Abstract
Introduction
Proper anatomic tuberosity reduction and restoration of humeral height during surgical treatment of proximal humerus fractures leads to fewer complications and better outcomes. In the presence of significant displacement and comminution in proximal humerus fractures, the assessment of the correct tuberosity position and humeral height can be challenging. The goal of this cadaveric study was to provide new and useful measurements for intraoperative guidance of proper tuberosity position and humeral height when treating proximal humerus fractures with open reduction internal fixation, anatomic hemiarthroplasty, or reverse total shoulder arthroplasty.
Methods
A total of 28 cadaveric shoulders were dissected with a deltopectoral approach. The distance between the insertion of the supraspinatus tendon and the superior aspect of the deltoid tendon was measured (cuff to deltoid distance [CDD]). Secondly, the distance between the superior aspects of the pectoralis major tendon to the medial aspect of the anatomic neck (PND) was measured. Further, we sought to determine if these measurements would correlate to patient height and differ between gender.
Results
The average age of the donors was 65.3 years (64% male). The CDD and PND were 87.6 ± 10.6 and 16.6 ± 6.9 mm, respectively (mean ± standard deviation). There were no differences between females and males for the CDD (86.9 ± 9.4 vs. 87.2 ± 15.2 mm, P = .96) and PND (16.3 ± 9.1 vs. 17.1 ± 5.9 mm, P = .76). There was no correlation between the cadaver height and CDD (R2 = 0.1) and PND (R2 = 0.3).
Discussion
In this study, we describe 2 new measurement tools that can readily be applied intraoperatively during surgical treatment of proximal humerus fractures to aid in tuberosity reduction and humeral height assessment. These measurements were found to be independent of patient height and gender and can be used as a reference tool for most patients.
Keywords: Proximal humerus fractures, open reduction internal fixation, hemiarthroplasty, reverse shoulder arthroplasty, pectoralis major, anatomic neck, deltoid, rotator cuff insertion
Although surgical treatment of severe proximal humerus fractures can be treated with a variety of techniques that include open reduction with internal fixation (ORIF), hemiarthroplasty (HA), and reverse total shoulder arthroplasty (RSA), the literature supports that anatomic reduction and healing of the tuberosities can improve patient outcomes.9,10,14,21,23
In surgical treatment of proximal humerus factures with ORIF, anatomic reduction of the facture fragments can be difficult in the setting of displacement and comminution. The malunion of the tuberosities can occur from poor initial reduction, secondary fracture displacement from poor fixation, and/or poor protection/stability leading to secondary displacement.9 For proximal humerus malunions, anatomic shoulder arthroplasty has been shown to have improved patient outcomes, but can be complex when performing a corrective tuberosity osteotomy and has complications related to the risk of glenohumeral instability.14
The difficulty of tuberosity reduction and humeral component height is also a challenge when using HA as the primary surgical treatment option in complex proximal humerus fractures. Prior studies have shown poor results with HA when the tuberosities are not satisfactorily restored.4 To help guide humeral component placements in HA, anatomical studies have characterized the pectoralis major tendon and its relationship to the top of the humeral head as one tool for measuring humeral height during HA for proximal humerus fractures.20
Reverse shoulder arthroplasty has grown as a popular treatment option for elderly patients with proximal humerus fractures.19 The nonanatomic configuration of the RSA joint relies primarily on the deltoid muscle for motion,3 and the importance of anatomic tuberosity reduction and healing was initially underappreciated. However, as the literature for RSA has greatly expanded as a primary treatment option for proximal humerus fractures, it has been shown that anatomic fixation and healing of the greater tuberosity leads to better functional improvement.11,15,17,21 Although the reverse shoulder arthroplasty has been recently shown to lead to more predictable outcomes than HA,12,24 it still retains a higher risk of complication compared with a nonfracture setting, such as elective treatment of rotator cuff arthropathy.18
In both HA and RSA, humeral stem vertical positioning (height) is essential to allowing for anatomic restoration of the tuberosities because the tuberosity is attached to the implant. In addition, correct tuberosity positioning provides adequate soft tissue tension and prevents the complication of instability when using either HA or RSA for fracture treatment. Unfortunately, in the setting of proximal humerus fracture bone loss and comminution, it may be difficult to visually identify all the corresponding fracture fragments to achieve an anatomic reduction of the tuberosity. In such settings, usage and knowledge of important anatomic landmarks are helpful.
