Abstract
Aims: This study aimed to explore the function of NKCC1 in the proliferation, migration and invasion of Gastric cancer (GC) cells.
Materials and Methods: GC data extracted from the database was analyzed using molecular bioinformatics. The expression levels of NKCC1 in tissue samples from GC patients and GC cell lines were determined by Western blotting, qRT-PCR, and immunohistochemistry. Immunofluorescence was used to detect protein localization. The GC cell lines were transfected with NKCC1-shRNA or expression plasmid, and in vitro proliferation, invasion and migration were analyzed by the CCK8, wound healing and transwell tests.
Results: The NKCC1 mRNA level was significantly increased in GC tissues than that in normal gastric tissues (P = 0.0195). This phenomenon was further confirmed by the analysis of the TCGA-GTEx database that includes 408 gastric cancer tissues and 211 normal gastric tissues (P < 0.01). Furthermore, the increased level of NKCC1 was significantly correlated with Tumor size (P = 0.039), lymphatic node metastasis (P = 0.035) and tumor stage (P = 0.034). In vitro experiments confirmed that NKCC1 expression was higher in GC cells compared to that in GES-1 cells, and was mainly localized to the cytoplasm and membrane. NKCC1 silencing inhibited GC cell proliferation, invasion, migration and EMT, whereas its overexpression had the opposite effects. Furthermore, NKCC1 overexpression upregulated and activated JNK, and the targeted inhibition of JNK by SP600125 abrogated the pro-metastatic effects of NKCC1.
Conclusions: NKCC1 promotes migration and invasion of GC cells by MAPK-JNK/EMT pathway and can be a potential therapeutic target.
Keywords: Na+/K+/2CI- cotransporter 1 (NKCC1), gastric cancer, migration, invasion, epithelial-mesenchymal transition
Introduction
Gastric cancer (GC) originates from the gastric epithelial cells, and is one of the most commonly diagnosed malignancies and the third leading cause of cancer-related deaths worldwide 1-3. The occurrence and development of GC are controlled by multiple genes and factors. Furthermore, due to lack of sensitive non-invasive diagnostic indicators, most patients are diagnosed in the advanced stages. In addition, the post-surgery five-year survival rate of gastric cancer is only 20% 4 owing to the high rates of local and distal metastasis and postoperative recurrence 5-6. Therefore, it is vital to elucidate the mechanisms underlying GC metastasis in order to identify novel early diagnostic markers and therapeutic targets, and improve patient prognosis.
Na+/K+/2CI- cotransporter 1 (NKCC1) is a member of the SLC12 family of cationic chloride co-transporter membrane proteins, including NKCC1 and NKCC2 isotypes 7-8. NKCC1 transports Na, K, and Cl ions across epithelial and non-epithelial cells, thereby regulating cell proliferation, differentiation and metastasis 9-11. It is present in the stomach, esophagus, colorectal, liver, pancreas, lung, and other tissues and is aberrantly expressed in many tumors. High levels of NKCC1 in glioma tissues affect the shape, polarity, and adhesion of tumor cells 10. In addition, NKCC1 promotes the growth of GC cells 9, although its potential role in GC metastasis has not been elucidated.
Epithelial-mesenchymal transition (EMT) refers to the transformation of polar epithelial cells into metastatic mesenchymal cells. This process endows tumor cells with the ability to metastasize and invade distant tissues 12. It is initiated with the degradation or redistribution of connexins and dissociation of the β-catenin/E-cadherin complex, resulting in the loss of cell stability, polarity and intercellular adhesion, and cytoskeletal rearrangement from keratin to vimentin 13-15. The tumor cells acquire characteristics of embryonic mesenchymal cells and become more invasive following EMT, resulting in greater infiltration into adjacent tissues, blood, or lymphatic vessels and eventually distant metastasis 16-18. EMT is regulated by multiple pathways, including NF-kB, Wnt, TGF-β, PI3k/Ak, Ras/MAPK, Rho/Rac, and others 19-21. It is believed that a greater understanding of the underlying molecular mechanisms of EMT in GC can help identify novel therapeutic targets.
We found that NKCC1 was overexpressed in GC tissues. It promoted EMT in GC cells, and upregulated MMP2 and MMP9 through the MAPK-JNK signaling pathway. NKCC1 is a potential treatment target for GC.
