Abstract
Due to rapid urbanization, children today have fewer opportunities to interact with nature and this may result in a greater risk for developing stress and depression. Outdoor nature-related activities can enhance general well-being. However, the underlying mechanisms are not fully delineated. Here we recruited 54 preschool children to participate in a 10-week structured nature-related “Play&Grow” program. Following the intervention, children were assessed for connectedness to nature and perceived stress levels using validated questionnaires. Moreover, fecal serotonin level and gut microbiota profiles were measured by ELISA and 16S rDNA amplicon sequencing, respectively. Children were significantly more connected to nature after the intervention. Their gut microbiota altered, especially by modulating the abundance of Roseburia and the fecal-serotonin level. Moreover, we also observed a reduction in the overall perceived stress, particularly in the frequency of anger among these children. This study is the first to demonstrate the impact of nature-related activities on gut microbiota, fecal serotonin and psychosocial behaviour of preschool children. However, further mechanistic studies are needed to confirm the functional role of gut microbiota in the association between connectedness to nature and improved psychosocial behavior.
Subject terms: Environmental impact, Microbiome
Introduction
Aggressive behaviour in early childhood, a concerning behavioural problem, has not been taken seriously as a risk factor for violence later in life1. A survey of the U.S. population shows that the overall prevalence of inappropriate, intense, or poorly controlled anger is 7.8%2. Behavioural problems in children are likewise becoming a prominent issue, particularly, in fast-growing cities like Hong Kong3. As a previous study indicated, early intervention is vital as it has been found to lower the risk of developing lifelong mental disorders4.
An increased understanding of the brain-gut axis gives evidence to the assertion that the gut microbiome is not only an indicator, but also, a bi-directional influencing factor on mental disorders5. Palma’s research found that exposing rat pups to a stressor (i.e., being separated from their mothers) changed their gut microbiota, their stress response, and behaviour6. Exposure to the stressor significantly reduced Lactobacilli levels, in particular, a phenomenon also identified in humans during school examinations7 and in murine studies utilising prolonged restraint or a short-lasting social stressor8. An important neurologically active substance, gamma-aminobutyric acid (GABA), has been found to be produced by the gut microbiome9. Relatedly, GABA deficiency is a hallmark of anxiety disorders and major depression10. The metabolic by-products of gut microbiota, short-chain fatty acids (SCFAs), have also been found to have a potential contribution to depression phenotype11,12 despite more mechanistic information being needed. However, our understanding is still limited in regard to microbiota associated functional changeset in relationship with the mental health of children, despite these indicators.
Another possibly significant link between gut microbiota and behaviours is serotonin, or 5-hydroxytryptamine (5-HT), which is involved in the modulation of a variety of physiological and psychological processes13,14. Gut microbiota can both produce and modulate the host’s biosynthesis of serotonin15,16. Using germ-free animal models, Yano et al. elegantly demonstrated that microbiota promoted 5-HT biosynthesis from the colonic enterochromaffin cells (EC)15, although animal experiments suggest that fecal serotonin can have a pro-inflammatory role and stabilise the gastro-intestinal barrier17.
The relationship between intestinal microbiota, brain development, and behaviour has been examined previously18, but only a limited number of studies have included exposure to the natural outdoor environment19. According to Wilson’s biophilia theory20, natural outdoor environments and greenness surrounding environments have been shown to be associated with positive health outcomes21, including improved psychological well-being22, and decreased the risk of psychiatric disorders23, and attention-deficit/hyperactivity disorder symptoms24. The increased exposure to natural environments and animals is an important determinant of individual gut microbiome25,26, skin microbiome27 and salivary microbiome composition28. Thus, exposing children to a higher bacterial load in the natural environment by encouraging them to play outside may be a reasonable way to increase the diversity of their intestinal microbiota19.
The “Play&Grow” early environmental education programme, with its unique ‘Connectedness to Nature’ component, was designed to increase biophilia and its positive health outcomes for preschoolers. This intervention allows interaction with the natural outdoor world and has proven to be effective in encouraging healthy lifestyle behaviours in families with preschoolers in prior experiments29,30. Moreover, the intervention increases the vegetable consumption of children31. The main objective of this trial was therefore to investigate the impact of the “Play&Grow” intervention on the intestinal microbiome (both taxa and predicted functions), gut serotonin level, and the psychological well-being of 2–5 years old children.
Results
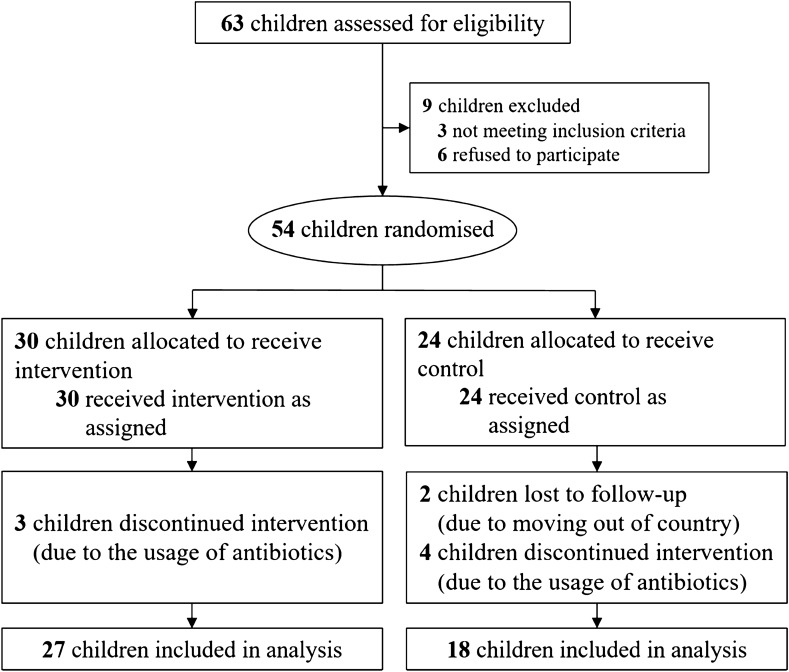
In total, fifty-four participant families were randomly assigned to the intervention (IG) (n = 30) or control groups (CG) (n = 24). Forty-five participants completed the pre- and post-intervention questionnaires and 84% of them provided the fecal samples (n = 27 in the IG and n = 18 in the CG) (Fig. 1). The demographics of the two groups are listed in Supplementary Table 1. Comparisons on demographics between study groups and their respective dropouts revealed no significant difference at baseline, indicating no impact of the dropout on the results of the study (Supplementary Table 1a and 1b).