Our cadaveric study set out to provide a consistent and reliable measurement that surgeons could use intraoperatively to assist in determining proper anatomic reduction of tuberosity fractures and achievement of native humeral height in the setting of operative fixation and/or arthroplasty. We hypothesized that the distance between the superior aspect of the deltoid insertion and the anterior portion of the supraspinatus footprint (superior edge of the greater tuberosity)—the cuff to deltoid distance (CDD)—can be a reliable marker for re-establishing the native anatomic relationship between tuberosity and humeral shaft. Further, we hypothesized that a similar relationship can be identified between the superior aspect of the pectoralis major tendon and the medial aspect of the humeral anatomic neck (PND) to appreciate the native humeral height. In the setting of severe proximal humerus fractures, knowledge of this anatomic relationship can be used by surgeons to achieve proper humeral height positioning, anatomic reduction of tuberosities, and native tensioning balance of the deltoid and rotator cuff muscles.
Materials and methods
We dissected 28 adult shoulders in 20 fresh cadavers. Of the cadavers, 18 were male and 9 were female, with 1 unknown gender. The mean age of morality was 65.3 years. The cadaver height was 169.1 cm inches and the weight was 79.6 kg on average. Causes of mortality included chronic obstructive pulmonary disease (2), cancer (11), dementia (2), cerebrovascular disease (2), cardiovascular disease (4), and infection (2), with 4 deaths from unknown causes. None of the cadavers had prior shoulder surgery that would affect the measurements in this study (Table I).
Table 1.
Demographics of cadaveric specimens: gender, laterality, age, CDD and PND measurements, height, and COD
| Gender | R/L | Age (yr) | CDD (mm) | PND (mm) | Height (inches) | COD | |
|---|---|---|---|---|---|---|---|
| 1 | F | L | 84 | 93.00 | 23.72 | 62 | COPD |
| 2 | F | R | 48 | 89.95 | 8.35 | 59 | Cancer |
| 3 | M | R | 76 | 80.91 | 30.13 | 66 | COPD |
| 4 | M | R | 56 | 72.26 | 9.83 | 68 | Cancer |
| 5 | M | L | 56 | 73.57 | 10.81 | 68 | Cancer |
| 6 | M | R | 49 | 85.13 | 24.53 | 75 | Cancer |
| 7 | M | L | 49 | 91.12 | 25.03 | 75 | Cancer |
| 8 | F | R | 83 | 86.82 | 15.54 | 62 | Cancer |
| 9 | F | NA | 58 | 71.89 | 9.26 | NA | NA |
| 10 | F | L | NA | 78.23 | 11.26 | NA | NA |
| 11 | F | R | 80 | 101.06 | 9.54 | 61 | Dementia |
| 12 | F | L | 80 | 85.49 | 9.28 | 61 | Dementia |
| 13 | M | L | 88 | 74.88 | 19.24 | 62 | CVD |
| 14 | M | R | 88 | 83.37 | 17.63 | 65 | CVD |
| 15 | M | R | 44 | 83.42 | 11.86 | 69 | Infection |
| 16 | M | L | 44 | 83.77 | 19.85 | 69 | Infection |
| 19 | F | R | 71 | 96.78 | 30.81 | 68 | Cancer |
| 20 | NA | NA | NA | 99.96 | 10.11 | NA | NA |
| 21 | M | L | 62 | 81.28 | 12.75 | 63 | CVD |
| 22 | M | R | 62 | 90.97 | 18.51 | 63 | CVD |
| 23 | M | L | 57 | 88.41 | 19.14 | 69 | Stroke |
| 24 | M | R | 57 | 98.19 | 9.23 | 69 | Stroke |
| 26 | M | R | 60 | 77.65 | 13.46 | 69 | Cancer |
| 27 | M | L | 65 | 82.88 | 22.95 | 70 | Cancer |
| 28 | M | R | 63 | 109.99 | 16.48 | 70 | Cancer |
| 29 | M | L | 63 | 102.71 | 11.13 | 70 | Cancer |
| 30 | M | R | 98 | 109.21 | 14.39 | 68 | COPD |
| 31 | F | L | 57 | 79.31 | 28.86 | 64 | NA |
F, female; M, male; L, left; R, right; CDD, cuff to deltoid distance; PND, pectoralis tendon to medial anatomic neck distance; COD, cause of death; COPD, chronic obstructive pulmonary disease; NA, not available; CVD, cardiovascular disease.
