Abstract
Introduction
A prerequisite for a satisfying functional result in the treatment of an irreparable rotator cuff rupture is a significant reduction of shoulder pain and better range of motion with an increase in anatomic glenohumeral joint stability.
Purpose
Prospective study to examine the outcome after superior capsular reconstruction using a porcine extracellular matrix dermal graft. A special emphasis was primarily on the functional outcome, secondarily on radiographic shoulder changes, that superior capsular reconstruction might yield.
Methods
Clinical results were evaluated using the Constant score and Western Ontario Rotator Cuff (WORC) index over a 2-year period. All patients had magnetic resonance imaging (MRI) of the injured shoulder after 1 year. Graft integration and durability were qualitatively estimated as well as any graft deterioration or resorption.
Results
Thirteen patients with 13 superior capsular reconstructions were included over a 3-year period. Mean age was 61 years (range 50-70) at the time of surgery. At final follow-up (mean 24 months, range 23-32), the mean Constant score had improved from an average of 24.9-55.7 points. The mean WORC index had increased from a percentage average of 32.3%-61.9%. Eleven of 13 grafts were intact on follow-up MRI.
Conclusion
Our hypothesis was that successful implantation of a dermal xenograft would correlate with both better functional outcome and stabilized glenohumeral radiographic features. We saw a group of patients with variable but significant increases in functional results and in general with limited pain and with an intact xenograft on an MRI scan. We did not find a positive correlation between functional outcome scores and graft durability nor with single cuff tendon defects vs. larger rotator cuff defects.
This study suggests that a superior capsular reconstruction can yield results that are comparable or superior to other known salvage treatment options in patients with large to massive rotator cuff defects without significant cuff tear arthropathy. The hypothesis that superior capsular reconstruction can be a relevant treatment method for irreparable rotator cuff tears could not be refuted despite a fairly low patient inclusion number. With these results, selected patients can be considered for a different treatment than reverse shoulder arthroplasty, débridement, or tendon transfer.
Keywords: Shoulder, superior capsular reconstruction, rotator cuff, chronic tendon rupture, arthroscopy
A great number of patients with a chronic irreparable superior rotator cuff rupture seek treatment.2,31 An irreparable superior rotator cuff rupture can be evaluated on a magnetic resonance imaging (MRI) scan showing retraction of a tendon remnant to the glenohumeral joint line with fatty degeneration grade 3 or 4 according to Goutallier and atrophy of the rotator cuff muscle under the tangent line according to Gerber.13,15,28 Such a defect can also be evaluated with shoulder arthroscopy where the tendon remnant typically is found to be rigid, leaving a large defect in the upper part of the rotator cuff.
During shoulder abduction, this contributes to instability with cranial displacement of the glenohumeral fulcrum and collision of the humeral head against the underside of the acromion.20,28 Attempts to mobilize retracted tendon tissue with interval slides12,19,20 must often be abandoned, or sometimes only a single-row fixation of a tight supraspinatus tendon can be achieved. According to Burkhart, it is possible to surgically close these chronic defects in at least 70% of cases.20 Other sources describe these defects more often as being irreparable28 and recommend that repair of retracted ruptured supraspinatus tendons >3 cm should not be attempted.18,27 Typically, symptoms are pain, loss of strength, and active range of motion reduced to <90° abduction. Conservative treatment such as supervised strengthening of the deltoid muscle and the remaining posterior rotator cuff can yield some pain alleviation, especially for patients aged ≥70 years, whereas patients aged <70 years attain less satisfying results because of increased functional demands and a greater desire for a functionally acceptable shoulder function.7,17,20,28,31
Current treatment options are débridement and smoothening of the underside of the acromion without violating the coracoacromial ligament, tenotomy of the long head of the biceps, partial tendon suture to reduce the size of the defect or transposition of the latissimus dorsi tendon, a salvage operation seldomly performed.1,6,11,25,30 Because neither of these techniques yield satisfying results for a nongeriatric age group, a growing number of reverse shoulder arthroplasties (RSAs) are being implanted despite intact joint cartilage.20 In a younger patient population of 45-65 years of age, the limited durability of an arthroplasty should be considered as well as a relatively high complication rate with morbidity and infection being serious and costly.20 Therefore, there is a need for a surgical method that is joint preserving and can re-create glenohumeral biomechanics, that is, a stable glenohumeral articular fulcrum.