Figure 1.
Flowchart of the RCT.
Increase in connectedness to nature
The 10-week intervention resulted in increased connectedness to nature (CN) within the IG children. The total CN score in the IG increased significantly post-intervention (p = 0.04, 3.54 vs. 3.85), compared to the CG (p = 0.20, 4.03 vs. 4.04) (Supplementary Fig. 1a). Specifically, the scores for one of the factors, “Responsibility towards Nature” (RN) significantly increased from pre- to post-intervention (p = 0.003, 3.33–3.67) (Supplementary Fig. 1b).
Fecal serotonin & improvement of psychosocial behaviour
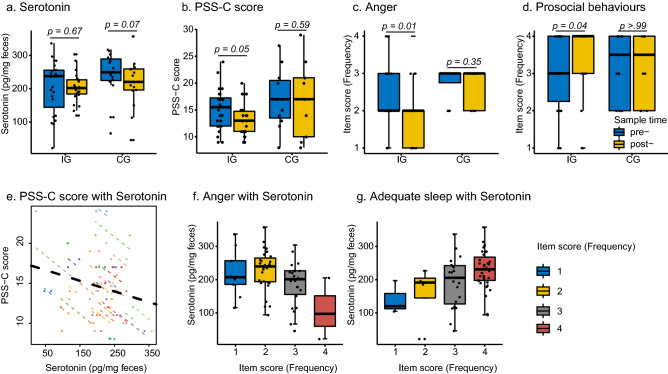
A stable level of fecal serotonin was observed in the IG while a decreasing trend was found in the CG (p = 0.07, 249.36–220.60 pg/mg of feces) (Fig. 2a). Overall score related to the perceived Stress Scale for Children (PSS-C) and specific scores of anger frequency and prosocial behaviour improved significantly post-intervention (p = 0.05, p = 0.01 and 0.04, respectively, Fig. 2b–d) compared with the control group. We further separated the participants into three groups according to the changes in their PSS-C scores between pre- and post-intervention. A total of 65% of participants in IG (n = 13) have decreased PSS-C scores compared with 22% in the CG group (n = 2, Fisher's Exact Test p = 0.01). Especially, three children with the highest anger score improved (from level 4 to level 2) following the intervention. In addition, three other children who had higher anger scores of level 3 also decreased to level 2. Following the intervention, the two children with the lowest prosocial behaviour scores improved (Fig. 2d). Fecal serotonin was found to have a negative correlation with the overall PSS-C score (Repeated measures correlation, r = −0.45, p value = 0.02, Fig. 2e). It was also found to have an increasing trend of association with sleep frequency (Jonckheere-Terpstra test: JT = 832, p value < 0.01), and have a decreasing trend with anger frequency (Jonckheere-Terpstra test: JT = 475.5, p value = 0.01) (Fig. 2f–g). The relationship of fecal serotonin with the frequency of anger was independent to the age of the children (Supplementary Table 2).
Figure 2.
Change in serotonin and child psychosocial measurements. (a) Change in fecal serotonin between IG (n = 23) and CG (n = 15), pre- and post-intervention. (b) Change in PSS-C score in child psychosocial measurements between IG (n = 20) and CG (n = 9). (c) Change in score of anger in child psychosocial measurements between IG (n = 24) and CG (n = 12). (d) Change in score of prosocial in child psychosocial measurements between IG (n = 24) and CG (n = 12). (e) Association between serotonin and PSS-C score. (f) Association between serotonin and anger frequency. (g) Association between serotonin and adequate sleep. In (a) to (d) blue colour indicated pre-samples, yellow colour indicated post-samples. In (e), different colour of node and dotted-line indicated different individual, and the black dotted-line indicated the overall association. In (f) and (g), four colour indicated four degrees of anger and adequate sleep frequency.
Changes in the gut microbiome
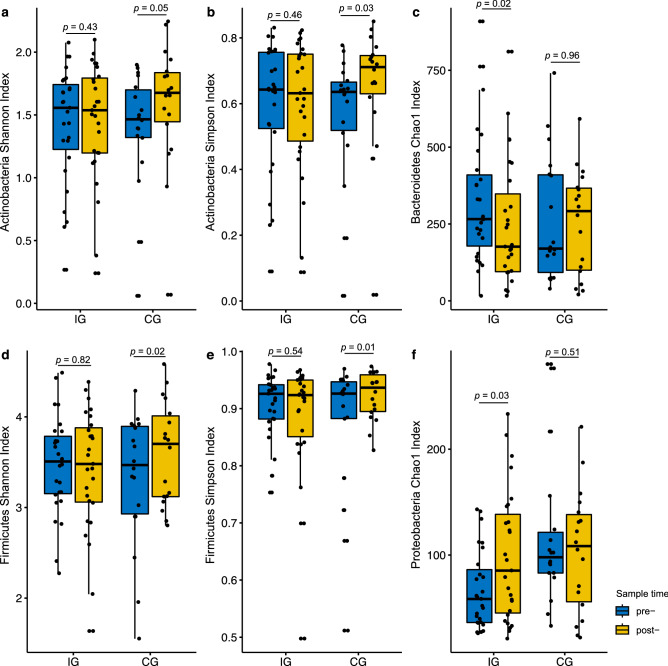
An average of 49,577 quality-filtered reads (per sample) were generated (Supplementary Table 3) and a total of 14 phylum, 176 genus, and 255 species were detected in gut microbiomes of the participants. The most abundant phyla of gut microbiota were Firmicutes, Bacteroidetes, and Proteobacteria (Median abundance: 0.44, 0.27, and 0.11%) (Supplementary Fig. 2). Although no notable difference in the overall alpha diversity between two time points both in IG and CG groups, the participants from the IG with decreased PSS-C score exhibited a significantly higher gut microbiota richness. (Supplementary Fig. 3). The richness of Bacteroidetes and Proteobacteria phyla changed significantly in the IG after the intervention. The Chao1 richness of Bacteroidetes significantly decreased (p < 0.01), while that of Proteobacteria increased (p = 0.03). On the other hand, there were no significant changes in the diversity of Actinobacteria and Firmicutes (both Shannon and Simpson indices) in the IG compared with the increased diversity in the CG following the intervention (Fig. 3).