The shoulder specimens were secured in an upright position to simulate a beach chair position. Dissection was performed using a standard deltopectoral incision. The pectoralis major tendon and deltoid insertion were identified and preserved. The clavipectoral fascia was divided, and the subscapularis tendon was exposed. A biceps tenotomy was performed along with a subscapularis peel to expose the glenohumeral joint. The subsequent anatomic measurements were made by 2 board certified orthopedic surgeons with advanced training in shoulder arthroplasty.
Cuff to deltoid distance measurements
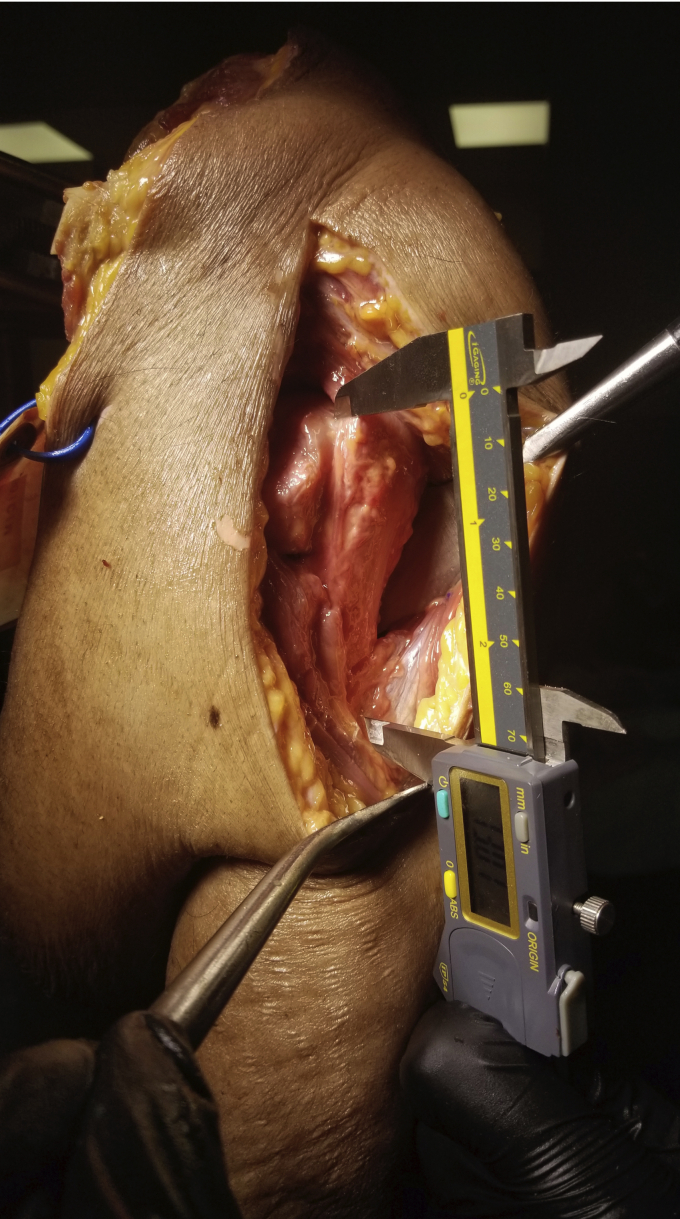
The anterior aspect of the supraspinatus footprint was marked using a surgical marker. The highest point at which the deltoid tendon inserts in neutral rotation was identified and marked. The distance between these 2 points was designated the CDD. This distance was measured with a digital caliper while the shoulder was in neutral rotation (Fig. 1).
Figure 1.
Clinical depiction of the cuff to deltoid distance measurement. This measurement is from the anterior aspect of the supraspinatus footprint to the highest point of the deltoid tendon insertion at neutral rotation.