Prior attempts at patch grafting, “bridging,” between the humerus and the retracted tendon remnant had not demonstrated convincing results.11,27 Patch grafting technique has since been modified so that the graft is fixed to the top of the glenoid in order to create a passive capsule-emulating structure that could prevent cranial humeral head migration.8 This patch graft technique, superior capsular reconstruction (SCR; Arthrex, Naples, FL, USA) has been used since 2007 and has shown promising short-term results in a few publications.5,22,23
In the first published series, the graft material was autologous fascia lata,8 but as this graft only has a low breakage strength of 180 N, a human dermal extracellular matrix (ECM) allograft is used in the USA. In Europe, this graft is not approved for use. Instead, a porcine ECM graft, used for several years in heart valve surgery, has shown itself to be immunologically compatible. This graft, which is an extracellular matrix surgical mesh (Arthrex DX Reinforcement Matrix; Arthrex, CE number 550398), in comparison has a breakage strength of 550 N21 and is the one used in this study.
If there were a significant correlation with SCR surgery and improvement of function and pain, this technique would be a strong choice for the patient aged <70 years, in good general health condition, still working, and with a desire to maintain a good shoulder and arm function. This could lessen costs for society and for patients with a greater functional level with less need of supportive measures and a higher individual quality of life and better function.
In this prospective study, we evaluate outcome after the use of SCR with a double-layered porcine ECM graft in patients with a chronic irreparable posterosuperior defect in the rotator cuff. This study has specifically evaluated this technique clinically and radiographically with an MRI scan during a longer follow-up period.
Materials and methods
During a 3-year period from October 2015 to January 2018, we used the Extracellular DX Reinforcement Matrix Mesh (Arthrex) for patients not older than 70 years with a chronic irreparable posterosuperior 1- or 2-tendon rotator cuff tear and with glenohumeral cartilage changes not more severe than Hamada type 313 and with no history of alchohol or drug abuse, mental inhability, or noncompliance. The extent of a tendon defect and its irreparability was verified either because the patient had undergone a prior shoulder arthroscopy that documented the defect and irreparability or it was estimated using an MRI scan that demonstrated a retracted rotator cuff remnant, Goutallier grade 3 or 4 fatty degeneration, and posterosuperior muscle atrophy inferior to the coracoid-scapula spine level, that is, a positive tangent sign.15 Also, an inclusion criterion for these patients was failure of improvement after 3 months of training of the anterior deltoid muscle and posterior rotator cuff supervised by a physiotherapist.29
The operation was performed either purely arthroscopically (12 patients) or arthroscopically with mini-open lateral fixation of the graft (1 patient). All tendon remnants were released and examined as to whether a tendon suture could be performed before an SCR xenograft was implanted. In one further patient besides the described 15 patients in this paper, a rotator cuff suture could be performed. The patients were followed prospectively for 2 years with functional outcome scores and MRI scan (see Table I). If an MRI scan was performed preoperatively, this was done maximally 6 months prior to the operation. All patients had an MRI scan approximately 12 months after the operation in order to evaluate graft durability and glenohumeral joint stability.
Table I.
Outcome evaluation plan
| Preoperative | Postoperative 6 mo | Postoperative 12 mo | Postoperative 24 mo | |
|---|---|---|---|---|
| MRI scan | x and/or prior arthroscopy | x | ||
| Constant score | x | x | x | x |
| WORC index | x | x | x |
MRI, magnetic resonance imaging; WORC, Western Ontario Rotator Cuff.
All written surveys were filled in at each visit to the outpatient clinic. When calculating strength for the Constant score, this was measured in 90° of scaption over 5 seconds and repeated 3 times using the best score. If the arm could not be abducted to 90°, strength was measured in a position of maximal abduction.14 An Isobex isometric dynamometer was used. Thus, active range of movement in flexion and abduction was measured for all patients and a Constant score was calculated for both arms for comparison. The Western Ontario Rotator Cuff (WORC) index reflects the patient's own perception of changes in health status and thus is an indicator of the success of treatment. The questionnaire contains 21 items each with a visual analog response option for the following 5 domains: pain and physical symptoms (6 questions), sport and recreation (4 questions), work (4 questions), lifestyle function (4 questions), and emotions (3 questions). Like Constant score values, the score values are adjusted and reported as a percentage of an ideal normal function.24
Operative technique and after treatment
The patients were operated by a team of 3 surgeons, 2 surgeons per procedure, the first author being the primary surgeon in all cases. The surgical procedure was performed according to the recommendations published by Hirahara17 and Burkhart.4 However, in our group, we fold the 1.5-mm-thick graft in 2 layers and whipstitch it on the remaining 3 sides with a nonresorbable suture, FiberWire 0 (Arthrex). We do not apply glue between the layers to avoid increasing brittleness of the material. We repair any infraspinatus and subscapularis tears if possible and use soft anchors medially on the glenoid and thread the medial anchor sutures extracorporeally, pulling the graft into place superiorly on the glenoid neck as a single-row fixation. The lateral fixation is then performed using a retrograde suture-passing device and completed as a double-row fixation using the Speedbridge anchor system (Arthrex).