Figure 3.
Alpha diversity changes between pre- and post-intervention in IG (n = 27) and CG (n = 18). (a) Shannon index of Actinobacteria. (b) Simpson index of Actinobacteria. (c) Chao1 index of Bacteroidetes. (d) Shannon index of Firmicutes. (e) Simpson index of Firmicutes. (f) Chao1 index of Proteobacteria. Blue colour indicated pre-samples, yellow colour indicated post-samples.
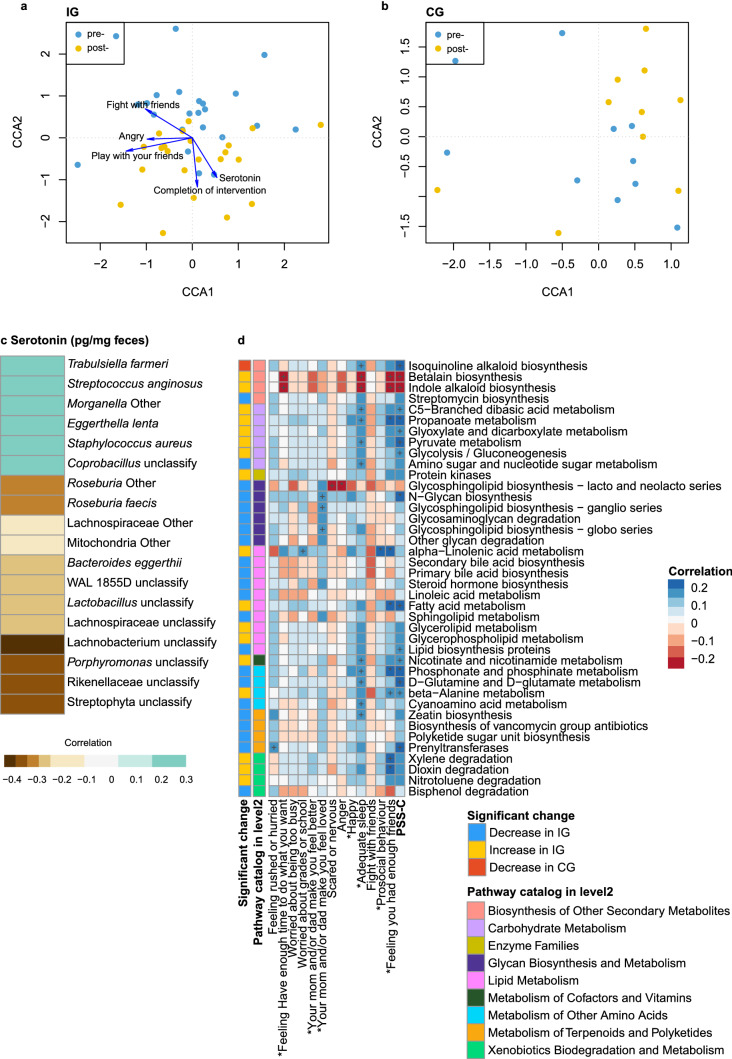
No significant difference in microbial community structure was observed between the IG and the CG at baseline; however, a clear difference between pre- and post-measurements in both IG and CG groups was observed based on the Canonical Correspondence Analysis (CCA) results. Several factors including completion of the intervention, serotonin levels, and anger frequency, were identified to influence the gut microbiota of children in the IG but not in the CG (Fig. 4a, b). Changes in certain species were found post-intervention (Supplementary Fig. 4). Interestingly, an increased abundance of Roseburia was shown in the CG but not in the IG (Supplementary Fig. 4g). The abundance of an unknown species of Bacteroides, Parabacteroides distasonis, an unclassified Acidaminococcus, an unclassified Dialister, and an unclassified Bilophila decreased in the IG, while an unclassified species of Blautia increased in the IG.
Figure 4.
Associations between serotonin, stress items and gut microbiome. (a) and (b) Canonical Correlation Analysis (CCA) in CG and IG samples. Blue node indicated the pre-intervention samples; yellow node indicated the post- samples. (c) Heatmap of correlations between serotonin and gut microbiota species. Only the significant associated factors on gut microbiota in spearman correlation were listed. Colour in each cell of the heatmap indicated spearman correlation of serotonin and species abundance. Only the p value of association less than 0.05 were chosen for display in this figure. (d) Associations between gut microbiota functions and stress items, and changes in microbiota functions by the intervention. The heatmap indicated the spearman correlation between metabolic functions of gut microbiota and children’s stress items. +p value less than 0.1; *p value less than 0.05; **p value less than 0.01. *in the front of the column name indicated we used the reversed score rather than the original score.
Correlation between bacterial, serotonin and psychosocial behaviours
Among the microbiota that were significantly correlated with fecal serotonin levels, six of the species showed positive correlation, while the other twelve correlated negatively (Fig. 4c). Although statistically not significant, there was a negative trend of correlation between fecal serotonin level and Shannon diversity index of Bacteroidetes (p = 0.07, ρ = −0.21) and Chao1 richness index of Firmicutes (p = 0.09, ρ = −0.19), but a positive correlation with Proteobacteria richness (p = 0.06, ρ = 0.22) (Supplementary Fig. 5).
