Abstract
Objective
Low‐dose methotrexate (LD‐MTX), a cornerstone in the treatment of rheumatoid arthritis, is associated with a moderately increased risk of anemia, leukopenia, and skin cancers, but the risks of myelosuppression and malignancy during LD‐MTX use remain incompletely described. We examined the risks of cytopenias and skin cancers among patients taking LD‐MTX versus placebo in a large randomized controlled trial (RCT).
Methods
We prespecified secondary analyses of a double‐blind, placebo‐controlled RCT that included adults with known cardiovascular disease and diabetes or metabolic syndrome in the United States and Canada. Subjects were randomly allocated to LD‐MTX (20 mg/week maximum) or placebo. All subjects received folic acid (1 mg daily for 6days/week). We assessed the frequency of blindly adjudicated hematologic and malignant adverse events (AEs).
Results
A total of 2391 subjects were randomized to LD‐MTX (mean dosage 14.9 mg/week), and 2395 were randomized to placebo. During follow‐up, in the LD‐MTX arm, simultaneous two‐line cytopenias (n = 92 [3.9%]) or pancytopenia (n = 13 [0.54%]) were infrequent. Pancytopenia developed as soon as 4 months and as late as 3.5 years after beginning LD‐MTX, though the latter subject had been recently diagnosed with multiple myeloma. Overall skin cancer risk was increased in users of LD‐MTX compared with users of placebo, which driven largely by a statistically significant increased risk of squamous cell skin cancer (hazard ratio [HR] 3.31; 95% confidence interval [CI] 1.63‐6.71). Melanoma was increased in LD‐MTX, but this was not statistically significant (HR 2.33; 95% CI 0.60‐9.01).
Conclusions
Among subjects using LD‐MTX, simultaneous two‐line cytopenias and pancytopenia were uncommon. We found more cases of skin cancer, particularly squamous cell carcinomas, in the LD‐MTX arm than the placebo arm.
INTRODUCTION
Methotrexate (MTX) was originally developed as aminopterin for pediatric acute lymphoblastic leukemia in the 1940s (1). Several decades later, clinicians began using low‐dose MTX (LD‐MTX) for systemic inflammatory conditions, such as psoriasis and rheumatoid arthritis. However, bone marrow toxicity and associated cytopenias proved to be an important adverse event (AE) of weekly LD‐MTX for chronic nononcologic use. In the first decades of LD‐MTX use, pancytopenia was reported to affect 1.4% to 2% of patients (2, 3) and to be fatal in 17% of cases (4). Folic acid supplementation has been part of standard practice since the late 1990s, but its effect on the risk of bone marrow toxicity and associated cytopenias remains unclear (4).
Furthermore, the effect of LD‐MTX on cancer risk remains incompletely understood. The widespread use of high‐dose MTX to treat various cancers, including breast cancer and some lymphomas, might make it seem as though LD‐MTX has a neutral or beneficial effect on cancer. Observational data in patients with rheumatoid arthritis suggest otherwise, as standardized incidence ratios appear elevated for LD‐MTX considering any cancer, melanoma, lung cancer, and non‐Hodgkin lymphoma (5). Moreover, several epidemiologic studies have found an increased risk of skin cancers among patients using LD‐MTX compared with those taking other disease‐modifying antirheumatic drugs for systemic inflammatory diseases (5, 6).
To address these issues in a contemporary setting with routine folic acid supplementation, we followed subjects initiating LD‐MTX in a double‐blind placebo‐controlled randomized controlled trial (RCT), the Cardiovascular Inflammation Reduction Trial (CIRT), which examined whether LD‐MTX may protect against subsequent cardiovascular events in patients with known cardiovascular disease. The trial did not find cardiovascular benefit (7). We prespecified analyses of several adjudicated AEs of interest, including cytopenias and malignancies. AEs affecting the liver, including liver function test abnormalities, have been reported outside of this paper (8). In our prior paper, we described a similar risk of malignancies between LD‐MTX and placebo but an increased risk of skin cancers (8). This finding is further examined herein.
PATIENTS AND METHODS
Study population and design
CIRT was a double‐blind, placebo‐controlled RCT that was stopped prematurely because of lack of efficacy regarding cardiovascular endpoints (8). Enrollment began in 2013, all study drug was terminated in April 2018, and final safety visits took place through December 2018. We prespecified safety analyses in CIRT, and AEs of interest were blindly adjudicated as part of the trial. The primary study population for the AE analyses was the randomized population. Potentially eligible patients had a known history of myocardial infarction or multivessel coronary artery disease plus diabetes or metabolic syndrome; known systemic rheumatic disease was exclusionary. Other exclusions included a history of non–basal cell malignancy, myeloproliferative disease, or treatment for lymphoproliferative disease in the past 5 years. Subjects were required to meet the following laboratory minimums at screening: hematocrit (HCT) of 32%, white blood cell (WBC) count of 3500/μl, and platelet (PLT) count of 75 000/μl.
