Abstract
Background
Persistent anterior shoulder pain is an under-reported complication after reverse total shoulder arthroplasty (RTSA). The purpose of this study was to determine the effectiveness of open conjoint tendon release in patients with anterior shoulder pain due to conjoint tendinitis after RTSA.
Methods
Open conjoint tendon release was performed by the senior author from June 2014 to November 2018 in patients with persistent anterior shoulder pain after RTSA. Patients were evaluated preoperatively and at a minimum of 1 year postoperatively by phone interview with patient-reported outcome scores including a visual analog scale score for pain and the American Shoulder and Elbow Surgeons score.
Results
We evaluated 11 of 12 patients (92% follow-up) at a minimum of 1 year (average, 27 ± 11 months) after conjoint tendon release. American Shoulder and Elbow Surgeons and visual analog scale pain scores improved from 29.0 ± 22.1 and 7.3 ± 2.0, respectively, preoperatively to 58.2 ± 30.6 and 3.1 ± 3.5, respectively, postoperatively, after open conjoint tendon release (P = .02 and P = .003, respectively). Of the patients, 45% (5 of 11) reported improvement but with some coracoid pain after the release whereas 55% (6 of 11) reported no coracoid pain after the release. No complications occurred as a result of the release, and no patients required reoperation.
Conclusion
Our results suggest that conjoint tendinitis may be a cause of persistent postoperative anterior shoulder pain after RTSA and open conjoint tendon release is a successful treatment.
Keywords: Reverse total shoulder arthroplasty, complications, conjoint tendon release, postoperative pain, conjoint tendinitis, functional outcomes
In recent years, reverse total shoulder arthroplasty (RTSA) has become the most commonly performed shoulder arthroplasty procedure9; it is indicated for rotator cuff tear arthropathy, glenohumeral osteoarthritis with a compromised rotator cuff, complex proximal humeral fractures, pseudoparalysis due to irreparable rotator cuff tear in elderly persons, and revision shoulder arthroplasty.2,3,5,6,7,19,20,22,26, 27, 28,32,33 Postoperative complications after RTSA include infection, instability, hardware component loosening, acromial and scapular spine fractures, neurologic injury, and scapular notching.2,9,12 We have identified another infrequent complication in our cohort of patients after RTSA, persistent anterior shoulder pain, the cause of which can be difficult to determine.
RTSA in the Grammont style involves a translation of the glenohumeral joint's center of rotation (COR) both medially and inferiorly.4,13,27,29 The COR after a traditional Grammont RTSA is fixed at the center of the glenosphere, which is bound tightly to the glenoid and more medial than the native COR.15,31 This shift in the COR is thought to be important for deltoid function.17,18 The shift lengthens the deltoid muscle, which is thought to increase the deltoid's mechanical moment arm and thus its ability to abduct and flex the humerus.17,23 Increased deltoid tension, however, may be a cause of acromial and scapular spine fractures or neurologic injury.1,2,8,16,21,24,25,34 Other structures are tensioned as well, including the short head of the biceps brachii and the coracobrachialis. It is unclear whether lengthening of these muscles has any consequences for the function of these muscles or whether lengthening these muscle-tendon units can create pathology within them. Certainly, muscle lengthening could theoretically create tendinitis within these muscles, which could lead to persistent anterior shoulder pain after RTSA.
The purpose of this study was to determine whether isolated short head of the biceps brachii tendon and coracobrachialis tendon release from the coracoid process in patients with persistent anterior shoulder pain after RTSA could reliably decrease pain and improve functional outcomes without complication or compromise of the function of the shoulder.
Materials and methods
Patient inclusion
This study was a retrospective case series. We identified all patients within the (R.Z.T.) electronic medical record who underwent an open conjoint tendon release procedure for persistent pain after RTSA during the years 2014-2018. The senior author (R.Z.T.) performed all of the procedures. The indications for the surgical procedure included persistent anterior shoulder pain directly over the short head of the biceps brachii and coracobrachialis either at the insertion on the coracoid process or along the tendon distally. All patients had tenderness to palpation directly over the conjoint tendon just distal to the coracoid process, and this tenderness represented the patients' primary symptomatic complaint. All patients underwent an evaluation for infection and showed negative inflammatory marker results (C-reactive protein level and erythrocyte sedimentation rate) and negative aspiration findings prior to the procedure. Radiographs showed no evidence of acromial, scapular, or coracoid fracture. All patients had a functioning deltoid with no clinical evidence of a neurologic injury. Only patients in whom the procedure had been performed >1 year prior to the time of study initiation were considered for inclusion in the study.