One or more nonresorbable convergence sutures are then placed between the graft and the posterior cuff tissue. The graft medially covers a sagittal area from the base of the coracoid process to the posterior cuff and laterally from the top of the intertubercular sulcus to the posterior cuff. We aim to avoid any torque of the graft to avoid graft rupture and hold it manually to avoid damage from metal instruments. The graft is secured with the arm in neutral position with no rotation. An abduction sling is used for 4 weeks, and the shoulder is passively mobilized for the first 6 weeks postoperatively. Patients start active range of movement 6 weeks after surgery.
The shoulder was kept unloaded for 4 months. The patients were advised that loading is not the aim of the operation. In addition, the patients were referred to rehabilitation supervised by local municipal physiotherapists outside the hospital. The rehabilitation program was designed according to the recommendations by Burkhart.4 At follow-up, clinical results were evaluated using the Constant score and WORC index. Postoperative MRI scans were evaluated separately by authors A.U. and M.R. A 100% interobserver consistency was found in their evaluation of graft integrity.
Results
Fifteen shoulders were operated on in 14 patients. Eight had a total rupture of the supraspinatus with a total or subtotal infraspinatus tendon rupture and 7 had an isolated total rupture of the supraspinatus tendon. Two patients were excluded. One patient with a 2-tendon defect developed a subacute postoperative infection where the graft had to be removed as an emergency procedure.
Later on, this patient had a second successful SCR procedure. A second patient with a 2-tendon defect had significant pain and deteriorating function over a year and a nontraumatically ruptured SCR. He had an RSA after 16 months that yielded a very satisfying result. Thus, the group included 13 patients, 7 with a 1-tendon defect and 6 with a 2-tendon defect. Of the 2-tendon defects, 4 infraspinatus tendon ruptures could be repaired, whereby the concomitant SCR covered a 1-tendon defect in those cases. There were 10 men and 3 women. Mean age was 61 years (range 50-70) at the time of surgery. All patients were available at all times of follow-up for a clinical and radiographic review to evaluate the effect of the operation. The mean follow-up period was 24 months (range 23-32).
In 11 of the 11 (100%) patients with intact SCRs at the time of the postoperative MRI, the humeral head had migrated cranially, resting the xenograft against the undersurface of the acromion with a thickness of approximately 3-4 mm in all cases. No arthritis greater than Hamada grade 3 was seen on a follow-up MRI scan. At follow-up, the final mean Constant score and WORC index values for the whole group, respectively, had increased from 25 (range 19-41) to 56 (range 15-82) and from 32% (range 8%-51%) to 62% (range 22%-84%). The relevant data on the 13 included patients are presented in Table II. Subscores of pain and elevation from the Constant score are presented in Table III. The elevation subscore improved or stabilized in all included patients except patient 2. The pain subscore rose in all patients. Hence, the operation improved either one or both parameters in all patients except one.
Table II.
Patients and results
| Case no. | Sex | Age at operation (yr) | Size of tendon defect∗ | Constant score/WORC index, preoperative | Constant score, 6 mo postoperative | Constant score/WORC index, 12 mo postoperative | MRI scan, 12 mo postoperative† | Constant score/WORC index, 24 mo, postoperative | Clinical outcome based on patient satisfaction/percent increase in Constant score and WORC index preoperatively to final follow-up |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 54 | SS+IS | 20/8.2% | 26 | 71/56.1% | Intact graft at 12 mo | 38/47.9% | Painfree Satisfied Constant score increase: 90% WORC index increase: 484% |
| 2 | F | 50 | SS | 25/51.4% | 29 | 14/21.9% | Intact graft at 12 mo | 15/22.0% | Not satisfied. RSA implanted after 2.5 yr Constant score increase: 0% WORC index increase: 0% |
| 3 | M | 61 | SS | 22/31.0% | 9/ | 51/70.9% | Graft ruptured at 5 mo | 71/62.4% | The patient was noncompliant. He was satisfied with his end result. Constant score increase: 222% WORC index increase: 101% |
