Abstract
Background
Given the active research on targeted therapy using tyrosine kinase (TK) inhibitors (TKIs) in the field of oncology, further studies have recently been conducted to evaluate their use in autoimmune disorders. Based on immunological investigations, previous studies have suggested that granulomatous meningoencephalomyelitis (GME) and necrotizing encephalomyelitis (NE) are similar to multiple sclerosis (MS), which is a human autoimmune demyelinating central nervous system disease.
Objectives
Considering this perspective, we hypothesized that canine GME and NE have significant expression of one or more TKs, which are associated with human MS pathogenesis.
Methods
To determine the possible use of conventional multi‐targeted TKIs as a treatment for canine GME and NE, we characterized the immunohistochemical expression of platelet‐derived growth factor receptor (PDGFR)‐α, PDGFR‐ß, vascular endothelial growth factor receptor (VEGFR)‐2, c‐Abl and c‐Kit in GME and NE samples.
Results
Histological samples from four dogs with GME and three with NE were retrieved. All samples stained positive for PDGFR‐ß (7/7 [100%]). PDGFR‐α and c‐Kit were expressed in 3/7 (42.8%) samples each. c‐Abl was identified in 2/7 (28.5%) samples; no sample showed VEGFR‐2 (0%) expression. Co‐expression of TKs was identified in 6/7 (85.7%) dogs.
Conclusions
All samples were positive for at least one or more of PDGFR‐α, PDGFR‐ß, c‐Kit and c‐Abl, which are known as the target TKs of conventional multi‐targeted TKIs. Their presence does suggest that these TKs may play a role in the pathogenesis of GME and NE. Therefore, multi‐targeted TKIs may provide benefits in the treatment of canine GME and NE by suppressing the activity of these TKs.
Keywords: granulomatous meningoencephalitis, multiple sclerosis, necrotizing encephalitis, tyrosine kinase, tyrosine kinase inhibitor
All GME and NE samples expressed one or more of the known targets of conventional multi‐targeted TKIs, suggesting that these kinases may play a role in the pathogenesis and development of GME and NE. Furthermore, multi‐targeted therapy with TKIs may provide benefits in the treatment of canine GME and NE by suppressing the activity of these TKs.
1. INTRODUCTION
Granulomatous meningoencephalitis (GME) and necrotizing encephalitis (NE) are common idiopathic inflammatory diseases of the central nervous system (CNS) in dogs (Talarico and Schatzberg, (2010)). A histopathologic diagnosis is generally not available for ante‐mortem examination; meningoencephalomyelitis of unknown aetiology (MUE) has been used as an umbrella term that encompasses GME and NE before histopathologic confirmation (Adamo, Rylander, & Adams, 2007). It usually involves the brain, spinal cord and/or meninges, and clinical signs typically depend upon the location and severity of the CNS lesions (Granger, Smith, & Jeffery, 2010). A presumptive diagnosis in the clinical setting is usually achieved through a multimodal approach that includes signalment, advanced imaging and cerebrospinal fluid analysis (Talarico & Schatzberg, 2010). The exact pathogenesis of GME and NE is still not fully understood; however, it has long been assumed to involve autoimmune‐mediated disorders, and based on immunopathological analysis, several studies have suspected common aberrant immune responses against CNS tissues (Park, Uchida, & Nakayama, 2014; Suzuki et al., 2003; Uchida, Park, Tsuboi, Chambers, & Nakayama, 2016).
Systemic medical therapy using a combination of glucocorticoids and immunosuppressive agents is the current mainstay of treatment of MUE in dogs, despite the less‐well‐understood pathogenic mechanisms (Dewey, 2015; Granger et al., 2010; Jung et al., 2007). The reported immunosuppressive drugs include cytosine arabinoside, azathioprine, lomustine, procarbazine, mycophenolate mofetil, leflunomide and cyclosporine (Adamo et al., 2007; Barnoon et al., 2016; Flegel et al., 2011; Levine, Fosgate, Porter, Schatzberg, & Greer, 2008; Talarico & Schatzberg, 2010; Zarfoss et al., 2006). Although the efficacy of these drugs varies from patient to patient, the overall reported median survival time for canine MUE treated using combination therapy is approximately one to 2 years (Granger et al., 2010). This is due to a lack of understanding of the exact treatment mechanism; furthermore, most of these therapies are dependent only on conventional immunosuppressants, which can have serious adverse side effects such as gastrointestinal problems and secondary infection. Therefore, new treatment strategies that target the specific pathways involved in GME and NE pathogenesis are needed.
Tyrosine kinases (TKs) are key mediators of normal cell signalling that tightly regulate cell survival, growth and cell proliferation (Natoli et al., 2010). They are abnormally activated in malignant cancers and inflammatory processes, which is referred to as the dysregulation of TKs. Some studies on this issue have been undertaken in human and veterinary medicine, and targeted therapy using TK inhibitors (TKIs) has shown efficacy in the treatment of some malignant tumors (Bavcar & Argyle, 2012; London, 2009). TKIs inhibiting a broad spectrum of TKs have been widely investigated in many clinical settings. The most effective approach to date has been identified for small‐molecule TKIs that block the ATP‐binding site of TKs. TKIs often induce toxicities that target normal cells which require TK pathways for cell survival and proliferation (e.g. bone marrow, liver, and gastrointestine). However, a mechanism of targeted action different from the conventional chemotherapy or immunomodulatory therapy results in a much higher specificity toward target cells and a less general toxicity. It thus appears to be relatively safe and well tolerated in humans, dogs and cats (London, 2009). Generally used, multi‐targeted small‐molecule TKIs in veterinary medicine include imatinib, toceranib and masitinib, of which toceranib and masitinib have been officially approved for use in the United States and the European Union. Imatinib is a selective inhibitor of c‐Abl, platelet‐derived growth factor (PDGF) receptor (PDGFR) α and ß, c‐Kit, c‐fms, Lck, Flt‐3 and MAPK activities (Deininger, Buchdunger, & Druker, 2005; Manley et al., 2010), whereas masitinib targets PDGFR α and ß, c‐Kit and Flt‐3 receptor (Dubreuil et al., 2009). Toceranib phosphate has inhibitory activity against c‐Kit, vascular endothelial growth factor receptor‐2 (VEGFR‐2), PDGFR α and ß, Flt‐3 receptor and FAK pathway (Leblanc et al., 2012).
Recently, the role of TKs in autoimmune disorders, especially rheumatoid arthritis and multiple sclerosis (MS), has been investigated in numerous animal models and in human medicine (Akashi et al., 2011; Azizi & Mirshafiey, 2013; Coffey et al., 2012). MS is an inflammatory disease of the CNS in young adult humans, characterized by the CD4 + Th1 cell‐mediated autoimmune response (Sospedra & Martin, 2005; Viglietta, Baecher‐Allan, Weiner, & Hafler, 2004). TKs play an important role in multiple signal transductions in the pathogenesis of MS (Azizi et al., 2014). The efficacy of TKIs in MS has already been demonstrated, and clinical trials have attempted to evaluate safety and clinical benefit (Azizi, Goudarzvand, Afraei, Sedaghat, & Mirshafiey, 2015; Mirshafiey, Ghalamfarsa, Asghari, & Azizi, 2014; Vermersch et al., 2012).
