Abstract
Background
As per some cadaveric studies, blood flow in posterosuperior rotator cuff tendons improves in the abducted shoulder position compared with the neutral position. In a clinical post–rotator cuff repair scenario, the impact of abduction on altered blood flow in and around the posterosuperior rotator cuff tendons is unknown in terms of clinical outcomes and structural healing.
Materials and methods
This study included 42 eligible patients aged between 40 and 70 years with clinically diagnosed and radiologically confirmed rotator cuff tears undergoing arthroscopic rotator cuff repair. Patients were randomly allocated to undergo application of either an abduction brace (group 1) or an arm pouch (group 2). On postoperative day 1, power Doppler scanning was performed on the index shoulder in adduction and 30° of abduction in each patient; the allocated treatment (abduction brace or arm pouch) was then applied. Power Doppler scanning was repeated at 6 weeks in the immobilization position assigned to the patient (abduction or adduction). The vascular flow in 6 regions was noted as per the criteria of Fealy et al. A visual analog scale score was assessed preoperatively and at 1, 3, 6, 12, and 56 weeks postoperatively. Clinical assessment was performed with the Constant-Murley score at 1 year, and structural healing of the cuff was assessed using ultrasonography at 3 and 12 months.
Result
On the first postoperative day, blood flow was significantly higher in all 6 areas of the shoulder in group 1 than in group 2. The mean total vascular score was significantly higher in group 1 than in group 2 on postoperative day 1 (P = .0001) and remained so at 6 weeks (P = .0001). However, significantly higher vascular flow was noted only in the peribursal region at 6 weeks in group 1 (P = .04). No significant difference in the visual analog scale score was noted between the 2 groups at any given point of follow-up. Furthermore, no clinical and structural healing differences were noted between the 2 groups at final follow-up.
Conclusion
Higher blood flow in and around the posterosuperior rotator cuff owing to an abducted shoulder position with an abduction brace in the first 6 weeks postoperatively fails to offer any advantage in terms of lower pain levels, better clinical scores, or superior cuff healing.
Keywords: Rotator cuff, arthroscopic repair, vascularity, abduction brace, pain, healing, doppler ultrasonography
Rotator cuff tears are a common pathology, with an increasing number of repairs being performed arthroscopically. The clinical and structural outcomes of a repair are dependent on multiple factors.25 One of the critical factors that affects postoperative outcomes is healing of the repaired rotator cuff tendon onto the footprint.9 Multiple factors affect tendon healing onto the footprint, such as the age of the patient, retraction and quality of tendon, fatty infiltration, atrophy of rotator cuff tendon, type of repair, vascularity of tendon, and tension of the repaired construct at the time of repair. Although most of the factors have been widely studied in the literature, very little attention in clinical studies has been paid to the vascularity of the tendons, which may have a significant role in tendon healing. Rathbun and Macnab23 concluded that abducting the shoulder results in greater filling of the vessels in the supraspinatus insertion compared with the adducted shoulder. Many cadaveric and animal studies have concluded that an abducted arm has a mechanical advantage. In a human cadaveric study, Reilly et al24 showed that 30° of abduction reduced load and gap formation at the repair site of the supraspinatus tendon. In an ovine model, Andres et al2 concluded that abduction results in decreased contact pressures over the footprint. In a magnetic resonance imaging study of human cadavers, Bey et al3 found that the strain values in the supraspinatus remain low at lower angles of abduction but considerably increase at 60° of abduction. Furthermore, Davidson and Rivenburgh6 reported that an increased amount of passive tension at the repair site, measured during surgery, is associated with a higher level of postoperative pain. In a human cadaveric model, Hawthorne et al15 found that an abduction pillow reduces the tension in the repaired supraspinatus tendon. Furthermore, it may appear that, postoperatively, keeping the shoulder at lower angles of abduction might help reduce pain levels owing to lesser strain. However, 2 recent studies have reported that the application of an abduction brace does not provide any significant pain relief.11,17
From clinical and biomechanical perspectives, it appears that a postoperatively abducted shoulder (using an abduction brace) would result in increased blood flow in and around the rotator cuff tendons and that, perhaps, an abducted shoulder would have better healing potential at the footprint owing to reduced stress and gap formation. However, there is hardly any evidence in the literature assessing the role of altered blood flow in clinical situations with varying shoulder abduction positions and its impact on rotator cuff healing.
The aim of this study was to evaluate the effect of shoulder immobilization in 30° of abduction (with an abduction brace) and 0° of adduction (with the arm in the neutral position by the side of the chest in an arm pouch), in a post–rotator cuff repair scenario, on the vascular flow pattern in and around the posterosuperior rotator cuff as the principal outcome; pain levels, clinical scores, and cuff healing were assessed as secondary outcome parameters. We hypothesized that immobilization in an abduction brace at 30° of abduction for 6 weeks postoperatively would result in increased blood flow around the posterosuperior rotator cuff, which would help relieve pain and improve clinical outcomes and cuff healing.
Materials and methods
Study design, patient selection, and randomization
This prospective randomized controlled trial evaluated patients with rotator cuff tears undergoing arthroscopic repair. The inclusion criteria were patients aged between 40 and 70 years with clinically diagnosed and radiologically confirmed full-thickness posterosuperior rotator cuff tears undergoing all-arthroscopic rotator cuff repair. The exclusion criteria were as follows: partial cuff tear, grade 4 fatty infiltration and severe atrophy of the cuff, glenohumeral osteoarthritis, adhesive capsulitis, concomitant labral repair, partial cuff repair, irreparable cuff tear, and Lafosse type IV or V subscapularis tear. Patients were informed about the trial, and appropriate informed consent was obtained.
Patients were randomly allocated to 1 of the 2 treatment arms using a block randomization method with a ratio of 1:1 and a block size of 8. The random allocation code was provided to the patient, and the code key was kept with an independent observer (S. Madi) whose job was to enter all the data, vascular scores, and sonographic reports in the records. The key holder was not involved in clinical assessment, decision making, or follow-up assessment. The key holder revealed the allocation code after the conclusion of the study, before the data analysis. The operating surgeon and radiologist remained blinded to the group assignment. Each follow-up assessment, as well as the final clinical assessment, was performed by another surgeon in our unit, who did not have access to any other data or any involvement with the preoperative evaluation, surgical intervention, or randomization.
Sample size
Apart from the study by Fealy et al,8 who described vascular flow in and around the repaired rotator cuff area, no previous study has compared vascular flow in various positions of the shoulder. Hence, we calculated the sample size according to the visual analog scale (VAS) described in a previous study performed by Tashjian et al.27 According to an a priori power analysis using an α of .05, power (1 – β) of 80%, and expected difference in the VAS pain score (0-10) of 1.4 between the groups, 17 patients had to be included in each group. Taking a maximum 20% dropout rate into consideration, we decided to include a minimum of 21 patients in each arm.
Preoperative assessment
A detailed history was taken and a thorough clinical evaluation was performed by a single senior surgeon; the findings were recorded on a standardized shoulder assessment form. Preoperatively, the Constant-Murley score was recorded and the level of pain was assessed on a VAS from 0 to 10.