Subjects completed an active run‐in phase of 5 to 8 weeks, using 10 to 15 mg of weekly LD‐MTX with 1 mg folic acid supplementation on the other 6 days. Those that tolerated LD‐MTX without AEs and no or minimal AEs on HCT, WBC, and PLT counts during the active run‐in were eligible for randomization to oral LD‐MTX or placebo. After randomization, the initial weekly dose of LD‐MTX (or matching placebo) was 15 mg; after 16 weeks, if patients tolerated study drug and laboratory monitoring met prespecified thresholds, the study drug (LD‐MTX or placebo) was increased to 20 mg weekly. Subjects were monitored for possible LD‐MTX toxicity through regular central laboratory monitoring every 4 to 8 weeks and study visits every 12 to 16 weeks during follow‐up. A computerized algorithm helped monitor laboratory values, and a titration algorithm was followed during the trial (see Supplemental Figure 1). In addition, board‐certified rheumatologists served as medical monitors if laboratory values reached critical levels or sites sought advice. Monitors would instruct sites how to downtitrate the study drug during periods of AEs or laboratory abnormalities. These monitors remained blinded to study treatment unless sites requested unblinding.
Hematology and skin cancer outcomes
The adjudicated hematology AEs included cytopenias detected by central laboratories plus occasional routine blood work from study sites. Abnormal cell count definitions were prespecified according to the central laboratory thresholds; mild (hemoglobin <13.5 for men or 10 to <12 g/dl for women, PLT <145‐75 k/μl, and WBC <4.0‐3.0 k/μl), moderate (hemoglobin 8‐10 g/dl, PLT 50‐75 k/μl, and WBC 2‐2.99 k/μl), and severe (hemoglobin <8 g/dl, PLT <50 k/μl; WBC <2 k/μl) reductions were categorized according to the Common Terminology Criteria for AEs (version 5.0) (9). Additionally, sites reported AEs at scheduled visits; AE reports, reasons sites noted for temporary or permanent study drug discontinuation, and central laboratory monitoring values were searched to identify hematologic AEs.
The cancer AEs included all malignancies, with premalignant diagnoses excluded. These AEs were prespecified and blindly adjudicated based on medical records using a standardized form (see Supplemental Figure 2) and were supervised by a hematology‐oncology subspecialist (N.B.). Cancer outcomes were considered definite if pathology reports noted malignancy, probable if treatment for cancer was documented but no definite pathology was available, and possible if cancer was mentioned but no pathology or cancer treatment was described in the medical record.
Statistical analyses
The analyses examined the frequency, rate, risk difference, and relative rate of hematology and skin cancer AEs. The primary analyses considered all first events occurring after randomization and followed a modified intention‐to‐treat strategy that censored subjects 180 days after the subject’s last reported dose of the study drug.
Relative rates for hematology and malignancy AEs were estimated using the proportional hazards regression model. Proportional hazard assumptions were tested using Schoenfeld residuals (10). Prespecified secondary analyses included the frequency, risk difference, and relative risk of multiple cytopenias occurring simultaneously, with two cell lines having at least mild reductions and/or pancytopenia or all three cell lines having at least mild reductions. Finally, we assessed the effect of LD‐MTX on hemoglobin in the subgroup of patients with mild or moderate anemia at baseline; this is based on the theory that some adults with anemia have chronic inflammation, which is possibly treated with LD‐MTX (11).
We had previously found that, among subjects taking LD‐MTX, the risk of any skin cancer was increased (8). Therefore, we examined the rate, 3‐year risk, risk difference, and relative risk of the skin cancer subtypes (basal and squamous cell carcinomas and melanoma). Furthermore, we examined the overall risk of skin cancer by age (<65 years versus ≥65 years), biological sex, and race (white versus nonwhite).
All analyses were conducted using SAS (version 9.4).
RESULTS
Of 9321 subjects screened, 4786 were randomized. The 2391 participants randomized to LD‐MTX took an average of 14.9 mg (±4.5 mg) weekly, and the remaining 2395 took an average of 15.3 mg (±4.3 mg) placebo. Baseline characteristics were well balanced (see Table 1).
Table 1.