Materials and methods
Patients and sample collection
The study was approved by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University. All the patients were diagnosed with gastric cancer by electronic gastroscope biopsy, excluding other systematic tumors or tumor metastasis to the stomach, and the patients did not receive chemotherapy or radiotherapy before operation. All primary tumor and paired normal gastric epithelial tissues were obtained from 95 pathologically confirmed GC patients who underwent surgical resection at our center between May 2010 and January 2019. The tissue samples were flash frozen and stored at -80°C for further analysis. Informed consent was acquired from all patients.
Database information acquisition
The Cancer Genome Atlas project database was analysis by GEPIA2 software (http://gepia.cancer-pku.cn/) to determine expression levels of NKCC1 in GC. The RNA-seq expression data of 408 gastric cancer tissue specimens and 211 normal gastric tissues in the database (Log FC > 1, p-value < 0.01) were also analyzed.
Cell culture
Human GC cell lines (HGC-27, BGC-823, SGC-7901, MKN-28, AGS, and MGC-803) were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Normal gastric epithelial cell GES-1 was obtained from Shanghai Fu Xiang Biotechnology Co. Ltd. The GES-1 and AGS cells were proliferated in DMEM (Gibco-BRI, USA) supplemented with 10% FBS (Gibco-BRI, USA), while the others cells were grown in complete RPMI1640 medium. All cells were cultured under 5% CO2 at 37°C.
qRT-PCR
Total RNA was extracted using the NucleoZOL RNA Isolation Kit (Genecompany, Germany) and reverse transcribed using the PrimerScript RT Kit (Takara, China) as per the manufacturers' instructions. The relative amounts of NKCC1 and GAPDH (internal control) mRNAs were measured by qRT-PCR in the ABI (7500) RT-PCR System (Applied Biosystems, USA) using the SYBR Green QRT-PCR Kit (Takara, China). The primers were used: NKCC1 forward 5′-TAAAGGAGTCGTGAAGTTTGGC-3′ and reverse 5′-CTTGACCCACAATCCATGACA-3′; GAPDH forward 5′-TGACTTCAACAGCGACACCCA-3′ and reverse 5′-CACCCTGTTGCTGTAGCCA AA-3′. All samples were tested in triplicate.
Western blotting
Proteins were extracted from cells and tissues using RIPA lysis buffer (Thermo Fisher Scientific) supplemented with protease inhibitors (Solarbio) and determined using the Bradford protein assay kit (Beyotime). Cell lysates containing equal amount of proteins (30 µg) were separated via SDS/PAGE gels and transferred to 0.22 µm PVDF membranes (Merck Millipore Ltd). Blots were blocked with 5% milk for 30min at 37 °C, and incubated with primary antibodies, including E-cadherin (CST. No, 3195S, 1:1000), Vimentin (CST. No, 5741S, 1:1000), snail (CST, no, 3879S, 1:1000), MMP2 (CST. No, 40994S, 1:1000), MMP9 (CST. no, 13667S, 1:1000), NKCC1 (Proteintech, no,13884-1-AP 1:10000), JNK (Santa Cruz, no, 7345, 1:200), p-JNK (Santa Cruz, no, 6254, 1:200), ERK (ABclonal, no, A19630, 1:1000), p-ERK (ABclonal, no, APO485, 1:1000), P38 (ABclonal, no, A4771, 1:1000), p-P38 (ABclonal, no,AP05261:1000), and GAPDH (Cell Signaling Technology, 1:10000). The blots were probed with secondary antibody (Cell Signaling Technology 1:5000 dilution) for 30min at 37 °C. The positive bands were detected by ECL kit (Pierce).
Constructs and vectors
NKCC1-shRNA (sh1: 5'-CCAGCACTACTATTATGATAC-3', sh2: 5'-GGTGATTTCGTCATAGGAACA-3', sh3: 5'-CCAATTATCTCAAACTTCTTC-3' shNC: 5'-ACAGAAGCGATTGTTGATC-3') and expression plasmid were synthesized by Genecopoeia, Inc. (Guangzhou, China). The GC cells were transfected with suitable constructs using Lipofectamine 3000 (Thermo Fisher Scientific, USA) as per the manufacturer's instructions. After culturing for 48 h, stable transfectants were screened using polybrene, and the expression levels of target protein and mRNA were analyzed.