Previous studies based on immunological investigations have suggested that GME and NE are similar to human autoimmune demyelinating CNS diseases such as MS (Greer et al., 2010; Munana & Luttgen, 1998; Talarico & Schatzberg, 2010), and that they share many clinical and neuropathological characteristics of neuroinflammation. Based on this perspective, we hypothesized that canine GME and NE involve significant expression of one or more TKs, which are associated with human MS pathogenesis. Therefore, to determine the possible use of conventional multi‐targeted TKIs (e.g. imatinib, toceranib and masitinib) as treatments for canine GME and NE, we characterized the immunohistochemical expression of PDGFR‐α, PDGFR‐ß, VEGFR‐2, c‐Abl and c‐Kit in GME and NE samples.
2. MATERIALS AND METHODS
2.1. Subjects and tissue samples
Formalin‐fixed (in 4% paraformaldehyde in phosphate‐buffered saline [PBS]), paraffin‐embedded sections from four GME and three NE (total 7) samples were obtained from the pathology files at the Gyeongsang National University Animal Medical Center (Jinju, Republic of Korea) and Konkuk University Veterinary Pathology Laboratory (Seoul, Republic of Korea) (Table 1). The data retrieved from the medical records included signalment data and definitive histopathologic diagnoses. For all samples, the corresponding hematoxylin and eosin‐stained slides were reviewed by two pathologists to confirm the diagnosis and to separate the lesioned tissue from areas of normal tissue.
TABLE 1.
Signalment characteristics and definitive diagnoses of dogs enrolled in this study
| Dog | Breed | Gender | Age (years) | Diagnosis |
|---|---|---|---|---|
| 1 | Mixed Breed Dog | F | 10 | GME |
| 2 | Pomeranian | F | 2 | GME |
| 3 | Chihuahua | M | 1 | GME |
| 4 | Maltese | F | 8 | GME |
| 5 | Miniature Poodle | F | 2 | NE |
| 6 | Maltese | F | 4 | NE |
| 7 | Shih Tzu | F | 8 | NE |
Abbreviations: GME, granulomatous meningoencephalitis; NE, necrotizing encephalitis.
2.2. Immunohistochemical staining
Immunohistochemical staining was performed on 5 µm paraffin‐embedded sections. Sections were mounted on positively charged glass slides. The slides were then deparaffinized in xylene and rehydrated in ethanol. Hydrogen peroxide (3%) in phosphate‐buffered saline (PBS) was applied to inhibit endogenous peroxidase activity for 20 min at room temperature. Slides were then washed three times with PBS. Antigen retrieval was performed subsequently using the heat‐induced epitope retrieval method for 20 min with a citric acid buffer at pH 6.0 for PDGFR‐α/ PDGFR‐ß/VEGFR‐2/c‐Abl and an ethylenediaminetetraacetic acid (EDTA) buffer at pH 9.0 for c‐Kit. After antigen retrieval, the slides were cooled for 20 min and washed three times in PBS. The slides were then incubated with 5% goat serum (protein blocking agent) in PBS for 30 min to reduce nonspecific binding. Next, the sections were incubated with the appropriate diluted antibodies: polyclonal rabbit anti‐PDGFR‐α (dilution 1:2,000; Lifespan BioSciences) for 120 min, polyclonal rabbit anti‐PDGFR‐ß (dilution 1:700; Abcam) for 120 min, polyclonal rabbit anti‐VEGFR‐2 (dilution 1:100; Abcam) for 90 min, polyclonal rabbit anti‐c‐Abl (dilution 1:800; Santa Cruz Biotechnology) for 90 min and polyclonal affinity isolated rabbit anti‐c‐Kit (dilution 1:300; DAKO, Glostrup, Denmark) for 180 min at room temperature. The sections were washed in PBS and stained with 3,3'‐diaminobenzidine tetrahydrochloride colorimetric reagent (DAB, Vector Laboratories). Finally, sections were counterstained with Gill's hematoxylin for 5 s, dehydrated through an ethanol series and mounted with Permount (Fisher, Scientific, Grand Island).
Appropriate known antigen‐positive tissues were used as positive controls (including canine cerebellar Purkinje cells for PDGFR‐α, canine renal tubule for PDGFR‐ß, canine mammary adenocarcinoma for VEGFR‐2 and c‐Abl and canine mast cell tumor tissue for c‐Kit) to confirm antibody staining. Negative control experiments were carried out by replacing the primary antibody with the antibody dilution buffer.
2.3. Immunohistochemical evaluation
Immunohistochemical evaluation assessed the staining positivity of each TK and the staining intensity and distribution of TK‐positive cells based on previously described methods (Walters, Martin, Price, & Sula, 2018). Overall staining intensity was graded as no immunostaining, weak immunostaining or moderate and intense immunostaining at ×40 magnification and given a score ranging from 0 to 3 (Table 2). The distribution of positively stained cells (% cells affected) was evaluated in randomly selected five separate fields at ×200 magnification and was graded as follows: 0%, 1%–9%, 10%–50% or 51%–100% and given a score ranging from 0 to 3 (Table 2). The immunohistochemical results were reviewed by two pathologists.
TABLE 2.
Immunohistochemistry scoring protocol
| Parameter on × 40 magnification | Intensity score | Parameter on × 200 magnification | Distribution score | ||
|---|---|---|---|---|---|
| Staining intensity | No immunostaining | 0 | Staining distribution | 0% | 0 |
| Weak | 1 | 1%–9% | 1 | ||
| Moderate | 2 | 10%–50% | 2 | ||
| Intense | 3 | 51%–100% | 3 |
2.4. Statistical analysis
Fisher's exact tests were used to determine if the immunohistochemical evaluation was statistically associated with sample groups (GME and NE) for each of the five TKs. Statistical analysis was performed using SPSS 19 software statistics, and P‐values < .05 were considered statistically significant.
3. RESULTS
3.1. Sample demographics
Tissue samples were available for this study from seven dogs that had died from MUE. Affected tissue samples were obtained after necropsy, and a definitive diagnosis was achieved by histopathologic examination. Of these seven samples, four were from dogs with GME and three with NE (Table 1). Six different breeds were present, and all the breeds were small breeds. The median age was five years (range, 1–10 years). Six dogs were female (85.7%), and one dog was male (14.3%).
3.2. Immunohistochemistry
Affected brain tissues were separated from areas of normal brain tissue and used for immunohistochemical staining. Table 3 summarizes the immunohistochemical results of all seven tissue samples.
TABLE 3.
Immunohistochemistry scorings of cases enrolled in this study
| Dog | PDGFR‐α | PDGFR‐ß | VEGFR−2 | c‐Abl | c‐Kit | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| I | D | I | D | I | D | I | D | I | D | |
| 1 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 2 | 2 |
| 2 | 2 | 2 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | 0 | 0 | 3 | 3 | 0 | 0 | 2 | 2 | 0 | 0 |
| 5 | 0 | 0 | 3 | 1 | 0 | 0 | 0 | 0 | 2 | 2 |
| 6 | 1 | 1 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7 | 2 | 3 | 2 | 3 | 0 | 0 | 1 | 1 | 1 | 1 |
Abbreviations: D, Distribution; I, Intensity; PDGFR, Platelet‐Derived Growth Factor Receptor; VEGFR, Vascular Endothelial Growth Factor Receptor.