| 4 | M | 61 | SS+IS | 19/25.0% | 75/ | 90/73.1% | Intact graft at 12 mo | 70/74.0% | Painfree Satisfied Constant score increase: 268% WORC index increase: 196% |
| 5 | M | 55 | SS+IS | 21/22.9% | 21 | 57/58.1% | Intact graft at 7 mo | 77/79.1% | Constant score increase: 266% WORC index increase: 245% |
| 6 | F | 61 | SS+IS | 30/31.7% | 37 | 39/66.3% | Intact graft at 16 mo | 58/83.6% | Constant score increase: 93% WORC index increase: 164% |
| 7 | M | 70 | SS | 41/39.0% | 59 | 62/85.9% | Intact graft at 11 mo | 75/72.2% | Constant score increase: 82% WORC index increase: 85% |
| 8 | M | 68 | SS+IS | 19/43.9% | 20 | 51/54.4% | Intact graft at 13 mo | 50/57.5% | Constant score increase: 163% WORC index increase: 31% |
| 9 | M | 63 | SS | 22/49.1% | 20 | 36/35.1% | Intact graft at 13 mo | 35/51.7% | Constant score increase: 62% WORC index increase: 5% |
| 10 | M | 60 | SS+IS | 25/10.2% | 46 | 55/47.3% | Intact graft at 12 mo | 51/51.0% | Constant score increase: 104% WORC index increase: 400% |
| 11 | M | 70 | SS | 24/38.2% | 42 | 40/55.4% | Intact graft at 12 mo | 46/60.9% | Constant score increase: 92% WORC index increase: 59% |
| 12 | M | 54 | SS | 19/28.0% | 37 | 65/29.6% | Graft ruptured at 3 mo | 6/65.0% | Constant score increase: 194% WORC index increase: 132% |
| 13 | M | 63 | SS | 37/41.5% | 43 | 56/73.1% | Intact graft at 13 mo | 82/77.7% | Constant score increase: 121% WORC index increase: 87% |
F, female; M, male; SS, supraspinatus tendon; IS, infraspinatus tendon; WORC, Western Ontario Rotator Cuff; MRI, magnetic rsonance imaging; CS, Constant score; RSA, reverse shoulder arthroplasty.
When a total or a subtotal rupture of an infraspinatus tendon was identified besides a supraspinatus tendon rupture, this was defined as a 2-tendon defect whether the infraspinatus rupture could be repaired or not.
Tendon status, graft durability, any graft retraction and grading of glenohumeral arthrosis.
Table III.
Constant subscores for flexion and pain
| Case no. | Sex | Age at operation (yr) | Flexion (°)∗ / Pain score (maximum 15 points)† |
|||
|---|---|---|---|---|---|---|
| Preoperative | 6 mo postoperative | 12 mo postoperative | 24 mo postoperative | |||
| 1 | F | 54 | 105/4 | 75/7 | 165/15 | 105/15 |
| 2 | F | 50 | 75/5 | 45/8 | 45/2 | 15/7 |
| 3 | M | 61 | 75/5 | 45/2 | 105/15 | 165/15 |
| 4 | M | 61 | 75/5 | 165/15 | 165/15 | 165/15 |
| 5 | M | 55 | 15/2 | 45/8 | 135/10 | 165/9 |
| 6 | F | 61 | 45/6 | 45/15 | 45/14 | 75/15 |
| 7 | M | 70 | 135/7 | 95/7 | 135/12 | 135/12 |
| 8 | M | 68 | 45/5 | 75/3 | 105/10 | 105/12 |
| 9 | M | 63 | 45/8 | 45/7 | 75/7 | 75/10 |
| 10 | M | 60 | 45/6 | 75/14 | 105/14 | 120/15 |
| 11 | M | 70 | 45/10 | 75/15 | 75/12 | 75/15 |
| 12 | M | 54 | 75/0 | 75/12 | 135/9 | 135/13 |
| 13 | M | 63 | 75/4 | 75/12 | 105/8 | 165/15 |
F, female; M, male.
The flexion intervals were registered from the Constant score form. In the form, the intervals are divided in the following categories of flexon: 0°-30°, 31°-60°, 61°-90°, 91°-120°, 121°-150°, and 151°-180°. The flexion was estimated within a 15° interval.
The pain score presented was registered from the Constant score. In the form, the score reflects the most severe pain experienced during normal daily activities over a 24-hour period. 15 points means no pain and 0 points reflects the maximal level.
Complications
Ten of the included 13 patients did not experience complications. Shoulder improvement was gradual and several patients reported how this slow improvement was mentally and emotionally taxing. They were encouraged by a fairly expedient relief of pain while improvements in function developed slowly. One patient (no. 2; see Table II) had an RSA after the 2-year follow-up because she was not satisfied with her end result. One patient (no. 3) had an SCR after a 1-tendon defect with an initial very good result but after 6 months, the graft ruptured due to heavy lifting. Another patient (no. 12) with a 1-tendon defect experienced a graft rupture after 2 months when loading his shoulder following an initial period of improvement in pain. However, after an initial setback in pain and elevation, these 2 patients went on to improve to a good result functionally and pertaining to pain relief (see Tables II and III). Thus, the clinical failures were the 2 excluded ones and patient 2, that is, 3/15.