Cell proliferation assay
CCK-8 assay (CCK-8 SAB biotech. College Park, MD, USA) was used for cell proliferation detection. Following the manufacturer's protocol. The Cells were seeded into 6-well plates at a density of 1.0×105 cells per well and incubated for 24 h in medium supplemented with 5% FBS, and incubated at 37 °C with 5% CO2. At 24 h after transfection, cells were digested with trypsin and seeded in triplicate into 96-well plates (3×104 cells/ well). Each well was incubated with 10 μl/well of Cell Counting Kit-8 solution for 2 h daily for 5 days. Optical density at 450 nm was measured on a microplate reader. Three independent experiments were performed.
Immunofluorescence
Cells were fixed with 4% polymerized formaldehyde (Solarbio) for 15 min and then permeabilized with 0.1% Triton-X100 (Solarbio) for another 15 min. The samples were incubated overnight with anti-E-cadherin and anti-vimentin antibodies (Protein Tech, 1:50) at 4 °C. After incubating with the secondary antibody (1:200) in 5% BSA (Solarbio) for 1 h at 37 °C, the cells were counterstained with DAPI for 25 min. The cells were observed using a fluorescence microscope and photographed (200× magnification).
Wound-healing assay
Cells (5×105) were seeded in a 6-well plates and cultured cells reached 85% confluence. The monolayer was scratched longitudinally across the plates with a sterile p200 pipette tip to simulate a wound. The cellular debris was removed by PBS. Cell migration was observed 0, 12, and 24 h after wounding.
Cell invasion and migration assays
The suitably transfected GC cells were plated in the top chambers of Corning Incorporated Cell Culture Inserts (#3422; Franklin Lakes, New Jersey, USA) or Corning Incorporated BioCoat™ Matrigel™-coated Invasion Chamber (#3422) in 2% FBS, and the lower chambers were placed with media containing 5% FBS as the chemoattractant. The cells were incubated at 37 °C in a humidified atmosphere containing 5% CO2 for 24 h or 48h. The migrated cancer cell on the lower surface were fixed with 95% alcohol, and stained using 0.1% crystal violet (Solarbio). The number of invaded cells was counted and photographed (100× magnification) under a phase contrast microscope (four random fields per well).
Statistical analysis
Experiments data are summarized as mean ± SD of three times repeation. GraphPad Prism 5 (GraphPad Software, Inc, USA) was used to statistical analysis. The differences between groups were compared using two-tail Student's t test and variance. P values were less than 0.05 were considered statistically significant.
Results
NKCC1 is overexpressed in primary GC tissues
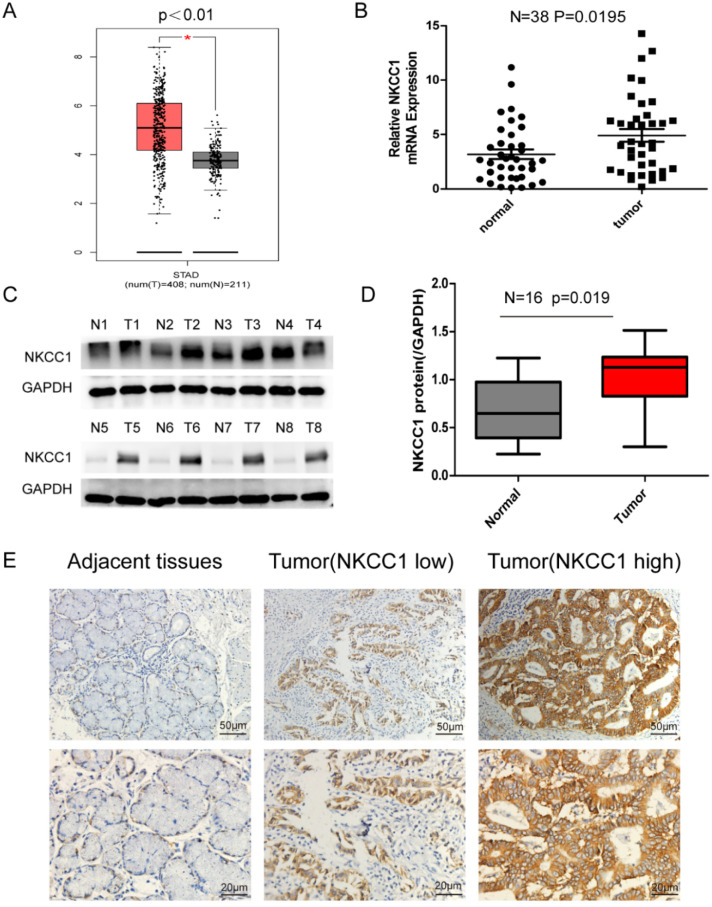