PDGFR‐ß was expressed in seven of seven (100%) cases, including four cases of GME and three of NE (Figure 1 and Table 4). All samples showed moderate to intense staining intensity; three of seven (42.9%) had moderate expression, and four of seven (57.1%) had intense expression. In three (42.9%) cases, between 1% and 9% of cells expressed PDGFR‐ß, whereas in two (28.6%) samples, 10%–50% of cells and in two (28.6%) samples, 51%–100% of cells expressed PDGFR‐ß.
FIGURE 1.
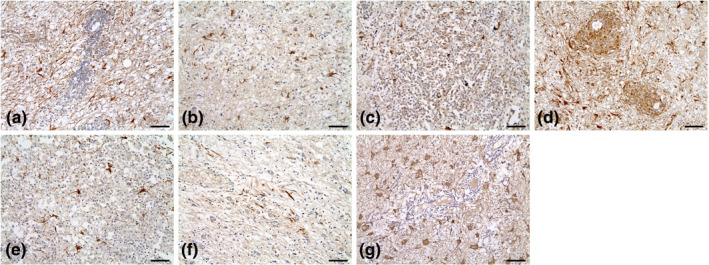
Immunohistochemical expression of PDGFR‐ß for each brain parenchymal tissue sample (a to g; cases 1 to 7). PDGFR‐β expression was relatively moderate to intense in all samples. The majority of the samples had > 10% staining distribution (distribution score ≥ 2). Magnification, ×200; Scale bar size, 70 μm
TABLE 4.
TK expression in seven samples
| Expression of TK | Expression | GME (n = 4) | NE (n = 3) | Total (n = 7) | % | |
|---|---|---|---|---|---|---|
| PDGFR‐α | Expression (total) | 1 (1/4) | 2 (2/3) | 3 (3/7) | 42.9 | |
| Staining intensity | Weak (1) | 0 | 1 | 1 | 14.3 | |
| Moderate (2) | 1 | 1 | 2 | 28.6 | ||
| Intense (3) | 0 | 0 | 0 | |||
| Staining distribution | 1%–9% (1) | 0 | 1 | 1 | 14.3 | |
| 10%–50% (2) | 1 | 0 | 1 | 14.3 | ||
| 51%–100% (3) | 0 | 1 | 1 | 14.3 | ||
| PDGFR‐ß | Expression (total) | 4 (4/4) | 3 (3/3) | 7 (7/7) | 100 | |
| Staining intensity | Weak (1) | 0 | 0 | 0 | ||
| Moderate (2) | 2 | 1 | 3 | 42.9 | ||
| Intense (3) | 2 | 2 | 4 | 57.1 | ||
| Staining distribution | 1%–9% (1) | 1 | 2 | 3 | 42.9 | |
| 10%–50% (2) | 2 | 0 | 2 | 28.6 | ||
| 51%–100% (3) | 1 | 1 | 2 | 28.6 | ||
| VEGFR−2 | Expression (total) | 0 (0/4) | 0 (0/3) | 0 (0/7) | 0 | |
| Staining intensity | Weak (1) | 0 | 0 | 0 | ||
| Moderate (2) | 0 | 0 | 0 | |||
| Intense (3) | 0 | 0 | 0 | |||
| Staining distribution | 1%–9% (1) | 0 | 0 | 0 | ||
| 10%–50% (2) | 0 | 0 | 0 | |||
| 51%–100% (3) | 0 | 0 | 0 | |||
| c‐Abl | Expression (total) | 1 (1/4) | 1 (1/3) | 2 (2/7) | 28.6 | |
| Staining intensity | Weak (1) | 0 | 1 | 1 | 14.3 | |
| Moderate (2) | 1 | 0 | 1 | 14.3 | ||
| Intense (3) | 0 | 0 | 0 | |||
| Staining distribution | 1%–9% (1) | 0 | 1 | 1 | 14.3 | |
| 10%–50% (2) | 1 | 0 | 1 | 14.3 | ||
| 51%–100% (3) | 0 | 0 | 0 | |||
| c‐Kit | Expression (total) | 1 (1/4) | 2 (2/3) | 3 (3/7) | 42.9 | |
| Staining intensity | Weak (1) | 0 | 1 | 1 | 14.3 | |
| Moderate (2) | 1 | 1 | 2 | 28.6 | ||
| Intense (3) | 0 | 0 | 0 | |||
| Staining distribution | 1%–9% (1) | 0 | 1 | 1 | 14.3 | |
| 10%–50% (2) | 1 | 1 | 2 | 28.6 | ||
| 51%–100% (3) | 0 | 0 | 0 |
PDGFR‐α was expressed in three of seven (42.9%) cases, including one of GME and two of NE (Figure 2 and Table 4). Of these, one of seven (14.3%) had weak expression, and two of seven (28.6%) had moderate expression. In one (14.3%) case between 1% and 9% of cells expressed PDGFR‐α; in one (14.3%) case, 10%–50% of cells and in another (14.3%), 51%–100% of cells expressed PDGFR‐α.
FIGURE 2.
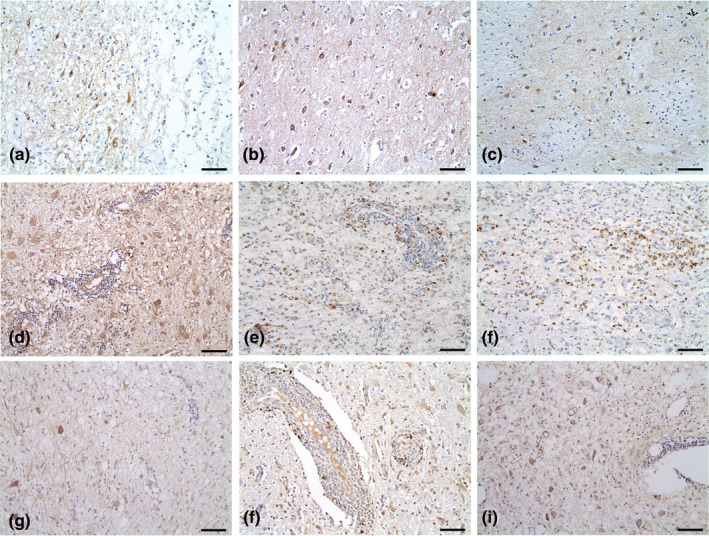
Expression of PDGFR‐α (A to C; cases 2, 6 and 7), c‐kit (d to f; cases 1, 5 and 7) and c‐Abl (g to h; cases 4 and 7) for each brain parenchymal tissue sample. All TK‐expressing samples showed mild to moderate staining intensity (intensity score 1 to 2). All except one (c; Case 7, Distribution score 3) of the samples had 1%–50% staining distribution (distribution score 1 to 2). Magnification, ×200; Scale bar size, 70 μm
c‐Kit was expressed in three of seven (42.9%) cases, including one of GME and two of NE (Figure 2 and Table 4). Of these, one of seven (14.3%) had weak expression, and two of seven (28.6%) had moderate expression. In one dog (14.3%), between 1% and 9% of cells, and in two (28.6%) dogs, between 10% and 50% of cells expressed c‐Kit.
c‐Abl was identified in two of seven (28.6%) cases, including one of GME and one of NE (Figure 2 and Table 4). Among these, one of seven (14.3%) had weak staining, and one (14.3%) had moderate staining intensity. In one case (14.3%), between 1% and 9% of cells and in another case (14.3%), between 10% and 50% of cells expressed c‐Abl.
