Abstract
We assessed soil fungal diversity at two sites on Deception Island, South Shetland Islands, Antarctica using DNA metabarcoding analysis. The first site was a relatively undisturbed area, and the second was much more heavily impacted by research and tourism. We detected 346 fungal amplicon sequence variants dominated by the phyla Ascomycota, Basidiomycota, Mortierellomycota and Chytridiomycota. We also detected taxa belonging to the rare phyla Mucoromycota and Rozellomycota, which have been difficult to detect in Antarctica by traditional isolation methods. Cladosporium sp., Pseudogymnoascus roseus, Leotiomycetes sp. 2, Penicillium sp., Mortierella sp. 1, Mortierella sp. 2, Pseudogymnoascus appendiculatus and Pseudogymnoascus sp. were the most dominant fungi. In addition, 440,153 of the total of 1,214,875 reads detected could be classified only at the level of Fungi. In both sampling areas the DNA of opportunistic, phytopathogenic and symbiotic fungi were detected, which might have been introduced by human activities, transported by birds or wind, and/or represent resident fungi not previously reported from Antarctica. Further long-term studies are required to elucidate how biological colonization in the island may be affected by climatic changes and/or other anthropogenic influences.
Subject terms: Environmental sciences, Environmental impact, Environmental microbiology, Soil microbiology
Introduction
The pristine environments of Antarctica are used as field laboratories to support taxonomic, ecological, evolutionary and biotechnological studies. Antarctic environments experience multiple extreme conditions including low temperatures, acidic and alkaline pH, ultra-oligotrophic conditions, freeze–thaw cycles, salinity stress, desiccation, wind abrasion and high radiation levels1 and, for these reasons, offer unique opportunities to study the diversity of fungi2.
In the latter part of the Twentieth Century, the Antarctic Peninsula region was one of the regions of the planet most affected by climatic changes. Deception Island, located in the South Shetland Islands is one of very few active volcanoes in the Antarctic Treaty area. Two summer-only research stations are presently active on the island (the Argentinian Decepción and Spanish Gabriel de Castilla). In addition, a shore-based whaling station operated in Whalers Bay in the early Twentieth Century3. The combination of unique geology, history, biota and aesthetic values, as well as the active presence of multiple national operators, underlie the designation of the entire island as an Antarctic Specially Managed Area (ASMA 4). In addition, Deception Island includes two Antarctic Specially Protected Areas (ASPAs), designated as ASPAs 140 (terrestrial, formed of multiple sub-sites) and 145 (marine). Deception Island is one of the best-known locations in Antarctica, visited by both researchers and tourists4, with more than 55,489 tourists visiting the island in the summer of 2018–2019 (https://iaato.org/tourism-statistics-327mnsyd), which generates pressure on its ecosystems. The island is an exceptional location even within Antarctica, as it is a young volcanic island formed less than 100 kya5 and still in the process of biological colonization.
The majority of mycological studies in Antarctica to date have focused on cultivable species, mainly represented by taxa of the phylum Ascomycota and its anamorphs, followed by Basidiomycota, Mortierellomycota, Mucoromycota, Chytridiomycota and Glomeromycota2. In Antarctica, different fungal assemblages contribute to complex ecological networks, including saprophytic, mutualistic and parasitic taxa, all of which are able to survive under various extreme environmental conditions6,7. However, despite the recognized importance of fungal diversity in Antarctica, few studies have applied metabarcoding approaches using high throughput sequencing (HTS). The present study aimed to characterize and compare fungal diversity assessed using metabarcoding in soil at two sites on Deception Island, (1) a relatively undisturbed site within the terrestrial Antarctic Specially Protected Area (ASPA) 140 and (2) a disturbed site in Whalers Bay subject to considerable visitor pressure and hence greater human impact.
Methods
Soil sampling
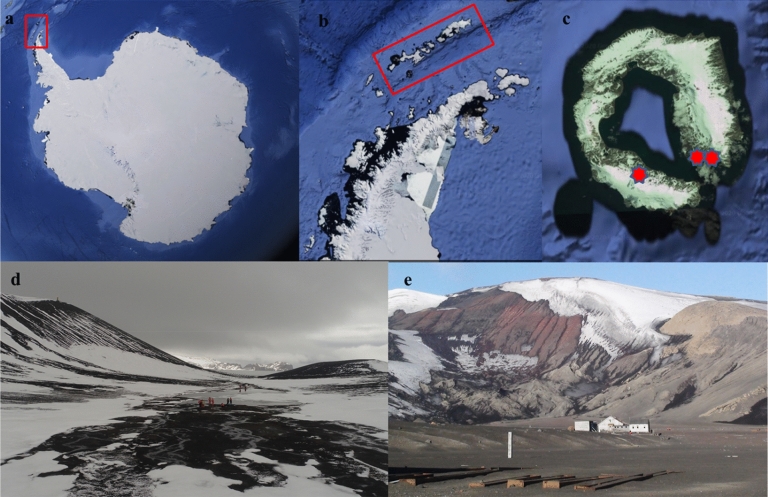
Soil samples were collected from two sites on Deception Island, South Shetland Islands (Fig. 1). The first was within an Antarctic Specially Protected Area (ASPA) close to Crater Lake [ASPA 140, subsite B], which has relatively low impact from researchers and is not accessible for tourism. The second site was in Whalers Bay, which includes the area of the historical whaling station and former UK research station on the island, and is formally declared a Historic Monument. It is one of the most popular visitor sites in Antarctica for both tourists and national operator personnel. The distance between the two sites is approximately 5 km. Superficial soil samples (approximately 5 cm depth and ca 250 g each) were collected using sterile spatulas and immediately placed in sterilized WhirlPak bags (Sigma-Aldrich, USA) kept at − 20 °C until processing. Seven (non-composite) samples from each site (obtained a minimum of 10 m from each other) were collected for use in DNA studies, totaling 14 samples in total.