NKCC1 was upregulated in the 408 GC tissues compared to the 211 normal gastric epithelial tissues (P < 0.01; Figure 1A) in TCGA datasets as per GEPIA (http://gepia.cancer-pku.cn/). Consistent with this, NKCC1 mRNA levels were significantly higher in the gastric tumors relative to the paired adjacent normal tissues in our cohort (Fig. 1B), and 87.5% of the GC tissues overexpressed NKCC1 protein compared to the paired adjacent normal samples (Fig. 1C, D). Representative immunohistochemistry images showed in situ NKCC1 expression in the tumor (Fig. 1E). As shown in Table 1, the clinical relevance of high NKCC1 expression was significantly associated with GC Tumor size (P = 0.039), TNM stage (P = 0.034) and lymphatic node metastasis (P = 0.035) but did not show any significant correlation to age, gender, tumor localization and differentiation. Altogether, NKCC1 is significantly upregulated in GC tissues and can be a potential oncogenic factor.
Figure 1.
Expression of NKCC1 in Gastric cancer tissues and normal Gastric tissues. A. NKCC1 mRNA levels in 408 GC tissues and 211 para-tumor tissues in TCGA database. B. NKCC1 mRNA levels in 38 GC tissues and paired adjacent tissues. C-D. Western blotting showing NKCC1 protein expression levels in GC tissues and para-tumor tissues with GAPDH as the internal reference control and the quantification of the three independent repeated experiment were shown (n=16, p=0.019). E. Representative immunohistochemistry images showing in situ NKCC1 expression in the tumor, magnification - 200X, Scale bars: 50 µm, magnification - 400X, Scale bars: 20 µm.
Table 1.
Correlation between NKCC1 expression and patients clinicopathological characteristics in 95 GC patients
| Parameters | Total case | NKCC1 | P value | |
|---|---|---|---|---|
| 95 | High expression (N=50) | Low expression (N=45) | ||
| Age (years) | 0.745 | |||
| ≥68 | 46 | 25 | 21 | |
| <68 | 49 | 25 | 24 | |
| Gender | 0.959 | |||
| Male | 72 | 38 | 34 | |
| Female | 23 | 12 | 11 | |
| Tumor size | 0.039* | |||
| ≥6.5m | 40 | 26 | 14 | |
| <6.5 cm | 55 | 24 | 31 | |
| Lymphatic node metastasis | 0.035* | |||
| Negative | 32 | 12 | 20 | |
| Positive | 63 | 38 | 25 | |
| Invasion depth (T) | 0.845 | |||
| T1-2 | 12 | 6 | 6 | |
| T3-4 | 83 | 44 | 39 | |
| N | 0.810 | |||
| N1-2 | 41 | 21 | 20 | |
| N3-4 | 54 | 29 | 25 | |
| M | 1.000 | |||
| M0 | 91 | 48 | 43 | |
| M1 | 4 | 2 | 2 | |
| TNM stage | 0.034* | |||
| I-II | 42 | 17 | 25 | |
| III-IV | 53 | 33 | 20 | |
NOTE: The values are statistically significant (*** P <0.001; ** P <0.01; * P <0.05);
The 8thTNM Classification of Malignant Tumors proposed by the AJCC/UICC.
The mean age at diagnosis is 68 years in patients with GC. Samples are divided into two groups based on the mean age.
The mean tumor size was 6.5 cm. They were divided into two groups based on the mean tumor size.