VEGFR‐2 was not detected in any of the GME and NE cases (0%) (Table 4).
There was no statistically significant association between GME and NE samples in terms of expression positivity for each of the five TKs (all p > .05).
Co‐expression of TKs was identified in six dogs (85.7%). One dog (14.3%) expressed three TKs; five dogs (71.4%) expressed two TKs; in one case (14.3%), only one TK (PDGFR‐ß) was expressed. Of the co‐expressed samples with PDGFR‐ß expression, three cases (42.9%) showed co‐expression of PDGFR‐α, three cases (42.9%) showed co‐expression of c‐Kit and two cases (28.6%) showed co‐expression of c‐Abl.
4. DISCUSSION
It is well known that TKs play an important role in the pathogenesis of malignant tumors and inflammatory processes and in human and veterinary medicine. To date, many novel TKIs are being increasingly developed by pharmaceutical companies, and several TKIs have been officially approved for the treatment of human and animal cancers. With active research on targeted therapy using TKIs in the field of oncology, further studies have recently been conducted to evaluate their use in autoimmune disorders. Among the autoimmune diseases, neuroinflammation in MS is one of the most promising research fields for TK and TKI applications (Liu et al., 2014). Despite this ongoing research on the application of TKIs in human and animal models of MS, the role of TKs and their efficacy in canine GME and NE have not yet been evaluated.
We have attempted to assess the expression of PDGFR‐α, PDGFR‐ß, VEGFR‐2, c‐Abl and c‐Kit in canine GME and NE using immunohistochemical staining. These TKs are the therapeutic targets for widely used multi‐targeted TKIs (e.g. imatinib, toceranib and masitinib) in veterinary medicine. Based on the expression of these TKs, we tried to show the applicability of TKIs in therapy for GME and NE and surmised their potential role in disease pathogenesis. To the authors’ knowledge, this is the first study to describe the expression of TKs in canine GME and NE.
In this current study, two different subtypes were compared, and there was no significant difference in the expression positivity of each TKs between GME and NE. All samples stained positive for PDGFR‐ß (100%), with the majority of samples exhibiting relatively intense staining and broad distribution. This result was more evident than the expression of other TKs, and there was no significant difference in the PDGFR‐ß positivity between GME and NE. PDGFR‐α, which is the isoform receptor of PDGFR‐ß, was expressed in 42.9% of the samples, but was relatively less intensely and broadly expressed than PDGFR‐ß. Based on these results, we suspected that each PDGFR has a different role in the disease process. These two receptor isoforms bind differently with each PDGF ligand and elicit its biological activity through signal transduction (Claesson‐Welsh, 1994). Although the specific role of the two different PDGFRs in the autoimmune disease process is still under investigation, astrocyte proliferation largely depends on signalling mediated by both PDGFRs, and strong upregulation of PDGFRs was well described by a previous study of experimental autoimmune encephalomyelitis (EAE), a mouse model of MS (Koehler et al., 2008; Luo & Miller, 1999).
Astrocytes are specialized supportive glial cells that are thought to play a number of active roles in the brain, including the physical structuring of the CNS, metabolism and synaptic transmission, as well as responding to pathological insults (Nair, Frederick, & Miller, 2008; Sofroniew & Vinters, 2010; Williams, Piaton, & Lubetzki, 2007). The proliferation of astrocytes (reactive astrogliosis) has an intimate association with the pathogenesis of CNS diseases in several ways. Tissue healing impairment and scar formation by reactive astrogliosis are well‐recognized contributing factors of MS deterioration (Crespo et al., 2011). The compromise of the blood‐brain barrier (BBB) is another negative pathogenic process of astrogliosis (Williams et al., 2007). Astrogliosis also contributes to the disruption of immune responses and glutamate homeostasis in CNS (Karpiak, Bridges, & Eyer, 2001; Nair et al., 2008; Werner, Pitt, & Raine, 2001). Similarly, the activation of astrocytes is closely related to the pathology of canine MUE (Coates & Jeffery, 2014). Astrocytes were considered as a common target cell type of autoantibodies in GME and NE, suggesting a vital role in the autoimmune CNS disease process (Matsuki et al., 2004; Matsuki, Takahashi, Yaegashi, Tamahara, & Ono, 2009). According to this perspective, we could also observe the area of prominent inflammatory cell infiltration around the astrocytes and these astrocytes showed high expression levels of PDGFRs (Figures 1 and 2a–c). Therefore, the overexpression of PDGFRs and the related astrocyte activation are thought to play a significant role in canine GME and NE pathogenesis.
A few studies investigating PDGFR have demonstrated that upregulated PDGFR‐α promotes oligodendrocyte maturation and remyelination (Maeda et al., 2001; Wilson, Scolding, & Raine, 2006), suggesting a beneficial effect of PDGFR‐α in the MS disease process. In contrast, recent studies have emphasized that PDGFR‐ß is crucial for vascular stabilization and that aberrant expression of this receptor contributes to the pathogenesis of immune‐mediated disorders, including EAE (Carmeliet, 2005; Crespo et al., 2011; Zhang et al., 2009). Thus, it is speculated that the overexpression of PDGFR‐ß in the lesions could have a more detrimental effect on disease status than that of PDGFR‐α. In addition, given the strong expression of PDGFR‐ß in all samples of our study, we surmised that this TK could be involved in key aspects of the pathogenesis of GME and NE, and targeted therapy for overexpressed PDGFR‐ß may have potential as an effective treatment modality for GME and NE. However, further studies will be required to fully understand the relationship between the expression of each PDGFR and immune‐mediated CNS inflammation in dogs.