Importantly, an unknown species of Roseburia, which increased in the CG post-intervention, was significantly negatively correlated with fecal serotonin levels (p = 0.01, ρ = −0.31) (Fig. 4c), as was another species under the same genus, Roseburia faecis (p = 0.01, ρ = −0.31). The association between serotonin and Roseburia could be future conformed by the logistic regression (Supplementary Table 4). Moreover, Trabulsiella farmeri, Streptococcus anginosus, Eggerthella lenta, and Staphylococcus aureus showed positive correlations.
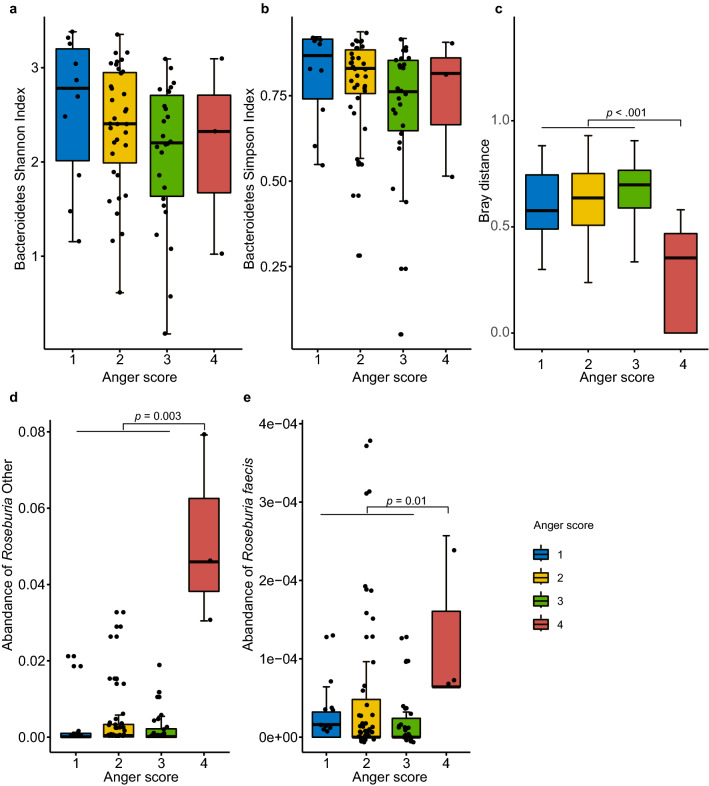
The overall PSS-C score was negatively correlated with Chao1 richness (p = 0.03, ρ = −0.27, Supplementary Fig. 6a), Shannon diversity (p = 0.03, ρ = −0.26, Supplementary Fig. 6b), and Simpson diversity (p = 0.05, ρ = −0.24, Supplementary Fig. 6b–c) of Bacteroidetes. A similar trend was observed for Shannon diversity of Proteobacteria (p = 0.07, ρ = −0.23, Supplementary Fig. 6d) although statistically not significant. Besides, Shannon and Simpson diversity of Bacteroidetes showed a decreasing trend according to anger frequency (Jonckheere-Terpstra test: JT = 674, p value = 0.02; JT = 666, p value = 0.02, Fig. 5a, b). Although no notable difference was detected in overall alpha diversity, children with the highest anger frequency shared more similar gut microbial community when compared to children with fewer anger problems (Fig. 5c). On a species level, two species of Roseburia were significantly higher in the microbiome of children who had the highest anger frequency (Fig. 5d, e).
Figure 5.
Associations between gut microbiota and Anger frequency. (a) Association between Shannon index of Bacteroidetes and anger frequency. (b) Association between Simpson index of Bacteroidetes and anger frequency. (c) Association between bray distance of species level and anger frequency. (d) Association between the abundance of Roseburia Other species and anger frequency. (e) Association between the abundance of Roseburia faecis species and anger frequency. Four colours indicated four degrees of anger frequency.
Alteration in predicted microbiota functions
A total of 273 microbiota functional categories (at KEGG level-3, www.kegg.jp/kegg/kegg1.html) were predicted using PICRUSt. Under the functional category of “Metabolism”, 125 metabolic functions were detected with the highest abundance in “Purine metabolism.” Among those metabolic functions, we observed more functional changes in the IG (p < 0.05), in which 22 pathways decreased and 17 pathways increased. Comparatively, only a single pathway decreased (Supplementary Fig. 5) in the control group. At KEGG level-2, a significant correlation was observed between the overall PSS-C score and various amino acid metabolisms covering Phosphonate and phosphinate, d-Glutamine and d-glutamate, and beta-Alanine. Furthermore, the overall PSS-C score was positively correlated with the metabolism of carbohydrates including C5-Branched dibasic acid, Propanoate, Glyoxylate and dicarboxylate, Pyruvate, and Glycolysis/Gluconeogenesis. Betalain and Indole alkaloid biosynthesis, which have been increased by intervention, was negatively correlated with the total PSS-C score as well as the other sub-scores of the PSS-C questionnaire (Fig. 4d). However, none of these altered pathways showed a significant correlation to the fecal serotonin level in general.
Discussion
To investigate the impact of Connectedness to Nature (CN), we conducted a randomized trial and subsequently studied its impact on changes in gut microbiota and serotonin as well as the psychosocial behaviour of children. In this study, we observed that early environmental education intervention significantly reduced the overall perceived stress, particularly anger frequency among preschool children, modulated the abundance of certain gut microbiota, and in contrast to the respective control condition, did not reduce gut serotonin levels. The “Play&Grow” programme was successful in modulating the microbiome within the IG after regular exposure to nature over the course of 2 months. Similarly, a recent study in an adult population demonstrated that dipping hands in soil daily for 2 weeks changed the skin and gut microbiomes of the study’s participants, indicating a correlation between natural interactions and the diversity of the gut microbiota32. In particular, our study discovered that exposure to natural bacteria moderated the diversity of the Actinobacteria and Firmicutes phyla, decreased the alpha diversity of the Bacteroidetes phylum, and increased the alpha diversity of Proteobacteria. We also observed inter-individual variation in changing gut microbiota diversity due to the intervention, and it might be related to the variation of exposure intensity among individuals.