Baseline Characteristics in CIRT
| Characteristics | Low‐Dose Methotrexate (n = 2391) | Placebo (n = 2395) |
|---|---|---|
| Female sex, n (%) | 461 (19.3) | 437 (18.2) |
| Age at enrollment, median (IQR), yr | 65.5 (59.5‐71.6) | 65.9 (59.7‐71.6) |
| Race, n (%) | ||
| White | 2008 (84.0) | 2059 (86.0) |
| Black or African American | 194 (8.1) | 156 (6.5) |
| Asian | 89 (3.7) | 92 (3.8) |
| American Indian or Alaska Native | 6 (0.25) | 7 (0.29) |
| Native Hawaiian or other Pacific Islander | 4 (0.17) | 6 (0.25) |
| Multiple | 15 (0.6) | 9 (0.38) |
| Other | 75 (3.1) | 66 (2.8) |
| Body mass index, median (IQR), kg/m2 | 31.6 (28.1‐35.7) | 31.3 (28.0‐35.5) |
| Weekly study drug dosage, mg, mean (SD) | 14.5 (±4.5) | 15.3 (±4.3) |
| Diabetes, n (%) | 1620 (67.8) | 1615 (67.4) |
| Current cigarette use, n (%) | 267 (11.2) | 270 (11.3) |
| Alcohol use, n (%) | ||
| Rarely or never | 1487 (62.2) | 1473 (61.50) |
| ≤ 1 drink/week | 514 (21.5) | 520 (21.71) |
| >1 drink/week | 390 (16.3) | 402 (16.8) |
| Aspirin use, n (%) | 1861 (77.8) | 1807 (75.4) |
| Other NSAID use, n (%) | 217 (9.1) | 195 (8.1) |
| Other anticoagulant use, n (%) | 570 (23.8) | 560 (23.4) |
| Oral corticosteroid use, n (%) | 27 (1.1) | 22 (0.9) |
| Hemoglobin, median (IQR), g/dl | 14.1 (13.0‐15.0) | 14.1 (13.0‐15.0) |
| White blood cell count, median (IQR), k/μl | 7.0 (6.0, 8.3) | 7.0 (5.9, 8.3) |
| Platelet count, k/μl | 233.0 (188.0‐260.0) | 221.0 (185.0‐261.0) |
IQR, interquartile range; NSAID, nonsteroidal anti‐inflammatory drug.
Results of the individual anemia, leukopenia, and thrombocytopenia analyses have been reported in brief (8) and are summarized in Table 2. Simultaneous two‐line cytopenia was uncommon. In the LD‐MTX arm, 92 subjects (3.9%) experienced two‐line cytopenia compared with 70 (2.9%) in the placebo arm. The hazard ratio (HR) for two‐line cytopenia was 1.31 (95% confidence interval [CI] 0.96‐1.79), and the risk difference over 3 years was 0.0196% (95% CI 0.0043‐0.0349).
Table 2.
Frequency and Relative Rates of Hematologic Abnormalities Skin Cancers During the Randomized Phase of the CIRT
| Low‐Dose Methotrexate | Placebo | Risk Difference | Hazard Ratio (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|
| N (%)* | Rate (95% CI) | 3‐Yr Rate (95% CI) | N (%)* | Rate (95% CI) | 3‐Yr Rate (95% CI) | |||
| Anemia | 722 (30.2) | 20.9 (19.4 to 22.4) | 0.37 (0.35 to 0.40) | 555 (23.2) | 15 (13.8 to 16.3) | 0.30 (0.28 to 0.33) | 0.07 (0.04 to 0.11) | 1.36 (1.22 to 1.52) |
| Thrombocytopenia | 204 (8.5) | 4.7 (4.1 to 5.4) | 0.12 (0.10 to 0.14) | 256 (10.7) | 6.1 (5.4 to 6.9) | 0.14 (0.12 to 0.16) | −0.02 (−0.04 to 0.01) | 0.78 (0.65 to 0.93) |
| Leukopenia | 220 (9.2) | 5.1 (4.4 to 5.8) | 0.12 (0.11 to 0.14) | 152 (6.4) | 3.5 (2.9 to 4.0) | 0.08 (0.07 to 0.10) | 0.04 (0.02 to 0.06) | 1.46 (1.19 to 1.80) |
| Any skin cancer | 53 (2.2) | 1.16 (0.89 to 1.51) | 0.04 (0.03 to 0.05) | 26 (1.1) | 0.57 (0.39 to 0.83) | 0.02 (0.01 to 0.03) | 0.0218 (0.0102 to 0.0334) | 2.05 (1.28 to 3.28) |