c‐Kit expression was found in a moderate number of samples (42.9%, 3/7) with relatively moderate staining intensity and distribution in this study. Up to now no studies have ever tried to evaluate the role of c‐Kit in canine immune‐mediated CNS inflammation. However, in the last few years, several articles have been devoted to the study of c‐Kit in autoimmune disorders including MS and EAE, and the role of c‐Kit in autoimmune disease pathogenesis through mast cells is well described (Benoist & Mathis, 2002; Piconese et al., 2011). c‐Kit is a stem cell factor receptor and acts as a key controller receptor for mast cell development and functioning (Maeda, Nishiyama, Ogawa, & Okumura, 2010; Maeda et al., 2001). The high expression of c‐kit in mast cells enhances their cell development, degranulation and release of chemokines and cytokines (Safavi et al., 2015). Mast cells are a dense cell population in the meninges and near the vessels involved in MS and EAE (Sayed, Christy, Quirion, & Brown, 2008). Mast cells play an important role in CNS demyelination and the initial entrance of myelin‐specific T‐cells by disrupting BBB permeability in the initial phases of MS (Bebo, Yong, Orr, & Linthicum, 1996; Hershko & Rivera, 2010; Sayed, Christy, Walker, & Brown, 2010). Their other contribution to the disease process is releasing large amounts of proinflammatory mediators and recruiting other inflammatory cells; therefore, they play a critical role in sustaining CNS inflammation (Kinet, 2007). For instance, the presence of mast cells and the percentage of degranulated mast cells in the CNS have been found to correlated with the severity and clinical onset of immune‐mediated neuroinflammatory disease (Brenner, Soffer, Shalit, & Levi‐Schaffer, 1994; Secor, Secor, Gutekunst, & Brown, 2000). Although it has not yet been studied in NE, a study on GME showed mast cell infiltration in the lesions in all cases (20/20), and a significantly larger number of mast cells was found in dogs with the acute form of the disease (Demierre et al., 2001). Based on these perspectives, we hypothesized that mast cells might also influence the disease pathogenesis and clinical status of canine GME and NE. Although mast cells were not evaluated in this study, c‐Kit expression was clearly identified in partial samples with moderate intensity and distribution. Therefore, targeted therapies directed at blocking c‐Kit and/or mast cell action, in conjunction with the standard treatment of disease, may provide more efficacious treatment options for GME and NE. Furthermore, conventional multi‐targeted TKIs mentioned above such as imatinib, toceranib and mastinib, which can directly block both c‐Kit and PDGFR‐ß may also be a meaningful aid in treatment of GME and NE. However, the relevant c‐Kit and mast cell functions in these diseases have remained elusive, and further well‐designed studies are required to confirm the clinical importance of these factors in canine GME and NE.
c‐Abl is a member of the Src family of non‐receptor TKs and participates in diverse signalling pathways that govern cellular proliferation, differentiation and apoptosis. c‐Abl has been shown to play a fundamental role in the development and function of the CNS (Koleske et al., 1998), and several studies have been conducted to reveal its role in various neurological diseases. However, the association of c‐Abl with immune‐mediated CNS diseases has not yet been studied. Although one recent study has suggested the role of c‐Abl in autoimmunity (Veillette, Rhee, Souza, & Davidson, 2009), further studies are needed to help clarify the role of this TK in canine MUE.
VEGF is a multifunctional mediator of blood vessel physiology, including vasculogenesis and angiogenesis. VEGF induces endothelial‐specific mitogenesis and vascular hyperpermeability and acts as a chemoattractant to inflammatory cells (Clauss, 2000). A previous study of autoimmune CNS disease suggested that the overexpression of VEGF may exacerbate the inflammatory response by multiple bioactivities: increased vascular permeability, BBB breakdown, the expression of major histocompatibility complex (MHC), the upregulation of adhesion molecules on endothelial cells and macrophage chemoattraction (Proescholdt, Jacobson, Tresser, Oldfield, & Merrill, 2002). Particularly, the BBB has been considered an important pathologic site in CNS autoimmunity (Gay & Esiri, 1991; Moor, de Vries, de Boer, & Breimer, 1994). BBB disruption and perivascular abnormality have also been considered typical features of canine GME and NE, and these abnormalities are known as key factors in the disease process (Plummer, Wheeler, Thrall, & Kornegay, 1992; Sorjonen, 1990). VEGFR‐2, the receptor of VEGF, was not detected in GME and NE samples in this study. The VEGFR‐2 expression was not detected even in the sites with marked perivascular mononuclear cell infiltration (Figure 1a,d,g), suggesting that the results might be more meaningful. According to this result, although various factors are known to affect the vascular and BBB integrity of the CNS, it may be suggested that VEGF is not always associated with the pathogenesis of GME and NE. Moreover, it is unlikely that therapy targeted to VEGFR will always be rewarding for subjects with GME and NE.
The relatively small sample size and selective sampling from individual tissue sections were the most marked limitations of this study. The small sample size limited sufficient comparisons of the expression rates of each TK and made a sufficient comparison between the two subgroups difficult. Furthermore, as the samples used in the study were collected from the most prominent lesion in each case and were evaluated in randomly selected microscopic fields, there was a clear limit to representing the TK expression in the entire specimen. Another weakness of this study was the lack of information about the histopathologic features, treatment and survival of the subjects. The changes in TK expression pattern according to histopathological feature, treatment used and clinical course are expected to be meaningful in canine GME and NE. Thus, further large retrospective studies with detailed medical records are needed to clarify the role of TKs.
5. CONCLUSION
Known targets of conventional multi‐targeted TKIs were found to be expressed in canine GME and NE in this study. It was found that all samples exhibited positive immunostaining for at least one TK, and various co‐expression patterns were identified in each of the seven cases analysed. Although positive expression of PDGFR‐α, PDGFR‐ß, c‐Kit and c‐Abl does not suggest specific functional activities, their presence does suggest that these kinases may play a role in the pathogenesis and development of GME and NE. Furthermore, PDGFR‐ß staining was significantly more intense and diffuse than that of other TKs in all samples, indicating that PDGFR‐ß may be involved in key aspects of the disease. We thus conclude that multi‐targeted therapy with TKIs may provide benefits in the treatment of canine GME and NE by suppressing the activity of these TKs. An investigation of the clinical application of TKIs in dogs with GME and NE should also be pursued in the future.
AUTHOR CONTRIBUTION
Joong‐Hyun Song: Conceptualization; Data curation; Investigation; Methodology; Writing‐original draft. Do‐Hyeon Yu: Formal analysis; Funding acquisition; Supervision. Tae‐Sung Hwang: Data curation; Resources; Visualization. Byung‐Joon Seung: Data curation; Investigation; Resources.Jung‐Hyang Sur: Data curation; Investigation; Visualization. Young Joo Kim: Data curation; Validation; Visualization. Dong‐In Jung: Investigation; Resources; Supervision; Writing‐review & editing.
