Abstract
Objective
The coronavirus disease 2019 (COVID‐19) pandemic has led to rapid research and reporting on potential preventatives and treatments for the disease, including the drug hydroxychloroquine (HCQ). Despite a lack of robust evidence to support the use of HCQ for treatment of COVID‐19, it was publicly endorsed and received widespread media coverage and public interest. The purpose of this report is to describe and contextualize the surges in public interest, demand, and adoption of HCQ for treatment of COVID‐19 and outline implications for future public health policy and practice.
Methods
Using national and global events and Google Trends data as a measure of public interest, we describe the timeline and trends in the emergence of, interest in, and adoption of HCQ as a treatment of COVID‐19. We additionally review reports on public demand for HCQ for treatment of COVID‐19 and impacts on medication access among patients with indicated uses.
Results
Public interest and demand for HCQ surged in the United States and globally following endorsements from public officials and enaction of policies to facilitate off‐label use of HCQ for treatment of COVID‐19. Surges in demand for HCQ led to multiple documented shortages and barriers to accessing HCQ treatment for patients with indicated uses for HCQ. Although there have been reversals in policies to support HCQ use for treatment of COVID‐19 in some regions, others have continued or expanded recommended uses.
Conclusion
Insights from the global response to HCQ and COVID‐19 can be used to inform prudent decision‐making in the future to prevent premature action and promote informed and equitable responses to promote public health.
Introduction
There is an urgent need for pharmaceutical therapies to combat the coronavirus disease 2019 (COVID‐19) pandemic. Despite a lack of robust evidence to support its effectiveness (1), advocacy and adoption of hydroxychloroquine (HCQ) as a prophylactic and treatment of COVID‐19 led to surges in media coverage, public interest, and demand in the United States and internationally.
This rush to judgment and uptake of HCQ was followed by major reversals in guidance and practice after emerging research failed to support purported benefits for COVID‐19 (1). Premature action to expand access to HCQ for COVID‐19 resulted in shortages, and other barriers to accessing treatment for patients with indicated uses for the drug may have magnified inequalities in health care access and health disparities (2, 3).
In this article, we describe the emergence of HCQ as a potential therapy for COVID, present data on the public’s seeking of this information over time and across regions, and discuss implications for public health policy and practice. We focus on the recent developments surrounding HCQ and COVID‐19 with the hope that these insights can be used to avoid clamor and upset around emerging treatments and inform prudent decision‐making moving forward.
The concept of international norm progression proposed by Finnemore and Sikkink (4) provides a useful framework to outline the developments in advocacy and adoption of HCQ for treatment of COVID‐19 and resulting implications:
-
Norm emergence and entrepreneurs:
US President Donald Trump propelled HCQ into the spotlight, capturing the attention of media, consumers, and political actors both nationally and internationally after publicly supporting HCQ and driving policy actions to enable its use for COVID‐19.
Didier Raoult is another prominent norm entrepreneur and scientific figure who promoted the use of HCQ for treatment of COVID‐19. Raoult is the director of the Institut Hospitalo‐Universitaire Méditerranée Infection in France and senior author on the preliminary study by Gautret et al (5). Raoult made claims about the efficacy of HCQ on news and social media in advance of published peer‐reviewed research findings (6).
President Trump may have driven the major spike in interest at the international level, but it was preceded by and justified with claims made by Raoult because he cited Raoult’s research in his promotion of HCQ.
Summaries of preliminary research and anecdotal claims supporting HCQ applications for treatment of COVID‐19 were further propagated through a variety of public figures and media channels.
-
Norm cascade:
HCQ as a potential and supported treatment of COVID‐19 was an emerging norm and justification for policy action (eg, emergency use authorization [EUA], Centers for Disease Control and Prevention coronavirus treatment guidance).
Government announcements, policy statements, and aid disbursement further contributed to the legitimization of HCQ use for treatment of COVID‐19. The claims in these official announcements varied; some explicitly stated that evidence on HCQ use in COVID‐19 was limited. For example, in the US Food and Drug Administration’s (FDA’s) EUA, the recommendation of HCQ for treatment of COVID‐19 in national guidelines was based on “limited in‐vitro and anecdotal clinical data” (7).