Figure 1.
Satellite images (a–c) (obtained in Google Earth Pro, 2019) and sites were the soil where sampled. (a) Antarctic Continent with the northern Antarctic Peninsula inside the red rectangle, (b) Antarctic Peninsula with the South Shetland Islands archipelago inside the red rectangle, (c) Deception Island with the sites *ASPA 140 and **Whalers Bay, (d) Antarctic Specially Protected Area 140 subsite B (protected area close to Crater Lake—62° 06′ 08.6′′ S; 57° 55′10.4′′ W), and (e) Whalers Bay (non-protected area, WB—62° 58′ 52.0′′ S; 60° 39′ 52.9′′ W). Photos (d,e) by L.H. Rosa.
DNA extraction and analysis, and fungal identification
Total DNA was extracted from environmental samples using the QIAGEN Power Soil Kit, following the manufacturer’s instructions. Extracted DNA was used as template for generating PCR-amplicons. The internal transcribed spacer 2 (ITS2) of the nuclear ribosomal DNA was used as a DNA barcode for molecular species identification8,9. PCR-amplicons were generated using the universal primers ITS3 and ITS410 and were sequenced by high-throughput sequencing at Macrogen Inc. (South Korea) on an Illumina MiSeq sequencer, using the MiSeq Reagent Kit v3 (600-cycle) following the manufacturer’s protocol.
Raw fastq files were filtered using BBDuk version 38.34 (BBMap—Bushnell B.—sourceforge.net/projects/bbmap/) to remove Illumina adapters, known Illumina artifacts, and PhiX Control v3 Library. Quality read filtering was carried out using Sickle version 1.33 -q 30-l 5011, to trim 3′ or 5′ ends with low Phred quality score, and sequences smaller than 50 bp were also discarded. Remaining sequences were imported to QIIME2 version 2019.10 (https://qiime2.org/) for bioinformatics analyses12. The qiime2-dada2 plugin is a complete pipeline that was used to filter, dereplicate, turn paired-end fastq files into merged, and remove chimeras13. Taxonomic assignments were determined for amplicon sequence variants (ASVs) using qiime2-feature-classifier14 classify-sklearn against the UNITE fungal ITS database version 7.215 and trained with Naive Bayes classifier and a confidence threshold of 98.5%.
Many factors, including extraction, PCR and primer bias, can affect the number of reads16, and thus lead to misinterpretation of abundance17. However, Giner et al.18 concluded that such biases did not affect the proportionality between reads and cell abundance, implying that more reads are linked with higher abundance19,20. Therefore, for comparative purposes we used the number of reads as a proxy for relative abundance.
Fungal diversity and distribution
To quantify species diversity, richness and dominance, we used the following indices: (1) Fisher’s α, (2) Margalef’s and (3) Simpson’s, respectively. The numbers of reads of each amplicon sequence variant (ASV) were used to quantify the fungal taxa present in the soils sampled, where fungal ASVs > 6000 were considered dominant and ≤ 1000 minor components (rare) within the fungal community. Species accumulation curves were assessed using the Mao Tao index. All diversity index calculations were performed using PAST, version 1.9021. Results were obtained with 95% confidence, and bootstrap values were calculated from 1000 iterations. Venn diagrams were prepared according to Bardou et al.22 to illustrate the comparison of fungal assemblages present in the two sampling areas.
Results
Fungal taxonomy
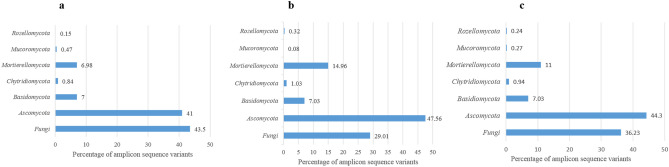
We detected 346 soil fungal amplicon sequence variants (ASVs) in the samples from the two sites on Deception Island (Suppl. Table 1). Ascomycota, Basidiomycota, Mortierellomycota and Chytridiomycota dominated the fungal assemblages of both sites at phylum level (Fig. 2). We also detected representatives of the generally rare phyla Mucoromycota and Rozellomycota, which occurred at moderate dominance in both sites. The ASVs identified as Cladosporium sp., Pseudogymnoascus roseus, Leotiomycetes sp. 2, Penicillium sp., Mortierella sp. 1, Mortierella sp. 2, Pseudogymnoascus appendiculatus and Pseudogymnoascus sp. were most dominant at genus/species level (with > 30,000 reads). A further 65 ASVs were moderately dominant (> 1000 reads). Twenty-three fungal ASVs could be assigned to only higher hierarchical levels (phylum, class, order or family) when compared with known DNA sequences deposited in the UNITE DNA database15 and might represent taxa above the species level new to science and new records for Antarctica. In addition, 440,153 of the total of 1,214,875 reads detected (262,844 in the ASPA and 177,309 in Whalers Bay) could only be classified at the level of Fungi.