Song J‐H, Yu D-H, Hwang T‐S, et al. Expression of platelet‐derived growth factor receptor‐α/ß, vascular endothelial growth factor receptor‐2, c‐Abl, and c‐Kit in canine granulomatous meningoencephalitis and necrotizing encephalitis. Vet Med Sci. 2020;6:965–974. 10.1002/vms3.314
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.314
REFERENCES
- Adamo, P. F. , Rylander, H. , & Adams, W. M. (2007). Ciclosporin use in multi‐drug therapy for meningoencephalomyelitis of unknown aetiology in dogs. Journal of Small Animal Practice, 48, 486–496. 10.1111/j.1748-5827.2006.00303.x [DOI] [PubMed] [Google Scholar]
- Akashi, N. , Matsumoto, I. , Tanaka, Y. , Inoue, A. , Yamamoto, K. , Umeda, N. , … Ito, S. (2011). Comparative suppressive effects of tyrosine kinase inhibitors imatinib and nilotinib in models of autoimmune arthritis. Modern Rheumatology, 21, 267–275. 10.3109/s10165-010-0392-5 [DOI] [PubMed] [Google Scholar]
- Azizi, G. , Goudarzvand, M. , Afraei, S. , Sedaghat, R. , & Mirshafiey, A. (2015). Therapeutic effects of dasatinib in mouse model of multiple sclerosis. Immunopharmacology and Immunotoxicology, 37, 287–294. 10.3109/08923973.2015.1028074 [DOI] [PubMed] [Google Scholar]
- Azizi, G. , Haidari, M. R. , Khorramizadeh, M. , Naddafi, F. , Sadria, R. , Javanbakht, M. H. , … Mirshafiey, A. (2014). Effects of imatinib mesylate in mouse models of multiple sclerosis and in vitro determinants. Iranian Journal of Allergy, Asthma and Immunology, 13, 198–206. [PubMed] [Google Scholar]
- Azizi, G. , & Mirshafiey, A. (2013). Imatinib mesylate: An innovation in treatment of autoimmune diseases. Recent Patents on Inflammation & Allergy Drug Discovery, 7, 259–267. [DOI] [PubMed] [Google Scholar]
- Barnoon, I. , Shamir, M. H. , Aroch, I. , Bdolah‐Abram, T. , Srugo, I. , Konstantin, L. , & Chai, O. (2016). Retrospective evaluation of combined mycophenolate mofetil and prednisone treatment for meningoencephalomyelitis of unknown etiology in dogs: 25 cases (2005–2011). Journal of Veterinary Emergency and Critical Care, 26, 116–124. 10.1111/vec.12399 [DOI] [PubMed] [Google Scholar]
- Bavcar, S. , & Argyle, D. J. (2012). Receptor tyrosine kinase inhibitors: Molecularly targeted drugs for veterinary cancer therapy. Veterinary and Comparative Oncology, 10, 163–173. 10.1111/j.1476-5829.2012.00342.x [DOI] [PubMed] [Google Scholar]
- Bebo Jr, B. F. , Yong, T. , Orr, E. L. , & Linthicum, D. S. (1996). Hypothesis: A possible role for mast cells and their inflammatory mediators in the pathogenesis of autoimmune encephalomyelitis. Journal of Neuroscience Research, 45, 340–348. [DOI] [PubMed] [Google Scholar]
- Benoist, C. , & Mathis, D. (2002). Mast cells in autoimmune disease. Nature, 420, 875–878. 10.1038/nature01324 [DOI] [PubMed] [Google Scholar]
- Brenner, T. , Soffer, D. , Shalit, M. , & Levi‐Schaffer, F. (1994). Mast cells in experimental allergic encephalomyelitis: Characterization, distribution in the CNS and in vitro activation by myelin basic protein and neuropeptides. Journal of the Neurological Sciences, 122, 210–213. [DOI] [PubMed] [Google Scholar]
- Carmeliet, P. (2005). Angiogenesis in life, disease and medicine. Nature, 438, 932–936. [DOI] [PubMed] [Google Scholar]
- Claesson‐Welsh, L. (1994). Platelet‐derived growth factor receptor signals. Journal of Biological Chemistry, 269, 32023–32026. [PubMed] [Google Scholar]
- Clauss, M. (2000). Molecular biology of the VEGF and the VEGF receptor family. Seminars in Thrombosis and Hemostasis, 26, 561–569. 10.1055/s-2000-13213 [DOI] [PubMed] [Google Scholar]
- Coates, J. R. , & Jeffery, N. D. (2014). Perspectives on meningoencephalomyelitis of unknown origin. The Veterinary Clinics of North America. Small Animal Practice, 44, 1157–1185. 10.1016/j.cvsm.2014.07.009 [DOI] [PubMed] [Google Scholar]
- Coffey, G. , DeGuzman, F. , Inagaki, M. , Pak, Y. , Delaney, S. M. , Ives, D. , … Sinha, U. (2012). Specific inhibition of spleen tyrosine kinase suppresses leukocyte immune function and inflammation in animal models of rheumatoid arthritis. Journal of Pharmacology and Experimental Therapeutics, 340, 350–359. 10.1124/jpet.111.188441 [DOI] [PubMed] [Google Scholar]
- Crespo, O. , Kang, S. C. , Daneman, R. , Lindstrom, T. M. , Ho, P. P. , Sobel, R. A. , … Robinson, W. H. (2011). Tyrosine kinase inhibitors ameliorate autoimmune encephalomyelitis in a mouse model of multiple sclerosis. Journal of Clinical Immunology, 31, 1010–1020. 10.1007/s10875-011-9579-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deininger, M. , Buchdunger, E. , & Druker, B. J. (2005). The development of imatinib as a therapeutic agent for chronic myeloid leukemia. Blood, 105, 2640–2653. 10.1182/blood-2004-08-3097 [DOI] [PubMed] [Google Scholar]
- Demierre, S. , Tipold, A. , Griot‐Wenk, M. E. , Welle, M. , Vandevelde, M. , & Jaggy, A. (2001). Correlation between the clinical course of granulomatous meningoencephalomyelitis in dogs and the extent of mast cell infiltration. The Veterinary Record, 148, 467–472. 10.1136/vr.148.15.467 [DOI] [PubMed] [Google Scholar]
- Dewey, C. W. (2015). Encephalopathies: Disorders of the brain In Dewey C. W. (Ed.), Practical guide to canine and feline neurology, 3rd ed. (pp. 141–236). New Jersey: John Wiley & Sons. [Google Scholar]
- Dubreuil, P. , Letard, S. , Ciufolini, M. , Gros, L. , Humbert, M. , Casteran, N. , … Hermine, O. (2009). Masitinib (AB1010), a potent and selective tyrosine kinase inhibitor targeting KIT. PLoS One, 4, e7258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegel, T. , Boettcher, I. C. , Matiasek, K. , Oevermann, A. , Doherr, M. G. , Oechtering, G. , & Henke, D. (2011). Comparison of oral administration of lomustine and prednisolone or prednisolone alone as treatment for granulomatous meningoencephalomyelitis or necrotizing encephalitis in dogs. Journal of the American Veterinary Medical Association, 238, 337–345. 10.2460/javma.238.3.337 [DOI] [PubMed] [Google Scholar]
- Gay, D. , & Esiri, M. (1991). Blood‐brain barrier damage in acute multiple sclerosis plaques. An Immunocytological Study. Brain., 114, 557–572. 10.1093/brain/114.1.557 [DOI] [PubMed] [Google Scholar]