In each patient, a plain radiograph of the shoulder (true anteroposterior and outlet view) was obtained to evaluate osteoarthritis of the glenohumeral joint and the type of acromion (Bigliani classification).4 Magnetic resonance imaging of the affected shoulder was performed to confirm the cuff tear (retraction), stage of atrophy (Thomazeau classification, stage 1-3),28 and grade of fatty infiltration (Goutallier classification, grade 0-4).12
Surgical procedure
All patients were operated on by a single senior surgeon. Each patient was operated on while under general anesthesia and an interscalene block in the sloppy lateral decubitus position with the affected upper limb attached to a limb positioner (Spider 2; Smith & Nephew, Andover, MA, USA) in neutral rotation and 20°-30° of abduction. After standard skin preparation and draping, diagnostic arthroscopy of the affected shoulder joint was performed from a standard posterior portal. An anterior portal was made just above the subscapularis tendon in the rotator interval. The biceps tendon was tenotomized if found to be significantly frayed, flat, split, or damaged. Open subpectoral tenodesis was performed if the patient demanded this procedure preoperatively, in manual laborers, and in patients aged < 50 years. The biceps was left alone if found to be healthy with normal pulleys. Regarding the subscapularis tendon, Lafosse type I tears were débrided whereas type II and III tears were repaired with a single anchor (double or triple loaded) by a modified Mason-Allen repair technique. After the subscapularis repair was completed, the scope was shifted to the subacromial space. Standard subacromial bursal excision was performed with a power shaver and radiofrequency device. Bony acromioplasty was performed only if there was a Bigliani type III acromion or an acromial spur associated with a Bigliani type II acromion.
After bursectomy and acromioplasty, the supraspinatus and infraspinatus tendons were assessed for the following characteristics: shape, size, retraction, and reparability onto the footprint. Tear size was measured in an anteroposterior direction using a graduated probe and categorized as small, medium, large, or massive as per the DeOrio and Cofield classification.7 Then, the margin of the cuff was held with a suture retriever (Arthrex, Naples, FL, USA) to assess its tensionless reducibility, adequate coverage, and repairability over the footprint. If the cuff was found to be retracted, the standard releases were performed until optimum coverage of the footprint (>80%) was obtained. Apical traction suture was applied in the case of an L-shaped or reverse L–shaped cuff tear for traction while the tendon was released from subacromial adhesions or the paralabral capsule. The sclerosed bone over the greater tuberosity was gently dusted using a burr until minimal bleeding ensued. If the footprint did not reveal gentle bleeding spots, microfracture over the footprint was performed using a straight awl after anchor placement. The type of cuff repair performed was determined based on the indications. Usually, small- to medium-size tears underwent single-row repair whereas large tears were repaired by a double-row suture bridge technique.
Single-row repair technique
For single-row repair, double-loaded suture anchors (4.5-mm Corkscrew anchor or 5.0-mm polyetheretherketone anchor; Arthrex) were deployed in the middle of the tuberosity. Two or three suture anchors were used depending on the size of the tear. The tear was repaired using the standard modified Mason-Allen technique.
Double-row suture bridge repair (transosseous-equivalent) technique
For double-row suture bridge repair, 2 to 3 double-loaded suture anchors (4.5-mm Corkscrew anchor or 5.0-mm polyetheretherketone anchor; Arthrex) were used for the medial row and were inserted just lateral to the cartilage margin. Mattress bites were taken in the cuff just lateral to the musculotendinous junction and tied in sequence. One limb each from the mattress sutures was brought laterally down to the lateral aspect of the greater tuberosity to create a suture bridge construct (transosseous equivalent) with the use of 1 or 2 lateral-row knotless anchors (4.75-mm SwiveLock; Arthrex) in the standard fashion. In the case of an L-shaped or reverse L–shaped tear, 1 to 3 side-to-side intratendinous sutures were placed and tied.
All the intraoperative findings were recorded on a standardized shoulder assessment form, and patients were given a neutral arm pouch for support for the night. This was converted to an abduction brace or arm pouch after power Doppler sonography.
Postoperative power Doppler assessment (Fealy et al criteria): 0 and 6 weeks
On the first postoperative day, each patient underwent power Doppler ultrasonography (Philips Healthcare, Amsterdam, The Netherlands) of the index shoulder in both the neutral position and 30° of abduction (with a standard abduction brace kept in the ultrasonography room) to assess the vascular flow in and around the rotator cuff in both positions of the index shoulder. Power Doppler sonography was performed using the scanner’s low-flow sensitivity settings, which were maintained for all patients. According to Fealy et al,8 power Doppler analysis in the rotator cuff repair setting involves the assessment of a total of 6 key areas in and around the repaired rotator cuff: (1) peribursal (above the peribursal fat stripe); (2) peritendinous (deep to the fat stripe and along the periphery of the repair); (3) musculotendinous (within the repair); (4) intratendinous (within the repair); (5) pericortical (along the cortical margins); and (6) suture anchor (along the suture anchor site). As per Fealy et al, subjective scoring was used to assess the blood flow in each region as either absent (0), sparse (1), moderate (2), or prominent (3) both in neutral adduction and in 30° of abduction of the shoulder with the shoulder in a standard abduction brace. Once the patient returned to the ward, he or she was given an abduction brace (group 1) or a neutral arm pouch (group 2) according to the allocated group as per the randomization arm and was discharged. The surgeon and radiologist remained blinded to the group to which the patient was allotted.
Every patient returned at the end of 6 weeks and underwent repeated power Doppler ultrasonography. Again, power Doppler scanning was performed in both positions of the shoulder by the same radiologist, and the vascular score was given to the independent observer (S. Madi), who remained blinded to the randomization. Both scores (in adduction and in abduction) were entered into the system. Because the same radiologist performed power Doppler scanning of each patient both times in both positions, blinding of the radiologist regarding the patient’s group allocation was ensured. Nevertheless, although the final assessment of the vascular scores in various areas was considered at 6 weeks, the scores were considered as per group allotment only (ie, scores in abduction for group 1 and scores in adduction for group 2) and other, nonrelevant scores were discarded.
All the vascular flow images were stored on hard disk, and each vascular assessment was later reread by a second musculoskeletal radiologist to ensure acceptable interobserver variation. However, the findings recorded by the principal radiologist were considered in the final assessment.
The structural healing of the repaired cuff was assessed twice: at 3-month follow-up and at 1-year follow-up. The ultrasound report findings were broadly classified into 3 categories: type I, normal thickness with homogeneously hyperechoic tendon, partial hypo-echogenicity, or heterogeneous echogenicity or insufficient thickness without discontinuity, indicating complete healing; type II, presence of minor discontinuity or a focal partial defect, indicating a partial tear; and type III, presence of significant discontinuity or a full-thickness tear. Gartsman et al10 and Gwark et al13 deployed similar criteria for ultrasound assessment of the postoperative healing status of the cuff.
Postoperative rehabilitation and pain relief measures
In all patients, a structured rehabilitation protocol was started in the postoperative period. The shoulder was immobilized for 6 weeks in an arm pouch or abduction brace as per the group allocation, and only elbow and finger movements, along with scapular isometrics, were encouraged. For pain relief, each patient was given a 100-mg tablet of aceclofenac (sustained release) to be taken as a single tablet at night for 10 days. After 10 days, patients were asked to take a 60-mg tablet of etoricoxib when necessary (with a maximum of 10 tablets to be used in the next 4 weeks). Patients were asked to apply a local cold pack three to four times a day. After 6 weeks, passive mobilization of the shoulder was started. At the end of 8 weeks, active-assisted movements were initiated, followed by active movements. At the end of 3 months, ultrasound of the shoulder was performed in all cases to ascertain the healing status of the cuff over the footprint. Furthermore, cuff-strengthening exercises were initiated with a TheraBand device (TheraBand, Akron, OH, USA). Return to full activity including sports activity was reserved until the end of 8-12 months depending on the return of movement, strength, and integrity of the cuff.