HCQ support cascaded into consumer interest and behavior; Internet searches indicative of online shopping/purchase interest in HCQ rose substantially in March 2020 (8), as did the number of new US patients who were prescribed HCQ (9).
-
Norm internalization:
Widespread and uniform acceptance of HCQ as a treatment of COVID‐19 has not been fully realized, with developments in the United States indicating a reversal in policy (eg, the FDA revoked the EUA). However, White House officials reportedly pressured the FDA to reinstate the EUA (10), indicating conflicting positions and messages on HCQ use.
Officials in several other countries announced further commitment to HCQ use for COVID‐19. For example, Indian public officials recommended HCQ for high‐risk COVID‐19 cases (11), which has been described as an unjustified and concerning state endorsement (12).
In Brazil, government officials endorsed HCQ treatment for children and pregnant women in early COVID‐19 cases (13). In early July, Brazilian President Jair Bolsonaro confirmed that he had tested positive for COVID‐19 and claimed he benefited from treatment with HCQ (14).
Regardless of the duration and extent of norm integration, premature support and uptake of HCQ for treatment of COVID‐19 spurred global actions and market upsets. These may have afforded new opportunities for governments and transnational companies to influence global markets and governance processes, potentially reinforcing power structures and asymmetries (15). The rapid discourse and mobilization also impacted public interest in HCQ, as we demonstrate below.
Materials and Methods
To present and contextualize trends in the general public’s seeking of HCQ‐related information, we used Google Trends data to develop a timeline of public search interest in HCQ and relevant events occurring in the United States. Google Trends is an online portal where users can assess search patterns across time and geographic ranges. Google is the most popular search engine in the world, and Google Trends offers a free, publicly available tool to assess public search patterns for a specific term or topic (16). Google Trends data can be used to understand information‐seeking behaviors and have been shown to correlate with and complement other measures of information sharing (eg, Twitter) (17). Online search data can also be used to predict consumer behavior; prior research has outlined correlations between Google search volumes and use rates for prescription drugs (18, 19). Google Trends data have recently been used to understand interest, concerns, and behaviors related to the COVID‐19 pandemic (20, 21).
We extracted Google Trends search data for the term “hydroxychloroquine” in the medication topic between January 1, 2020, and August 1, 2020. The search was conducted on August 9, 2020, and default search settings were used, except for searching within the medication topic. Google Trends groups related search terms within search topics, so the medication topic was used to include search terms other than “hydroxychloroquine” with the same meaning (eg, Plaquenil) or non‐English search terms. Data on relative search volume (RSV) and top related queries were extracted for the United States, for worldwide subregions, and globally. RSV represents a normalized value (0‐100) for the proportion of searches conducted for a specific topic within a geography and time range (22). RSVs that were less than one or not reported were recoded as zero for visualization. Countries that did not have RSV data available are reported in Supplementary Table 1.
Results
Figure 1 outlines a timeline of events related to HCQ use for treatment of COVID‐19 and US and global search interest in HCQ. As the Google Trends data show, interest in HCQ rose substantially following public announcements supporting use for treatment of COVID‐19.
Figure 1.
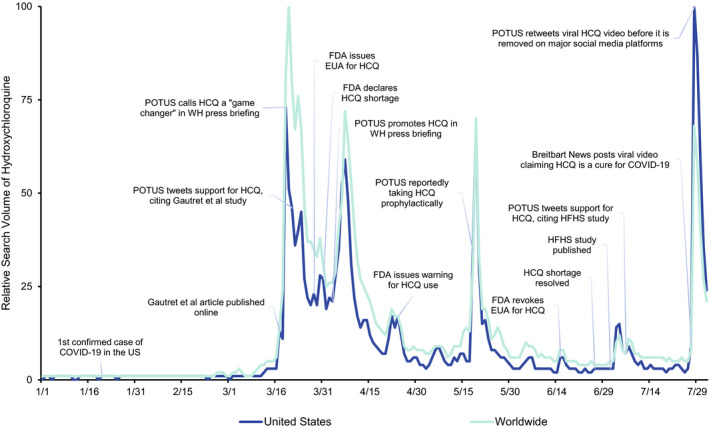
Events and relative search volume for HCQ in the United States and worldwide, January 1, 2020, to August 1, 2020. COVID‐19, coronavirus disease 2019; EUA, emergency use authorization; FDA, US Food and Drug Administration; HCQ, hydroxychloroquine; HFHS, Henry Ford Health System; POTUS, President of the United States; WH, White House.