| Basal cell | 19 (0.8) | 0.41 (0.26 to 0.64) | 0.01 (0.01 to 0.02) | 14 (0.6) | 0.30 (0.18 to 0.51) | 0.01 (0.01 to 0.02) | 0.0038 (−0.0032 to 0.0108) | 1.35 (0.68 to 2.68) |
| Squamous cell | 33 (1.4) | 0.72 (0.51 to 1.01) | 0.02 (0.02 to 0.03) | 10 (0.4) | 0.22 (0.12 to 0.40) | 0.01 (0.00 to 0.01) | 0.0165 (0.0085 to 0.0245) | 3.31 (1.63 to 6.71) |
| Melanoma | 6 (0.3) | 0.15 (0.07 to 0.32) | 0.01 (0.00 to 0.01) | 3 (0.1) | 0.07 (0.02 to 0.20) | 0.00 (0.00 to 0.00) | 0.0041 (0.0009 to 0.0073) | 2.33 (0.60 to 9.04) |
| Any skin cancer | ||||||||
| Age <65 yr | 13 (1.1) | 0.55 (0.32 to 0.95) | 0.02 (0.01 to 0.03) | 5 (0.4) | 0.22 (0.09 to 0.53) | 0.01 (0.00 to 0.02) | 0.01156 (0.00160 to 0.02152) | 2.48 (0.88 to 6.98) |
| Age ≥65 yr | 40 (3.2) | 1.79 (1.32 to 2.43) | 0.06 (0.04 to 0.08) | 21 (1.7) | 0.90 (0.59 to 1.38) | 0.03 (0.02 to 0.04) | 0.03285 (0.01285 to 0.05284) | 1.97 (1.16 to 3.36) |
| Male | 44 (2.3) | 1.19 (0.89 to 1.60) | 0.04 (0.03 to 0.06) | 25 (1.3) | 0.67 (0.45 to 0.99) | 0.02 (0.01 to 0.03) | 0.02165 (0.00868 to 0.03461) | 1.78 (1.09 to 2.91) |
| Female | 9 (2.0) | 1.02 (0.53 to 1.95) | 0.03 (0.02 to 0.07) | 1 (0.2) | 0.12 (0.02 to 0.84) | 0.01 (0.00 to 0.07) | 0.02275 (0.00422 to 0.04129) | 8.76 (1.04 to73.72) |
| White race | 51 (2.5) | 1.32 (1.00 to 1.73) | 0.04 (0.03 to 0.06) | 26 (1.3) | 0.66 (0.45 to 0.96) | 0.02 (0.01 to 0.03) | 0.02442 (0.01106 to 0.03779) | 2.01 (1.25 to 3.22) |
| Nonwhite race | 2 (0.5) | 0.28 (0.07 to 1.13) | 0.01 (0.00 to 0.04) | 0 (0.0) | ‐ | 0.00 (0.00 to 0.00) | 0.00894 (0.00224 to 0.01563) | ‐ |
CIRT, Cardiovascular Inflammation Reduction Trial;
N includes first events of a given type. Rates are per 100 person‐years. Three‐year rate reflects the cumulative incidence percent risk. Risk difference is calculated from the 3‐year cumulative incidence percent risk.
Pancytopenia was numerically more common among patients randomized to LD‐MTX but was rare in both arms. Thirteen participants (0.5%) taking LD‐MTX and six (0.3%) taking placebo experienced pancytopenia during the trial; the HR was 2.15 (95% CI 0.82‐5.66), and the 3‐year risk difference was 0.0043% (range −0.0001 to 0.0095). We examined the 13 cases of LD‐MTX–associated pancytopenia in detail (Table 3). Pancytopenia developed as soon as 4 months and as late as 3.5 years after beginning LD‐MTX, though the latter subject had been diagnosed with multiple myeloma 1 week before. The median age of subjects who developed pancytopenia was 70.5 years (interquartile range [IQR] 69‐75), compared with a median age of 65.6 years (IQR 59.7‐71.8) in the entire LD‐MTX arm. The PLT counts in all 13 cases showed only mild reductions. Nine of these cases were limited to mild decreases across all three cell lines, three had moderate anemia and moderate leukopenia with mild thrombocytopenia, and one had severe anemia with mild leukopenia and mild thrombocytopenia. Four subjects continued LD‐MTX without a temporary stop or dose change, five continued at a reduced dose, one resumed after a temporary stop, and three discontinued permanently. All subjects recovered.
Table 3.