Data collection
The following data were collected via a review of the electronic medical record: patient age, sex, body mass index, American Society of Anesthesiologists score, tobacco use, type of arthroplasty prior to conjoint tendon release, overall number of prior surgical procedures before conjoint tendon release, preoperative American Shoulder and Elbow Surgeons (ASES) score, and preoperative visual analog scale (VAS) score for pain.
A total of 12 patients were identified who met the inclusion criteria. The patients were contacted via telephone. Eleven of 12 patients were able to be contacted and had a minimum of 1-year follow-up. On contact, patients were asked whether they still had pain localized to the coracoid (yes or no) and were asked to complete the ASES score and VAS score for pain.
Surgical technique
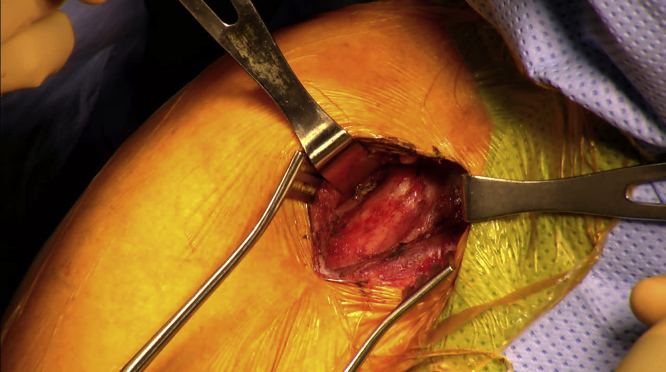
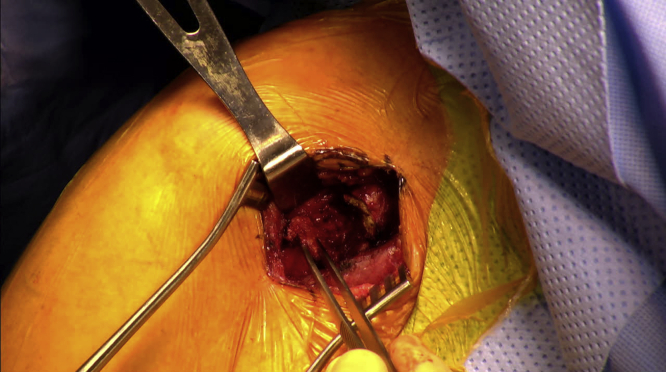
All procedures were performed with patients in the beach-chair position. A 4-cm incision was made in the superior segment of the existing deltopectoral incision (Fig. 1). The deltoid was retracted laterally, and the pectoralis was retracted medially. The conjoint tendon and coracoid tip were identified, and dissection was carried out posterior to the conjoint tendon at the level of the coracoid (Fig. 2). The short head of the biceps brachii and coracobrachialis tendons were then completely released from the coracoid tip using cautery. The released tendons were dissected distally several centimeters to ensure a tension-free release. After release, the tendons would typically retract 1-2 cm from the tip of the coracoid (Fig. 3).
Figure 1.
Proximal aspect of deltopectoral incision marked for conjoint tendon release.
Figure 2.
Exposed coracoid tip and conjoint tendon.
Figure 3.
Complete release of conjoint tendon being held with forceps and allowed to retract.
Statistical analysis
Statistical analyses were performed using Excel X (Microsoft, Redmond, WA, USA). Descriptive statistics were calculated, including demographic characteristics and functional outcomes (ASES and VAS pain scores). Comparisons between preoperative and postoperative measures were performed using the paired t test. Statistical significance was evaluated at the .05 level.
Results
We were able to contact 11 of 12 patients at a minimum of 1 year after conjoint tendon release, with a mean follow-up period (± standard deviation) of 27.3 ± 11.3 months (range, 14-46 months) postoperatively. Of the 11 patients, 82% (9 of 11) were women. Demographic characteristics, including tobacco use, age, body mass index, and American Society of Anesthesiologists score, are detailed in Table I. The conjoint tendon release was, on average, each patient's third procedure (standard deviation, ±1.5) on the affected shoulder, including non-arthroplasty procedures. Four of 11 releases were performed after a revision RTSA, whereas 7 of 11 RTSAs were primary arthroplasties. The senior author performed the RTSA (either primary or revision) in 82% of patients (9 of 11), and the conjoint tendon release procedure was performed at an average of 25.8 ± 23.8 months (range, 3-72 months) after the RTSA.