Knockdown of NKCC1 inhibited the proliferation, migration and invasion of GC cell lines
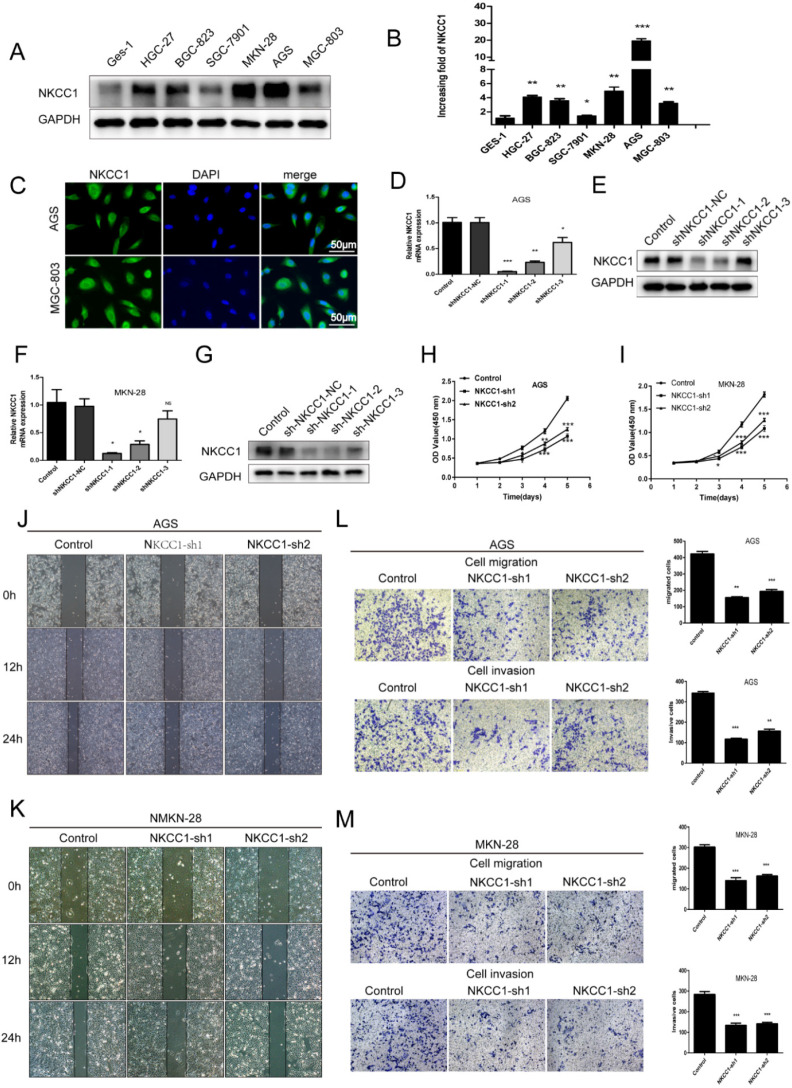
To elucidate the potential biological function of NKCC1 in GC development, the expression of NKCC1 in GC cell lines (HGC-27, BGC-823, SGC-7901, MGC-803, AGS, MKN-28) was first evaluated by Western-blot and qRT-PCR. NKCC1 protein and mRNA were markedly upregulated in the GC cells compared with GES-1 cells (Fig. 2A, B). In addition, NKCC1 was mainly localized in the vesicles and plasma membrane in AGS and MGC-803 cells (Fig. 2C). This is particularly important because the exact function of NKCC1 varies depending on its intra- or extracellular localization. To further demonstrate the role of NKCC1 in GC progression, NKCC1 was knocked down in AGS and MKN-28 lines (Fig. 2D-G). As Figure 2H and I illustrate, stable knockdown of NKCC1 obviously suppressed growth of GC cells in vitro in the CCK-8 assays. As Figure 2J and K illustrate, stable knockdown of NKCC1 decreased migration of GC cells in the wound healing assay. Furthermore, the transwell assay showed an obvious reduction in the motility and invasiveness of the NKCC1-knockdown GC cells compared to the control cells (Fig. 2L M). These results implied that knocking down NKCC1 inhibited the proliferation, invasiveness and metastasis of GC cells.
Figure 2.
Knockdown of NKCC1 inhibited the proliferation, migration and invasion of GC cells. A-B. NKCC1 protein and mRNA levels were observed in the GC cell lines and GES-1 cells. C. Representative immunofluorescence images showing localization of NKCC1 on the plasma membrane of the GC cells (magnification - 200X, Scale bars: 50 µm). D-G. NKCC1 mRNA and protein levels in GC cells transfected with shNKCC1 and scrambled control. H-I. The proliferation capacities were detected by CCK8 assays in GC cells transfected with shNKCC1 and scrambled control. J-K. Representative images of wound healing assay showing the in vitro migration of control and shNKCC1 GC cells (magnification - 40X, Scale bars: 200 µm). L-M. Transwell assay showing in vitro migration and invasion of control and shNKCC1 GC cells (magnification - 100X, Scale bars: 100 µm). * P < 0.05; ** P < 0.01; *** P < 0.001.