Velles-Colomer et al. has recently reported an important association between depression, lower quality of life, and Bacteroides in the large Flemish Gut Flora Project33. Our controlled intervention allows us to suggest that exposure to bacteria from natural environmental could be beneficial to behavioural outcomes; however, this intriguing possibility needs further investigation. According to the natural history of gut microbiome development in early-life, the variation in the abundance of Bacteroides species among individuals is large due to different exposure levels and other factors associated with early life (e.g. birth mode and breastfeeding)34,35. Thus, the impact of our intervention on these particular taxa might be hindered by various factors. Despite this, we observed less individual variation of Bacteroides taxa following the intervention, which implies a movement toward modulation of the gut microbiome could be related to increased exposure to the natural environment and connectedness to nature34,36. During the first years of life, exposure to the natural microbial experience is therefore an important moderator for normal developmental patterns of gut microbiota and behaviour.
The current study showed positive changes in the psychosocial scores of participants in the IG after the “Play&Grow” intervention. This is in agreement with the systematic review of studies that found a beneficial impact of exposure to the natural environment on negative emotions (i.e., anger and sadness)37,38. Moreover, living in close proximity to forests is known to have salutogenic effects on the amygdala39. Likewise, the “Play&Grow” programme significantly improved the overall perceived stress, particularly anger frequency and prosocial behaviour of participants, which suggests a link between these behaviours and the bacterial load encountered by the participants. This is a new area of research, lacking the human data (although preclinical studies are promising); comparison of our results with other studies on similar age groups is therefore difficult. Studies in germ-free (GF) animals showed that GF mice were significantly socially impaired, yet this deficit could be repaired following bacterial colonisation, suggesting that gut microbiota affects psychosocial behaviour40.
Identification of the bacteria that is involved in these new mechanisms may help to understand the nature of the psychosocial consequences of bacterial alterations early in life. Our intervention decreased the prevalence of Parabacteroides distasonis, which has been positively associated with exposure to social stressors in mice41. A lower abundance of Roseburia has been reported in schizophrenics, but the opposite trend was found in people with mood disorders42,43. In this study, we also found that our intervention somewhat maintained Roseburia, whose abundance increased over time in the CG. Our results suggest that exposure to bacteria in nature may control the growth of Roseburia, resulting in a stabilization of gut serotonin levels. This indicates that the changes to the gut microbiota following exposure to natural environments may influence behaviours of preschool children. The gut microbiota related serotonin (5HT) pathway has already been proposed in animal models; but it has not yet been well-studied in human subjects, especially in children.
The “Play&Grow” programme’s components align with biophilia theory. Both structured and unstructured nature experiences (i.e., playing with leaves and soil) and educational messaging (i.e., “it is ok to get your hands dirty with soil”) increased children’s contact with the natural environment and associated health outcomes. It strengthened the idea that nature is a “playground” and provided an opportunity for highly-urbanized children to reconnect to nature and interact with the microbiome of the natural environment.
Our intervention may be seen as an extension of the “Hygiene Hypothesis” also known as the “Microbiota Hypothesis”, which suggests that exposure to bacteria and other pathogens may actually be beneficial to the education and development of the immune system44–49. Studies have reported that reduced exposure to microbes in modern urban societies may increase vulnerability to neurodevelopmental disorders (including autism spectrum disorders (ASD) and schizophrenia) and stress-related psychiatric disorders (including anxiety and mood disorders)46,50–53. Our study also showed this association, indicating children with the highest anger frequency had the least inter-individual variation in gut microbial community and the highest levels of Roseburia species.
This study investigated a possible relationship between fecal serotonin, the gut microbiome, and psychosocial behaviours. While a trend towards decreased fecal serotonin level was observed only in the CG, serotonin levels stayed stable in the IG. Moreover, serotonin is negatively correlated with the diversity of Bacteroidetes, Firmicutes, and Proteobacteria, as well as with the abundance of Roseburia. A recent study identified Roseburia as potential serotonin producers; however, the degradation pathway of serotonin by the bacteria is still not yet fully elucidated33. Hence, our study indicates that the Play&Grow intervention in preschool children increased connectedness to nature, improved children’s moods, especially with respect to their anger levels, as well as changed the gut microbiome (especially the abundance of Roseburia), and in contrast to the respective control group, did not decrease gut serotonin levels. But due to the limitation of database and software, we didn’t find the significant difference related to serotonin metabolism, so the associations need to be further confirmed by more targeted studies.
Besides the changes observed in microbial taxa, we also identified that our intervention significantly altered the microbiota functions within participants of the IG when compared with those in the CG. Among the functional changes, the most noteworthy one was a rise in Indole alkaloid biosynthesis, which also has a negative correlation with the PSS-C score, has been previously reported as a potential drug for depression and anxiety54. Another remarkable functional change was the decreased abundance of metabolic functions related to the biosynthesis of glycosphingolipid. A previous study also reported an increase in glycosphingolipid biosynthesis in Chinese children with autism spectrum disorders55, indicating a potential disadvantage to an abundance of glycosphingolipid. In general, the functional role of glycosphingolipids in the central nervous system have been detected56, but mechanistic study is needed to explore their roles in the behavioural development of children.
This is a pioneer study that links a nature-related intervention with psychosocial outcomes and modulating gut-microbiota-associated serotonin levels, suggesting a potential pathway to highlight the benefit of connectedness to nature. While our data are encouraging, certain limitations should be mentioned. Although there is a diurnal fluctuation of serotonin levels in the blood, this is still unobserved in fecal samples. Our study did not take account of the fecal sample collection time in order to have more flexibility for participants. Moreover, the sample size and the duration of the intervention may have significantly influenced the results of our study. Due to the limitation of the 16S rRNA reference database, our study could not provide species-level resolution for some taxa. Further studies with a larger sample size coupled with metagenomic sequencing are warranted. Despite these limitations, the findings of this pilot trial might be important in contributing to further related investigations.