No vascular or neurologic complications were registered. No clinically significant shoulder contracture was seen. Other complications were the ones described for the excluded 2 procedures. From this, it can be learned that patience, mental stamina, as well as compliance and a strong understanding of the procedure is highly important for the patient for the operation to be successful.
Statistics
Because of the small sample size, the distributions of data cannot be regarded to be normal. Also, there were only 13 patients with an SCR, which is too small a sample for a statistically viable comparative study. The study was underpowered. Therefore, the results are reported in a descriptive way. The WORC index offers a subjective evaluation of quality of life following treatment, and we found it to be useful as a supplement to the Constant score. Comparing the preoperative scores to the scores at the 2-year follow-up, all registered scores are much above the recognized MCID levels. This indicates that the procedure precipitated a significant positive clinical difference in all patients except patient 2.16,20
Discussion
This study found that improvements in functional scores were generally satisfying. The increases in score aggregates are equal to or better than several published studies with an interpositional dermal graft.2,4,5,12,13,17,22,28,29,31 The improvements were generally sustainable over the 2-year follow-up period. The scores from this follow-up period may support that the SCR procedure in selected patients operated on in dedicated surgical teams may experience sustainable longer-term good to very good results.
Other reconstructive salvage procedures are RSA and latissimus dorsi tendon transfer. In RSA for rotator cuff arthropathy, several studies have found comparable improvements in functional scores. However, the infection rates and revision rates are considerable, varying from 5% to 52%.9, 10, 11,26 In latissimus dorsi transfer for rotator cuff arthropathy, several studies have found significant relief of pain. The improvement in function based on a variety of functional scores varies between 25%-130% after varying follow-up periods. Also, the tendon rupture rate is reported as fairly high, from 10%-44%.1,11,25,30
All patients had undergone extensive physiotherapy, and the majority also had had at least one prior shoulder operation. The patients had pain ascribed to the continuous anterosuperior instability of the joint with subluxation and collision of the humeral head against the inferior acromion with a substantial lack of elevation. It was not found that the patients realistically would profit either from further rehabilitation, local steroid injections, and more analgesics or from a less extensive surgical procedure such as a biceps tenotomy, partial tendon repair, or débridement.
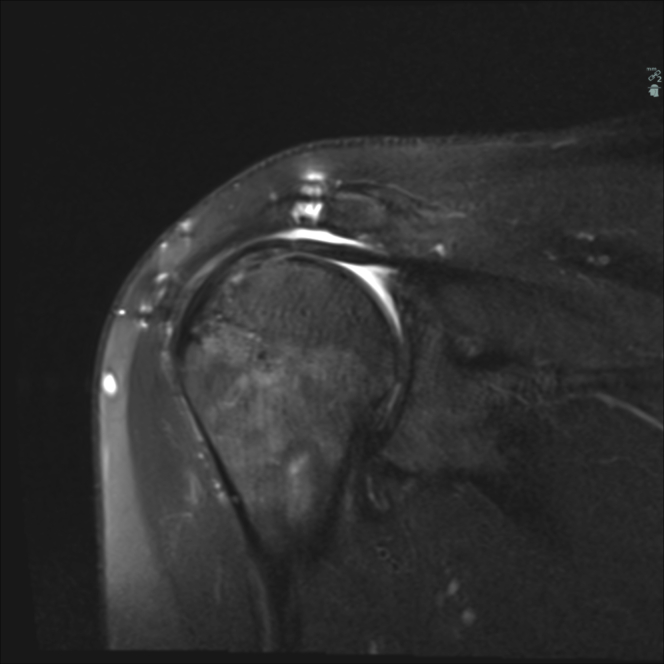
The patients would have been selected for an RSA or a latissimus dorsi transfer if no SCR graft could have been offered. SCR is purported to have potential biomechanical benefits by reconstructing a static stabilizing capsular fulcrum to let the remaining cuff tissue act as check reins to dynamically stabilize the joint and act as indirect elevators of the arm with the deltoid muscle.1,3 In our study, follow-up MRI scans were performed to document the integrity and position of the xenograft. None of the postoperative MRI scans displayed an anatomically stabilized glenohumeral joint. In all cases, the humeral head was cranially displaced in relation to the glenoid joint surface, with the xenograft directly interposed between the humeral head and the inferior acromion. Thus, in our material, the SCR graft radiographically seems to be an interpositional graft and not a structure that yields a stable glenohumeral joint. Judged from the postoperative MRI scans at 1 year postoperatively, the graft was intact in 11 cases. The radiographic migration of the humeral head was apical as on the MRI scans for patients with an intact graft and with full graft contact with the undersurface of the acromion (Fig. 1).