Table I.
Demographic
| Variable | n, Mean | SD (%) |
|---|---|---|
| Age, yr | 67.1 (10.5) | 10.5 |
| BMI | 30.4 (6.8) | 6.8 |
| Time from primary RTSA to conjoint tendon release, mo | 25.8 (23.8) | 23.8 |
| Female sex | 9 | 9 (81.8) |
| Tobacco use | ||
| Current | 0 (0) | |
| Former | 4 (36.4) | |
| Never smoker | 7 (63.6) | |
| ASA score | ||
| 1 | 2 (18.2) | |
| 2 | 3 (27.3) | |
| 3 | 5 (45.5) |
SD, standard deviation; BMI, body mass index; RTSA, reverse total shoulder arthroplasty; ASA, American Society of Anesthesiologists.
Discrete variables are presented as number (percentage), whereas continuous variables are presented as mean (SD).
Patient-reported outcomes
An improvement in the ASES score greater than the minimal clinically important difference (MCID) for patients after shoulder arthroplasty occurred in 5 of 11 patients (45%), and an improvement in the VAS pain score greater than the MCID for patients after shoulder arthroplasty was noted in 9 of 11 patients (82%).30 There were significant improvements from preoperatively to postoperatively in the ASES score (29.0 ± 22.1 vs. 58.2 ± 30.6, P = .02) and VAS score for pain (7.3 ± 2.0 vs. 3.1 ± 3.5, P = .003) (Table II). On the postoperative VAS, 55% of patients (6 of 11) reported a score of 0 or 1 for pain. Of the patients, 55% (6 of 11) reported complete resolution of their anterior shoulder pain whereas 45% (5 of 11) reported some residual anterior pain over the coracoid. No intraoperative or postoperative complications occurred, and no patients required further surgery on the shoulder.
Table II.
Preoperative and postoperative patient-reported outcomes for each patient with 1-year follow-up
| Preoperative |
Time to follow-up, mo | Postoperative |
Change |
||||
|---|---|---|---|---|---|---|---|
| ASES score | VAS pain score | ASES score | VAS pain score | ASES score | VAS pain score | ||
| Patient 1 | 3.3 | 10 | 46 | 18 | 8 | 14.7 | –2 |
| Patient 2 | 13.3 | 8 | 26 | 15 | 8 | 1.7 | 0 |
| Patient 3 | 85 | 2 | 17 | 96.7 | 0 | 11.7 | –2 |
| Patient 4 | 27 | 7 | 36 | 86 | 0 | 59 | –7 |
| Patient 5 | 48.3 | 8 | 24 | 93 | 0 | 44.7 | –8 |
| Patient 6 | 36.7 | 6 | 38 | 63.3 | 1 | 26.6 | –5 |
| Patient 7 | 18.3 | 8 | 28 | 36.7 | 5 | 18.4 | –3 |
| Patient 8 | 16.7 | 8 | 40 | 81.7 | 0 | 65 | –8 |
| Patient 9 | 26.7 | 7 | 17 | 35 | 6 | 8.3 | –1 |
| Patient 10 | 20 | 8 | 14 | 78.3 | 0 | 58.3 | –8 |
| Patient 11 | 23.3 | 8 | 14 | 36.7 | 6 | 13.4 | –2 |
| Mean ± SD | 29 ± 22.1 | 7.3 ± 2 | 27.3 ± 11.3 | 58.2 ± 30.6 | 3.1 ± 3.5 | 29.3 ± 23.1 | –4.2 ± 3.1 |
ASES, American Shoulder and Elbow Surgeons; VAS, visual analog scale; SD, standard deviation.
Discussion
A substantial amount of literature exists regarding complications of RTSA; however, there is limited information on the treatment of persistent postoperative anterior shoulder pain after RTSA without another underlying diagnosis. As RTSA with a Grammont-style prosthesis involves distalization of the humerus, it tensions the conjoint tendon and, in our experience, can contribute to residual anterior shoulder pain. In this cohort of patients, isolated release of the entire conjoint tendon without repair led to a significant reduction in pain and improved function. This procedure was rapid, did not have any functional consequence for the shoulder, and did not result in complications or reoperations.
