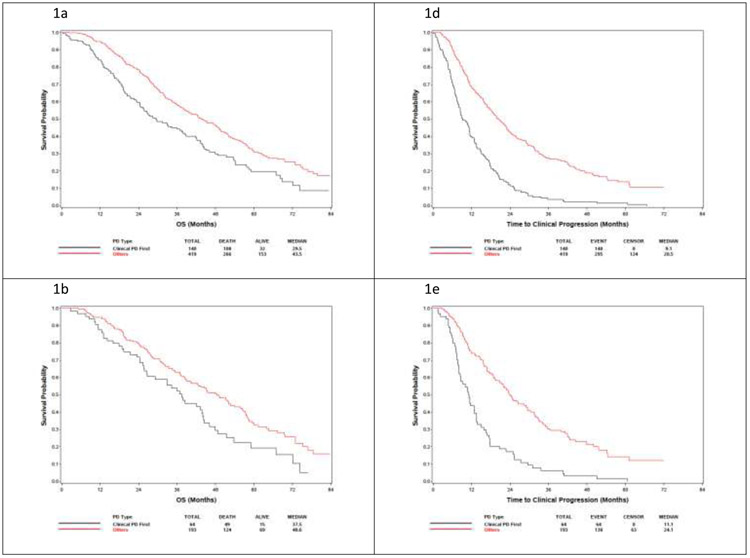
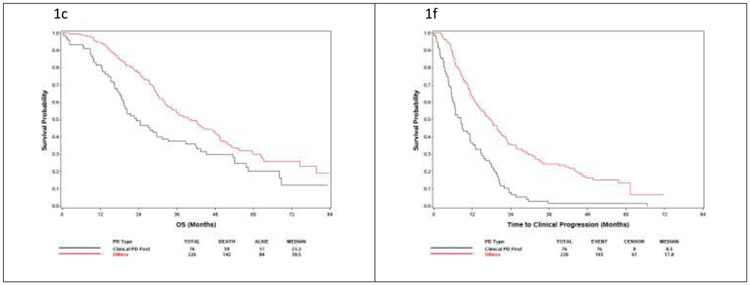
Time to clinical progression from randomization was inferior in the clinical progression cohort (Table 3 and Fig. 1). In the clinical progression group, median time to clinical progression was 9.1 (95% confidence interval [CI]: 7.8–11.4) mo compared with 20.5 (95% CI: 18.2–22.9) mo in patients who had PSA progression with/without clinical progression. In the docetaxel arm, the corresponding values for time to progression were 11.1 (95% CI: 8.0–13.5) and 24.1 (95% CI: 20.7–29.1) mo. In the ADT-alone arm, the values were 8.4 (95% CI: 5.7–11.3) and 17.8 (95% CI: 14.2–20.1) mo, respectively.
Fig. 1 –
Overall survival from randomization and time to progression on study therapy by treatment type. OS in (A) clinical progression versus all other progression patterns in the entire cohort, (B) docetaxel-treated patients, and (C) ADT-treated patients. (D—F) Time from study entry to progression on study as stratified by the type of progression. ADT = androgen deprivation therapy; OS = overall survival; PD = progressive disease.
3.2. OS from randomization and date of progression
OS was measured both from randomization and from the date of progression to death. The OS from randomization of patients with clinical progression appears to be shorter than that of patients with any pattern of PSA progression (Table 4 and Fig. 1). Patients with clinical progression had OS of 29.5 (95% CI: 24.3–37.9) mo compared with 43.5 (95% CI: 38.7–49.1) mo in patients with PSA progressive disease. It is notable that the CIs are nonoverlapping, but no formal statistical comparison of these groups is provided given the unplanned, exploratory nature of this analysis.