Overexpression of NKCC1 enhances the proliferation, migration and invasion of GC cell lines
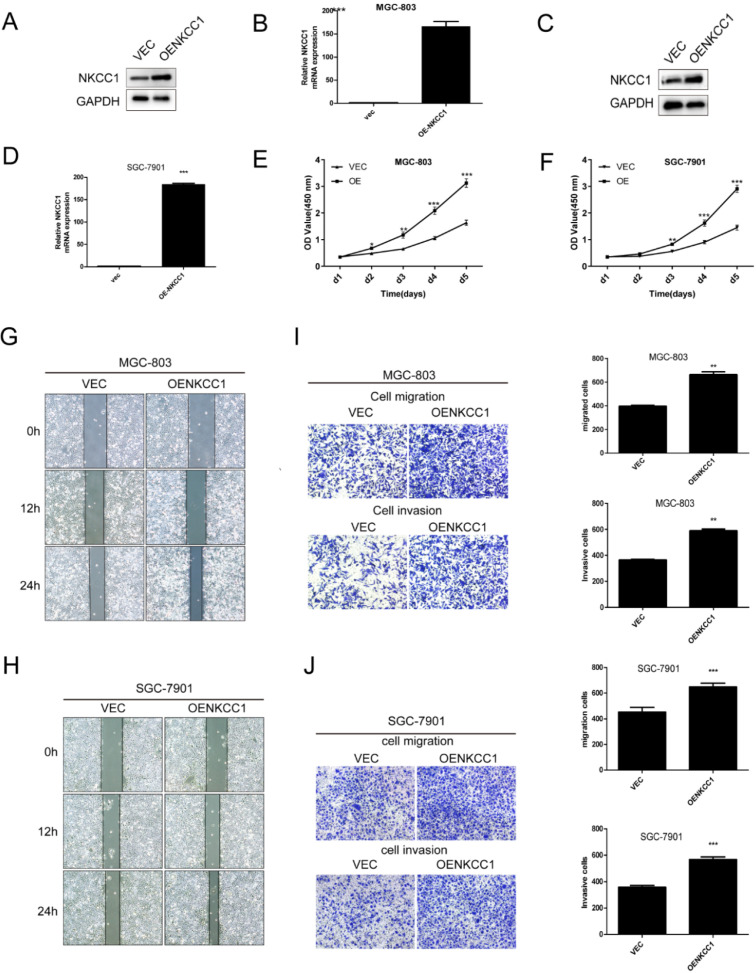
NKCC1 was overexpressed in MGC-803 and SGC-7901 cells (Fig. 3A-D). In contrast, stable overexpressed of NKCC1 significantly accelerated cell growth (Fig. E-F). and markedly increased migration of GC cells in the wound healing assay (Fig. 3G, H), and the transwell assay indicated a significant increase in the migration and invasiveness of the overexpressed NKCC1 GC cells compared to the control cells (Fig. 3I, J). Taken together, intracellular NKCC1 enhances the proliferation, invasiveness and metastasis of GC cells.
Figure 3.
Overexpress of NKCC1 enhances the proliferation, invasion and migration of GC cells. A-D. NKCC1 protein and mRNA levels in GC cells transfected with NKCC1 expression plasmid. E-F. The proliferation capacities were detected by CCK8 assays in GC cells transfected with the control or the NKCC1-overexpressing plasmid. G-H. Wound healing assay showing the in vitro migration of control and NKCC1-overexpressing GC cells (magnification - 40X, Scale bars: 200 µm). I-J. Transwell assay showing in vitro invasion and migration of control and NKCC1-overexpressing GC cells (magnification - 100X, Scale bars: 100 µm). ** P < 0.01; *** P < 0.001.