Search interest in HCQ in the United States and globally remained low through February 2020. The first major surge in search interest occurred in March 2020, following a White House press briefing in which the President described HCQ as a “game changer” for COVID‐19 treatment; global HCQ search interest reached its highest peak on March 20. On March 28, the FDA issued an EUA to open HCQ supplies from the Strategic National Stockpile for treatment in patients with COVID‐19 and, days later, declared a national shortage of HCQ.
Another spike in search interest occurred in late May, following an announcement that the President was reportedly taking HCQ prophylactically for COVID‐19. Search interest was highest in the United States in late July, following a viral video of a press conference held by a group of doctors critical of the pandemic response. The video featured depictions of HCQ as a “cure” for COVID‐19, which eliminated the need for masks and social distancing measures. The video was posted on news and social media platforms and shared by public figures, including the President. Despite major social media companies making quick efforts to remove the video from their platforms the same day, it had already been viewed millions of times and continued to be reposted after the original was taken down. On Facebook alone, the original video had more than 17 million views before it was removed (23).
Figure 2 outlines similar trends, with varying peaks in HCQ search interest among a subset of countries with prominent figures and events supporting use for treatment of COVID‐19. These trends highlight the international implications of premature support for HCQ among researchers and public officials. Figure 3 shows global comparisons in HCQ interest, with high RSVs in the United States, Brazil, and India, which also had support from political leadership for treatment of COVID‐19 during the search period. Of note, these three countries have had the highest confirmed cases and deaths from COVID‐19 through October 2020 (24).
Figure 2.
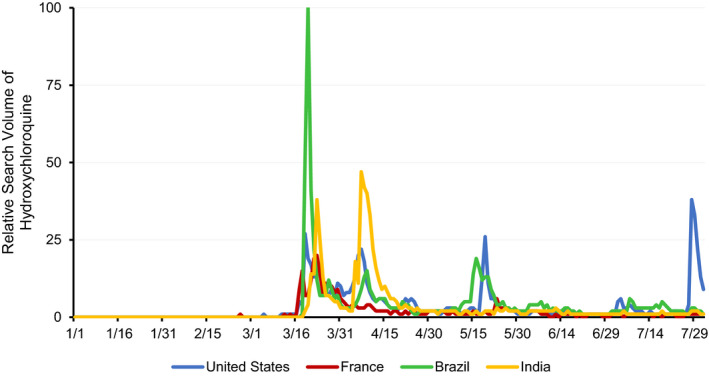
Relative search volume for hydroxychloroquine in Brazil, France, India, and the United States, January 1, 2020, to August 1, 2020.
Figure 3.
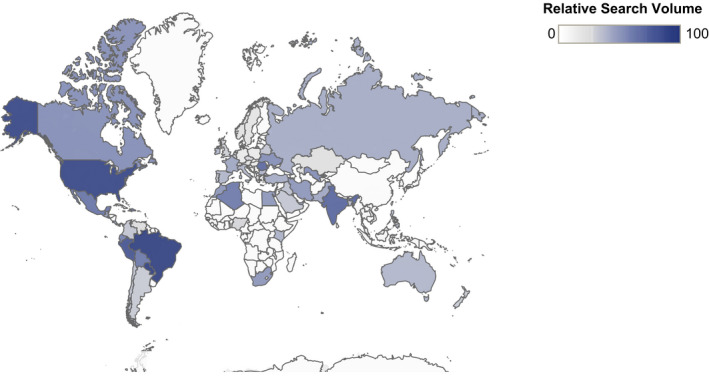
Relative search volume for hydroxychloroquine by country, January 1, 2020, to August 1, 2020.