Pectoralis tendon to medial anatomic neck measurements
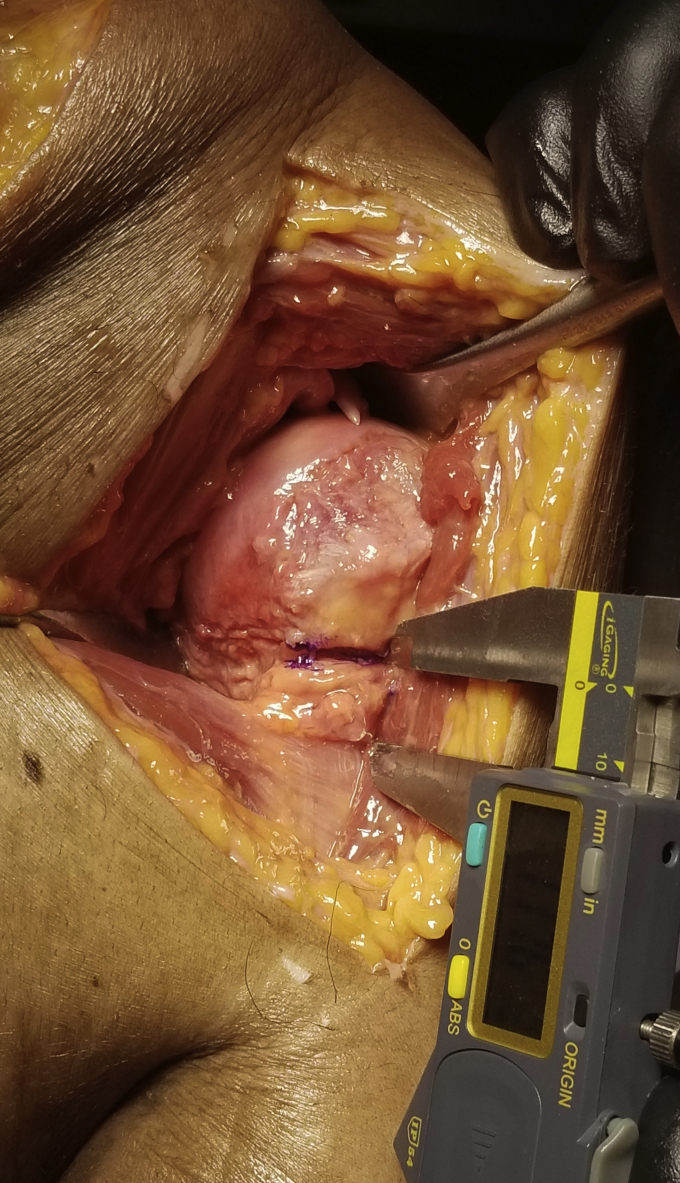
With the shoulder in neutral position, the superior aspect of the pectoralis tendon was identified. The medial aspect of the anatomic neck at the margin of the articular surface (representing the medial aspect of a standard humeral head cut) was then identified. From this anatomic neck position, a horizontal line is drawn in a lateral direction that is parallel to the pectoralis tendon. The linear distance between both sites was measured using a digital caliper. This was designated the PND distance (Fig. 2).
Figure 2.
Measurement of the distance between the superior edge of the pectoralis tendon and the medial edge of the anatomic neck. A straight line is drawn from the medial border of the anatomic neck. The distance between this line and the pectoralis tendon is measured.
Statistical analysis
Statistical analysis was performed and the Anderson-Darling normality test was used to confirm that the distances measured in our sample had a normal distribution. The Student t-test was used to establish if there was any significant difference between both shoulders and gender groups. Significance was set at P < .05. Pearson correlation was used to determine whether there was a linear correlation between the height of the patients and the measured distances.
Results
Cuff to deltoid distance
There were a total of 28 cadaveric specimens for analysis with 20 specimens being matched pairs from 10 donors. The mean distance from the rotator cuff insertion to the deltoid insertion (CDD) was 87.6 ± 10.6 mm. There was a low amount of correlation between the CDD and the cadaver height in both matched and unmatched analysis (R2 = 0.10, P = .63). Between gender groups, there was no significant difference between females vs. males (86.9 ± 9.4 vs. 87.2 ± 15.2 mm, P = .96).