Table 4 –
Overall survival (from randomization) by disease progression type
| Clinical PD first | Current PSA and clinical PD PSA PD then clinical PD PSA PD only |
|
|---|---|---|
| OS (both arms) | ||
| N | 140 | 419 |
| Number of deaths | 108 | 266 |
| Median (mo) | 29.5 | 43.5 |
| 95% CI | (24.3, 37.9) | (38.7, 49.1) |
| OS (docetaxel arm) | ||
| N | 64 | 193 |
| Number of deaths | 49 | 124 |
| Median (mo) | 37.5 | 48.6 |
| 95% CI | (26.5, 44.0) | (40.3, 55.8) |
| OS (ADT-alone arm) | ||
| N | 76 | 226 |
| Number of deaths | 59 | 142 |
| Median (mo) | 23.3 | 39.5 |
| 95% CI | (18.8, 31.4) | (34.4, 47.3) |
ADT = androgen deprivation therapy; CI = confidence interval; OS = overall survival; PD = progressive disease; PSA = prostate-specific antigen.
OS is defined as the time from randomization to death from any cause or the date last known to be alive.
OS in patients on the docetaxel arm was 37.5 (95% CI: 26.5–44.0) mo in the clinical progression group and 48.6 (95% CI: 40.3–55.8) mo in the PSA progression group. In the ADT-alone arm, the respective median OS periods were 23.3 (95% CI: 18.8–31.4) and 39.5 (95% CI: 34.4–47.3) mo. To assess the clinical course after progressing in ECOG3805, we analyzed the OS after the first progression on trial (Supplementary Table 4). The median OS after progression was 15.7 (95% CI: 13.2–24.3) mo in patients with clinical progression alone and 20.3 (95% CI: 18.1–22.0) mo in patients with PSA clinical progression. In docetaxel-treated patients, the postprogression median OS was 22.7 (95% CI: 13.6–30.0) mo in the clinical progression group and 17.6 (95% CI: 14.9–21.6) mo in the PSA progression group. The patients treated with ADT alone had median OS after progression of 13.6 (95% CI: 11.0–18.1) mo in the clinical progression group and 20.9 (95% CI: 18.8–24.2) mo in the PSA progression group.
The clinical courses of individuals on trial and post-trial survival are illustrated in the swimmers plots in Figure 2. In particular, Figures 2B and 2D show the timing of PSA progression relative to subsequent clinical progression.
Fig. 2 –
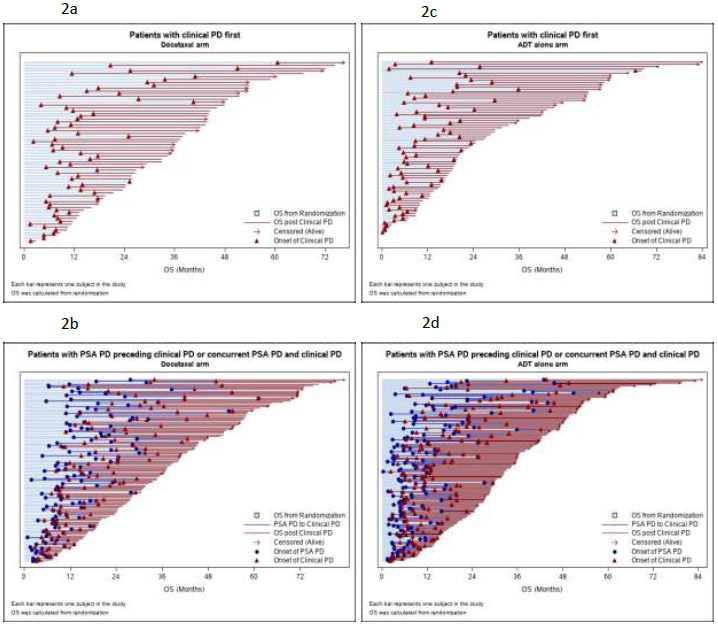
Swimmers plots of on-study and post-study survival patients by study arm and progression type. (A) Patients with clinical progression first in the docetaxel arm. (B) Patients with PSA progression preceding clinical progression or with concurrent PSA progression and clinical progression in the docetaxel arm. (C) Patients with clinical progression first in the ADT-alone arm. (D) Patients with PSA progression preceding clinical progression or with concurrent PSA progression and clinical progression in the ADT-alone arm. ADT = androgen deprivation therapy; OS = overall survival; PD = progressive disease; PSA = prostate-specific antigen.