Figure 2.
Percentage of fungal amplicon sequence variants (ASVs) at phylum level identified from soil of Deception Island, South Shetland Islands. (a) Fungal assemblage of soil in ASPA 140 (non-impacted site), (b) fungal assemblage of soil in Whalers Bay (impacted site), and (c) total soil fungal community of both sites.
Fungal diversity
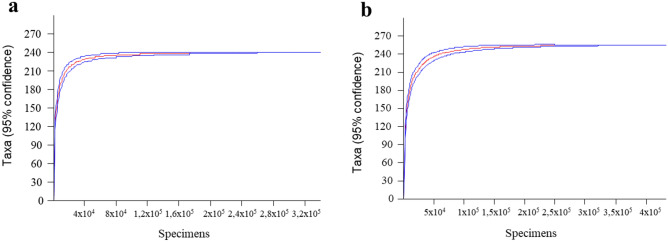
The Mao Tao rarefaction curves reached asymptote for both fungal assemblages (Fig. 3), indicating that the data provided a good description of the diversity present. The fungal assemblages of both sites displayed high diversity, richness, and dominance indices (Table 1) when compared with studies of cultivable fungi present in Antarctic soils23,24. That of Whalers Bay had the highest values of each index.
Figure 3.
Rarefaction curves for samples from (a) ASPA 140 sampling site and (b) Whalers Bay site (impacted area) on Deception Island, South Shetland Islands. Blue lines represent confidence limits.
Table 1.
Diversity indices of fungal assemblages present in soils of the Antarctic Specially Protected Area (ASPA) 140 and Whalers Bay sampling sites on Deception Island, as indicated by numbers of amplicon sequence variants (ASVs) and compared with diversity results of cultivable fungi present in soils of Antarctica.
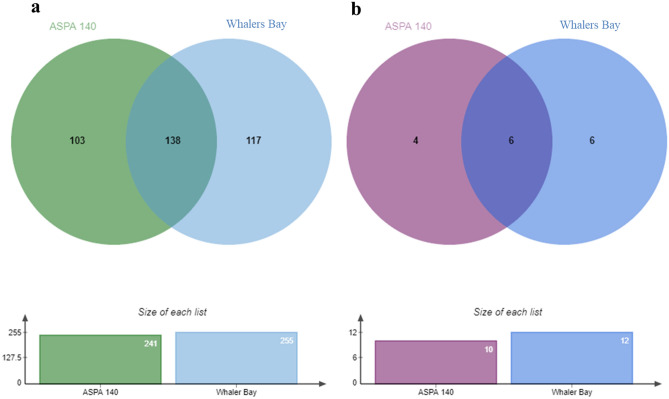
Of the fungal ASVs characterized, 103 were present only in ASPA 140, 117 in Whalers Bay, with 138 common to both (Fig. 4a), indicating that a small majority of the diversity at both sites was shared between them. The ecological assemblage profiles of exclusive or shared fungi between the two sites did not display significant differences. In both sites the DNA of both cosmopolitan and Antarctic endemic fungi was detected (Suppl. Table 1).
Figure 4.
(a) Venn diagram showing the total and (b) dominant (those with > 6000 reads) fungal taxa distribution between ASPA 140 (non-impacted) and Whalers Bay (impacted) sampling areas.
When the dominant fungi (> 6000 reads) were compared between the two sites (Fig. 4b), Malassezia restricta, Mortierella fimbricystis and M. antarctica occurred only in the ASPA samples, and Leucosporidiella creatinivora, Cleistothelebolus nipigonensis, Thelebolus globosus, Colletotrichum sp. 1 and Leotiomycetes sp. 2 only in the Whalers Bay samples. Pseudogymnoascus roseus, P. appendiculatus, Pseudogymnoascus sp., Cladosporium sp., Mortierella sp. 2 and Penicillium sp. were present in both areas.