Pain following RTSA is common. After RTSA, the VAS score for pain at minimum 2-year follow-up was 1.8 ± 2.2 and the average ASES score was 72.7 ± 20.8.30 Anterior shoulder pain due to conjoint tendinitis has been previously described but never in the setting of RTSA.14 Within our study, conjoint tendon release significantly improved pain and function in patients with anterior shoulder pain after RTSA. However, whereas conjoint release can significantly improve pain and function, patients on average still appear to have slightly inferior outcomes to those of an uncomplicated primary RTSA, although standard deviations overlap for both ASES scores and VAS pain scores, with the current data supporting no statistically significant differences.30
Other potential techniques for the treatment of persistent anterior conjoint tendon–based pain may be considered, including lengthening of the tendon in a step-cut fashion or in situ release using the “pie-crust” technique.10,11 Both of these methods may better preserve the function of the short head of the biceps brachii and coracobrachialis compared with complete release, although pain may continue with these techniques because of some continuity of the tendon. Complete release did not appear to result in an increased risk of complications or reoperations in the current cohort of patients. Neither the pie-crust technique nor the lengthening procedure has been performed by the senior author.
This study has limitations. It was a retrospective study with only 1-year minimum follow-up. Because the release procedure only involves the soft tissue, we believe that 1 year is enough time for patients to experience improvement and that the extent of improvement would be sustainable at that time point. Thus, no predefined algorithm was used to guide treatment. Our sample size was small, as this procedure is uncommon. Consequently, both primary and revision procedures were included, and because of the rarity of the operation, any exclusion based on indication would have severely restricted the sample size. Our study presents results from short- to mid-term follow-up. We identified that only 45% of patients showed improvement in the ASES score greater than the MCID but >80% showed improvement in the VAS score greater than the MCID. Therefore, while not perfect, conjoint tendon release does give an option for patients to achieve significant pain improvement with low morbidity. Finally, we did not include any specific measure of elbow or shoulder flexion strength to document whether this procedure resulted in measurable weakness. With longer follow-up, patient-reported function and pain may change.
Conclusion
Our results suggest that conjoint tendinitis may be a cause of persistent postoperative anterior shoulder pain after RTSA and open conjoint tendon release can be successful at reducing pain and improving functional outcomes.
Disclaimer
Robert Z. Tashjian is a paid consultant for Zimmer/Biomet, Wright Medical, and Mitek; has stock in Conextions, INTRAFUSE, Genesis, and KATOR; receives intellectual property royalties from Wright Medical, Shoulder Innovations, and Zimmer/Biomet; receives publishing royalties from the Journal of Bone and Joint Surgery and Springer; and serves on the editorial board of the Journal of American Association of Orthopaedic Surgeons and Shoulder & Elbow.
Peter N. Chalmers is a paid consultant for Mitek and Arthrex; serves on the editorial board of the Journal of Shoulder and Elbow Surgery; is a paid speaker for DePuy; and receives intellectual property royalties from DePuy.
The other authors, their immediate families, and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Footnotes
The work for this manuscript was performed at the University of Utah, Salt Lake City, UT, USA.
This study was approved by the authors' institutional review board under protocol no. 00046622.