4. Discussion
This analysis shows that clinical progression at low PSA levels is a relatively common phenomenon, with over 10% of patients developing progressive disease below the protocol criteria for PSA progression of a PSA level of at least 2 ng/ml. Among patients who never achieved PSA complete responses of <0.2 ng/ml at nadir, 22–31% experienced clinical progression of their disease before meeting PSA criteria for progression. The data also suggest that patients with clinical progression in the absence of PSA progression have inferior OS from the time of randomization. While these findings are derived from post hoc analyses, it adds to prior reports identifying the limitations of PSA as a marker of disease progression. However, these data implying that clinical progression alone occurs earlier and is associated with shorter OS suggest that patients who have clinical progression alone may have a worse biology from the outset.
Our work highlights the phenomenon of PSA-radiographic discordance in mHSPC and emphasizes the disparate nature of PSA as it pertains to biological behavior and standard imaging assessments. The design of ECOG3805 reflects that, in 2004, the consensus among the cooperative group and National Cancer Institute (NCI) prostate cancer leaders and other NCI leaders was for imaging only at baseline and then at the time of mCRPC emergence, as evidenced by PSA progression or clinical symptoms. The frequency of clinical progression without a preceding rise in PSA in our analysis calls this approach into question.
The clinical progression population is somewhat heterogeneous since it includes patients with increasing bone pain, radiographic progression, and clinical deterioration. In addition, progression could be established based on imaging for causes unrelated to the study, which nonetheless demonstrated progression of the prostate cancer. Nevertheless, radiographic progression was the dominant driver of clinical progression across all groups and did not vary in relation to the pattern of PSA progression.
This analysis is limited by being an unplanned, post hoc analysis. This analysis would have benefited from imaging obtained at standardized intervals, as is standard for modern industry-sponsored but not co-operative group trials of mHSPC. The data on PSA and progression were not collected in a way so as to optimize the analysis performed here, and the definition of PSA-radiographic discordance is not standardized. In real-world practice, the definition of clinical progression without PSA progression also depends on the definition of PSA rise. In this analysis, we used the definition of PSA progression that was prespecified in the study written in 2004. However, the practicing clinician may reasonably argue that a rise of 50% above nadir is an arbitrary distinction (indeed, the current PCWG3 guideline is 25% and a minimum starting level of 1 rather than 2 ng/ml [9]), and this lower threshold is reasonable to trigger “for-cause” imaging in a real-world setting. For the time being, no formal definition of nonrising PSA exists. Nevertheless, it is clear that PSA discordant radiographic or clinically progressive disease is a relatively frequent event that should be considered in clinical care and clinical trial conduct. Given the frequency of skeletal related events (SRE) in metastatic prostate cancer, the availability of interventions that can reduce SREs [17], and the significant prognostic implications of abdominal visceral metastases [18], the rationale for including imaging in the surveillance of metastatic prostate cancer is clear. What is unclear is which imaging modalities should be used and at what intervals.
The other recent randomized trials of combination therapy for mHSPC reflect the lack of consensus regarding the role of surveillance imaging. The STAMPEDE study, similar to ECOG3805, reported a comparison of ADT plus docetaxel with ADT alone, as well as a comparison of ADT plus abiraterone with ADT alone. In these studies, imaging was assessed at baseline and then repeated at 24 wk for patients with metastases or low PSA at baseline [7,19]. Conversely, the LATITUDE study investigating ADT with or without docetaxel specified imaging at baseline and every 4 mo [5]. Harmonized recommendations for monitoring of patients on mHSPC trials are clearly needed.
5. Conclusions
In summary, this dataset confirms that clinical progression with modest or no PSA progression occurs in about a quarter of patients with mHSPC, similar to what has been reported in mCRPC. It also confirms that progression can occur at low PSA values, undermining the sole use of PSA as a trigger for imaging. Clinical progression without a rising PSA value clearly denotes a more aggressive initial disease course with inferior OS from the start of therapy. Future studies should incorporate advanced imaging, such as whole-body magnetic resonance imaging and prostate-specific membrane antigen positron emission tomography imaging, as well as prospectively measure the relationship between PSA and radiographic progression.