The patterns of occurrence of rare taxa (those detected with reads ≤ 1000) in both sites indicated similarities in their ecological status between the assemblages, with the presence of human and animal opportunistic and plant pathogenic taxa (Table 2). In the heavily human impacted Whalers Bay a proportion of the identifiable fungi detected have previously been reported as being opportunistically associated with humans and animals (16 taxa) or phytopathogenic (16). In the soil of ASPA 140, 13 human and animal opportunistic and 12 phytopathogenic taxa were detected. Aspergillus sydowii, Curvularia lunata, Malassezia dermatis, M. globosa, M. restricta, M. sympodialis, Rhodotorula mucilaginosa and Trichosporon asahii (human and animal associated), and Aspergillus niger, Colletotrichum annellatum, Curvularia lunata, Gibberella tricincta, G. zeae, Herpotrichia juniper, Nigrospora oryzae, Thanatephorus cucumeris and Cleistothelebolus nipigonensis (phytopathogenic) were detected in both sites. We also detected the presence of DNA of 11 lichenized fungi, of which five (Lecidea cancriformis, Psoroma tenue, Trimmatothelopsis smaragdula, Verrucaria alpicola and V. margacea) occurred in both sites.
Table 2.
Ecological status of the uncultured fungi recovered from different soil samples of Deception Island, Antarctic Peninsula.
| Ecological status | Site/number of reads | |
|---|---|---|
| ASPA 140a | Whalers Bay | |
| Opportunistic human and animal pathogens | ||
| Aphanoascus keratinophilus | 0 | 19 |
| Aspergillus sydowii | 427 | 112 |
| Aspergillus terreus | 160 | 0 |
| Blastobotrys proliferans | 0 | 12 |
| Candida parapsilosis | 0 | 60 |
| Curvularia lunata | 91 | 29 |
| Cutaneotrichosporon smithiae | 0 | 64 |
| Cyphellophora pluriseptata | 24 | 0 |
| Exophiala cancerae | 43 | 0 |
| Magnusiomyces capitatus | 0 | 4 |
| Malassezia dermatis | 36 | 70 |
| Malassezia globosa | 5831 | 689 |
| Malassezia restricta | 11,413 | 3719 |
| Malassezia slooffiae | 35 | 0 |
| Malassezia sympodialis | 364 | 171 |
| Malassezia yamatoensis | 0 | 65 |
| Papiliotrema laurentii | 0 | 358 |
| Pseudallescheria boydii | 21 | 0 |
| Pyrenochaeta keratinophila | 0 | 8 |
| Rhodotorula mucilaginosa | 2565 | 2663 |
| Sporothrix brasiliensis | 0 | 46 |
| Trichosporon asahii | 158 | 68 |
| Plant pathogens | ||
| Aspergillus niger | 292 | 18 |
| Colletotrichum annellatum | 802 | 127 |
| Colletotrichum brevisporum | 0 | 16 |
| Colletotrichum cliviae | 0 | 2524 |
| Curvularia lunata | 91 | 29 |
| Fusarium asiaticum | 0 | 43 |
| Fusarium oxysporum | 0 | 139 |
| Fusarium solani | 0 | 115 |
| Gibberella intricans | 14 | 0 |
| Gibberella tricincta | 4 | 42 |
| Gibberella zeae | 32 | 278 |
| Herpotrichia juniper | 598 | 1074 |
| Mycosphaerella tassiana | 0 | 54 |
| Nigrospora oryzae | 2 | 5 |
| Peniophora albobadia | 5 | 0 |
| Pestalotiopsis trachicarpicola | 0 | 12 |
| Pyrenochaeta keratinophila | 0 | 8 |
| Thanatephorus cucumeris | 23 | 182 |
| Volutella consors | 26 | 0 |
| Cleistothelebolus nipigonensis | 980 | 12,637 |
| Fungi able to form lichen thalli | ||
| Lecidea cancriformis | 129 | 21 |
| Lecidea sp. | 0 | 19 |
| Parmelina sp. | 64 | 0 |
| Placopsis sp. | 35 | 0 |
| Psoroma hypnorum | 24 | 0 |
| Psoroma tenue | 590 | 205 |
| Trimmatothelopsis smaragdula | 73 | 177 |
| Verrucaria alpicola | 2305 | 985 |
| Verrucaria humida | 0 | 24 |
| Verrucaria margacea | 17 | 30 |
| Verrucaria nodosa | 253 | 0 |
In bold taxa detected in soil of both sites.
aASPA = Antarctic Specially Protected Areas.
Discussion
Fungal taxonomy and diversity
In Antarctica, around 1000 fungal species have been described through studies of the macro- and/or micromorphology of colonies and fruiting bodies, and DNA sequencing of mycelia of cultivable fungi25. However, according to Amann et al.26 and Rappe and Giovannoni27, just 0.01–1% of the microbial life present in a given habitat can be characterized using cultivation methods. Magnuson and Lasure28 suggested that a rather lower proportion (70–90%) of soil fungi cannot be obtained using culturing methods. Blackwell29 and Taylor et al.30 estimated that, including fungi detected by their environmental DNA, the Kingdom Fungi might include between 5.1 and 6 million species worldwide, respectively.