Postoperative data collection
In all patients, the following data were collected: (1) vascular flow assessment on day 1 (both neutral position and shoulder abduction) and at the end of 6 weeks (abduction in group 1 and neutral position in group 2); (2) VAS score at the end of weeks 1, 3, 6, 12, and 56 (1 year); (3) Constant-Murley score at the end of 1 year; and (4) ultrasonographic assessment of healing of the repaired cuff at the end of 3 months and 1 year. All patients completed a minimum of 1 year of follow-up.
Statistical analysis
Statistical analysis was performed using the SPSS program (version 20.0; IBM, Armonk, NY, USA). The results were considered significant at P < .05. Equal distributions of baseline characteristics were evaluated using the Student t test for continuous variables and the χ2 test for categorical variables. Although the vascular flow score (0, 1, 2, or 3) is an ordinal variable, Fealy et al8 calculated the total vascular score in each patient in all 6 regions, converting the total score in each patient as a continuous variable, and calculated the mean difference at various time intervals. We analyzed the vascular scores in 2 ways: The vascular scores in all 6 regions in each patient were summed, converting the scores into a continuous variable, and the independent t test was used to assess the difference between the mean vascular scores of the 2 groups on day 1 and at 6 weeks. Furthermore, the vascular scores in each region were used as ordinal variables, and the difference between the 2 groups was calculated using the nonparametric Wilcoxon test. VAS score and Constant-Murley score analysis at various intervals between the 2 groups was performed using the independent t test. The difference in structural healing between the 2 groups was assessed using the χ2 test.
Results
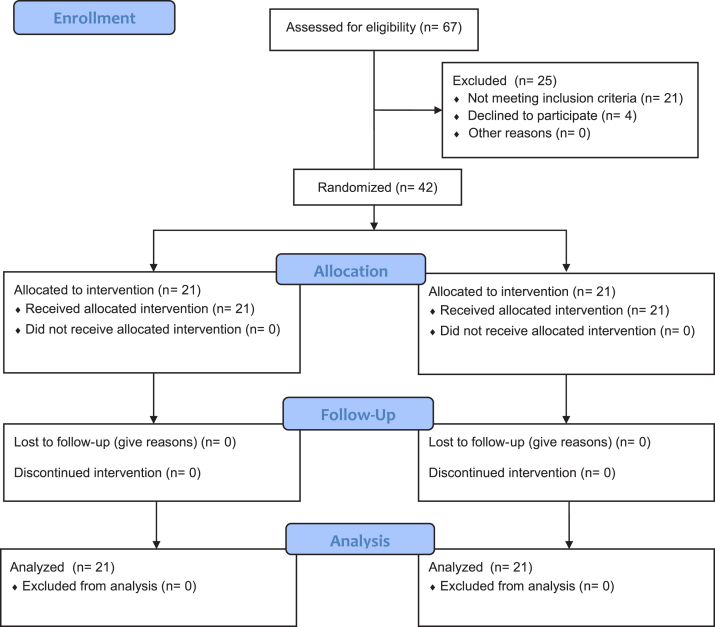
A total of 42 cases (21 in each group) were included in the trial, with no dropouts or loss to follow-up (Fig. 1) up to 1 year. The baseline characteristics were similar in both groups (Table I). No patient in group 1 or 2 reported noncompliance with the assigned brace.
Figure 1.
CONSORT (Consolidated Standards of Reporting Trials) flow diagram.
Table I.
Baseline characteristics and intraoperative operative findings of both treatment groups
| Variable | Group 1 (abduction brace) (n = 21) | Group 2 (arm pouch) (n = 21) | P value |
|---|---|---|---|
| Age, mean ± SD (range), yr | 55.8 ± 7.8 (40-68) | 55.5 ± 10.5 (42-70) | .93 |
| Sex, n | |||
| Male | 13 | 9 | .35 |
| Female | 8 | 12 | |
| Side, n | |||
| Right | 17 | 16 | .7 |
| Left | 4 | 5 | |
| Etiology of tear, n | |||
| Traumatic | 12 | 12 | >.999 |
| Degenerative | 9 | 9 | |
| Tendons torn, n | |||
| Supraspinatus | 7 | 7 | .93 |
| Supraspinatus and partial infraspinatus | 6 | 7 | |
| Supraspinatus and infraspinatus | 8 | 7 | |
| Shape of tear, n | |||
| Crescent | 14 | 11 | .63 |
| L | 4 | 6 | |
| U | 3 | 4 | |
| Size of posterosuperior cuff tear: Cofield grade 1/2/3/4, n | 3/7/5/6 | 1/9/6/5 | .69 |
| Condition of supraspinatus, n | |||
| Atrophy: stage 1/2/3 | 17/4/0 | 16/5/0 | .72 |
| Fatty degeneration: stage 0/1/2/3/4 | 8/10/3/0/0 | 9/8/4/0/0 | .84 |
| Condition of infraspinatus, n | |||
| Atrophy: stage 1/2/3 | 18/3/0 | 15/6/0 | .45 |
| Fatty degeneration: stage 0/1/2/3/4 | 9/9/3/0/0 | 10/7/4/0/0 | .77 |
| Subscapularis tear, n | 9 | 11 | |
| Type of acromion, n | |||
| Type II | 15 | 11 | .15 |
| Type II with spur | 6 | 7 | |
| Biceps tenotomy, n | 13 | 13 | |
| Footprint advancement, n | 2 | 3 | |
| Delamination in infraspinatus, n | 5 | 3 | |
| Delamination in supraspinatus, n | 0 | 2 | |
| Footprint microfracture, n | 16 | 15 | |
| Type of repair, n | |||
| Single row | 11 | 7 | .35 |
| DRSB | 10 | 14 | |
| Diabetes mellitus, n | 6 | 4 | |
| Preoperative CM score, mean (SD) | 32.43 (5.22) | 30.25 (5.46) | .12 |
| Preoperative VAS score, mean (SD) | 8.27 (1.22) | 8.15 (1.21) | .8 |
| Smoking, n | 4 | 3 |
SD, standard deviation; DRSB, double-row suture bridge; CM, Constant-Murley; VAS, visual analog scale.