References
- 1.Ball C.M. Neurologic complications of shoulder joint replacement. J Shoulder Elbow Surg. 2017;26:2125–2132. doi: 10.1016/j.jse.2017.04.016. [DOI] [PubMed] [Google Scholar]
- 2.Barco R., Savvidou O.D., Sperling J.W., Sanchez-Sotelo J., Cofield R.H. Complications in reverse shoulder arthroplasty. EFORT Open Rev. 2017;1:72–80. doi: 10.1302/2058-5241.1.160003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barlow J.D., Jamgochian G., Wells Z., Bateman D.K., Schmerfeld A.A., Abboud J.A. Reverse shoulder arthroplasty is superior to hemiarthroplasty for cuff tear arthropathy with preserved motion. Arch Bone Jt Surg. 2020;8:75–82. doi: 10.22038/abjs.2019.38427.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berliner J.L., Regalado-Magdos A., Ma C.B., Feeley B.T. Biomechanics of reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24:150–160. doi: 10.1016/j.jse.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Boileau P., Gonzalez J.F., Chuinard C., Bicknell R., Walch G. Reverse total shoulder arthroplasty after failed rotator cuff surgery. J Shoulder Elbow Surg. 2009;18:600–606. doi: 10.1016/j.jse.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Boileau P., Watkinson D., Hatzidakis A.M., Hovorka I. Neer Award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006;15:527–540. doi: 10.1016/j.jse.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Bufquin T., Hersan A., Hubert L., Massin P. Reverse shoulder arthroplasty for the treatment of three- and four-part fractures of the proximal humerus in the elderly: a prospective review of 43 cases with a short-term follow-up. J Bone Joint Surg Br. 2007;89:516–520. doi: 10.1302/0301-620X.89B4.18435. [DOI] [PubMed] [Google Scholar]
- 8.Chalmers PN, Boileau P, Romeo AA, Tashjian RZ. Revision reverse shoulder arthroplasty. J Am Acad Orthop Surg 2019;27:426–36. 10.5435/JAAOS-D-17-00535. [DOI] [PubMed]
- 9.Chalmers PN, Salazar DH, Romeo AA, Keener JD, Yamaguchi K, Chamberlain AM. Comparative utilization of reverse and anatomic total shoulder arthroplasty: a comprehensive analysis of a high-volume center. J Am Acad Orthop Surg 2018;26:e504–10. 10.5435/JAAOS-D-17-00075. [DOI] [PubMed]
- 10.Claret-Garcia G., Montañana-Burillo J., Tornero-Dacasa E., Llusá-Pérez M., Popescu D., Combalia-Aleu A. Pie crust technique of the deep medial collateral ligament in knee arthroscopy: ultrasound and anatomic study. J Knee Surg. 2019;32:764–769. doi: 10.1055/s-0038-1668125. [DOI] [PubMed] [Google Scholar]
- 11.Clarke H.D., Scuderi G.R. Correction of valgus deformity in total knee arthroplasty with the pie-crust technique of lateral soft-tissue releases. J Knee Surg. 2004;17:157–161. doi: 10.1055/s-0030-1248215. [DOI] [PubMed] [Google Scholar]
- 12.Friedman R.J., Barcel D.A., Eichinger J.K. Scapular notching in reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2019;27:200–209. doi: 10.5435/JAAOS-D-17-00026. [DOI] [PubMed] [Google Scholar]
- 13.Henninger H.B., King F.K., Tashjian R.Z., Burks R.T. Biomechanical comparison of reverse total shoulder arthroplasty systems in soft tissue-constrained shoulders. J Shoulder Elbow Surg. 2014;23:e108–e117. doi: 10.1016/j.jse.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Karim M.R., Fann A.V., Gray R.P., Neale D.F., Escarda J.D. Enthesitis of biceps brachii short head and coracobrachialis at the coracoid process: a generator of shoulder and neck pain. Am J Phys Med Rehabil. 2005;84:376–380. doi: 10.1097/01.phm.0000159973.97391.c9. [DOI] [PubMed] [Google Scholar]
- 15.Lädermann A., Edwards T.B., Walch G. Arm lengthening after reverse shoulder arthroplasty: a review. Int Orthop. 2014;38:991–1000. doi: 10.1007/s00264-013-2175-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lädermann A., Lübbeke A., Mélis B., Stern R., Christofilopoulos P., Bacle G. Prevalence of neurologic lesions after total shoulder arthroplasty. J Bone Joint Surg Am. 2011;93:1288–1293. doi: 10.2106/JBJS.J.00369. [DOI] [PubMed] [Google Scholar]
- 17.Lädermann A., Walch G., Lubbeke A., Drake G.N., Melis B., Bacle G. Influence of arm lengthening in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21:336–341. doi: 10.1016/j.jse.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 18.Lädermann A., Williams M.D., Melis B., Hoffmeyer P., Walch G. Objective evaluation of lengthening in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18:588–595. doi: 10.1016/j.jse.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Levy J., Frankle M., Mighell M., Pupello D. The use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty for proximal humeral fracture. J Bone Joint Surg Am. 2007;89:292–300. doi: 10.2106/JBJS.E.01310. [DOI] [PubMed] [Google Scholar]