Pancytopenia Cases within the LD‐MTX Arm of the CIRT
| Case | Age (yr) | Sex | LD‐MTX Dose at Time of Event (mg/wk) | Duration of LD‐MTX Use before Event (mo) | BMI (kg/m2) | Alcohol Use at Baseline | Tobacco Use at Baseline | Aspirin Use at Baseline | Other Anticoagulant Use at Baseline | Hgb (g/dl) | WBC (k/μl) | PLT (k/μl) | Study Drug Change | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| At Baseline | At Time of Event | At Baseline | At time of Event | At Baseline | At time of Event | |||||||||||
| 1 | 74 | M | 20 | 31.4 | 30.5 | Never or <1 time/mo | No | Yes | Yes, clopidogrel | 13.8 | 13.4 | 4.7 | 3.9 | 160 | 142 | Continued |
| 2 | 71 | M | 20 | 6.0 | 27.4 | 2‐3 times/wk | No | Yes | No | 14.2 | 13.2 | 5.9 | 3.9 | 150 | 144 | Decreased to 15 mg/wk |
| 3 | 69 | M | 15 | 21.9 | 27.2 | 2‐3 times/wk | No | Yes | No | 13.7 | 13.3 | 5.1 | 3.5 | 176 | 136 | Decreased to 10 mg/wk |
| 4 | 69 | F | 20 | 8.0 | 27.1 | Never or <1 time/mo | No | Yes | Yes, dipyridamole | 10.7 | 9.6 | 5.1 | 2.8 | 222 | 123 | Temporary stop; resumed after 10 weeks |
| 5 | 68 | M | 20 | 41.4 | 31.6 | Never or <1/mo | No | Yes | No | 12.7 | 7.0 | 5.9 | 3.5 | 219 | 143 | Permanent stop |
| 6 | 67 | F | 15 | 4.0 | 19.4 | Never or <1/mo | Yes | No | Yes, clopidogrel | 12.6 | 11.1 | 4.5 | 3.4 | 164 | 138 | Decreased to 10 mg/wk |
| 7 | 76 | M | 20 | 17.8 | 35.4 | 1‐3 times/mo | No | No | Yes, clopidogrel | 13.9 | 12.3 | 4.7 | 3.6 | 170 | 137 | Continued |
| 8 | 75 | M | 20 | 29.8 | 34.6 | Never or <1 time/mo | No | Yes | Yes, clopidogrel | 14.3 | 13.4 | 5.9 | 3.4 | 158 | 133 | Decreased to 15 mg wk |
| 9 | 83 | M | 20 | 13.6 | 27.3 | 1‐3 times/mo | No | Yes | No | 13.7 | 12.4 | 5.0 | 3.7 | 167 | 141 | Continued |
| 10 | 70 | M | 20 | 13.8 | 33.7 | Never or <1 time/mo | No | Yes | Yes, clopidogrel | 12.9 | 8.6 | 6.0 | 2.4 | 246 | 112 | Permanent stop |
| 11 | 69 | F | 20 | 24.7 | 40.2 | Never or <1 time/mo | No | Yes | Yes, clopidogrel | 12.0 | 9.5 | 4.8 | 2.3 | 203 | 124 | Permanent stop |
| 12 | 73 | M | 20 | 7.9 | 25.5 | 2‐3 times/wk | No | Yes | Yes, apixaban | 13.5 | 13.4 | 4.3 | 3.1 | 151 | 138 | Decreased to 15 mg/wk |
| 13 | 75 | M | 20 | 30.0 | 26.0 | 1 time/wk | No | Yes | No | 14.4 | 13.2 | 4.3 | 3.8 | 157 | 142 | Continued |
CIRT, Cardiovascular Inflammation Reduction Trial; F, female; Hgb, hemoglobin; LD‐MTX, low‐dose methotrexate; M, male; PLT, platelet count; WBC, white blood cell count.
Figure 1 presents median hemoglobin levels and platelet and leukocyte counts over time in the two treatment groups. The baseline values represent the end of the active run‐in period immediately before randomization. Median hemoglobin decreased over the study in both arms, with a 0.40 g/dl (IQR −0.90 to 0.10) decrease in the LD‐MTX arm and a 0.10 g/dl (IQR −0.60 to 0.30) decrease in the placebo arm. WBC counts did not change between baseline and the end of follow‐up in the LD‐MTX arm (median change 0.00 k/μlL; IQR −0.90 to 0.80) and increased by 0.20 k/μL in the placebo arm (IQR −0.70 to 1,10). The median PLT count did not change in the LD‐MTX arm between baseline and final study visits (median change 0.00 k/μL; IQR −18 to 16) but decreased by 9 k/μL (IQR −26 to 9) in the placebo arm.
Figure 1.