NKCC1 induced EMT and increased MMP2/9 expression in GC cell
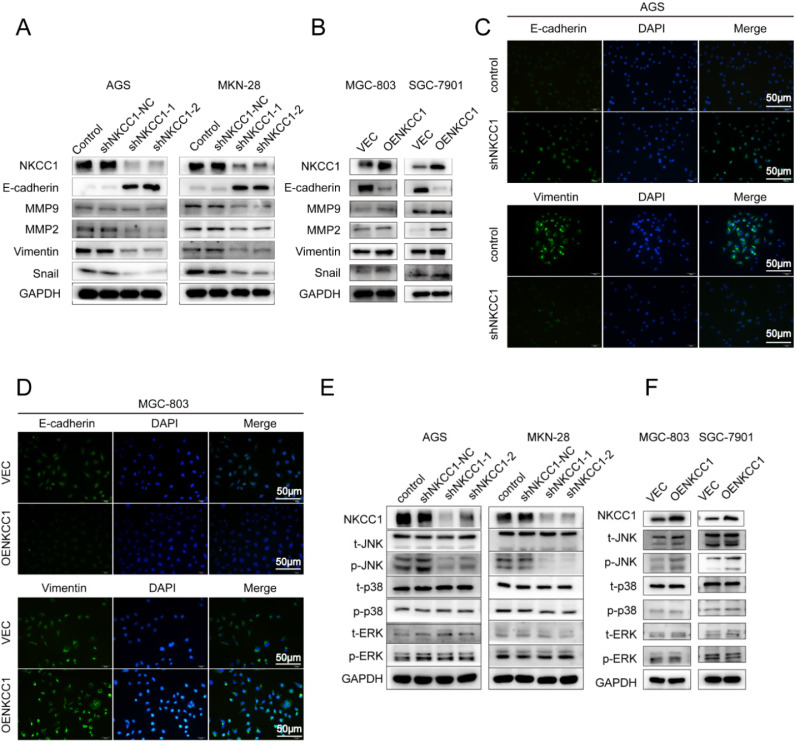
EMT and extracellular matrix remodeling are critical for cancer cell migration and invasion and are thus key to metastasis 22. Therefore, we analyzed the levels of EMT-related proteins in the NKCC1-knocked down and overexpressing GC cells. As shown in Figure 4A, knocking down NKCC1 downregulated the mesenchymal marker EMT-related transcription factors Snail and Vimentin, and ECM proteins MMP-2 and MMP-9, and upregulated epithelial E-cadherin. In contrast, overexpression of NKCC1 upregulated Vimentin, Snail, MMP-2 and MMP-9 but downregulated E-cadherin (Fig. 4B). Thus, NKCC1 promotes EMT of GC cells.
Figure 4.
NKCC1-induced EMT activates the MAPK-JNK pathway in GC cells. Western blotting showing expression levels of EMT makers in A. NKCC1-knockdown GC cells and B. NKCC1-overexpressing GC cells. C-D. Representative immunofluorescence images showing in situ expression of E-cadherin and vimentin in GC cells treated as above (magnification - 200X, Scale bars: 50 µm). E-F. Immunoblots showing expression levels of JNK/p-JNK, ERK/p-ERK and p38/p-p38 in E. NKCC1-knockdown GC cells and F. NKCC1-overexpressing GC cells.