The majority of mycological studies carried out to date on Deception Island have focused on cultivable fungal diversity. Gonçalves et al.31 reported seven fungal taxa present in freshwater in Crater Lake, Held and Blanchette32 reported 68 taxa on historic wooden structures in Whalers Bay, Figueredo et al.4 identified 17 taxa from soil samples from Fumarole Bay and de Menezes et al.33 reported 14 taxa from snow. Baeza et al.34 used culture-independent techniques to characterize fungal diversity in soils from various different sites in Antarctica, including some samples obtained from the same locations on Deception Island as studied here. They reported 33 taxa, many identified only to genus level, a much lower total than the 346 distinct taxa detected here. Only 10 genera were reported in both studies (Candida, Exophila, Herpotrichia, Lecidea, Malassezia, Merozyma, Pseudogymnoascus, Psoroma, Thelebolus and Verrucaria). Baeza et al.32 reported the most abundant taxa to be Verticillium sp., Xanthophyllomyces dendrorhous, Malassezia restricta and Circinaria fruticulosa, differing from the dominant taxa detected in our study (Cladosporium sp., P. roseus, Leotiomycetes sp., Penicillium sp., Mortierella sp. 1, Mortierella sp. 2, P. appendiculatus and Pseudogymnoascus sp.). Our study differs from that of Baeza et al.34 in sample size, techniques used, and PCR bias. Despite these differences, our data confirm the presence of a much higher fungal diversity than reported in previous studies. The observation that many of ASVs could only be classified to higher taxonomic levels, with a significant proportion only to the Kingdom Fungi, suggests that it is likely that Antarctica hosts many as yet unrecognised fungal taxa.
Using number of reads as a proxy measure of abundance, Ascomycota was the dominant phylum detected, followed by Basidiomycota, Mortierellomycota and Chytridiomycota. Previous studies of fungal diversity in Antarctic soil have demonstrated the same overall pattern of dominant fungal phyla detected here6,7,24,35,36. However, we also detected the presence of taxa from the phyla Mucoromycota and Rozellomycota, which are not commonly reported in Antarctic soils. Although these phyla have global distributions they are poorly known from Antarctica, when compared with Ascomycota, Basidomycota and Mortierellomycota, and are generally regarded as rare2.
Members of the genera Cladosporium, Penicillium and Mortierella dominated the assemblages detected in this study. Cladosporium and Penicillium include cosmopolitan species detected in Antarctica. Cladosporium is one of the largest genera of dematiaceous hyphomycetes37, with global distribution. It includes species with many different characteristics, including saprophytic and phytopathogenic taxa38. In Antarctica, Cladosporium are often associated with the availability of organic matter, such as in moss carpets39,40 and the native flowering plant Colobanthus quitensis (Kunth.) Bartl. (Caryophyllaceae)41. They are broadly distributed in Antarctica, indicating versatility in adaptation to the extreme conditions of the continent, and have been reported from soil, snow, ice, seawater and marine sediments, freshwater and lake sediments, plants and animals2.
Pseudogymnoascus (syn. Geomyces) have been often described from cold habitats of Arctic, alpine, temperate and Antarctic regions2,42–44. In Antarctica, Pseudogymnoascus is widely distributed and has been reported from both terrestrial and marine ecosystems, including soils24,42,45, mosses39,40,46, as an endophyte of C. quitensis41, as algicolous fungi of macroalgae47,48, in freshwater lakes31 and in the lichenosphere49. Taxonomic studies of Pseudogymnoascus draw attention to P. destructans, causative agent of the lethal disease white-nose syndrome (WNS) in bats of temperate regions50. Further studies are required to elucidate if genetic material of this genus detected here belongs to the P. destructans group.
The genus Mortierella (Mortierellomycota), whose members are also known as “snow moulds”, includes some species often reported in Antarctica. Species of this genus have been reported in association with mosses39,40, lichens49, soils24,51, freshwater31, macroalgae52 and in the rhizosphere of Deschampsia antarctica Desv. (Poaceae)23.
Considering specifically the rare taxa detected in the Deception Island fungal assemblages, the sequence data of several taxa detected from Whalers Bay matched fungi previously reported as opportunistically associated with humans and animals or able to cause plant diseases. Amongst these, M. dermatis, R. mucilaginosa and T. asahii (human and animal opportunistic) and C. lunata, G. intricans, G. zeae and H. juniper (phytopathogenic) were present in both sampling areas. Although present at apparently low frequency, these fungi merit further attention. For example, de Menezes et al.33 reported a high density of cultivable R. mucilaginosa in Antarctic snow, a fungus capable of growing at 37 °C and that displays resistance against the antifungal compound fluconazole, and which may represent a health risk for immunosuppressed persons. In this context, Whalers Bay is a very popular visitor site, including by many elderly tourists likely with weaker immune systems, who may therefore come into contact with the resident microorganisms including those reported as opportunistic disease agents. However, further studies are necessary to assess the risk of infection from resident fungi during a visit to Whalers Bay.