Additionally, search queries combining “hydroxychloroquine” and “Trump” were in the top 10 related queries (ie, top searches among people who also searched for HCQ) in the United States and worldwide, indicating that actions from the President sparked interest and had broad influence during the search period. Both Donald Trump and Didier Raoult were listed in the top search topics related to HCQ worldwide. Of potential concern, search terms related to purchasing HCQ rose over the course of the search period in the United States (eg, “buy hydroxychloroquine”) and globally (eg, “hydroxychloroquine for sale” and “hidroxicloroquina comprar,” which is Spanish for “buy hydroxychloroquine”). Additional research is warranted to understand whether this interest could be indicative of unlawful online marketing and sale of HCQ.
The full scope of impacts that medication shortages have had on patients taking HCQ for systemic lupus erythematosus (SLE) and other indicated uses is not yet clear. Emerging research has found patient‐reported barriers to filling prescriptions, interruptions in HCQ treatment, and emotional stress and anxiety related to medication access during the COVID‐19 pandemic (2, 25). A survey by the Lupus Research Alliance found that among 334 respondents who were already taking HCQ for lupus, more than 36% reported that they had issues refilling their HCQ prescription or were not able to refill their HCQ prescription at all (26). Another study conducted by the Lupus Foundation of America surveyed 2855 patients with lupus and found that 90% were taking HCQ to manage their lupus. More than half of respondents taking HCQ reported difficulties accessing their prescription between March and May 2020 (27). Reports of HCQ shortages and concerns about rheumatology patients’ access have also been reported in Canada (28) and countries across Europe (2) and the Middle East (29).
Although we do not yet know the full extent of the impact that barriers to HCQ access and shortages have had on patients, serious adverse effects can result from withdrawal in patients with SLE, for which HCQ is a cornerstone of treatment (30). Both SLE and COVID‐19 disproportionately impact racial and ethnic minority populations (31, 32), so interrupted access to HCQ may have further exacerbated existing disparities in health care access and health outcomes.
Beyond impacting access for patients with indicated uses, widespread adoption of HCQ in clinical practice may have further reinforced beliefs in its effectiveness and legitimacy as a treatment of COVID‐19 (33); this response could undermine recruitment and retention for clinical trials that seek to establish such evidence because patients may be reluctant to be randomly assigned to a condition other than the one they believe is effective (34). Conflicting information about HCQ circulating through media and public discourse has also reportedly led to difficulties in recruiting and enrolling participants into clinical trials (35). Additionally, the rapid endorsement of HCQ as a treatment of COVID‐19 in official guidelines and clinical practice, followed by reversals in policies and positions, may undermine public trust in research and evidence‐based recommendations (36).
Discussion
How do we react in times of uncertainty? Moving forward with skeptical optimism and an abundance of caution is a reasonable first step. Rapid endorsement of HCQ for treatment of COVID‐19 precluded sound evidence while still generating international attention and adoption in policy and practice. The COVID‐19 response to HCQ is unlikely to be a onetime scenario because iterative cycles of interest in emerging treatments will undoubtedly present again (37).
Given widespread online access and worldwide impacts and upheaval caused by COVID‐19, the rapid health care responses and collaboration across fields and international borders is possibly at its highest since the surge of the HIV/AIDS epidemic. Although swift action and progress to address the immediate threats of the COVID‐19 pandemic should be commended, it is important that we move forward in a conscious manner, guided by an evidence base that comes from high‐quality research, not from rushed judgments based on preliminary studies, perspectives from individual scientists, or pressure from political leaders. Public‐ and private‐sector health care institutions, organizations, and individual actors should establish transparent guidelines to evaluate and allocate emerging treatments in an ethical, evidence‐based manner. Responses should also consider the broader context in which the pandemic has taken place and capitalize on this opportunity to critically examine and address social and political factors that reinforce inequities and support equitable and sustainable advancements throughout the COVID‐19 pandemic (38, 39).