Conclusion
In our early environmental educational intervention study, we demonstrated the impact of nature-related activities on gut microbiota, fecal serotonin, and in improving psychosocial behaviour of preschool children. The impact included the changes in the diversity of microbiota, modulation of Roseburia abundance, prevention of a downregulation of fecal serotonin levels, and the improvement of psychosocial behaviours of children. Our findings suggest that the gut microbiota can be a target for further studies on behavioural modifications and mental health interventions in a wider perspective. Further mechanistic studies are needed to confirm the mechanistic contribution of gut microbiota into the association between connectedness to nature and improved psychosocial behaviour.
Material and methods
Informed consent and ethics
This study was approved by the human research ethics committee (HREC) of Hong Kong University, and written informed consent was obtained from all of the participants’ parents or legal guardians and the participants themselves. Trial registration: ClinicalTrials.gov, NCT02715544. Registered 22 March 2016. All methods were performed in accordance with the relevant guidelines and regulations by HREC.
Intervention design
A two-arm, randomized controlled trial (RCT) with masked outcome assessment—“Play&Grow”—was developed as a family-oriented, early environmental education programme for families with preschool children at the University of Hong Kong29,30. Primarily, the “Play&Grow” programme aims to reconnect preschoolers to nature and induce changes in health behaviours and outcomes by having outdoor activities that promote exposure to nature. On previous studies and calculation principles and methods57, the sample size (α = 0.05, power of 0.8) needed to detect the effect of the “Play&Grow” intervention on other related outcomes was 100 families in each group of RCT (including an allowance for 20% attrition). However, the sample size of this proof-of-principle study was not determined by a formal power calculation given the exploratory nature of the study. The assumption was that at least 30 children in total would be recruited from two groups: intervention group (IG) or Control group (CG). They were measured twice: before and after the intervention and the samples analysed for gut microbiota.
Participants
Children, aged two to five years, were recruited to participate in this programme, together with their main caregivers, via online advertisement at the beginning of 2018. The exclusion criteria were children from non-local families, children who were on antibiotic therapy in the two months prior to the start of the programme, and children with chronic health conditions.
Procedures
Consenting families (n = 54) were randomly assigned to the Intervention (IG)(n = 30) or Control Groups (CG)(n = 24) using a random computer-generated number from 0 to 1 before the start of the trial, which was in April-June 2018. Participants were assigned to the IG if their number was > 0.5, and to the CG if their number was < 0.5. During the randomization process, no stratification or blocking were performed. All participants completed the pre- and post-intervention questionnaires, and 84% provided fecal samples (n = 27 in the IG (3 lost to antibiotic usage during the study, or failure to complete the study) and n = 18 in the CG (6 lost to antibiotic usage during the study, a failure to complete the study, or were lost to follow up) (Fig. 1). The demographics of the two groups are listed in Supplementary Table 1. The study aims and hypothesis were not discussed with any participants; they were only informed that they would be participating in a family health promotion programme. All measurements were done pre- and post-study on both groups, and all related research assistants, the questionnaire typist, and study statisticians were not informed of the group allocations, measurements and outcomes.
Intervention group (IG)
The intervention was held once per week for ten consecutive weeks, from June 2018, in public parks throughout the Hong Kong SAR. Topics and elements of the programme were developed prior to the start of the intervention and were discussed in detail29. Each session, led by a pair of research assistants, included a guided nature activity that promoted “hands-on” experiences with materials found in nature. These activities were specifically designed for IG participants to provide them an opportunity to come into contact with the microbiome of the natural environment. Some additional “homework”, such as collecting nature subjects, making nature art, and growing plants, was given to the families in the IG to further increase their interaction with the outdoor environment and its bacteria. A package of healthy lifestyle recommendations published by the Hong Kong government did not have any special dietary change recommendations and was available to all caregivers in Hong Kong58.
Control group (CG)
In order to eliminate potential confound factors, i.e. changes in gut microbial composition as a result of any dietary changes, both groups were reminded of the resource mentioned above58.
Harms
This study focused on exposing children to the nature environment and did not contain any clinical procedure/intervention. As a result, no considerable harm or unintended effect were noted during the trial.
Outcome measurement
All measurements were conducted prior to and after the 10-week intervention. Detailed procedures for children’s psychosocial measurement, assessment for connectedness to nature (CN), serotonin measurement, and gut microbiota analysis were indicated in the Supplemental material.
Statistical analysis
With the recommended pipeline in QIIME, the relative abundance of OTUs was summarised at phylum, genus and species level. Microbial alpha diversity was calculated by Chao1, Shannon, and Simpson indices. The differences between two time-points within groups were identified by the Wilcoxon signed-rank test (R package stats), including the difference of CN factor scores, microbiota diversity based on OTU, and species level, microbiota alpha diversity for each of the phylum, serotonin, and stress levels. Jonckheere-Terpstra test was used to test the trend (R package clinfun). The association between microbiota diversity, species, and stress levels with serotonin levels was measured by Spearman's rank correlation and logistic regression (R package stats) by category the continue number by the median. The association between PSS-C score with serotonin levels was measured by repeated measures correlation coefficient (R package rmcorr). CCA was performed based on species profile of the IG and the CG, separately (R package vegan).
Supplementary Information
Acknowledgements
The authors would like to thank all “Play&Grow” staff members and the families who participated in the programme, as well as Darren C.L. Chan and Kanchana Poonsuk for their contributions in lab procedures. The authors would further like to thank Kristopher Jordan for scrupulous editing and review. The study is supported by General Research Grant, nr 17108217.
Author contributions
T.S. initiated, designed and conducted the intervention, as well as participated in data interpretation. W.H.G.C contributed in questionnaire data collection. S.L did microbiome data analysis. T.H.M contributed to the study design and led microbiome sequencing, data analysis, and interpretation. All authors participated in writing the manuscript. All authors read and approved the final manuscript.