The high-throughput sequencing methodology used in the current study allowed detection of the DNA of a range of fungal taxa able to form the lichenized fungal associations, but without their thalli being visibly present in the soils sampled. Although the lichen diversity of mainland Antarctica and adjacent islands is generally well-known53, that of Deception Island specifically is less well studied, with 70 species currently reported53. Among the species whose fungal DNA was detected in the current study, V. alpicola, T. smaragdula, Parmelina sp., V. nodosa, V. humida and V. margacea are first records for both Deception Island and Antarctica generally. The dominant DNA detected in both sampled areas was that of V. alpicola. According to Shivarov et al.54 this species is known only from Europe (Austria, Great Britain, Germany, Italy, Norway, Romania, Switzerland). Trimmatothelopsis smaragdula is a circumpolar sub-Arctic and alpine species55. Verrucaria humida is another European lichen known from Wales, Norway, Germany and Poland, while V. margacea is widespread in Scandinavia, central and western European mountain ranges, and temperate areas in the Southern Hemisphere56 and V. nodosa is known only from Wales57. Lichens in the genera Psoroma, Lecidea and Placopsis are common in Deception Island and the South Shetland Islands generally.
Conclusions
DNA metabarcoding of soil fungal assemblages in samples obtained from ASPA 140 subsite B and Whalers Bay on Deception Island indicated the presence of a rich fungal diversity. The ‘rare’ fungal taxa detected in both areas included fungi reported as human and animal opportunistic and plant pathogens. The diversity detected may have been transported to Deception Island associated with human activities such as the historic whaling industry, research, tourism, through natural transport by birds or in the air column, or represent resident fungi not previously described. Further long-term studies are required to elucidate how biological colonization of the island may be affected by climatic changes and other anthropogenic influences.
Supplementary Information
Acknowledgements
This study received financial support from CNPq, PROANTAR, FAPEMIG, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES), INCT Criosfera 2. P. Convey is supported by NERC core funding to the British Antarctic Survey’s ‘Biodiversity, Evolution and Adaptation’ Team. We are also grateful for the generous support of the Spanish Polar Committee and its staff at Gabriel de Castilla base.
Author contributions
L.H.R. and P.E.A.S.C. conceived the study. T.H.S. and M.B.O. performed DNA extraction from soils. L.H.R., P.E.A.S.C., O.H.B.Z., M.S., P.C., M.C.S., C.A.R. analyzed the results and wrote the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-020-78934-7.
References
- 1.Convey P, et al. The spatial structure of Antarctic biodiversity. Ecol. Monogr. 2014;84:203–244. doi: 10.1890/12-2216.1. [DOI] [Google Scholar]
- 2.Rosa LH, et al. Fungi in Antarctica: diversity, ecology, effects of climate change, and bioprospection for bioactive compounds. In: Rosa LH, et al., editors. Fungi of Antarctica: Diversity, Ecology and Biotechnological Applications. Berlin: Springer; 2019. pp. 1–18. [Google Scholar]
- 3.Hart IB. Whaling in the Falkland Islands Dependencies, 1904–1931. Pequena: Newton St. Margarets; 2006. [Google Scholar]
- 4.Figueredo HM, et al. Diversity and ecology of cultivable fungi isolated from the thermal soil gradients in Deception Island Antarctica. Extremophiles. 2020;24:219–225. doi: 10.1007/s00792-019-01146-z. [DOI] [PubMed] [Google Scholar]
- 5.Smellie JL. Lithostratigraphy and volcanic evolution of Deception Island South Shetland Islands. Antarct. Sci. 2001;13:188–209. doi: 10.1017/S0954102001000281. [DOI] [Google Scholar]
- 6.Fell JW, et al. Biodiversity of micro-eukaryotes in Antarctic dry valley soils with <5% soil moisture. Soil Biol. Biochem. 2006;38:3107–3119. doi: 10.1016/j.soilbio.2006.01.014. [DOI] [Google Scholar]
- 7.Godinho VM, et al. Diversity and bioprospection of fungal community present in oligotrophic soil of continental Antarctica. Extremophiles. 2015;19:585–596. doi: 10.1007/s00792-015-0741-6. [DOI] [PubMed] [Google Scholar]
- 8.Chen S, et al. Validation of the ITS2 region as a novel DNA barcode for identifying medicinal plant species. PLoS ONE. 2010;5:e8613. doi: 10.1371/journal.pone.0008613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richardson RT, et al. Application of ITS2 metabarcoding to determine the provenance of pollen collected by honey bees in an agroecosystem. Appl. Plant Sci. 2015;3:1400066. doi: 10.3732/apps.1400066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White TJ, et al. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, et al., editors. PCR Protocols: A Guide to Methods and Applications. New York: Academic Press; 1990. pp. 315–322. [Google Scholar]
- 11.Joshi, N. A., Fass, J. N. Sickle: A Sliding-Window, Adaptive, Quality-Based Trimming Tool for FastQ files (Version 1.33) [Software]. https://github.com/najoshi/sickle. Accessed June 2020 (2011).