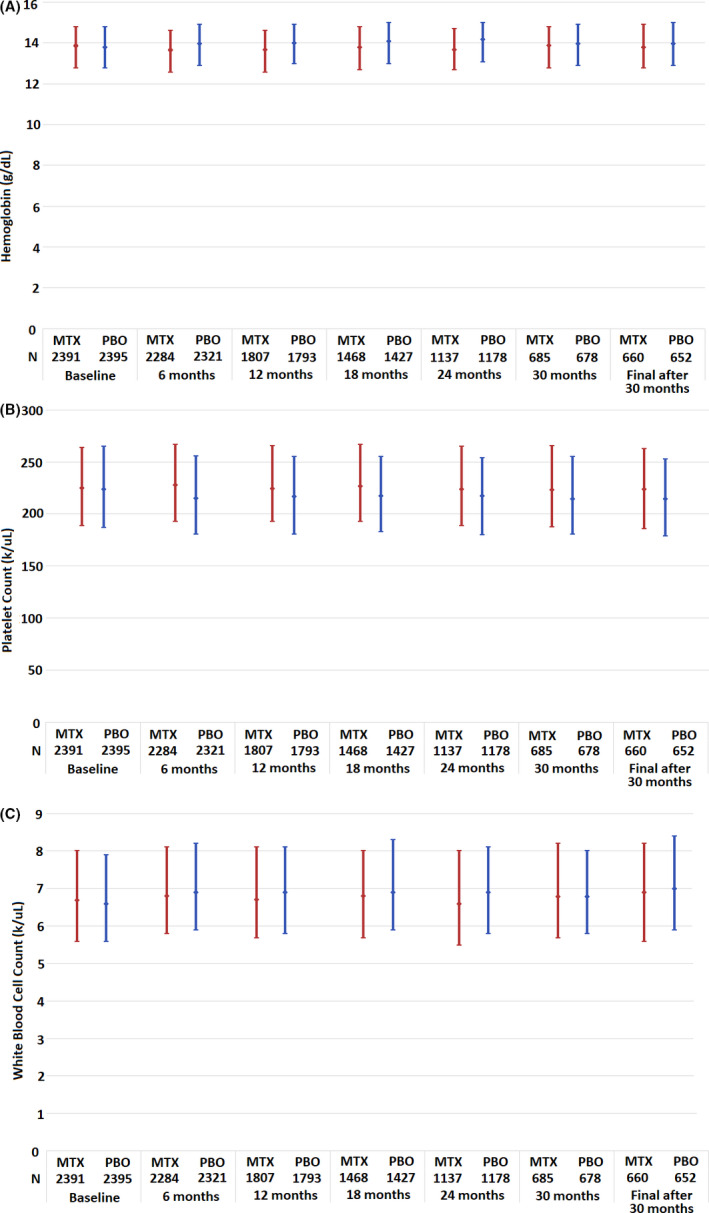
This figure represents the median (filled circle) and interquartile range (IQR) for hemoglobin (A), platelet count (B), and white blood cell (WBC) count (C) during the Cardiovascular Inflammation Reduction Trial. Compared with baseline, by the end of the study, the median hemoglobin value among patients randomized to low‐dose (LD) methotrexate (MTX) decreased by 0.4 g/dl (IQR −0.90 to 0.10) and decreased by 0.1 mg/dl (IQR −0.60 to 0.30) among those randomized to placebo (PBO). The median platelet count did not change in the LD‐MTX arm (IQR −18 to 16 k/μl) and decreased by 9 k/μl (IQR −26 to 9) among those randomized to PBO. The median WBC count did not change in the LD‐MTX arm (IQR −0.90 to 0.80) and increased by 0.2 k/μl (IQR −0.70 to 1.10) in the PBO arm.
We reported previously that the risk of any cancer was similar across both treatment arms, though the risk of skin cancer was increased for those taking LD‐MTX (8). In the present analyses, we examined the differences in risk for each type of skin cancer (basal, squamous, and malignant melanoma). No differences in risk were observed between LD‐MTX and placebo for cancers overall. There was a small, statistically significant increase in skin cancer risk for the LD‐MTX arm compared with the placebo arm (Table 3). The risk difference was 0.0218 (95% CI 0.0102‐0.0334), translating into an HR of 2.05 (95% CI 1.28‐3.28) (8).
We further examined the risk of any skin cancer event by age (<65 years versus ≥65 years), sex, and race (white versus nonwhite) (Table 3). Although the increased risk of skin cancer with LD‐MTX remained in the ≥65 years age group (HR 1.97; 95% CI 1.16‐3.36), the risk was increased but not statistically significant in the <65 years age group (HR 2.48; 96% CI 0.88‐6.98). The increased risk of skin cancer persisted among both men (HR 1.78; 95% CI 1.09‐2.91) and women (HR 8.76, 95% CI 1.04‐73.72). No cases of skin cancer were reported among nonwhite subjects who were randomized to placebo, and two were reported among nonwhite participants taking LD‐MTX.
Finally, the subgroup of patients with mild or moderate anemia at baseline were studied to see if LD‐MTX might improve hemoglobin levels in this group, which included 1507 participants. From this group, 258 subjects were female (17.12%), and the median age was 68 years (IQR 62‐73). The median hemoglobin value at the end of the study was unchanged relative to baseline (IQR −0.6 to 0.7 g/dl) among this subgroup.