- Granger, N. , Smith, P. M. , & Jeffery, N. D. (2010). Clinical findings and treatment of non‐infectious meningoencephalomyelitis in dogs: A systematic review of 457 published cases from 1962 to 2008. The Veterinary Journal, 184, 290–297. 10.1016/j.tvjl.2009.03.031 [DOI] [PubMed] [Google Scholar]
- Greer, K. A. , Wong, A. K. , Liu, H. , Famula, T. R. , Pedersen, N. C. , Ruhe, A. , … Neff, M. W. (2010). Necrotizing meningoencephalitis of Pug dogs associates with dog leukocyte antigen class II and resembles acute variant forms of multiple sclerosis. Tissue Antigens, 76, 110–118. 10.1111/j.1399-0039.2010.01484.x [DOI] [PubMed] [Google Scholar]
- Hershko, A. Y. , & Rivera, J. (2010). Mast cell and T cell communication; amplification and control of adaptive immunity. Immunology Letters, 128, 98–104. 10.1016/j.imlet.2009.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, D. I. , Kang, B. T. , Park, C. , Yoo, J. H. , Gu, S. H. , Jeon, H. W. , … Park, H. M. (2007). A comparison of combination therapy (cyclosporine plus prednisolone) with sole prednisolone therapy in 7 dogs with necrotizing meningoencephalitis. Journal of Veterinary Medical Science, 69, 1303–1306. 10.1292/jvms.69.1303 [DOI] [PubMed] [Google Scholar]
- Karpiak, V. C. , Bridges, R. J. , & Eyer, C. L. (2001). Organotins disrupt components of glutamate homeostasis in rat astrocyte cultures. J. Toxicol. Environ. Health A., 63, 273–287. 10.1080/15287390151143668 [DOI] [PubMed] [Google Scholar]
- Kinet, J. P. (2007). The essential role of mast cells in orchestrating inflammation. Immunological Reviews, 217, 5–7. 10.1111/j.1600-065X.2007.00528.x [DOI] [PubMed] [Google Scholar]
- Koehler, N. K. , Roebbert, M. , Dehghani, K. , Ballmaier, M. , Claus, P. , von Hoersten, S. , … Heidenreich, F. (2008). Up‐regulation of platelet‐derived growth factor by peripheral‐blood leukocytes during experimental allergic encephalomyelitis. Journal of Neuroscience Research, 86, 392–402. 10.1002/jnr.21497 [DOI] [PubMed] [Google Scholar]
- Koleske, A. J. , Gifford, A. M. , Scott, M. L. , Nee, M. , Bronson, R. T. , Miczek, K. A. , & Baltimore, D. (1998). Essential roles for the Abl and Arg tyrosine kinases in neurulation. Neuron, 21, 1259–1272. 10.1016/S0896-6273(00)80646-7 [DOI] [PubMed] [Google Scholar]
- Leblanc, A. K. , Miller, A. N. , Galyon, G. D. , Moyers, T. D. , Long, M. J. , Stuckey, A. C. , … Morandi, F. (2012). Preliminary evaluation of serial (18) FDG‐PET/CT to assess response to toceranib phosphate therapy in canine cancer. Veterinary Radiology & Ultrasound, 53, 348–357. 10.1111/j.1740-8261.2012.01925.x [DOI] [PubMed] [Google Scholar]
- Levine, J. , Fosgate, G. T. , Porter, B. , Schatzberg, S. J. , & Greer, K. (2008). Epidemiology of necrotizing meningoencephalitis in Pug dogs. Journal of Veterinary Internal Medicine, 22, 961–968. 10.1111/j.1939-1676.2008.0137.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Holdbrooks, A. T. , De Sarno, P. , Rowse, A. L. , Yanagisawa, L. L. , McFarland, B. C. , … Qin, H. (2014). Therapeutic efficacy of suppressing the Jak/STAT pathway in multiple models of experimental autoimmune encephalomyelitis. The Journal of Immunology, 192, 59–72. 10.4049/jimmunol.1301513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- London, C. A. (2009). Tyrosine kinase inhibitors in veterinary medicine. Topics in Companion Animal Medicine, 24, 106–112. 10.1053/j.tcam.2009.02.002 [DOI] [PubMed] [Google Scholar]
- Luo, J. , & Miller, M. W. (1999). Platelet‐derived growth factor‐mediated signal transduction underlying astrocyte proliferation: Site of ethanol action. Journal of Neuroscience, 19, 10014–10025. 10.1523/JNEUROSCI.19-22-10014.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda, K. , Nishiyama, C. , Ogawa, H. , & Okumura, K. (2010). GATA2 and Sp1 positively regulate the c‐kit promoter in mast cells. The Journal of Immunology, 185, 4252–4260. [DOI] [PubMed] [Google Scholar]
- Maeda, Y. , Solanky, M. , Menonna, J. , Chapin, J. , Li, W. , & Dowling, P. (2001). Platelet‐derived growth factor‐alpha receptor‐positive oligodendroglia are frequent in multiple sclerosis lesions. Annals of Neurology, 49, 776–785. [DOI] [PubMed] [Google Scholar]
- Manley, P. W. , Stiefl, N. , Cowan‐Jacob, S. W. , Kaufman, S. , Mestan, J. , Wartmann, M. , … Gallagher, N. (2010). Structural resemblances and comparisons of the relative pharmacological properties of imatinib and nilotinib. Bioorganic & Medicinal Chemistry, 18, 6977–6986. 10.1016/j.bmc.2010.08.026 [DOI] [PubMed] [Google Scholar]
- Matsuki, N. , Fujiwara, K. , Tamahara, S. , Uchida, K. , Matsunaga, S. , Nakayama, H. , … Ono, K. (2004). Prevalence of autoantibody in cerebrospinal fluids from dogs with various CNS diseases. Journal of Veterinary Medical Science, 66, 295–297. 10.1292/jvms.66.295 [DOI] [PubMed] [Google Scholar]
- Matsuki, N. , Takahashi, M. , Yaegashi, M. , Tamahara, S. , & Ono, K. (2009). Serial examinations of anti‐GFAP autoantibodies in cerebrospinal fluids in canine necrotizing meningoencephalitis. Journal of Veterinary Medical Science, 71, 99–100. 10.1292/jvms.71.99 [DOI] [PubMed] [Google Scholar]
- Mirshafiey, A. , Ghalamfarsa, G. , Asghari, B. , & Azizi, G. (2014). Receptor tyrosine kinase and tyrosine kinase inhibitors: New hope for success in multiple sclerosis therapy. Innovations in Clinical Neuroscience, 11, 23–36. [PMC free article] [PubMed] [Google Scholar]
- Moor, A. C. , de Vries, H. E. , de Boer, A. G. , & Breimer, D. D. (1994). The blood‐brain barrier and multiple sclerosis. Biochemical Pharmacology, 47, 1717–1724. 10.1016/0006-2952(94)90297-6 [DOI] [PubMed] [Google Scholar]
- Munana, K. R. , & Luttgen, P. J. (1998). Prognostic factors for dogs with granulomatous meningoencephalomyelitis: 42 cases (1982–1996). Journal of the American Veterinary Medical Association, 212, 1902–1906. [PubMed] [Google Scholar]
- Nair, A. , Frederick, T. J. , & Miller, S. D. (2008). Astrocytes in multiple sclerosis: A product of their environment. Cellular and Molecular Life Sciences, 65, 2702–2720. 10.1007/s00018-008-8059-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natoli, C. , Perrucci, B. , Perrotti, F. , Falchi, L. , Iacobelli, S. , Consorzio Interuniversitario Nazionale per Bio‐Oncologia (CINBO) . (2010). Tyrosine kinase inhibitors. Current Cancer Drug Targets, 10, 462–483. [DOI] [PubMed] [Google Scholar]