- 12.Bolyen E, et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019;37:852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Callahan BJ, et al. Holmes SP. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods. 2016;13:581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bokulich NA, et al. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome. 2018;6:90. doi: 10.1186/s40168-018-0470-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kõljalg U, et al. Towards a unified paradigm for sequence-based identification of fungi. Mol. Ecol. 2013;22:5271–5277. doi: 10.1111/mec.12481. [DOI] [PubMed] [Google Scholar]
- 16.Medinger R, et al. Diversity in a hidden world: potential and limitation of next-generation sequencing for surveys of molecular diversity of eukaryotic microorganisms. Mol. Ecol. 2010;19:32–40. doi: 10.1111/j.1365-294X.2009.04478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weber AA, Pawlowski J. Can abundance of protists be inferred from sequence data: a case study of Foraminifera. PLoS ONE. 2013;8:e56739. doi: 10.1371/journal.pone.0056739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giner CR, et al. Environmental sequencing provides reasonable estimates of the relative abundance of specific picoeukaryotes. Appl. Environ. Microbiol. 2016;82:4757–4766. doi: 10.1128/AEM.00560-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deiner K, et al. Environmental DNA metabarcoding: transforming how we survey animal and plant communities. Mol. Ecol. 2017;26:5872–5895. doi: 10.1111/mec.14350. [DOI] [PubMed] [Google Scholar]
- 20.Hering D, et al. Implementation options for DNA-based identification into ecological status assessment under the European Water Framework Directive. Water Res. 2018;138:192–205. doi: 10.1016/j.watres.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Hammer Ø, Harper DAT, Ryan PD. PAST: paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001;4:9. [Google Scholar]
- 22.Bardou P, et al. An interactive Venn diagram viewer. BMC Bioinform. 2014;15:293. doi: 10.1186/1471-2105-15-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonçalves, V. N. et al. Antibacterial, antifungal and antiprotozoal activities of fungal communities present in different substrates from Antarctica. Polar Biol. 38, 1143–1152 (2015).
- 24.Gomes, E. C. et al. Cultivable fungi present in Antarctic soils: taxonomy, phylogeny, diversity, and bioprospecting of antiparasitic and herbicidal metabolites. Extremophiles 22, 381–393 (2018). [DOI] [PubMed]
- 25.Bridge PD, Spooner BM. Non-lichenized Antarctic fungi: transient visitors or members of a cryptic ecosystem? Fungal Ecol. 2012;5:381–394. doi: 10.1016/j.funeco.2012.01.007. [DOI] [Google Scholar]
- 26.Amann RI, Ludwig W, Schleifer KH. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 1995;59:143–169. doi: 10.1128/MR.59.1.143-169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rappe MS, Giovannoni SJ. The uncultured microbial majority. Annu. Rev. Microbial. 2003;57:369–394. doi: 10.1146/annurev.micro.57.030502.090759. [DOI] [PubMed] [Google Scholar]
- 28.Magnuson, J. K., Lasure, L. L. Fungal diversity in soils as assessed by direct culture and molecular techniques. In Salt Lake: Abstracts from the 102nd General Meeting of the American Society for Microbiology, 19–23. http://www.pnnl.gov/biobased/docs/fungal_diversity.pdf. Accessed June 2020 (2002).
- 29.Blackwell M. The fungi: 1,2,3…5,1 million species? Am. J. Bot. 2011;98:426–438. doi: 10.3732/ajb.1000298. [DOI] [PubMed] [Google Scholar]
- 30.Taylor DE, et al. A first comprehensive census of fungi in soil reveals both hyperdiversity and fine-scale niche partitioning. Ecol. Monogr. 2014;84:3–20. doi: 10.1890/12-1693.1. [DOI] [Google Scholar]
- 31.Gonçalves VN, et al. Diversity and distribution of fungal communities in lakes of Antarctica. FEMS Microbiol. Ecol. 2012;82:459–471. doi: 10.1111/j.1574-6941.2012.01424.x. [DOI] [PubMed] [Google Scholar]
- 32.Held BW, Blanchette RA. Deception Island, Antarctica, harbors a diverse assemblage of wood decay fungi. Fungal Biol. 2017;121:145–157. doi: 10.1016/j.funbio.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 33.de Menezes GCA, et al. Diversity, distribution, and ecology of fungi in the seasonal snow of Antarctica. Microorganisms. 2019;7:445. doi: 10.3390/microorganisms7100445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baeza M, et al. Amplicon-metagenomic analysis of fungi from Antarctic terrestrial habitats. Front. Microbiol. 2017;8:2235. doi: 10.3389/fmicb.2017.02235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boyd WL, Staley JT, Boyd JW. Ecology of soil microorganisms of Antarctica. Antarctic soils and soil forming processes. Antarct. Res. 1966;8:125–129. [Google Scholar]
- 36.Adams BJ, et al. Diversity and distribution of Victoria Land biota. Soil Biol. Biochem. 2006;38:3003–3018. doi: 10.1016/j.soilbio.2006.04.030. [DOI] [Google Scholar]