Figure 1.
A postoperative magnetic resonance image at the 1-year follow-up illustrating the intact xenograft positioned between the cranially migrated humeral head and the acromion.
However, in all patients, we observed an intraoperative bounce-back (“anti-trampoline”) effect of the SCR graft at the end of each operation. Hence, the graft seemed stable in a passive setting at that time. With these functional and radiographic observations, the authors find that the graft possibly could function as a stabilizer in a dynamic setting and may also function as a cushion/interpositional graft. Generally, the patients achieved a good range of motion compared to their preoperative movement ranges, so we find it difficult to conclude that the graft would solely function as an interpositional graft only yielding pain relief. When evaluating the patients at the various times of follow-up, the authors had no reason to believe other than that the graft improved the dynamic function of the shoulders.
The functional failure rate was 3/15 procedures whereas the structural graft failure rate was 4/15 procedures. Two of those 4 patients were satisfied and the 2 others had a second procedure, 1 SCR and 1 RSA. Patient number 2 had a structurally intact SCR but was not satisfied and had an RSA implanted later on. Thus, a verified graft rupture did not necessarily have a negative impact correlation on functional scores. Four patients with a secondary procedure after the SCR index operation or with a graft rupture were identified within the first 6 of 15 patients. There was only one graft rupture among procedures 7-15 (patient no. 12). These results could reflect the learning curve of the surgical team.
Based on the scores, patient satisfaction was generally high. Most af the patients had a previous failed rotator cuff operation, which reflects on their low preoperative scores. Possible reasons for disappointing results of SCR in some studies with high rates of graft rupture could be a relatively small number of procedures performed per surgeon. It could also be due to inclusion of patients with massive rotator cuff defects, where the posterior cuff tissue could not be repaired or was of a functionally inferior quality. In our study, the 13 included patients had their procedures performed by the same primary surgeon, and most of the massive cuff defects had their infraspinatus tear repaired fully.
Early onset and correct postoperative rehabilitation is important to obtain a good outcome.4 In our study, the rehabilitation was supervised by local primary care physiotherapists. The quality of the rehabilitation was unknown and therefore questionable. However, the included patients qualitatively reported a high level of compliance.
The infection rate was 1/15 procedures. The rate is most likely lower than this in a larger series. The author group has performed 14 further SCR procedures at the time of submission with no other infection cases.
Conclusions
We saw a group of patients with limited or no pain, and with a fair range of movement. Our study contains a smaller number of patients and we therefore are unable to draw any certain conclusions or recommendations concerning the long-term treatment effect after this procedure. However, this study suggests that SCR can be an excellent choice for pain relief and functional improvement for patients with rotator cuff arthropathy and limited arthrosis. The results in our study suggest that SCR can yield considerable functional score gains that can be superior to the results after RSA or latissimus dorsi tendon transfer with a low rate of infection. Also, should the SCR fail, there is still the possibility of offering those other established salvage procedures. In most cases, SCR can be performed arthroscopically in a minimally invasive fashion, which has potential benefits for postoperative rehabilitation.
SCR is technically demanding and relatively expensive using many anchors besides the graft implant. In osteopenic bone, the surgeons need experience for intraoperative technical challenges in order to achieve a stable fixation of the dermal graft. Surgical technique, patient compliance and understanding, medical morbidity, and the quality of rehabilitation are all crucial factors for a successful result.
Our contention was that a successful SCR procedure would correlate with more anatomic radiographic joint features and provide significant improvement in functional scores. The radiographic features did not improve toward centering the head of the humerus in the cavitas. We find this important because those findings demonstrate a chronic dysfunction of the rotator cuff unit. To the author group, the graft seems to function as an interpositional graft rather than to re-create passive joint stability, yet the graft could possibly function as a stabilizer in a dynamic setting. Nevertheless, the shoulder scores and patient scores improved significantly in all included patients except one. If the presented tendency could be supported with a long-term, larger study, this could point surgeons toward choosing SCR as a surgical option for patients with the proposed inclusion criteria.
From the MRI scans, it cannot be assessed whether there was a biological integration of the xenograft to bone. Perhaps the xenograft will integrate, but to prove this it seems as if a graft-to-bone biopsy or an advanced angiographic study is necessary. A second look procedure with a biopsy for such a purpose is not found to be ethically justified to perform routinely.
Disclaimer
The authors, their immediate families, and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
The study has been approved by the Danish Patient Safety Authority with the study number SJ-531.