Pectoralis tendon to medical anatomic neck distance
The mean distance from the pectoralis tendon to the medial border of the anatomic neck (PND) was 16.6 ± 6.9 mm. Again, there was little correlation between this distance and the specimen height (R2 = 0.26, P = .21). Between gender groups, there was no significant difference between females vs. males (16.3 ± 9.1 vs. 17.1 ± 5.9 mm, P = .76).
Discussion
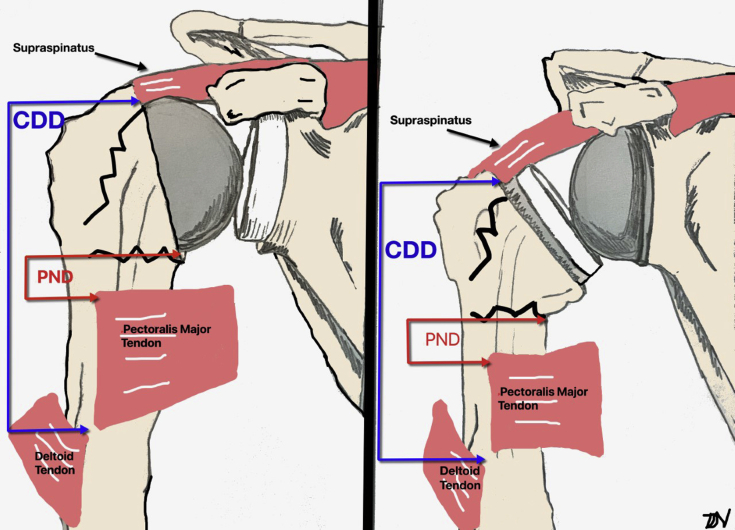
This cadaveric study describes 2 unique measurements that may be used in the setting of surgical treatment of proximal humeral fractures. We found that the CDD and PND were consistent measures that are not influenced by patient height or gender. This relative relationship between the anatomical sites can be clinically applied when performing ORIF, HA, or RSA for proximal humerus fractures (Fig. 3). By appreciating the anatomical relationships of the CDD and PND distance in complex proximal humerus fractures, a surgeon can potentially use this information as a reference to restore these relationships and subsequently achieve an appropriate tuberosity reduction and restoration of humeral height.
Figure 3.
Diagram of the cuff to deltoid distance (CDD) and pectoralis tendon to medial anatomic neck (PND) being used in the setting of hemiarthroplasty and reverse total shoulder arthroplasty.
Anatomic restoration of humeral height and the greater tuberosity is important in ORIF as inadequate reduction can lead to a symptomatic malunion.9 These malunions have been classified by Boileau et al5 and Beredjiklian et al2 to help guide potential treatment options. Malunion with upward malalignment of the greater tuberosity can lead to reduced abduction amplitude as it abuts the acromion, whereas posterior displacement of the greater tuberosity can lead to a loss of tension in the rotator cuff.9 The CDD and PND distances can be used as an additional tool when reducing tuberosity fracture fragments when there is significant comminution that affects visualization of fracture reduction in ORIF.
In the elderly population with proximal humerus fractures, the use of an arthroplasty option has grown with RSA surpassing HA between 2009 and 2016.8 This is likely from improvement in improved range of motion, clinical outcome scores, and rates of all-cause reoperation with RSA.1,22 Correspondingly, studies have shown RSA to have higher predictability and lower rate of complications as compared with HA and ORIF.16 HA outcomes are dependent on achieving anatomic reduction of the tuberosity fragments in order to recreate the native humeral offset and restore the native tension of the deltoid and rotator cuff muscles.10,23 Patients with a poor outcome after HA or open reduction internal fixation have also been shown to have improvements in pain relief and patient-reported outcomes when undergoing a conversion to an RSA.7,13,25 Despite this fact, tuberosity reduction and healing is still important and tuberosity malposition/malunion/nonunion in the setting of RSA leads to worse functional outcomes.16 To the best of our knowledge, this is the first study to identify landmarks and measurements (CDD and PND) that can guide intraoperative restoration of humeral height and anatomic reduction of tuberosities in the RSA setting.