Researchers should be proactive in communicating findings to the public in a manner that clearly outlines the limitations of current understanding and should be quick to address and combat misinformation that arises (40). Recent research on Twitter has found that there are more tweets with false information about COVID‐19, whereas tweets on science‐based evidence and fact‐checking have higher engagement and retweets (40), which offers promise for researchers to combat false information in the future. It is critical that we protect and promote public trust in evidence‐based guidance and decisions, particularly with the importance of diligent adherence to guidelines to prevent disease spread, and in hopeful anticipation of a vaccine for COVID‐19.
Findings from a recent randomized clinical trial did not reveal any reduction in symptom severity for patients with COVID‐19 treated with HCQ (41). Citing this study, Schluger (42) has made a call to learn from “the saga of hydroxychloroquine and COVID‐19” and move on with careful consideration in research for effective therapies. Sattui et al (43) also highlight the swinging pendulum of public discourse related to HCQ and COVID‐19, from early enthusiasm and support for HCQ as a cure to widespread skepticism. The authors provide an overview of major events and trends in US media mentions of HCQ between February 2020 and May 2020 (43). Trends in media mentions of HCQ showed some similarities with the search interest trends presented here, particularly following the emergence of HCQ in March and after influential events in May. Their conclusions add to and support the findings discussed here, underscoring the need for cohesive, evidence‐based public health communications to effectively respond to the pandemic. These insights also highlight opportunities to explore the utility of using Google Trends and other online data sources to better understand surges in interest and demand, which could prove valuable in predicting public responses to new information and protecting health care access during the COVID‐19 pandemic.
Moving forward, we must also protect public trust in science by communicating clear and accurate messages around emerging therapies while avoiding premature advocacy and resulting cascades into policy and practice. As new therapies are propelled into the COVID‐19 spotlight, additional protections are needed for patients with indicated uses for existing medications to ensure access to health care. Premature uptake of HCQ for treatment of COVID‐19 and its resulting widespread consequences is just one example that we can learn from and use to guide informed, equitable, and sustainable public health responses moving forward.
Author Contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Englund had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Englund, Sheikh.
Acquisition of data
Englund.
Analysis and interpretation of data
Englund, Kinlaw, Sheikh.
Supporting information
Table S1
Tessa R. Englund, PhD, MPH, Alan C. Kinlaw, PhD, Saira Z. Sheikh, MD: University of North Carolina at Chapel Hill.
Dr. Sheikh has served on an advisory board for GlaxoSmithKline and has grant funding from Pfizer. No other disclosures relevant to this article were reported.
References
- 1. Hernandez AV, Roman YM, Pasupuleti V, Barboza JJ, White CM. Update alert 3: hydroxychloroquine or chloroquine for the treatment or prophylaxis of COVID‐19 [published online ahead of print, 2020 Oct 21]. Ann Intern Med 2020. 10.7326/L20-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Owens B. Hydroxychloroquine side‐effects raise concerns for rheumatology patients. Lancet Rheumatol 2020;2:e390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kim AH, Sparks JA, Liew JW, Putman MS, Berenbaum F, Duarte‐García A, et al. A rush to judgment? Rapid reporting and dissemination of results and its consequences regarding the use of hydroxychloroquine for COVID‐19 [editorial]. Ann Intern Med 2020;172:819–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Finnemore M, Sikkink K. International norm dynamics and political change. Int Organ 1998;52:887–917. [Google Scholar]
- 5. Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, et al. Hydroxychloroquine and azithromycin as a treatment of COVID‐19: results of an open‐label non‐randomized clinical trial. Int J Antimicrob Agents 2020;56:105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6. Lecuit M. Chloroquine and COVID‐19, where do we stand? [editorial]. Med Mal Infect 2020;50:229–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hinton DM. Request for emergency use authorization for use of chloroquine phosphate or hydroxychloroquine sulfate supplied from the strategic national stockpile for treatment of 2019 coronavirus disease. URL: https://www.fda.gov/media/136534/download. Food and Drug Administration. March 28, 2020. [Google Scholar]
- 8. Liu M, Caputi TL, Dredze M, Kesselheim AS, Ayers JW. Internet searches for unproven COVID‐19 therapies in the United States [letter]. JAMA Intern Med 2020;180:1116–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bull‐Otterson L, Gray EB, Budnitz DS, Strosnider HM, Schieber LZ, Courtney J, et al. Hydroxychloroquine and chloroquine prescribing patterns by provider specialty following initial reports of potential benefit for COVID‐19 treatment ‐ United States, January‐June 2020. MMWR Morb Mortal Wkly Rep 2020;69:1210–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McGinley L, Dawsey J. Touting criticized study, White House presses FDA to authorize hydroxychloroquine – again. The Washington Post July 10, 2020. URL: https://www.washingtonpost.com/health/2020/07/10/peter‐navarro‐hydroxychloroquine‐coronavirus/.