References
- 1.Anastasopoulos P.P., Alexiadis G., Spyridonos S., Fandridis E. Latissimus dorsi transfer in posterior irreparable rotator cuff tears. Open Orthop J. 2017;11:77–94. doi: 10.2174/1874325001711010077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azevedo C., Angelo A., Vinga S. Arthroscopic superior capsular reconstruction with a minimally invasive harvested fascia Lata autograft produces good clinical results. Orth J Sports Med. 2018;6:1–13. doi: 10.1177/2325967118808242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burkhart S. Fluoroscopic comparison of kinematic patterns in massive rotator cuff tears. A suspension bridge model. Clin Orthop. 1992;284:144–152. [PubMed] [Google Scholar]
- 4.Burkhart S.S., Pranckun J.J., Hartzler R.U. Superior capsular reconstruction for the operatively irreparable rotator cuff tear: clinical outcomes are maintained 2 years after surgery. Arthroscopy. 2020;36:373–380. doi: 10.1016/j.arthro.2019.08.035. [DOI] [PubMed] [Google Scholar]
- 5.de Campos Azevedo C.I., Andrade R., Leiria Pires Gago Ângelo A.C., Espregueira-Mendes J., Ferreira N., Sevivas N. Fascia Lata autograft versus human dermal allograft in arthroscopic superior capsular reconstruction for irreparable rotator cuff tears: a systematic review of clinical outcomes. Arthroscopy. 2020;36:579–591.e2. doi: 10.1016/j.ar-thro.2019.08.033. [DOI] [PubMed] [Google Scholar]
- 6.Clark N.J., Elhassan B.T. The role of tendon transfers for irreparable rotator cuff tears. Curr Rev Musculoskelet Med. 2018;11:141–149. doi: 10.1007/s12178-018-9468-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Constant C.R., Gerber C., Roger J.H., Soejbjerg J.O., Gohlke F., Boileau P. A review of the Constant score: modifications and guidelines for its use. J Shoulder Elbow Surg. 2008;17:355–361. doi: 10.1016/j.jse.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 8.Deprés-Tremblay G., Chevrier A., Snow M., Hurtig M., Rodeo S., Buschmann M. Rotator cuff repair: a review of surgical techniques, animal models, and new technologies under development. J Shoulder Elbow Surg. 2016;25:2078–2085. doi: 10.1016/j.jse.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Ernstbrunner L., Suter A., Catanzaro S., Rahm S., Gerber C. Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears before the age of 60 years: long-term results. J Bone Joint Surg Am. 2017;99:1721–1729. doi: 10.2106/JBJS.17.00095. [DOI] [PubMed] [Google Scholar]
- 10.Gerber C., Canonica S., Catanzaro S., Ernstbrunner L. Longitudinal observational study of reverse total shoulder arthroplasty for irreparable rotator cuff dysfunction: results after 15 years. J Shoulder Elbow Surg. 2018;27:831–838. doi: 10.1016/J.JSE.2017.10.037. [DOI] [PubMed] [Google Scholar]
- 11.Greenspoon J., Petri M., Warth R., Millett P. Massive rotator cuff tears: pathomechanics, current treatment options, and clinical outcomes. J Shoulder Elbow Surg. 2015;24:1493–1505. doi: 10.1016/j.jse.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Gupta A.K., Hug K., Berkoff D.J., Boggess B.R., Gavigan M., Malley P.C. Dermal tissue allograft for the repair of massive irreparable rotator cuff tears. Am J Sports Med. 2012;40:141–147. doi: 10.1177/0363546511422795. [DOI] [PubMed] [Google Scholar]
- 13.Gupta A.K., Hug K., Boggess B., Gavigan M., Toth A.P. Massive or 2-tendon rotator cuff tears in active patients with minimal glenohumeral arthritis: clinical and radiographic outcomes of reconstruction using dermal tissue matrix xenograft. Am J Sports Med. 2013;41:872–879. doi: 10.1177/0363546512475204. [DOI] [PubMed] [Google Scholar]
- 14.Hackett E.S., Harilal D., Bowley C., Hawes M., Turner A.S., Goldman S.M. Evaluation of porcine hydrated dermis augmented repair in a fascial defect model. J Biomed Mater Res B Appl Biomater. 2011;96:134–138. doi: 10.1002/jbm.b.31751. [DOI] [PubMed] [Google Scholar]
- 15.Hamada K., Yamanaka K., Uchiyama Y., Mikasa T., Mikasa M. A radiographic classification of massive rotator cuff tear arthritis. Clin Orthop Relat Res. 2011;469:2452–2460. doi: 10.1007/s11999-011-1896-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris J., Pedroza A., Jones G. Predictors of pain and function in patients with symptomatic, atraumatic full-thickness rotator cuff tears. Am J Sports Med. 2012;40:359–366. doi: 10.1177/0363546511426003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirahara A.M., Andersen W.J., Panero A.J. Superior capsular reconstruction: clinical outcomes after minimum 2-year follow-up. Am J Orthop (Belle Mead NJ) 2017;46:266–278. [PubMed] [Google Scholar]