Vascular scores
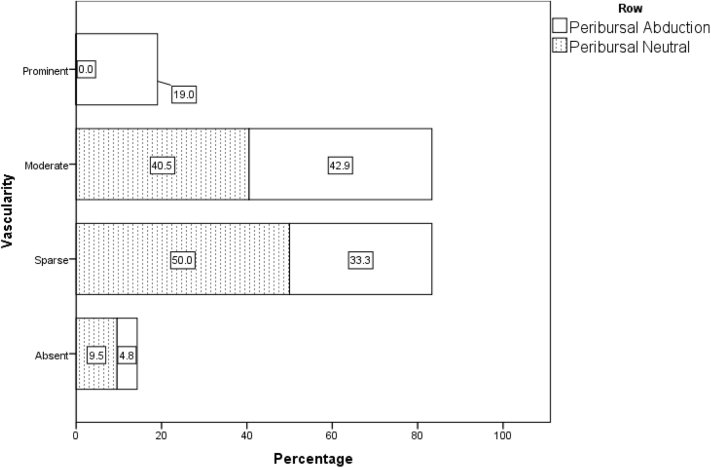
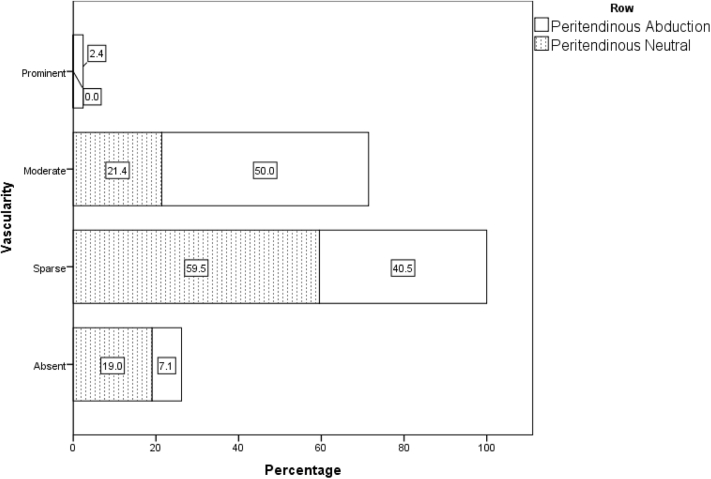
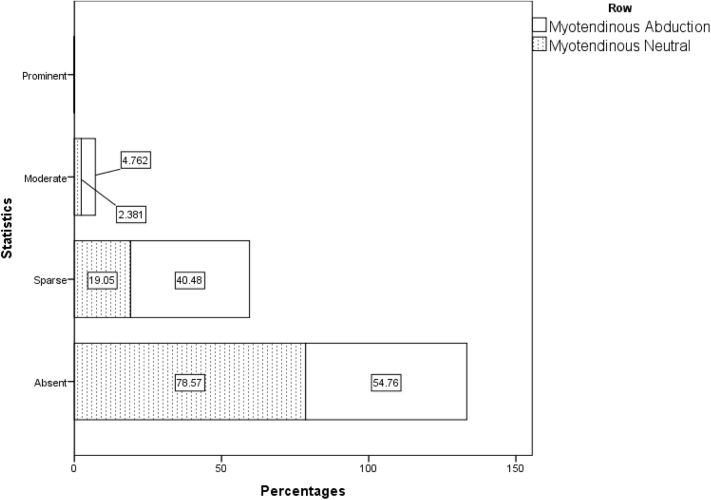
When all 42 patients who underwent power Doppler sonography in both the adducted and abducted positions on day 1 are taken into account, irrespective of their randomization status, the nonparametric Wilcoxon test, considering the vascular scores in 6 regions, revealed significantly higher blood flow for a 30° abducted shoulder compared with the neutral position (adduction) (Table II, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7). These differences were statistically significant in all 6 regions.
Table II.
Distribution of vascular pattern in all 42 patients on day 1 using power Doppler with shoulder kept in neutral position and 30° of abduction
| Variable | Cases, n (%) |
Median | P value | |||
|---|---|---|---|---|---|---|
| Absent (score of 0) | Sparse (score of 1) | Moderate (score of 2) | Prominent (score of 3) | |||
| Peribursal | ||||||
| Neutral | 4 (9.5) | 21 (50.0) | 17 (40.5) | 0 (0.0) | 1 | <.0001 |
| Abduction | 2 (4.8) | 14 (33.3) | 18 (42.9) | 8 (19.0) | 2 | |
| Peritendinous | ||||||
| Neutral | 8 (19.0) | 25 (59.5) | 9 (21.4) | 0 (0.0) | 1 | .001 |
| Abduction | 3 (7.1) | 17 (40.5) | 21 (50.0) | 1 (2.4) | 2 | |
| Myotendinous | ||||||
| Neutral | 33 (78.6) | 8 (19.0) | 1 (2.4) | 0 (0.0) | 0 | .008 |
| Abduction | 23 (54.8) | 17 (40.5) | 2 (4.8) | 0 (0.0) | 0 | |
| Intratendinous | ||||||
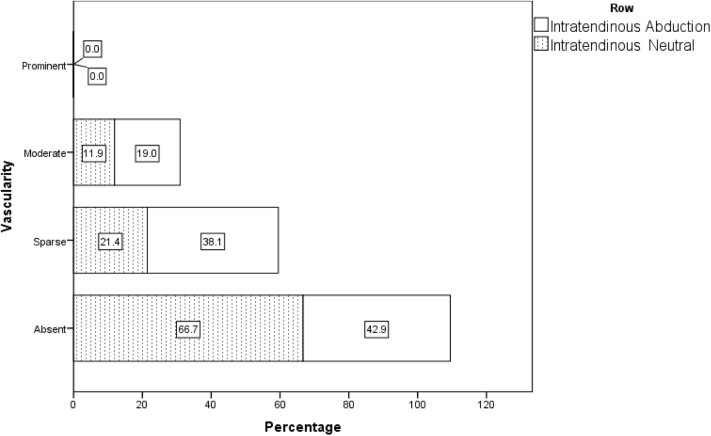
| Neutral | 28 (66.7) | 9 (21.4) | 5 (11.9) | 0 (0.0) | 0 | .005 |
| Abduction | 18 (42.9) | 16 (38.1) | 8 (19.0) | 0 (0.0) | 1 | |
| Pericortical | ||||||
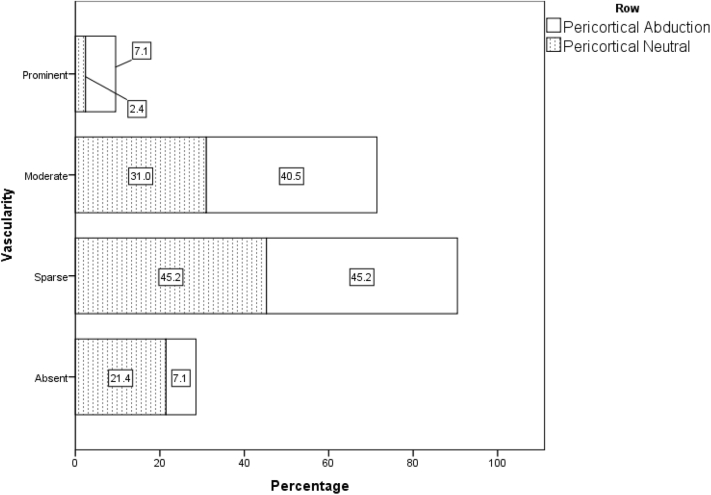
| Neutral | 9 (21.4) | 19 (45.2) | 13 (31.0) | 1 (2.4) | 1 | <.0001 |
| Abduction | 3 (7.1) | 19 (45.2) | 17 (40.5) | 3 (7.1) | 1 | |
| Suture anchor | ||||||
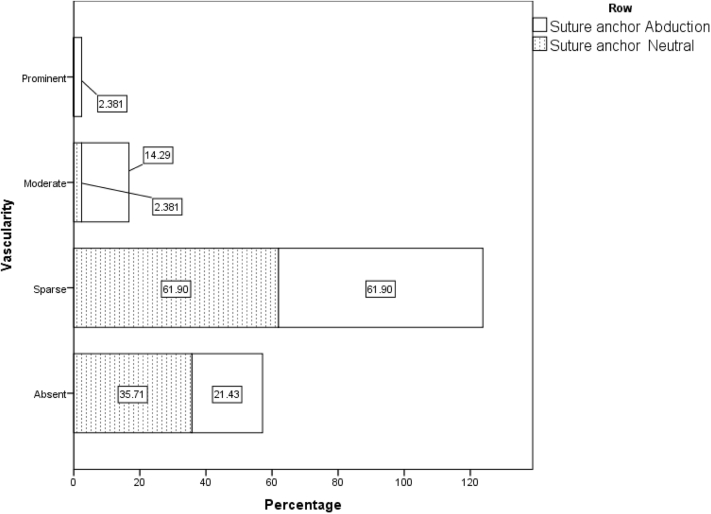
| Neutral | 15 (35.7) | 26 (61.9) | 1 (2.4) | 0 (0.0) | 1 | .001 |
| Abduction | 9 (21.4) | 26 (61.9) | 6 (14.3) | 1 (2.4) | 1 | |
Vascular flow was graded as per the criteria of Fealy et al.8 Nonparametric tests (Wilcoxon) revealed significant differences between the 6 vascular profile areas while the arm was in 2 different positions.