Supplementary Material
Take Home Message.
In the E3805 CHAARTED study, radiographic surveillance detected progressive disease before a rise in prostate-specific antigen in 25% of patients with metastatic hormone-sensitive prostate cancer.
Acknowledgments
Funding/Support and role of the sponsor: This study was coordinated by the ECOG-ACRIN Cancer Research Group (Peter J. O'Dwyer, MD, and Mitchell D. Schnall, MD, PhD—group cochairs) and supported by the NCI of the National Institutes of Health under the following award numbers: CA180820, CA180794, CA180790, CA180795, CA180799, CA180801, CA180802,CA180821, CA180833,CA180853, CA180867, CA180888, CA180801, CA189829. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Footnotes
Financial disclosures: Alan H. Bryce certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med 2015;373:737–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gravis G, Boher JM, Chen YH, et al. Burden of metastatic castrate naive prostate cancer patients, to identify men more likely to benefit from early docetaxel: further analyses of CHAARTED and GETUG-AFU15 studies. Eur Urol 2018;73:847–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kyriakopoulos CE, Chen YH, Carducci MA, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol 2018;36:1080–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chi KN, Agarwal N, Bjartell A, et al. Apalutamide for Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med 2019;381:13–24. [DOI] [PubMed] [Google Scholar]
- [5].Fizazi K, Tran N, Fein L, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med 2017;377:352–60. [DOI] [PubMed] [Google Scholar]
- [6].Davis ID, Martin AJ, Stockier MR, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med 2019;381:121–31. [DOI] [PubMed] [Google Scholar]
- [7].James ND, de Bono JS, Spears MR, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med 2017;377:338–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bubley GJ, Carducci M, Dahut W, et al. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol 1999;17:3461–7. [DOI] [PubMed] [Google Scholar]
- [9].Scher HI, Morris MJ, Stadler WM, et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol 2016;34:1402–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Leuprolide Study Group. Leuprolide versus diethylstilbestrol for metastatic prostate cancer. N Engl J Med 1984;311:1281–6. [DOI] [PubMed] [Google Scholar]
- [11].Bryce AH, Alumkal JJ, Armstrong A, et al. Radiographic progression with nonrising PSA in metastatic castration-resistant prostate cancer: post hoc analysis of PREVAIL. Prostate Cancer Prostatic Dis 2017;20:221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gillessen S, Attard G, Beer TM, et al. Management of patients with advanced prostate cancer: the report of the Advanced Prostate Cancer Consensus Conference APCCC 2017. Eur Urol 2018;73:178–211. [DOI] [PubMed] [Google Scholar]
- [13].Papandreou CN, Daliani DD, Thall PF, et al. Results of a phase II study with doxorubicin, etoposide, and cisplatin in patients with fully characterized small-cell carcinoma of the prostate. J Clin Oncol 2002;20:3072–80. [DOI] [PubMed] [Google Scholar]
- [14].Moore SR, Reinberg Y, Zhang G. Small cell carcinoma of prostate: effectiveness of hormonal versus chemotherapy. Urology 1992;39:411–6. [DOI] [PubMed] [Google Scholar]
- [15].Sartor O, Xu L, Shan M, et al. An exploratory analysis of alkaline phosphatase, lactate dehydrogenase, and prostate-specific antigen dynamics in the phase 3 ALSYMPCA trial with radium-223. Ann Oncol 2017;28:1090–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Evans CP, Higano CS, Keane T, et al. The PREVAIL study: primary outcomes by site and extent of baseline disease for enzalutamide-treated men with chemotherapy-naive metastatic castration-resistant prostate cancer. Eur Urol 2016;70:675–83. [DOI] [PubMed] [Google Scholar]
- [17].Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet 2011;377:813–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Pond GR, Sonpavde G, de Wit R, Eisenberger MA, Tannock IF, Armstrong AJ. The prognostic importance of metastatic site in men with metastatic castration-resistant prostate cancer. Eur Urol 2014;65:3–6. [DOI] [PubMed] [Google Scholar]
- [19].James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 2016;387:1163–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.