Data availability
The sequence data under this study are publicly available in the European Nucleotide Archive under accession number PRJEB34058. Dr. Tun had full access to all subjects’ meta-data and associated microbiota data in the study and takes responsibility of correspondence for data requests related to this study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Tanja Sobko and Suisha Liang.
Supplementary information
The online version contains supplementary material available at 10.1038/s41598-020-78642-2.
References
- 1.Hawes SW, et al. Chronic anger as a precursor to adult antisocial personality features: The moderating influence of cognitive control. J. Abnorm. Psychol. 2016;125:64–74. doi: 10.1037/abn0000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okuda M, et al. Prevalence and correlates of anger in the community: Results from a national survey. CNS Spectrosc. 2015;20:130–139. doi: 10.1017/S1092852914000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.FHB. Mental Health Review Report (Food and Health Bureau, HKSAR Government, 2017).
- 4.Kessler RC, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch. Gen. Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 5.Martin CR, Osadchiy V, Kalani A, Mayer EA. The brain-gut-microbiome axis. Cell. Mol. Gastroenterol. Hepatol. 2018;6:133–148. doi: 10.1016/j.jcmgh.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palma GD, et al. Microbiota and host determinants of behavioural phenotype in maternally separated mice. Nat. Commun. 2015 doi: 10.1038/ncomms8735. [DOI] [PubMed] [Google Scholar]
- 7.Knowles SR, Nelson EA, Palombo EA. Investigating the role of perceived stress on bacterial flora activity and salivary cortisol secretion: A possible mechanism underlying susceptibility to illness. Biol. Psychol. 2008;77:132–137. doi: 10.1016/j.biopsycho.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Galley J, et al. Exposure to a social stressor disrupts the community structure of the colonic mucosa-associated microbiota. BMC Microbiol. 2014;14:189. doi: 10.1186/1471-2180-14-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barrett E, Ross RP, O'Toole PW, Fitzgerald GF, Stanton C. gamma-Aminobutyric acid production by culturable bacteria from the human intestine. J. Appl. Microbiol. 2012;113:411–417. doi: 10.1111/j.1365-2672.2012.05344.x. [DOI] [PubMed] [Google Scholar]
- 10.Mohler H. The GABA system in anxiety and depression and its therapeutic potential. Neuropharmacology. 2012;62:42–53. doi: 10.1016/j.neuropharm.2011.08.040. [DOI] [PubMed] [Google Scholar]
- 11.Skonieczna-Zydecka K, et al. Faecal short chain fatty acids profile is changed in polish depressive women. Nutrients. 2018;10:66. doi: 10.3390/nu10121939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szczesniak O, Hestad KA, Hanssen JF, Rudi K. Isovaleric acid in stool correlates with human depression. Nutr. Neurosci. 2016;19:279–283. doi: 10.1179/1476830515y.0000000007. [DOI] [PubMed] [Google Scholar]
- 13.Lucki I. The spectrum of behaviors influenced by serotonin. Biol. Psychiatry . 1998;44:151–162. doi: 10.1016/S0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- 14.Asan E, Steinke M, Lesch KP. Serotonergic innervation of the amygdala: Targets, receptors, and implications for stress and anxiety. Histochem. Cell Biol. 2013;139:785–813. doi: 10.1007/s00418-013-1081-1. [DOI] [PubMed] [Google Scholar]
- 15.Yano J, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015;277:32–48. doi: 10.1016/j.bbr.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 17.Spohn SN, Mawe GM. Non-conventional features of peripheral serotonin signalling—The gut and beyond. Nat. Rev. Gastroenterol. Hepatol. 2017;14:412. doi: 10.1038/nrgastro.2017.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cryan JF, Dinan TG. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012;13:701. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 19.Sing D, Sing CF. Impact of direct soil exposures from airborne dust and geophagy on human health. Int. J. Environ. Res. Public Health. 2010;7:1205–1223. doi: 10.3390/ijerph7031205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kellert SR, Wilson EO. The Biophilia Hypothesis. London: Island Press; 1995. [Google Scholar]
- 21.Wells NM, Evans GW. Nearby nature: A buffer of life stress among rural children. Environ. Behav. 2003;35:311–330. doi: 10.1177/0013916503035003001. [DOI] [Google Scholar]
- 22.Kaplan R. Some psychological benefits of gardening. Environ. Behav. 1973;5:145–152. doi: 10.1177/001391657300500202. [DOI] [Google Scholar]
- 23.Engemann K, et al. Residential green space in childhood is associated with lower risk of psychiatric disorders from adolescence into adulthood. Proc. Natl. Acad. Sci. USA. 2019;116:5188–5193. doi: 10.1073/pnas.1807504116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang BY, et al. Association between greenness surrounding schools and kindergartens and attention-deficit/hyperactivity disorder in children in China. JAMA Netw. Open. 2019;2:e1917862. doi: 10.1001/jamanetworkopen.2019.17862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nielsen CC, et al. Natural environments in the urban context and gut microbiota in infants. Environ. Int. 2020;142:105881. doi: 10.1016/j.envint.2020.105881. [DOI] [PubMed] [Google Scholar]
- 26.Yatsunenko T, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lehtimaki J, et al. Skin microbiota and allergic symptoms associate with exposure to environmental microbes. Proc. Natl. Acad. Sci. USA. 2018;115:4897–4902. doi: 10.1073/pnas.1719785115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langgartner D, et al. Association of the salivary microbiome with animal contact during early life and stress-induced immune activation in healthy participants. Front. Psychiatry. 2020;11:353. doi: 10.3389/fpsyt.2020.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sobko T, Tse M, Kaplan M. A randomized controlled trial for families with preschool children—Promoting healthy eating and active playtime by connecting to nature. BMC Public Health. 2016;16:505–505. doi: 10.1186/s12889-016-3111-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sobko T, Jia Z, Kaplan M, Lee A, Tseng C-H. Promoting healthy eating and active playtime by connecting to nature families with preschool children: Evaluation of pilot study "Play&Grow". Pediatr. Res. 2017;81:572. doi: 10.1038/pr.2016.251. [DOI] [PubMed] [Google Scholar]