Figure 2.
Distribution of vascular flow pattern in peribursal region in abduction and adduction position in all 42 patients on day 1.
Figure 3.
Distribution of vascular flow pattern in peritendinous region in abduction and adduction position in all 42 patients on day 1.
Figure 4.
Distribution of vascular flow pattern in myotendinous region in abduction and adduction position in all 42 patients on day 1.
Figure 5.
Distribution of vascular flow pattern in intratendinous region in abduction and adduction position in all 42 patients on day 1.
Figure 6.
Distribution of vascular flow pattern in pericortical region in abduction and adduction position in all 42 patients on day 1.
Figure 7.
Distribution of vascular flow pattern in suture anchor region in abduction and adduction position in all 42 patients on day 1.
When we compared group 1 vs. group 2 on day 1, the mean total vascular score in all 6 regions was significantly higher in group 1 than in group 2 (P < .0001) and remained significantly higher in group 1 at the end of 6 weeks (P < .0001) (Table III). When we considered individual regions, the day 1 vascular flow details in both groups showed a higher blood flow trend in group 1 as compared with group 2, with prominent blood flow (score of 3) noted only in group 1 (Table IV). At 6 weeks, all 6 areas in both groups revealed a decrease in the total vascular score, as well as individual region flow (Table V). Nevertheless, 3 regions (peribursal, peritendinous, and pericortical) in both groups revealed a higher blood flow trend as compared with the remaining 3 areas at both times (day 1 and 6 weeks). At 6 weeks, statistical significance remained only in the peribursal region (P = .04). There was no difference in the total vascular flow score blood flow in any individual or all combined six regions with single- vs. double-row repair (P > .05). Between the 2 radiologists (principal radiologist and second radiologist), the interobserver reliability of the vascular score was good (Cronbach α = 0.82 [interclass correlation coefficient]).
Table III.
Mean vascular flow scores in both groups on day 1 and at end of 6 weeks
| Total vascular score timing | Vascular score, mean ± SD | P value |
|---|---|---|
| Day 1 | ||
| Group 1 (n = 21) | 7.14 ± 2.33 | <.0001 |
| Group 2 (n = 21) | 4.38 ± 1.50 | |
| End of 6 weeks | ||
| Group 1 (n = 21) | 5.24 ± 1.54 | <.0001 |
| Group 2 (n = 21) | 3.19 ± 1.57 |
SD, standard deviation.
Table IV.
Comparison of vascular flow pattern in 6 regions in both groups on day 1
| Variable | Group 1 (abduction brace, n = 21) |
Group 2 (arm pouch, n = 21) |
P value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Absent | Sparse | Moderate | Prominent | Total vascular score | Median | Absent | Sparse | Moderate | Prominent | Total vascular score | Median | ||
| Peribursal | 1 (4.8) | 9 (42.9) | 5 (23.8) | 6 (28.6) | 37 | 2 | 2 (9.5) | 11 (52.4) | 8 (38.1) | 0 (0.0) | 27 | 1 | .109 |
| Peritendinous | 2 (9.5) | 10 (47.6) | 8 (38.1) | 1 (4.8) | 29 | 1 | 4 (19.0) | 13 (61.9) | 4 (19.0) | 0 (0.0) | 21 | 1 | .086 |
| Myotendinous | 10 (47.6) | 9 (42.9) | 2 (9.5) | 0 (0.0) | 13 | 1 | 16 (76.2) | 4 (19.0) | 1 (4.8) | 0 (0.0) | 6 | 0 | .066 |
| Intratendinous | 7 (33.3) | 10 (47.6) | 4 (19.0 | 0 (0.0) | 18 | 1 | 15 (71.4) | 4 (19.0) | 2 (9.5) | 0 (0.0) | 8 | 0 | .022∗ |
| Pericortical | 1 (4.8) | 8 (38.1) | 9 (42.9) | 3 (14.3) | 35 | 2 | 7 (33.3) | 8 (38.1) | 6 (28.6) | 0 (0.0) | 20 | 1 | .011∗ |
| Suture anchor | 4 (19) | 16 (76.2) | 1 (4.8) | 0 (0.0) | 18 | 1 | 11 (52.4) | 10 (47.6) | 0 (0.0) | 0 (0.0) | 10 | 0 | .020∗ |
| Total vascular score | 0 | 62 | 58 | 30 | 150 | 0 | 50 | 42 | 0 | 92 | |||
Data for absent, sparse, moderate, and prominent blood flow are presented as number of patients (percentage). The nonparametric Wilcoxon test was used to compare the 2 groups.
Statistically significant difference.
Table V.
Comparison of vascular flow pattern in 6 regions in both groups at 6 weeks
| Variable | Group 1 (abduction brace, n = 21) |
Group 2 (arm pouch, n = 21) |
P value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Absent | Sparse | Moderate | Prominent | Total vascular score | Median | Absent | Sparse | Moderate | Prominent | Total vascular score | Median | ||
| Peribursal | 3 (14.3) | 9 (42.9) | 7 (33.3) | 2 (9.5) | 29 | 1 | 6 (28.6) | 12 (57.1) | 3 (14.3) | 0 (0.0) | 18 | 1 | .041∗ |
| Peritendinous | 5 (23.8) | 9 (42.9) | 6 (28.6) | 1 (4.8) | 24 | 1 | 8 (38.1) | 11 (52.4) | 2 (9.5) | 0 (0.0) | 15 | 1 | .092 |
| Myotendinous | 13 (61.9) | 8 (38.1) | 0 (0.0) | 0 (0.0) | 8 | 0 | 17 (81.0) | 4 (19.0) | 0 (0.0) | 0 (0.0) | 04 | 0 | .117 |
| Intratendinous | 10 (47.6) | 10 (47.6) | 1 (4.8) | 0 (0.0) | 12 | 1 | 15 (71.4) | 5 (23.8) | 1 (4.8) | 0 (0.0) | 07 | 0 | .145 |
| Pericortical | 3 (14.3) | 13 (61.9) | 5 (23.8) | 0 (0.0) | 23 | 1 | 8 (38.1) | 10 (47.6) | 3 (14.3) | 0 (0.0) | 16 | 1 | .105 |
| Suture anchor | 8 (38.1) | 12 (57.1) | 1 (4.8) | 0 (0.0) | 14 | 1 | 14 (66.7) | 7 (33.3) | 0 (0.0) | 0 (0.0) | 07 | 0 | .056 |
| Total vascular score | 0 | 61 | 40 | 9 | 110 | 0 | 49 | 18 | 0 | 67 | |||
Data for absent, sparse, moderate, and prominent blood flow are presented as number of patients (percentage). The nonparametric Wilcoxon test was used to compare the 2 groups.