Implant height and version are critically important for outcomes of both HA and RSA. The ability to restore ideal implant height during RSA can be challenging in the setting of complex proximal humerus fractures. Implant broaches can be used to estimate stem retroversion based on the epicondylar axis. However, ideal stem height is more difficult to determine. This same challenge is noted in the setting of HA for fracture. In an attempt to provide some guidance during HA for fracture, Murachovsky et al20 published a study evaluating the distance from the top of the humeral head to the top of the pectoralis tendon. Using 40 cadavers, they determined that the average distance from the top of the humeral head to the superior border of the pectoralis tendon was 5.6 cm.20 Unfortunately, this measurement does not assist in the setting of RSA for fracture as the relationship of the humerus and glenoid is altered in an RSA style implant system. With the increasing popularity of RSA use for proximal humerus fractures, similar references such as our described CDD and PND are needed.
Common complications after RSA include instability, scapular notching, infection, glenoid loosening, and others. The rate of clinical and radiographic complication has been reported between 10% and 67%.6,16 In the setting of fracture, the incidence of complication has been reported to be higher. Klug et al16 noted a complication rate of 22% in 51 patients who underwent RSA for fracture, with instability being the most common complication. To reduce the risk of instability, understanding the optimal positioning of the RSA prosthesis with more precise anatomic tuberosity placement may improve the balance soft tissue tensioning and achieve better outcomes and lower rates of instability. In an example where this is not achieved, an implant that is placed to low will likely result in poor soft tissue tension and nonanatomic tuberosity position. To adjust for this, one may have to use an excessively large polyethylene implant to make up this distance and achieve some form of soft tissue tension that is unbalanced and less predictable between the deltoid, supraspinatus, infraspinatus, and subscapularis. This unbalanced form of tensioning may be responsible for the higher risk of instability when using RSA in the setting of proximal humerus fractures. In contrast, placing the stem too high may lead to inability to reduce the components, excessive implant construct tightness putting the patient at risk for stress fractures, and excessive tensioning of the tuberosity fragment that may lead to secondary displacement.
This study is not without limitations. As with all cadaveric studies, the conditions may not perfectly simulate in vivo conditions. Surgical conditions and severe soft tissue injury and bone comminution may restrict the ability to make such measurements. The PND distance has also noted to have large amount of variability in its standard deviation in comparison to its mean value that may limit its application in clinical care. Although these measurements provide useful guides during shoulder arthroplasty for fracture, specific implant features may dictate alternative methods. In addition, other tools for estimation should be used in conjunction to verify position. Such tools include contralateral humeral measurements, intraoperative assessment of tensioning and tuberosity placement, intraoperative use of fluoroscopy, contact and positioning between the tuberosity and humeral shaft. The CDD and PND could be potentially measured on the contralateral shoulder for a greater degree of patient-specific precision. These contralateral measurements on computed tomography or magnetic resonance imaging would be subject to the imaging modalities’ inherent side effects and magnificent errors (radiation for computed tomography and magnification errors for magnetic resonance imaging) but would be an area of interest for future study.
In conclusion, we were able to determine a unique set of measurements between anatomic landmarks that are predictable and easy to identify at the time of surgery. We believe that using the CDD and PND can serve as an intraoperative guide in the setting of significant displacement and comminution of proximal humerus fracture fragments to help achieve improved anatomic reduction and/or placement of HA/RSA components. These measurements were found to be independent of patient height and gender and therefore may be applicable to the generalized population.
Conclusion
In this study, we describe the CDD and PND as 2 new measurement tools that can readily be applied intraoperatively during ORIF, HA, or RSA for management of proximal humerus fractures.
Disclaimer
The authors, their immediate families, and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Footnotes
Institutional review board approval was not required for this cadaver study.