- 11. National Task Force for COVID‐19 . Advisory on the use of hydroxy–chloroquine as prophylaxis for SARS‐CoV‐2 infection. URL: https://www.mohfw.gov.in/pdf/AdvisoryontheuseofHydroxychloroquinasprophylaxisforSARSCoV2infection.pdf.
- 12. Rathi S, Ish P, Kalantri A, Kalantri S. Hydroxychloroquine prophylaxis for COVID‐19 contacts in India [letter]. Lancet Infect Dis 2020;20:1118–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pedroso R, Arias T, Picheta R. Brazil swipes at FDA, pushes hydroxychloroquine for pregnant women and children. CNN June 16, 2020. URL: https://www.cnn.com/2020/06/16/americas/brazil‐hydroxychloroquine‐recommendations‐fda‐intl/index.html.
- 14. Londoño E, Andreoni M, Casado L. President Bolsonaro of Brazil tests positive for coronavirus. The New York Times July 7, 2020. URL: https://www.nytimes.com/2020/07/07/world/americas/brazil‐bolsonaro‐coronavirus.html.
- 15. Ottersen OP, Dasgupta J, Blouin C, Buss P, Chongsuvivatwong V, Frenk J, et al. The political origins of health inequity: prospects for change. Lancet 2014;383:630–67. [DOI] [PubMed] [Google Scholar]
- 16. NetMarketShare . Search engine market share. URL: https://www.netmarketshare.com/search‐engine‐market‐share.aspx.
- 17. Shin SY, Seo DW, An J, Kwak H, Kim SH, Gwack J, et al. High correlation of Middle East respiratory syndrome spread with Google search and Twitter trends in Korea. Sci Rep 2016;6:32920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Simmering JE, Polgreen LA, Polgreen PM. Web search query volume as a measure of pharmaceutical utilization and changes in prescribing patterns. Res Social Adm Pharm 2014;10:896–903. [DOI] [PubMed] [Google Scholar]
- 19. Schuster NM, Rogers MA, McMahon LF Jr. Using search engine query data to track pharmaceutical utilization: a study of statins. Am J Manag Care 2010;16:e215–9. [PMC free article] [PubMed] [Google Scholar]
- 20. Beytía P, Cruz Infante C. Digital pathways, pandemic trajectories. Using Google Trends to track social responses to COVID‐19. URL: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3571360.
- 21. Higgins TS, Wu AW, Sharma D, Illing EA, Rubel K, Ting JY, et al. Correlations of online search engine trends with coronavirus disease (COVID‐19) incidence: infodemiology study. JMIR Public Health Surveill 2020;6:e19702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Google Trends . FAQ about Google Trends data. URL: https://support.google.com/trends/answer/4365533?hl=en‐GB&ref_topic=6248052.
- 23. Giles C, Sardarizadeh S, Goodman J. Hydroxychloroquine: why a video promoted by Trump was pulled on social media. BBC News July 28, 2020. URL: https://www.bbc.com/news/53559938.
- 24. World Health Organization . WHO coronavirus disease (COVID‐19) dashboard. 2020. URL: https://covid19.who.int/.
- 25. Michaud K, Wipfler K, Shaw Y, Simon TA, Cornish A, England BR, et al. Experiences of patients with rheumatic diseases in the United States during early days of the COVID‐19 pandemic. ACR Open Rheumatol 2020;2:335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lupus Research Alliance . COVID‐19 caused hydroxychloroquine issues for third of lupus patients, new LRA survey finds. 2020. URL: https://www.lupusresearch.org/covid‐19‐caused‐hydroxychloroquine‐issues‐for‐third‐of‐lupus‐patients‐new‐lra‐survey‐finds/.