- Park, E. S. , Uchida, K. , & Nakayama, H. (2014). Establishment of a rat model for canine necrotizing meningoencephalitis (NME). Veterinary Pathology, 51, 1151–1164. 10.1177/0300985813519115 [DOI] [PubMed] [Google Scholar]
- Piconese, S. , Costanza, M. , Musio, S. , Tripodo, C. , Poliani, P. L. , Gri, G. , … Pedotti, R. (2011). Exacerbated experimental autoimmune encephalomyelitis in mast‐cell‐deficient Kit W‐sh/W‐sh mice. Laboratory Investigation, 91, 627–641. 10.1038/labinvest.2011.3 [DOI] [PubMed] [Google Scholar]
- Plummer, S. B. , Wheeler, S. J. , Thrall, D. E. , & Kornegay, J. N. (1992). Computed tomography of primary inflammatory brain disorders in dogs and cats. Veterinary Radiology & Ultrasound, 33, 307–312. 10.1111/j.1740-8261.1992.tb00148.x [DOI] [Google Scholar]
- Proescholdt, M. A. , Jacobson, S. , Tresser, N. , Oldfield, E. H. , & Merrill, M. J. (2002). Vascular endothelial growth factor is expressed in multiple sclerosis plaques and can induce inflammatory lesions in experimental allergic encephalomyelitis rats. Journal of Neuropathology and Experimental Neurology, 61, 914–925. 10.1093/jnen/61.10.914 [DOI] [PubMed] [Google Scholar]
- Safavi, F. , Li, H. , Gonnella, P. , Mari, E. R. , Rasouli, J. , Zhang, G. X. , & Rostami, A. (2015). c‐kit plays a critical role in induction of intravenous tolerance in experimental autoimmune encephalomyelitis. Immunologic Research, 61, 294–302. 10.1007/s12026-015-8624-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayed, B. A. , Christy, A. , Quirion, M. R. , & Brown, M. A. (2008). The master switch: The role of mast cells in autoimmunity and tolerance. Annual Review of Immunology, 26, 705–739. 10.1146/annurev.immunol.26.021607.090320 [DOI] [PubMed] [Google Scholar]
- Sayed, B. A. , Christy, A. L. , Walker, M. E. , & Brown, M. A. (2010). Meningeal mast cells affect early T cell central nervous system infiltration and blood‐brain barrier integrity through TNF: A role for neutrophil recruitment? The Journal of Immunology, 184, 6891–6900. 10.4049/jimmunol.1000126 [DOI] [PubMed] [Google Scholar]
- Secor, V. H. , Secor, W. E. , Gutekunst, C. A. , & Brown, M. A. (2000). Mast cells are essential for early onset and severe disease in a murine model of multiple sclerosis. Journal of Experimental Medicine, 191, 813–822. 10.1084/jem.191.5.813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew, M. V. , & Vinters, H. V. (2010). Astrocytes: Biology and pathology. Acta Neuropathologica, 119, 7–35. 10.1007/s00401-009-0619-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorjonen, D. (1990). Clinical and histopathological features of granulomatous meningoencephalomyelitis in dogs. Journal of the American Animal Hospital Association, 26, 141–147. [Google Scholar]
- Sospedra, M. , & Martin, R. (2005). Immunology of multiple sclerosis. Annual Review of Immunology, 23, 683–747. 10.1146/annurev.immunol.23.021704.115707 [DOI] [PubMed] [Google Scholar]
- Suzuki, M. , Uchida, K. , Morozumi, M. , Hasegawa, T. , Yanai, T. , Nakayama, H. , & Tateyama, S. (2003). A comparative pathological study on canine necrotizing meningoencephalitis and granulomatous meningoencephalomyelitis. Journal of Veterinary Medical Science, 65, 1233–1239. 10.1292/jvms.65.1233 [DOI] [PubMed] [Google Scholar]
- Talarico, L. R. , & Schatzberg, S. J. (2010). Idiopathic granulomatous and necrotising inflammatory disorders of the canine central nervous system: A review and future perspectives. Journal of Small Animal Practice, 51, 138–149. 10.1111/j.1748-5827.2009.00823.x [DOI] [PubMed] [Google Scholar]
- Uchida, K. , Park, E. , Tsuboi, M. , Chambers, J. K. , & Nakayama, H. (2016). Pathological and immunological features of canine necrotising meningoencephalitis and granulomatous meningoencephalitis. The Veterinary Journal, 213, 72–77. 10.1016/j.tvjl.2016.05.002 [DOI] [PubMed] [Google Scholar]
- Veillette, A. , Rhee, I. , Souza, C. M. , & Davidson, D. (2009). PEST family phosphatases in immunity, autoimmunity, and autoinflammatory disorders. Immunological Reviews, 228, 312–324. [DOI] [PubMed] [Google Scholar]
- Vermersch, P. , Benrabah, R. , Schmidt, N. , Zéphir, H. , Clavelou, P. , Vongsouthi, C. , … Hermine, O. (2012). Masitinib treatment in patients with progressive multiple sclerosis: A randomized pilot study. BMC Neurol., 12, 36 10.1186/1471-2377-12-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viglietta, V. , Baecher‐Allan, C. , Weiner, H. L. , & Hafler, D. A. (2004). Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. Journal of Experimental Medicine, 199, 971–979. 10.1084/jem.20031579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters, L. , Martin, L. , Price, J. , & Sula, M. M. (2018). Expression of receptor tyrosine kinase targets PDGFR‐β, VEGFR2 and KIT in canine transitional cell carcinoma. Veterinary and Comparative Oncology, 16, 117–122. [DOI] [PubMed] [Google Scholar]
- Werner, P. , Pitt, D. , & Raine, C. S. (2001). Multiple sclerosis: Altered glutamate homeostasis in lesions correlates with oligodendrocyte and axonal damage. Annals of Neurology, 50, 169–180. 10.1002/ana.1077 [DOI] [PubMed] [Google Scholar]
- Williams, A. , Piaton, G. , & Lubetzki, C. (2007). Astrocytes–friends or foes in multiple sclerosis? Glia, 55, 1300–1312. 10.1002/glia.20546 [DOI] [PubMed] [Google Scholar]
- Wilson, H. C. , Scolding, N. J. , & Raine, C. S. (2006). Co‐expression of PDGF alpha receptor and NG2 by oligodendrocyte precursors in human CNS and multiple sclerosis lesions. Journal of Neuroimmunology, 176, 162–173. [DOI] [PubMed] [Google Scholar]
- Zarfoss, M. , Schatzberg, S. , Venator, K. , Cutter‐Schatzberg, K. , Cuddon, P. , Pintar, J. , … Delahunta, A. (2006). Combined cytosine arabinoside and prednisone therapy for meningoencephalitis of unknown aetiology in 10 dogs. Journal of Small Animal Practice, 47, 588–595. 10.1111/j.1748-5827.2006.00172.x [DOI] [PubMed] [Google Scholar]
- Zhang, J. , Cao, R. , Zhang, Y. , Jia, T. , Cao, Y. , & Wahlberg, E. (2009). Differential roles of PDGFR‐alpha and PDGFR‐beta in angiogenesis and vessel stability. The FASEB Journal, 23, 153–163. [DOI] [PubMed] [Google Scholar]