References
- 1.Austin D.C., Torchia M.T., Cozzolino N.H., Jacobowitz L.E., Bell J.E. Decreased reoperations and improved outcomes with reverse total shoulder arthroplasty in comparison to hemiarthroplasty for geriatric proximal humerus fractures: a systematic review and meta-analysis. J Orthop Trauma. 2019;33:49–57. doi: 10.1097/BOT.0000000000001321. [DOI] [PubMed] [Google Scholar]
- 2.Beredjiklian P.K., Iannotti J.P., Norris T.R., Williams G.R. Operative treatment of malunion of a fracture of the proximal aspect of the humerus. J Bone Joint Surg Am. 1998;80:1484–1497. doi: 10.2106/00004623-199810000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Berliner J.L., Regalado-Magdos A., Ma C.B., Feeley B.T. Biomechanics of reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24:150–160. doi: 10.1016/j.jse.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Boileau P., Krishnan S.G., Tinsi L., Walch G., Coste J.S., Mole D. Tuberosity malposition and migration: reasons for poor outcomes after hemiarthroplasty for displaced fractures of the proximal humerus. J Shoulder Elbow Surg. 2002;11:401–412. doi: 10.1067/mse.2002.124527. [DOI] [PubMed] [Google Scholar]
- 5.Boileau P., Trojani C., Walch G., Krishnan S.G., Romeo A., Sinnerton R. Shoulder arthroplasty for the treatment of the sequelae of fractures of the proximal humerus. J Shoulder Elbow Surg. 2001;10:299–308. doi: 10.1067/mse.2001.115985. [DOI] [PubMed] [Google Scholar]
- 6.Cheung E., Willis M., Walker M., Clark R., Frankle M.A. Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2011;19:439–449. [PubMed] [Google Scholar]
- 7.Dezfuli B., King J.J., Farmer K.W., Struk A.M., Wright T.W. Outcomes of reverse total shoulder arthroplasty as primary versus revision procedure for proximal humerus fractures. J Shoulder Elbow Surg. 2016;25:1133–1137. doi: 10.1016/j.jse.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Dillon M.T., Prentice H.A., Burfeind W.E., Chan P.H., Navarro R.A. The increasing role of reverse total shoulder arthroplasty in the treatment of proximal humerus fractures. Injury. 2019;50:676–680. doi: 10.1016/j.injury.2019.01.034. [DOI] [PubMed] [Google Scholar]
- 9.Duparc F. Malunion of the proximal humerus. Orthop Traumatol Surg Res. 2013;99(Suppl.):S1–S11. doi: 10.1016/j.otsr.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Frankle M.A., Mighell M.A. Techniques and principles of tuberosity fixation for proximal humeral fractures treated with hemiarthroplasty. J Shoulder Elbow Surg. 2004;13:239–247. doi: 10.1016/s1058-2746(02)00041-1. [DOI] [PubMed] [Google Scholar]
- 11.Gallinet D., Cazeneuve J.F., Boyer E., Menu G., Obert L., Ohl X. Reverse shoulder arthroplasty for recent proximal humerus fractures: outcomes in 422 cases. Orthop Traumatol Surg Res. 2019;105:805–811. doi: 10.1016/j.otsr.2019.03.019. [DOI] [PubMed] [Google Scholar]
- 12.Gallinet D., Ohl X., Decroocq L., Dib C., Valenti P., Boileau P. Is reverse total shoulder arthroplasty more effective than hemiarthroplasty for treating displaced proximal humerus fractures in older adults? A systematic review and meta-analysis. Orthop Traumatol Surg Res. 2018;104:759–766. doi: 10.1016/j.otsr.2018.04.025. [DOI] [PubMed] [Google Scholar]
- 13.Holschen M., Siemes M.K., Witt K.A., Steinbeck J. Five-year outcome after conversion of a hemiarthroplasty when used for the treatment of a proximal humeral fracture to a reverse total shoulder arthroplasty. Bone Joint J. 2018;100-B:761–766. doi: 10.1302/0301-620X.100B6.BJJ-2017-1280.R1. [DOI] [PubMed] [Google Scholar]