- 27. Lupus Foundation of America . Lupus Foundation of America survey finds over half of respondents experienced issues accessing hydroxychloroquine during coronavirus pandemic. 2020. URL: https://www.lupus.org/news/lupus‐foundation‐of‐america‐survey‐finds‐over‐half‐of‐respondents‐experienced‐issues‐accessing?utm_source=LO&utm_medium=email&utm_campaign=HCQ_update&utm_content=button_cta.
- 28. Mendel A, Bernatsky S, Thorne JC, Lacaille D, Johnson SR, Vinet É. Hydroxychloroquine shortages during the COVID‐19 pandemic [published online ahead of print, 2020 May 20]. Ann Rheum Dis 2020. 10.1136/annrheumdis-2020-217835. [DOI] [PubMed] [Google Scholar]
- 29. Ziadé N, Hmamouchi I, El Kibbi L, Abdulateef N, Halabi H, Abutiban F, et al. The impact of COVID‐19 pandemic on rheumatology practice: a cross‐sectional multinational study. Clin Rheumatol 2020;39:3205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Aouhab Z, Hong H, Felicelli C, Tarplin S, Ostrowski RA. Outcomes of systemic lupus erythematosus in patients who discontinue hydroxychloroquine. ACR Open Rheumatol 2019;1:593–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Drenkard C, Lim SS. Update on lupus epidemiology: advancing health disparities research through the study of minority populations. Curr Opin Rheumatol 2019;31:689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tai DBG, Shah A, Doubeni CA, Sia IG, Wieland ML. The disproportionate impact of COVID‐19 on racial and ethnic minorities in the United States [published online ahead of print, 2020 Jun 20]. Clin Infect Dis 2020. 10.1093/cid/ciaa815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Addis A, Genazzani A, Trotta MP, Magrini N. Promoting better clinical trials and drug information as public health interventions for the COVID‐19 emergency in Italy [editorial]. Ann Intern Med 2020;173:654–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lenzer J. COVID‐19: US gives emergency approval to hydroxychloroquine despite lack of evidence. BMJ 2020;369:m1335. [DOI] [PubMed] [Google Scholar]
- 35. Stone W. Hydroxychloroquine debate interferes with recruiting research volunteers. NPR May 20, 2020. URL: https://www.npr.org/2020/05/20/859261838/hydroxychloroquine‐debate‐interferes‐with‐recruiting‐research‐volunteers.
- 36. Fickle experts to blame for public's vaccine skepticism. Boston Herald June 13, 2020. URL: https://www.bostonherald.com/2020/06/13/fickle‐experts‐to‐blame‐for‐vaccine‐skepticism/.
- 37. Alexander GC, Qato DM. Ensuring access to medications in the US during the COVID‐19 pandemic [viewpoint]. JAMA 2020;324:31–2. [DOI] [PubMed] [Google Scholar]
- 38. Sandset TJ, Heggen K, Engebretsen E. What we need is a sustainable politics of life [letter]. Lancet 2020;395:1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Horton R. Offline: a global health crisis? No, something far worse [comment]. Lancet 2020;395:1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pulido CM, Villarejo‐Carballido B, Redondo‐Sama G, Gómez A. COVID‐19 infodemic: more retweets for science‐based information on coronavirus than for false information. Int Sociol 2020;35:377–92. [Google Scholar]
- 41. Skipper CP, Pastick KA, Engen NW, Bangdiwala AS, Abassi M, Lofgren SM, et al. Hydroxychloroquine in nonhospitalized adults with early COVID‐19: a randomized trial. Ann Intern Med 2020;173:623–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schluger NW. The saga of hydroxychloroquine and COVID‐19: a cautionary tale [editorial]. Ann Intern Med 2020;173:662–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sattui SE, Liew JW, Graef ER, Coler‐Reilly A, Berenbaum F, Duarte‐García A, et al. Swinging the pendulum: lessons learned from public discourse concerning hydroxychloroquine and COVID‐19. Expert Rev Clin Immunol 2020;16:659–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