- 37.Bensch K, et al. Species and ecological diversity within the Cladosporium cladosporioides complex (Davidiellaceae, Capnodiales) Stud. Mycol. 2010;67:1–94. doi: 10.3114/sim.2010.67.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Torres DE, et al. Cladosporium cladosporioides and Cladosporium pseudocladosporioides as potential new fungal antagonists of Puccinia horiana Henn., the causal agent of chrysanthemum white rust. PLoS ONE. 2017;12:e0170782. doi: 10.1371/journal.pone.0170782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tosi S, Casado B, Gerdol R. Fungi isolated from Antarctic mosses. Polar Biol. 2002;25:262–268. doi: 10.1007/s00300-001-0337-8. [DOI] [Google Scholar]
- 40.Rosa LH, et al. Opportunistic fungal assemblages present on fairy rings spread on different moss species in the Antarctic Peninsula. Polar Biol. 2020;43:587–596. doi: 10.1007/s00300-020-02663-w. [DOI] [Google Scholar]
- 41.Rosa LH, et al. Endophytic fungi community associated with the dicotyledonous plant Colobanthus quitensis (Kunth) Bartl. (Caryophyllaceae) in Antarctica. FEMS Microbiol. Ecol. 2010;73:178–189. doi: 10.1111/j.1574-6941.2010.00872.x. [DOI] [PubMed] [Google Scholar]
- 42.Mercantini R, Marsella R, Cervellati MC. Keratinophilic fungi isolated from Antarctic soil. Mycopathologia. 1989;106:47–52. doi: 10.1007/BF00436926. [DOI] [PubMed] [Google Scholar]
- 43.Lorch JM, et al. A culture-based survey of fungi in soil from bat hibernacula in the eastern United States and its implications for detection of Geomyces destructans, the causal agent of bat white-nose syndrome. Mycologia. 2013;105:237–252. doi: 10.3852/12-207. [DOI] [PubMed] [Google Scholar]
- 44.Minnis AM, Lindner DL. Phylogenetic evaluation of Geomyces and allies reveals no close relatives of Pseudogymnoascus destructans, comb. nov., in bat hibernacula of eastern North America. Fungal Biol. 2013;117:638–649. doi: 10.1016/j.funbio.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 45.Arenz BE, Blanchette RA. Distribution and abundance of soil fungi in Antarctica at sites on the Peninsula, Ross Sea Region and McMurdo Dry Valleys. Soil Biol. Biochem. 2011;43:308–315. doi: 10.1016/j.soilbio.2010.10.016. [DOI] [Google Scholar]
- 46.Carvalho CR, et al. Fungi associated with the briosphere of the bipolar mosses Polytrichastrum alpinum and Polytrichum juniperinum in Antarctica. Polar Biol. 2020;43:545–553. doi: 10.1007/s00300-020-02658-7. [DOI] [Google Scholar]
- 47.Loque CP, et al. Fungal community associated with marine macroalgae from Antarctica. Polar Biol. 2010;33:641–648. doi: 10.1007/s00300-009-0740-0. [DOI] [Google Scholar]
- 48.Furbino LE, et al. Diversity patterns, ecology and biological activities of fungal communities associated with the endemic macroalgae across the Antarctic. Microb. Ecol. 2014;67:775–787. doi: 10.1007/s00248-014-0374-9. [DOI] [PubMed] [Google Scholar]
- 49.Santiago IF, et al. Lichenosphere: a protected natural microhabitat of the non-lichenised fungal communities living in extreme environments of Antarctica. Extremophiles. 2015;19:1087–1097. doi: 10.1007/s00792-015-0781-y. [DOI] [PubMed] [Google Scholar]
- 50.Lorch JM, et al. Experimental infection of bats with Geomyces destructans causes white-nose syndrome. Nature. 2011;480:376–378. doi: 10.1038/nature10590. [DOI] [PubMed] [Google Scholar]
- 51.Bridge PD, Newsham KK. Soil fungal community composition at Mars Oasis, a southern maritime Antarctic site, assessed by PCR amplification and cloning. Fungal Ecol. 2009;2:66–74. doi: 10.1016/j.funeco.2008.10.008. [DOI] [Google Scholar]
- 52.Godinho VM, et al. Diversity and bioprospecting of fungal communities associated with endemic and cold-adapted macroalgae in Antarctica. ISME J. 2013;7:1434–1451. doi: 10.1038/ismej.2013.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Øvestedal DO, Smith RIL. Lichens of Antarctica and South Georgia: A Guide to Their Identification and Ecology. Cambridge: Cambridge University Press; 2001. [Google Scholar]
- 54.Shivarov VV, Thüs H, Denchev CM. First records of two freshwater lichens, Hydropunctaria scabra and Verrucaria alpicola, from Bulgaria. Mycobiota. 2017;7:1–5. doi: 10.12664/mycobiota.2017.07.01. [DOI] [Google Scholar]
- 55.Thompson, J. American Arctic Lichens: The Microlichens. https://lichenportal.org/cnalh/taxa/index.php?taxon=127670&clid=1019. Accessed June 2020 (1997).
- 56.Thüs H, et al. Revision of the Verrucaria elaeomelaena species complex and morphologically similar freshwater lichens (Verrucariaceae, Ascomycota) Phytotaxa. 2015;197:161–185. doi: 10.11646/phytotaxa.197.3.1. [DOI] [Google Scholar]
- 57.Orange A. Four new species of Verrucaria (Verrucariaceae, lichenized Ascomycota) from freshwater habitats in Europe. Lichenologist. 2013;45:305–322. doi: 10.1017/S0024282912000898. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.