- 14.Jacobson J.A., Duquin T.R., Sanchez-Sotelo J., Schleck C.D., Sperling J.W., Cofield R.H. Anatomic shoulder arthroplasty for treatment of proximal humerus malunions. J Shoulder Elbow Surg. 2014;23:1232–1239. doi: 10.1016/j.jse.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 15.Jain N.P., Mannan S.S., Dharmarajan R., Rangan A. Tuberosity healing after reverse shoulder arthroplasty for complex proximal humeral fractures in elderly patients-does it improve outcomes? A systematic review and meta-analysis. J Shoulder Elbow Surg. 2019;28:e78–e91. doi: 10.1016/j.jse.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Klug A., Wincheringer D., Harth J., Schmidt-Horlohe K., Hoffmann R., Gramlich Y. Complications after surgical treatment of proximal humerus fractures in the elderly—an analysis of complication patterns and risk factors for reverse shoulder arthroplasty and angular-stable plating. J Shoulder Elbow Surg. 2019;28:1674–1684. doi: 10.1016/j.jse.2019.02.017. [DOI] [PubMed] [Google Scholar]
- 17.Luciani P., Farinelli L., Procaccini R., Verducci C., Gigante A. Primary reverse shoulder arthroplasty for acute proximal humerus fractures: a 5-year long term retrospective study of elderly patients. Injury. 2019;50:1974–1977. doi: 10.1016/j.injury.2019.09.019. [DOI] [PubMed] [Google Scholar]
- 18.Malik A.T., Bishop J.Y., Neviaser A.S., Beals C.T., Jain N., Khan S.N. Shoulder arthroplasty for a fracture is not the same as shoulder arthroplasty for osteoarthritis: implications for a bundled payment model. J Am Acad Orthop Surg. 2019;27:927–932. doi: 10.5435/JAAOS-D-18-00268. [DOI] [PubMed] [Google Scholar]
- 19.Mclean A., Price N., Graves S., Hatton A., Taylor F.J. Nationwide trends in management of proximal humeral fractures: an analysis of 77,966 cases from 2008 to 2017. J Shoulder Elbow Surg. 2019;28:2072–2078. doi: 10.1016/j.jse.2019.03.034. [DOI] [PubMed] [Google Scholar]
- 20.Murachovsky J., Ikemoto R.Y., Nascimento L.G., Fujiki E.N., Milani C., Warner J.J. Pectoralis major tendon reference (PMT): a new method for accurate restoration of humeral length with hemiarthroplasty for fracture. J Shoulder Elbow Surg. 2006;15:675–678. doi: 10.1016/j.jse.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 21.Ohl X., Bonnevialle N., Gallinet D., Ramdane N., Valenti P., Decroocq L. How the greater tuberosity affects clinical outcomes after reverse shoulder arthroplasty for proximal humeral fractures. J Shoulder Elbow Surg. 2018;27:2139–2144. doi: 10.1016/j.jse.2018.05.030. [DOI] [PubMed] [Google Scholar]
- 22.Peters P.M., Plachel F., Danzinger V., Novi M., Mardian S., Scheibel M. Clinical and radiographic outcomes after surgical treatment of proximal humeral fractures with head-split component. J Bone Joint Surg Am. 2020;102:68–75. doi: 10.2106/JBJS.19.00320. [DOI] [PubMed] [Google Scholar]
- 23.Rietveld A.B., Daanen H.A., Rozing P.M., Obermann W.R. The lever arm in glenohumeral abduction after hemiarthroplasty. J Bone Joint Surg Br. 1988;70:561–565. doi: 10.1302/0301-620X.70B4.3403598. [DOI] [PubMed] [Google Scholar]
- 24.Sebastia-Forcada E., Cebrian-Gomez R., Lizaur-Utrilla A., Gil-Guillen V. Reverse shoulder arthroplasty versus hemiarthroplasty for acute proximal humeral fractures. A blinded, randomized, controlled, prospective study. J Shoulder Elbow Surg. 2014;23:1419–1426. doi: 10.1016/j.jse.2014.06.035. [DOI] [PubMed] [Google Scholar]
- 25.Sebastia-Forcada E., Lizaur-Utrilla A., Cebrian-Gomez R., Miralles-Munoz F.A., Lopez-Prats F.A. Outcomes of reverse total shoulder arthroplasty for proximal humeral fractures: primary arthroplasty versus secondary arthroplasty after failed proximal humeral locking plate fixation. J Orthop Trauma. 2017;31:e236–e240. doi: 10.1097/BOT.0000000000000858. [DOI] [PubMed] [Google Scholar]