DISCUSSION
MTX is a drug used for cancer at high dosages, but LD‐MTX has an important role in treating patients with systemic rheumatic diseases. We found a clear increase in the risk of anemia and leukopenia among patients randomized to LD‐MTX versus placebo, albeit that most cases were very mild. However, we found a slightly reduced risk of thrombocytopenia among patients taking LD‐MTX. Simultaneous two‐line cytopenia and pancytopenia were numerically increased among subjects randomized to LD‐MTX, although these events did not reach statistical significance, as they were uncommon in both arms. In this placebo‐controlled, double‐blind randomized trial with blind adjudication of AEs, we found no increase in cancer overall but found an increase in skin cancers, particularly squamous cell carcinoma.
The results from the current analyses regarding pancytopenia reveal several important findings. Before the widespread use of folic acid supplementation, pancytopenia was estimated to occur in 1.4 to 2% of patients taking LD‐MTX for rheumatoid arthritis (2, 3). However, we conducted a meta‐analysis of 30 RCTs that included 3858 patients with rheumatoid arthritis taking LD‐MTX and folic acid supplementation, and no cases of pancytopenia were reported (12). In the current study, 13 cases (0.5%) of pancytopenia occurred within the LD‐MTX arm. These cases developed in older adults at a wide range of time points throughout follow‐up. They consisted mostly of mild cell count reductions, and all recovered. The platelet counts among these subjects ranged from 112 to 144 k/μl at the time of pancytopenia, and the study used 145 k/μl as the lower limit of normal (LLN). A laboratory that used a less conservative LLN might consider these values to be within the normal range.
Our findings support prior observational studies that noted an increase in skin cancer among patients taking LD‐MTX or other immunosuppressants. Similar to the findings of the current analyses, a United States–based registry study found an increased risk of nonmelanoma skin cancers compared with other nonbiologic treatments for rheumatoid arthritis (13). A large administrative claims‐based analysis among patients with psoriasis also found an increased risk of second nonmelanoma skin cancers with LD‐MTX, with an HR of 1.60 (95% CI 1.08‐2.37), but this included LD‐MTX used in combination with other immunosuppressive medications (6). The biologic basis for LD‐MTX causing skin cancers is not clear based on published literature; however, there are many reports of skin cancer risk associated with immunosuppression among the post‐transplant population (14). Based on our current findings, monitoring for skin cancer in LD‐MTX should be considered, especially in patients 65 years and older.
Although the risks for anemia and leukopenia were increased among subjects randomized to LD‐MTX, the absolute reductions in hemoglobin were small, and WBC counts did not change between baseline and end of follow‐up. Because the baseline values represent the end of the active run‐in period, the slight increase in WBC count and slight decrease in PLT count in the placebo arm reflect the effect of LD‐MTX discontinuation. The modest protective effect of LD‐MTX against thrombocytopenia is consistent with this decrease in the PLT count among subjects randomized to placebo.
Strengths of the current study include the inclusion of a placebo arm; prior analyses among patients with rheumatic diseases compare LD‐MTX with other active immunosuppressive agents. We blindly adjudicated the AEs, using standardized data collection forms, with a board‐certified hematologist‐oncologist. Moreover, the complete blood counts were conducted in centralized laboratories among a relatively large study cohort. However, patients were monitored frequently, so we may have identified more patients with hematologic abnormalities than would be typically found in a clinic population. All subjects were monitored at the same time points per protocol; thus, our results cannot address whether or how monitoring strategies might be optimized. The rates of abnormalities found in the current study were among patients who did not have systemic rheumatic diseases or psoriasis. These rates among patients with chronic inflammatory diseases could differ.
In conclusion, we found a substantial increase in the risk of skin cancer among subjects using LD‐MTX compared with placebo, most of which were squamous cell carcinomas. There were no risk increases observed for other cancers. The skin cancer finding may have particular clinical relevance when LD‐MTX is used in psoriasis and psoriatic arthritis, related conditions associated with increases in nonmelanoma skin cancer and malignant melanoma (15). There appeared to be a small decrease in PLT counts among patients using placebo compared with those using LD‐MTX, with a reduced risk of thrombocytopenia associated with LD‐MTX. This result was surprising and needs further study. Although we found a numerical doubling in the LD‐MTX arm of cases of both melanoma and pancytopenia, two of the most serious side effects of LD‐MTX, the events were too rare to reach statistical significance, despite this being the largest double‐blind, placebo‐controlled RCT of LD‐MTX to date. The current findings should prompt further investigation in this population.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual contact, and all authors approved the final version to be published. Dr. Solomon had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Paynter, Glynn, Ridker, Solomon.
Acquisition of data
Vanni, Berliner, Paynter, Glynn, Colls, Lu, Ridker, Solomon.
Analysis and interpretation of data
Vanni, Paynter, Glynn, MacFadyen, Xu, Ridker, Solomon.
Supporting information
Fig S1
Supplementary Material
ClinicalTrials.gov identifier: NCT01594333.
This work was supported by the National Institutes of Health (R01‐HL‐119718, U01‐HL‐101422, and U01‐HL‐101389).