Statistically significant difference.
Pain level
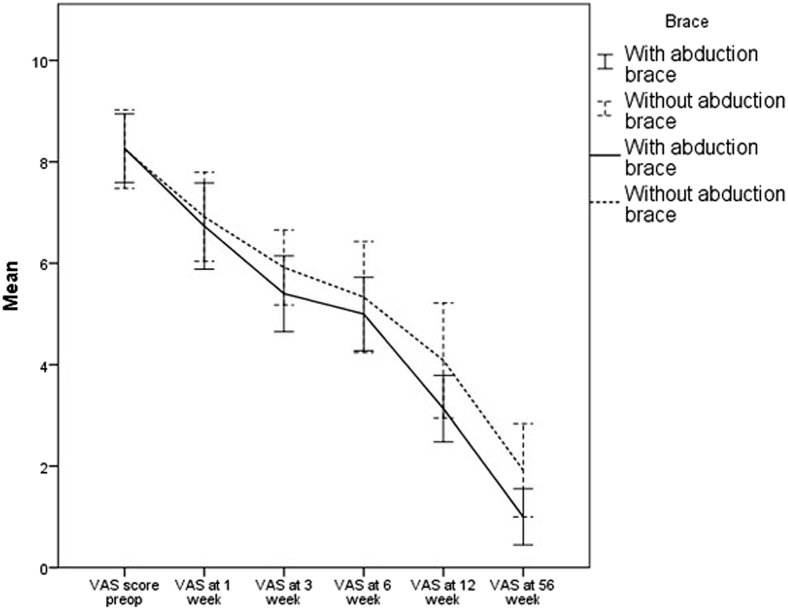
The pain levels (VAS score) significantly decreased in both groups between the assessments preoperatively and at the end of the 1-year postoperative period (56 weeks). Although group 1 had a slightly lower mean pain (VAS) score than group 2 at all designated follow-up points, the difference was not statistically significant (Table VI, Fig. 8).
Table VI.
Mean VAS score in groups 1 and 2 preoperatively and at 1, 3, 6, 12, and 56 weeks postoperatively
| VAS score, mean (SD) |
||||||
|---|---|---|---|---|---|---|
| Preoperatively | Postoperatively |
|||||
| 1 wk | 3 wk | 6 wk | 12 wk | 56 wk | ||
| Group 1 (abduction brace) | 8.27 (1.22) | 6.73 (1.53) | 5.40 (1.35) | 5.0 (1.30) | 3.13 (1.18) | 1.0 (1.0) |
| Group 2 (arm pouch in adduction) | 8.15 (1.21) | 6.62 (1.71) | 5.69 (1.37) | 5.08 (1.89) | 3.85 (1.90) | 1.92 (1.44) |
| P value | .80 | .85 | .57 | .90 | .24 | .06 |
VAS, visual analog scale; SD, standard deviation.
Figure 8.
Mean visual analog scale (VAS) scores in groups 1 and 2 preoperatively (preop) and 1, 3, 6, 12, and 56 weeks (1 year) postoperatively. The error bars represent 95% confidence intervals.
Clinical outcome
On comparison with the preoperative Constant-Murley scores, both groups revealed significant improvement in their final Constant-Murley scores. However, no difference in the Constant-Murley scores at final follow-up was found between the 2 groups (P = .36) (Table VII).
Table VII.
Summary of mean postoperative clinical outcome scores
| Functional score | Group 1 (n = 21) | Group 2 (n = 21) | P Value |
|---|---|---|---|
| CM, mean (SD) | 84.63 (8.33) | 82.93 (4.16) | .36 |
CM, Constant-Murley; SD, standard deviation.
Structural outcomes
When comparing structural healing assessed with ultrasonography between the 2 groups, we observed no difference in the cuff healing status at 3 months and at final follow-up at 1 year (Table VIII). A total of 3 patients had full-thickness tears: 1 patient in group 1 and 2 patients in group 2. Two patients were able to participate in their normal activities and had no specific complaints, whereas 1 patient (in group 1) reported difficulty sustaining the shoulder in mid abduction. However, the latter patient refused any surgical intervention.
Table VIII.
Healing pattern in both groups with single-row and DRSB technique at 3 months and 1 year detected by ultrasonography
| Type of repair | Healing status, n (%) |
Total | P value | ||
|---|---|---|---|---|---|
| Completely healed | Partial tear | Complete tear | |||
| Group 1 (n = 21) | |||||
| Single row (n = 11) | 10 (90.9) | 1 (9.1) | 0 | 11 | .366 |
| DRSB (n = 10) | 9 (90) | 0 | 1 (10) | 10 | |
| Group 2 (n = 21) | |||||
| Single row (n = 7) | 7 (100) | 0 | 0 | 7 | .417 |
| DRSB (n = 14) | 11 (78.5) | 1 (7.14) | 2 (14.2) | 14 | |
DRSB, double-row suture bridge.
Discussion
We found a significantly high vascular flow pattern in an abducted shoulder compared with an adducted shoulder (neutral by the chest) on day 1 and at 6 weeks. The blood flow was higher especially in 3 key areas: peribursal, peritendinous, and pericortical. However, there seems to be no additional clinical advantage of higher blood flow in an abducted shoulder in terms of improving pain scores, clinical outcomes, or healing rates of the cuff over the footprint compared with an adducted shoulder.
To our knowledge, our study is the first in the English-language literature that has compared the blood flow in and around the cuff repair site in an adducted and 30° abducted shoulder and further assessed the impact of altered blood flow on pain level, as well as clinical and structural outcomes. Rathbun and Macnab23 noted higher blood flow in the supraspinatus tendon and superior part of the infraspinatus (posterosuperior cuff) in an abducted cadaveric arm—possibly because an abducted arm avoids the “wringing out” phenomenon of the rotator cuff overlying the humeral head observed in the adducted position of the arm. They also noted that there were areas of avascularity in the rotator cuff if calcification and tendon rupture were present. Another observation by Rathbun and Macnab was that most of the supraspinatus tendon proximal to the rupture was avascular. Hence, it seems logical that keeping the shoulder in abduction after arthroscopic cuff repair may improve the circulation in and around the rotator cuff. Our study confirmed that compared with an adducted shoulder, an abducted position of the index shoulder improves the blood flow in and around the repaired cuff. Although overall blood flow remained high in the abduction group at both intervals, the blood flow in all 6 regions did not show similar patterns of difference at both intervals. On day 1, the Wilcoxon analysis of all 42 patients, irrespective of randomization, revealed significantly higher blood flow in all 6 regions in the abducted position. However, when the numbers in each group became smaller after randomization, the regional flow pattern was different at the 2 time intervals—day 1 and 6 weeks—as is evident in Tables IV and V. Perhaps, this anomaly can be explained by the fact that each group contains just half the patients, reducing the power of analysis. Hence, we believe that another randomized study with larger numbers in each group would be able to ascertain which of the 6 regions would continue to reveal higher flow consistently and which regions would show diminished flow.