- 31.Sobko T, Brown GTL, Cheng WHG. Does connectedness to nature improve the eating behaviours of pre-schoolers? Emerging evidence from the Play&Grow randomised controlled trial in Hong Kong. Appetite. 2020 doi: 10.1016/j.appet.2020.104781. [DOI] [PubMed] [Google Scholar]
- 32.Nurminen N, et al. Nature-derived microbiota exposure as a novel immunomodulatory approach. Fut. Microbiol. 2018;13:737. doi: 10.2217/fmb-2017-0286. [DOI] [PubMed] [Google Scholar]
- 33.Valles-Colomer M, et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 2019;4:623–632. doi: 10.1038/s41564-018-0337-x. [DOI] [PubMed] [Google Scholar]
- 34.Kennedy PJ, et al. Microbiome in brain function and mental health. Trends Food Sci. Technol. 2016;57:289–301. doi: 10.1016/j.tifs.2016.05.001. [DOI] [Google Scholar]
- 35.Yassour M, et al. Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci. Transl. Med. 2016;8:343ra381. doi: 10.1126/scitranslmed.aad0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borre YE, et al. Microbiota and neurodevelopmental windows: Implications for brain disorders. Trends Mol. Med. 2014;20:509–518. doi: 10.1016/j.molmed.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 37.Sampson TR, Mazmanian SK. Control of brain development, function, and behavior by the microbiome. Cell Host Microbe. 2015;17:565–576. doi: 10.1016/j.chom.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bowler DE, Buyung-Ali LM, Knight TM, Pullin AS. A systematic review of evidence for the added benefits to health of exposure to natural environments. BMC Public Health. 2010;10:456–456. doi: 10.1186/1471-2458-10-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuhn S, et al. In search of features that constitute an "enriched environment" in humans: Associations between geographical properties and brain structure. Sci. Rep. 2017;7:11920. doi: 10.1038/s41598-017-12046-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Desbonnet L, Clarke G, Shanahan F, Dinan TG, Cryan JF. Microbiota is essential for social development in the mouse. Mol. Psychiatry. 2013;19:146. doi: 10.1038/mp.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bailey MT, et al. Exposure to a social stressor alters the structure of the intestinal microbiota: Implications for stressor-induced immunomodulation. Brain Behav. Immun. 2011;25:397–407. doi: 10.1016/j.bbi.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen Y, et al. Analysis of gut microbiota diversity and auxiliary diagnosis as a biomarker in patients with schizophrenia: A cross-sectional study. Schizophr. Res. 2018 doi: 10.1016/j.schres.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 43.Jiang H, et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immunol. 2015;48:186–194. doi: 10.1016/j.bbi.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 44.Eberl G. The microbiota, a necessary element of immunity. C. R. Biol. 2018;341:281–283. doi: 10.1016/j.crvi.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 45.Nash MJ, Frank DN, Friedman JE. Early microbes modify immune system development and metabolic homeostasis—The "Restaurant" hypothesis revisited. Front. Endocrinol. 2017;8:349. doi: 10.3389/fendo.2017.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rook GA, Lowry CA, Raison CL. Hygiene and other early childhood influences on the subsequent function of the immune system. Brain. Res. 2015;1617:47–62. doi: 10.1016/j.brainres.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 47.Tanaka M, Nakayama J. Development of the gut microbiota in infancy and its impact on health in later life. Allergol. Int. 2017;66:515–522. doi: 10.1016/j.alit.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 48.Stein MM, et al. Innate immunity and asthma risk in amish and hutterite farm children. N. Engl. J. Med. 2016;375:411–421. doi: 10.1056/NEJMoa1508749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bobel TS, et al. Less immune activation following social stress in rural vs. urban participants raised with regular or no animal contact, respectively. Proc. Natl. Acad. Sci. USA. 2018;115:5259–5264. doi: 10.1073/pnas.1719866115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raison CL, Lowry CA, Rook GA. Inflammation, sanitation, and consternation: Loss of contact with coevolved, tolerogenic microorganisms and the pathophysiology and treatment of major depression. Arch. Gen. Psychiatry. 2010;67:1211–1224. doi: 10.1001/archgenpsychiatry.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rook GA, Lowry CA. The hygiene hypothesis and psychiatric disorders. Trends Immunol. 2008;29:150–158. doi: 10.1016/j.it.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 52.Rook GA, Lowry CA, Raison CL. Microbial 'Old Friends', immunoregulation and stress resilience. Evol. Med. Public Health. 2013;46–64:2013. doi: 10.1093/emph/eot004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lowry CA, et al. The microbiota, immunoregulation, and mental health: Implications for public health. Curr. Environ. Health Rep. 2016;3:270–286. doi: 10.1007/s40572-016-0100-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kochanowska-Karamyan AJ, Hamann MT. Marine indole alkaloids: Potential new drug leads for the control of depression and anxiety. Chem. Rev. 2010;110:4489–4497. doi: 10.1021/cr900211p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang M, Ma W, Zhang J, He Y, Wang J. Analysis of gut microbiota profiles and microbe-disease associations in children with autism spectrum disorders in China. Sci. Rep. 2018;8:13981. doi: 10.1038/s41598-018-32219-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu YH, Barnes S, Sun Y, Grabowski GA. Multi-system disorders of glycosphingolipid and ganglioside metabolism. J. Lipid. Res. 2010;51:1643–1675. doi: 10.1194/jlr.R003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhong B. How to calculate sample size in randomized controlled trial? J. Thorac. Dis. 2009;1:51–54. [PMC free article] [PubMed] [Google Scholar]
- 58.StartSmart@school.hk. Start Smart Parent Guide (Hong Kong Department of Health HKSAR, 2018).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequence data under this study are publicly available in the European Nucleotide Archive under accession number PRJEB34058. Dr. Tun had full access to all subjects’ meta-data and associated microbiota data in the study and takes responsibility of correspondence for data requests related to this study.