Kathleen M.M. Vanni, BA, Nancy Berliner, MD, Nina P. Paynter, PhD, Robert J. Glynn, PhD, Jean MacFadyen, BA, Joshua Colls, BS, Fengxin Lu, MD, Chang Xu, MS, Paul M. Ridker, MD, Daniel H. Solomon, MD: Brigham and Women’s Hospital, Boston, Massachusetts.
Drs. Ridker and Solomon contributed equally to this work.
Dr. Solomon receives research support unrelated to the present study from Abbvie, Amgen, Corrona, Genentech, Janssen, and Pfizer. Dr. Ridker receives research support unrelated to the present study from Kowa, Novartis, and Amarin and has served as a consultant to Corvidia, Inflazome, and CiviBioPharm. No other disclosures relevant to this article were reported.
REFERENCES
- 1. Farber S, Diamond LK. Temporary remissions in acute leukemia in children produced by folic acid antagonist, 4‐aminopteroyl‐glutamic acid. N Engl J Med 1948;238:787–93. [DOI] [PubMed] [Google Scholar]
- 2. Gutierrez‐Ureña S, Molina JF, García CO, Cuéllar ML, Espinoza LR. Pancytopenia secondary to methotrexate therapy in rheumatoid arthritis. Arthritis Rheum 1996;39:272–6. [DOI] [PubMed] [Google Scholar]
- 3. Williams HJ, Willkens RF, Samuelson CO, Alarcón GS, Guttadauria M, Yarboro C, et al. Comparison of low‐dose oral pulse methotrexate and placebo in the treatment of rheumatoid arthritis. Arthritis Rheum 1985;28:721–30. [DOI] [PubMed] [Google Scholar]
- 4. Van Ede AE, Laan RF, Rood MJ, Huizinga TW, van de Laar MA, van Denderen CJ, et al. Effect of folic or folinic acid supplementation on the toxicity and efficacy of methotrexate in rheumatoid arthritis: a forty‐eight week, multicenter, randomized, double‐blind, placebo‐controlled study. Arthritis Rheum 2001;44:1515–24. [DOI] [PubMed] [Google Scholar]
- 5. Solomon DH, Kremer JM, Fisher M, Curtis JR, Furer V, Harrold LR, et al. Comparative cancer risk associated with methotrexate, other non‐biologic and biologic disease‐modifying anti‐rheumatic drugs. Semin Arthritis Rheum 2014;43:489–97. [DOI] [PubMed] [Google Scholar]
- 6. Scott FI, Mamtani R, Brensinger CM, Haynes K, Chiesa‐Fuxench ZC, Zhang J, et al. Risk of nonmelanoma skin cancer associated with the use of immunosuppressant and biologic agents in patients with a history of autoimmune disease and nonmelanoma skin cancer. JAMA Dermatol 2016;152:164–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ridker PM, Everett BM, Pradhan A, MacFadyen JG, Solomon DH, Zaharris E, et al. Low‐dose methotrexate for the prevention of atherosclerotic events. N Engl J Med 2019;380:752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Solomon DH, Glynn RJ, Karlson EW, Lu F, Corrigan C, Colls J, et al. adverse effects of low‐dose methotrexate: a randomized trial. Ann Intern Med 2020;172:369–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. National Cancer Institute . Common terminology criteria for adverse events (CTCAE) version 5.0. Bethesda (MD): US Department of Health and Human Services; 2017. URL: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf. [Google Scholar]
- 10. Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometika 1982;69:239–41. [Google Scholar]
- 11. Berliner N. Anemia in the elderly. Trans Am Clin Climatol Assoc 2013;124:230–7. [PMC free article] [PubMed] [Google Scholar]
- 12. Vanni KM, Lyu H, Solomon DH. Cytopenias among patients with rheumatic diseases using methotrexate: a meta‐analysis of randomized controlled clinical trials. Rheumatology (Oxford) 2020;59:709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wolfe F, Michaud K. Biologic treatment of rheumatoid arthritis and the risk of malignancy: analyses from a large US observational study. Arthritis Rheum 2007;56:2886–95. [DOI] [PubMed] [Google Scholar]
- 14. Jensen P, Hansen S, Moller B, Leivestad T, Pfeffer P, Geiran O, et al. Skin cancer in kidney and heart transplant recipients and different long‐term immunosuppressive therapy regimens. J Am Acad Dermatol 1999;40:177–86. [DOI] [PubMed] [Google Scholar]
- 15. Chiesa Fuxench ZC, Shin DB, Ogdie Beatty A, Gelfand JM. The risk of cancer in patients with psoriasis: a population‐based cohort study in the health improvement network. JAMA Dermatol 2016;152:282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Supplementary Material