- 20.Longo U.G., Petrillo S., Berton A., Denaro V. Reverse total shoulder arthroplasty for the management of fractures of the proximal humerus: a systematic review. Musculoskelet Surg. 2016;100:83–91. doi: 10.1007/s12306-016-0409-0. [DOI] [PubMed] [Google Scholar]
- 21.Lowe J.T., Lawler S.M., Testa E.J., Jawa A. Lateralization of the glenosphere in reverse shoulder arthroplasty decreases arm lengthening and demonstrates comparable risk of nerve injury compared with anatomic arthroplasty: a prospective cohort study. J Shoulder Elbow Surg. 2018;27:1845–1851. doi: 10.1016/j.jse.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 22.Mangold D.R., Wagner E.R., Cofield R.H., Sanchez-Sotelo J., Sperling J.W. Reverse shoulder arthroplasty for rheumatoid arthritis since the introduction of disease-modifying drugs. Int Orthop. 2019;43:2593–2600. doi: 10.1007/s00264-019-04373-3. [DOI] [PubMed] [Google Scholar]
- 23.Monir J.G., Abeyewardene D., King J.J., Wright T.W., Schoch B.S. Reverse shoulder arthroplasty in patients younger than 65 years, minimum 5-year follow-up. J Shoulder Elbow Surg. 2020;29:E215–E221. doi: 10.1016/j.jse.2019.10.028. [DOI] [PubMed] [Google Scholar]
- 24.Neyton L., Erickson J., Ascione F., Bugelli G., Lunini E., Walch G. Grammont Award 2018: scapular fractures in reverse shoulder arthroplasty (Grammont style): prevalence, functional, and radiographic results with minimum 5-year follow-up. J Shoulder Elbow Surg. 2019;28:260–267. doi: 10.1016/j.jse.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Patterson D.C., Chi D., Parsons B.O., Cagle P.J., Jr. Acromial spine fracture after reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2019;28:792–801. doi: 10.1016/j.jse.2018.08.033. [DOI] [PubMed] [Google Scholar]
- 26.Rittmeister M., Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10:17–22. doi: 10.1067/mse.2001.110515. [DOI] [PubMed] [Google Scholar]
- 27.Rugg C.M., Coughlan M.J., Lansdown D.A. Reverse total shoulder arthroplasty: biomechanics and indications. Curr Rev Musculoskelet Med. 2019;12:542–553. doi: 10.1007/s12178-019-09586-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sebastiá-Forcada E., Cebrián-Gómez R., Lizaur-Utrilla A., Gil-Guillén V. Reverse shoulder arthroplasty versus hemiarthroplasty for acute proximal humeral fractures. A blinded, randomized, controlled, prospective study. J Shoulder Elbow Surg. 2014;23:1419–1426. doi: 10.1016/j.jse.2014.06.035. [DOI] [PubMed] [Google Scholar]
- 29.Tashjian RZ, Burks RT, Zhang Y, Henninger HB. Reverse total shoulder arthroplasty: a biomechanical evaluation of humeral and glenosphere hardware configuration. J Shoulder Elbow Surg 2015;24:e68–77. 10.1016/j.jse.2014.08.017. [DOI] [PubMed]
- 30.Tashjian RZ, Hung M, Keener JD, Bowen RC, McAllister J, Chen W, et al. Determining the minimal clinically important difference for the American Shoulder and Elbow Surgeons score, Simple Shoulder Test, and visual analog scale (VAS) measuring pain after shoulder arthroplasty. J Shoulder Elbow Surg 2017;26:144–8. 10.1016/j.jse.2016.06.007. [DOI] [PubMed]
- 31.Walker M., Brooks J., Willis M., Frankle M. How reverse shoulder arthroplasty works. Clin Orthop Relat Res. 2011;469:2440–2451. doi: 10.1007/s11999-011-1892-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Werner C.M., Steinmann P.A., Gilbart M., Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87:1476–1486. doi: 10.2106/JBJS.D.02342. [DOI] [PubMed] [Google Scholar]
- 33.Zavala J.A., Clark J.C., Kissenberth M.J., Tolan S.J., Hawkins R.J. Management of deep infection after reverse total shoulder arthroplasty: a case series. J Shoulder Elbow Surg. 2012;21:1310–1315. doi: 10.1016/j.jse.2011.08.047. [DOI] [PubMed] [Google Scholar]
- 34.Zmistowski B., Gutman M., Horvath Y., Abboud J.A., Williams G.R., Jr., Namdari S. Acromial stress fracture following reverse total shoulder arthroplasty: incidence and predictors. J Shoulder Elbow Surg. 2020;29:799–806. doi: 10.1016/j.jse.2019.08.004. [DOI] [PubMed] [Google Scholar]