Fealy et al8 observed significant blood flow in all areas around the rotator cuff repair site compared with the normal shoulder, followed by a gradual diminution in flow over time; we observed similar phenomena. However, they did not attempt to check the blood flow in different positions of the index shoulder. Moreover, they found the highest flow in the peritendinous area, whereas our study detected the highest flow in the peribursal area. Another contrasting finding was that the lowest vascularity score was observed in the pericortical area at all time points in their study whereas our study showed the third highest score in the pericortical region on day 1 and at 6 weeks. The difference between the 2 studies could be explained by 2 facts: (1) We performed microfracture in almost all cases, unless the footprint showed reasonable bleeding after gentle dusting by a power burr, whereas there is no detailed technical description of rotator cuff repair in the series of Fealy et al except that trough decortication was mentioned in brief. Hence, we believe that multiple microfracture holes at the footprint resulted in consistent, robust pericortical blood flow in our series. (2) The series of Fealy et al involved 11 surgeons performing rotator cuff repair using open, mini-open, and arthroscopic techniques in 33 patients, making the entire methodology quite variable, whereas all 42 patients in our series were operated on by a single surgeon using an all-arthroscopic technique. Thus, variable techniques, such as handling of tissues (soft tissue and bone) in a different fashion, as well as trough decortication, could result in variable blood flow patterns. Furthermore, Fealy et al described a high rate of persistent defects at the rotator cuff repair site (43%). Perhaps, a relatively lesser blood supply at the decorticated site compared with multiple microfracture holes could explain the persistent defects or retear rates as compared with our study, given that bone marrow stimulation techniques are known to reduce the retear rates after rotator cuff repair.1
Another hypothesis behind the provision of an abduction brace in the post–rotator cuff repair patient is that an abducted shoulder position in the postoperative period would result in lesser tension on the repaired cuff, which in turn would decrease postoperative pain. Davidson and Rivenburgh6 found that an increased amount of passive tension in the cuff could result in a higher level of postoperative pain. In a human cadaveric study, Hawthorne et al15 found that shoulder immobilization using a large abduction pillow producing 25° of abduction and 0° of neutral rotation can decrease the tension on the anterior and posterior sutures in the supraspinatus by 42% and 56%, respectively. Hence, Hawthorne et al recommended the use of an abduction pillow after cuff repair to reduce the tension at the repair site, which would also protect the repair while healing. Hatakeyama et al14 concluded that reduced stress in the repaired superior cuff in the abducted position is important in the early healing phase. In the surgical setting, a chronically torn rotator cuff tendon is often retracted medially, and passive tension is increased in the tendon.16 Furthermore, the posterosuperior cuff tendons are often delaminated, with the inferior delaminated layer edge being more medial than the superior layer.21 Repair of the medialized inferior-medial layer could further contribute to tension in the repaired construct. To mobilize the retracted tendon, adequate soft-tissue releases are performed to bring the torn lateral edge toward the footprint to achieve a tensionless repair. Despite sufficient soft-tissue releases and footprint medialization, the repair may remain under some “unwanted tension,” which could result in persistent pain and possibly failure of the construct.18,24,26 In a clinical study of 132 patients, Kim et al19 concluded that there is an inverse correlation of repair tension with cuff healing. In another clinical study, Park et al22 concluded that the possibility of a retear is higher with tension > 35 N. In all 42 patients in our series, we could achieve a relatively tensionless repair coupled with or without releases and medialization of the footprint.
In our series, although the VAS score was slightly lower in group 1 than in group 2 at most time points, the difference was not significant at any given point of follow-up. A similar observation has been made in 2 recent studies, implying the futility of an abduction brace in significantly minimizing postoperative pain.11,17 Hence, we conclude that the use of an abduction brace in the postoperative period will not make any difference in decreasing the pain levels if the repair is performed in a tensionless fashion. On the contrary, Conti et al5 reported better pain levels with the shoulder immobilized in an external rotation brace (not an abduction brace). Furthermore, in a Web-based survey of Arthroscopy Association of North America and American Orthopaedic Society for Sports Medicine members, 70% of respondents agreed that they prefer immobilization using an abduction brace with the arm in a neutral position.20 However, with the currently available evidence, the utility of using an abduction brace seems debatable. Finally, the rate of structural healing and clinical outcomes at 1 year were not different between the 2 groups in our study, signifying that there may not be any clinically significant utility of using an abduction brace in the post–rotator cuff repair setting as long as the repair has been performed in a tensionless fashion.
Study limitations
Like every study, our study has certain unavoidable limitations. First, the principal problem we faced was that the absence of a similar previous study led to a lack of data for calculating the number of patients required to adequately power the study. We had to take the data regarding the VAS score from a previous study to achieve minimum power.27 Hence, we recommend a larger randomized controlled trial with adequate power to establish the facts regarding altered blood flow in an abducted position and to establish its clinical utility beyond doubt. Second, there is no control group of healthy shoulders wherein vascular flow could have been assessed in adduction and abduction, and we do not have baseline preoperative vascular flow data for the participants. Third, the assessment of vascular flow by power Doppler itself is a subjective procedure. The findings of our study and that of Fealy et al8 do not match, especially the overall scores and areas of vascular predominance. A more objective method that is easily replicable is required to assess the vascular flow in various regions. Fourth, we did not analyze the difference in vascular flow between patients who underwent single-row repair and those who underwent double-row suture bridge fixation as the number of anchors and the double row itself could affect blood flow. Fifth, we could not evaluate the vascular flow alterations weekly between day 1 and 6 weeks because of compliance issues. A weekly assessment would have been more appropriate to understand the pattern of flow predominance, its sustenance, and its correlation with pain. However, it is difficult for patients to return every week for follow-up, as many are outstation patients. Sixth, we could not assess the impact of the number of anchors affecting the vascular score in the suture anchor group as the groups were too small to compare. Finally, although the pain level was assessed at 1, 3, 6, 12, and 56 weeks postoperatively, power Doppler scanning was not performed at each interval to assess the correlation between the blood flow scores and pain level. Hence, we recommend a larger, multicenter, level I trial with more patients and an objective method of vascular flow assessment to study the effect of blood flow alteration on the pain levels and clinical and structural outcomes after rotator cuff repair.
Conclusion
Even though application of an abduction brace, as compared with an arm pouch, results in increased blood flow in and around an all-arthroscopically repaired posterosuperior rotator cuff during the first 6 weeks postoperatively, it fails to establish any clinically significant value in terms of lesser pain levels, better clinical scores, or superior healing tendency.
Disclaimer
The authors, their immediate families, and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Footnotes
The Institutional Ethical Committee of Kasturba Hospital (Manipal, India) approved this study (IEC 779/2016).