- 18.Iannoti J.P., Codsi M.J., Kwon Y.W., Derwin K., Coccon J., Brems J.J. Porcine small intestine submucosa augmentation of surgical repair of chronic two-tendon rotator cuff tear. A randomized, controlled trial. J Bone Joint (Am) 2006;88:1238–1244. doi: 10.2106/JBJS.E.00524. [DOI] [PubMed] [Google Scholar]
- 19.Kissenberth M.J., Rulewicz G.J., Hamilton S.C., Bruch H.E., Hawkins R.J. A positive tangent sign predicts the repairability of rotator cuff tears. J Shoulder Elbow Surg. 2014;23:1023–1027. doi: 10.1016/j.jse.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 20.Kukkonen J., Kauko T., Vahlberg T., Joukainen A., Aärimaa V. Investigating minimal clinically important difference for Constant score in patients undergoing rotator cuff surgery. J Shoulder Elbow Surg. 2013;22:1650–1655. doi: 10.1016/j.jse.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Levy O., Mullett H., Roberts S., Copeland S. The role of anterior deltoid reeducation in patients with massive irreparable degenerative rotator cuff tears. J Shoulder Elbow Surg. 2008;17:863–870. doi: 10.1016/j.jse.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Mihata T., McGarry M., Pirolo J., Kinoshita Lee T. Superior capsule reconstruction to restore superior stability in irreparable rotator cuff tears: a biomechanical cadaveric study. Am J Sports Med. 2012;40:2248–2255. doi: 10.1177/0363546512456195. [DOI] [PubMed] [Google Scholar]
- 23.Mihata T., Lee T.Q., Watanabe C., Fukunishi K., Ohue M., Tsujimura T. Clinical results after SCR for irreparable rotator cuff tears. Arthroscopy. 2013;29:459–470. doi: 10.1016/j.arthro.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 24.Mihata T., Bui C., Akeda M., Cavagnaro M., Kuenzler M., Peterson A. A biomechanical cadaveric study comparing superior capsule reconstruction using fascia lata allograft with human dermal allograft for irreparable rotator cuff tear. J Shoulder Elbow Surg. 2017;26:2158–2166. doi: 10.1016/j.jse.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 25.Miniaci A., MacLeod M. Transfer of the latissimus dorsi muscle after failed repair of a massive tear of the rotator cuff. A two to five-year review. J Bone Joint Surg Am. 1999;81:1120–1127. doi: 10.2106/00004623-199908000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Mulieri P., Dunning P., Klein S., Pupello D., Frankle M. Reverse shoulder arthroplasty for the treatment of irreparable rotator cuff tear without glenohumeral arthritis. J Bone Joint Surg Am. 2010;92:2544–2556. doi: 10.2106/JBJS.I.00912. [DOI] [PubMed] [Google Scholar]
- 27.Danish Health Authority . Danish Health Authority; Copenhagen: 2013. National clinical guidance for diagnostics and treatment of patients with selected shoulder disabilities. ISBN: 978-87-7104-528-4. [Google Scholar]
- 28.Neumann J.A., Agonis M.H., Rickert K.D., Bradley K.E., Kremen T.J., Boggess B.R. Interposition dermal matrix xenografts: a successful alternative to traditional treatment of massive rotator cuff tears. Am J Sports Med. 2017;45:1261–1268. doi: 10.1177/0363546516683945. [DOI] [PubMed] [Google Scholar]
- 29.Pennington W.T., Bartz B.A., Pauli J.M., Walker C.E., Schmidt W. Arthroscopic superior capsular reconstruction with acellular dermal allograft for the treatment of massive irreparable rotator cuff tears: short-term clinical outcomes and the radiographic parameter of superior capsular distance. Arthroscopy. 2018;34:1764–1773. doi: 10.1016/j.arthro.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 30.Warner J.J., Parsons I.M., 4th Latissimus dorsi tendon transfer: a comparative analysis of primary and salvage reconstruction of massive, irreparable rotator cuff tears. J Shoulder Elbow Surg. 2001;10:514–521. doi: 10.1067/mse.2001.118629. [DOI] [PubMed] [Google Scholar]
- 31.Woodmass J.M., Wagner E.R., Borque K.A., Chang M.J., Welp K.M., Warner J.P. Superior capsule reconstruction using dermal allograft; early outcomes and survival. J Shoulder Elbow Surg. 2019;28:S100–S109. doi: 10.1016/j.jse.2019.04.011. [DOI] [PubMed] [Google Scholar]