References
- 1.Ajrawat P., Dwyer T., Almasri M., Veillette C., Romeo A., Leroux T. Bone marrow stimulation decreases retear rates after primary arthroscopic rotator cuff repair: a systematic review and meta-analysis. J Shoulder Elbow Surg. 2019;28:782–791. doi: 10.1016/j.jse.2018.11.049. [DOI] [PubMed] [Google Scholar]
- 2.Andres B.M., Lam P.H., Murrell G.A. Tension, abduction, and surgical technique affect footprint compression after rotator cuff repair in an ovine model. J Shoulder Elbow Surg. 2010;19:1018–1027. doi: 10.1016/j.jse.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Bey M.J., Song H.K., Wehrli F.W., Soslowsky L.J. Intratendinous strain fields of the intact supraspinatus tendon: the effect of glenohumeral joint position and tendon region. J Orthop Res. 2002;20:869–874. doi: 10.1016/s0736-0266(01)00177-2. [DOI] [PubMed] [Google Scholar]
- 4.Bigliani L.U., Morrison D.S., April E.W. The morphology of the acromion and its relationship to rotator cuff tears. Orthop Trans. 1986;10:216. [Google Scholar]
- 5.Conti M., Garofalo R., Castagna A. Does a brace influence clinical outcomes after arthroscopic rotator cuff repair? Musculoskelet Surg. 2015;99(Suppl 1):S31–S35. doi: 10.1007/s12306-015-0357-0. [DOI] [PubMed] [Google Scholar]
- 6.Davidson P.A., Rivenburgh D.W. Rotator cuff repair tension as a determinant of functional outcome. J Shoulder Elbow Surg. 2000;9:502–506. doi: 10.1067/mse.2000.109385. [DOI] [PubMed] [Google Scholar]
- 7.DeOrio J.K., Cofield R.H. Results of a second attempt at surgical repair of a failed initial rotator-cuff repair. J Bone Joint Surg Am. 1984;66:563–567. [PubMed] [Google Scholar]
- 8.Fealy S., Adler R.S., Drakos M.C., Kelly A.M., Allen A.A., Cordasco F.A. Patterns of vascular and anatomical response after rotator cuff repair. Am J Sports Med. 2006;34:120–127. doi: 10.1177/0363546505280212. [DOI] [PubMed] [Google Scholar]
- 9.Galanopoulos I., Ilias A., Karliaftis K., Papadopoulos D., Ashwood N. The impact of re-tear on the clinical outcome after rotator cuff repair using open or arthroscopic techniques—a systematic review. Open Orthop J. 2017;11:95–107. doi: 10.2174/1874325001711010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gartsman G.M., Drake G., Edwards T.B., Elkousy H.A., Hammerman S.M., O'Connor D.P. Ultrasound evaluation of arthroscopic full-thickness supraspinatus rotator cuff repair: single-row versus double-row suture bridge (transosseous equivalent) fixation. Results of a prospective, randomized study. J Shoulder Elbow Surg. 2013;22:1480–1487. doi: 10.1016/j.jse.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 11.Ghandour T.M., Ibrahim A., Abdelrahman A.A., Elgammal A., Hammad M.H. Does the type of shoulder brace affect postoperative pain and clinical outcome after arthroscopic rotator cuff repair? Arthroscopy. 2019;35:1016–1023. doi: 10.1016/j.arthro.2018.10.137. [DOI] [PubMed] [Google Scholar]
- 12.Goutallier D., Postel J.M., Bernageau J., Lavau L., Voisin M.C. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994;304:78–83. [PubMed] [Google Scholar]
- 13.Gwark J.Y., Sung C.M., Na J.B., Park H.B. Outcomes of arthroscopic rotator cuff repair in patients who are 70 years of age or older versus under 70 years of age: a sex- and tear size-matched case-control study. Arthroscopy. 2018;34:2045–2053. doi: 10.1016/j.arthro.2018.02.047. [DOI] [PubMed] [Google Scholar]
- 14.Hatakeyama Y., Itoi E., Pradhan R.L., Urayama M., Sato K. Effect of arm elevation and rotation on the strain in the repaired rotator cuff tendon. A cadaveric study. Am J Sports Med. 2001;29:788–794. doi: 10.1177/03635465010290061901. [DOI] [PubMed] [Google Scholar]
- 15.Hawthorne J.R., Carpenter E.M., Lam P.H., Murrell G.A.C. Effects of abduction pillows on rotator cuff repair: a biomechanical analysis. HSS J. 2018;14:114–122. doi: 10.1007/s11420-017-9592-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hersche O., Gerber C. Passive tension in the supraspinatus musculotendinous unit after long-standing rupture of its tendon: a preliminary report. J Shoulder Elbow Surg. 1998;7:393–396. doi: 10.1016/s1058-2746(98)90030-1. [DOI] [PubMed] [Google Scholar]
- 17.Hollman F., Wolterbeek N., Zijl J.A.C., van Egeraat S.P.M., Wessel R.N. Abduction brace versus antirotation sling after arthroscopic cuff repair: the effects on pain and function. Arthroscopy. 2017;33:1618–1626. doi: 10.1016/j.arthro.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 18.Jerosch J., Castro W.H. Belastung der Rotatorenmanschettennaht in Abhängigkeit von der Gelenkstellung [Stress on the rotator cuff sutures in relation to joint position] Z Orthop Ihre Grenzgeb. 1993;131:317–322. doi: 10.1055/s-2008-1040032. [in German] [DOI] [PubMed] [Google Scholar]
- 19.Kim D.H., Jang Y.H., Choi Y.E., Lee H.R., Kim S.H. Evaluation of repair tension in arthroscopic rotator cuff repair: does it really matter to the integrity of the rotator cuff? Am J Sports Med. 2016;44:2807–2812. doi: 10.1177/0363546516651831. [DOI] [PubMed] [Google Scholar]
- 20.Mollison S., Shin J.J., Glogau A., Beavis R.C. Postoperative rehabilitation after rotator cuff repair: a web-based survey of AANA and AOSSM members. Orthop J Sports Med. 2017;5 doi: 10.1177/2325967116684775. 2325967116684775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pandey V., Joseph C.J., Mathai N.J., Acharya K.K.V., Karegowda L.H., Willems W.J. Clinical and structural outcomes after arthroscopic repair of medium- to massive-sized delaminated and nondelaminated rotator cuff tears. Indian J Orthop. 2019;53:384–391. doi: 10.4103/ortho.IJOrtho_440_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park S.G., Shim B.J., Seok H.G. How much will high tension adversely affect rotator cuff repair integrity? Arthroscopy. 2019;35:2992–3000. doi: 10.1016/j.arthro.2019.05.049. [DOI] [PubMed] [Google Scholar]
- 23.Rathbun J.B., Macnab I. The microvascular pattern of the rotator cuff. J Bone Joint Surg Br. 1970;52:540–553. [PubMed] [Google Scholar]
- 24.Reilly P., Bull A.M., Amis A.A., Wallace A.L., Richards A., Hill A.M. Passive tension and gap formation of rotator cuff repairs. J Shoulder Elbow Surg. 2004;13:664–667. doi: 10.1016/j.jse.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 25.Saccomanno M.F., Sircana G., Cazzato G., Donati F., Randelli P., Milano G. Prognostic factors influencing the outcome of rotator cuff repair: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2016;24:3809–3819. doi: 10.1007/s00167-015-3700-y. [DOI] [PubMed] [Google Scholar]
- 26.Saul K.R., Hayon S., Smith T.L., Tuohy C.J., Mannava S. Postural dependence of passive tension in the supraspinatus following rotator cuff repair: a simulation analysis. Clin Biomech (Bristol, Avon) 2011;26:804–810. doi: 10.1016/j.clinbiomech.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Tashjian R.Z., Deloach J., Porucznik C.A., Powell A.P. Minimal clinically important differences (MCID) and patient acceptable symptomatic state (PASS) for visual analog scales (VAS) measuring pain in patients treated for rotator cuff disease. J Shoulder Elbow Surg. 2009;18:927–932. doi: 10.1016/j.jse.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 28.Thomazeau H., Rolland Y., Lucas C., Duval J.M., Langlais F. Atrophy of the supraspinatus belly. Assessment by MRI in 55 patients with rotator cuff pathology. Acta Orthop Scand. 1996;67:264–268. doi: 10.3109/17453679608994685. [DOI] [PubMed] [Google Scholar]