NKCC1 induced EMT of GC cells by activating the MAPK-JNK pathway
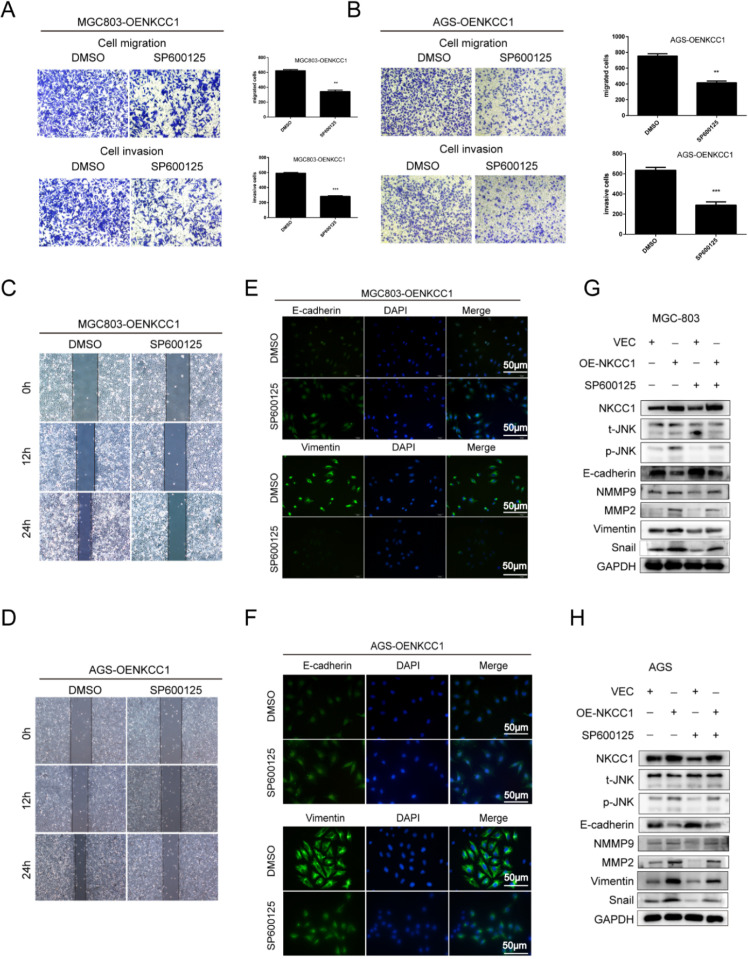
The MAPK-JNK/p38 pathway has been implicated in EMT, and the JNK pathway in particular is essential for the progression and maintenance of EMT-related phenotypes and cellular changes 23-24. Therefore, we analyzed the levels of the MAPK-JNK pathway proteins in GC cells following NKCC1 knockdown or overexpression. The p-JNK/JNK ratio was significantly reduced after silencing NKCC1 (Fig. 4E), while no significant change was observed in the phosphorylation of P38 and ERK. As expected, p-JNK/JNK increased markedly in the NKCC1-overexpressing cells whereas p-ERK and p-P38 were not affected (Fig. 4F). To determine whether the MAPK-JNK signaling pathway mediates the metastatic effects of NKCC1, the JNK inhibitor SP600125 (Selleckchem, Inc.) was dissolved in DMSO, we treated the NKCC1-overexpressing MGC-803 and AGS cells with 10 μM SP600125 and found that the invasion and migration of the cells were significantly inhibited (Fig. 5A-D). Immunofluorescent assay also confirmed the changes of EMT markers (vimentin and E-cadherin) after SP600125 treatment in MGC-803 and AGS cells (Fig. 5E-F). Consistent with this, SP600125 downregulated the mesenchymal markers and EMT-related transcription factors in the MGC-803 and AGS cells overexpressing NKCC1 and upregulated E-cadherin (Fig. 5G-H). Overall, NKCC1 regulates EMT by the MAPK- JNK pathway to promote GC invasion and migration.
Figure 5.
The MAPK-JNK pathway mediates the pro-metastatic effects of NKCC1 in GC cells. A-B. Transwell assay showing invasion and migration of MGC-803 and AGS cells treated as above (magnification - 100X, Scale bars: 100 µm). The experiments were repeated 3 times, and the results were summarized as mean ± SD (**, P < 0.01). C-D. Wound healing assay showing in vitro migration of NKCC1-overexpressing MGC-803 and AGS cells treated with JNK inhibitor SP600125 (magnification - 40X, Scale bars: 200 µm). E-F. Representative immunofluorescence images showing in situ expression of vimentin and E-cadherin in MGC-803 and AGS cells treated as above (magnification - 200X, Scale bars: 50 µm). G-H. Immunoblots showing expression levels of p-JNK, ERK, E-cadherin snail, vimentin, MMP2 and MMP9 in MGC-803 and AGS cells treated as above.
Discussion
NKCC1 is an active Na+/K+/ 2Cl- cotransporter, which regulates the changes in cell volume by controlling the intracellular content of water and sodium. Ion transporters and ion channels have recently gained attention for their potential tumorigenic roles. They regulate the intracellular content of water and ions and therefore control cell volume. The CI- channel (CLIC1) enhanced the invasion and migration of gastric and liver cancer cells 25-26, and K+/Ca2+ channels are frequently overexpressed in colon cancer 27-28. Ectopic expression of NKCCI induced the proliferation and phenotypic transformation of mouse fibroblasts 29. In addition, NKCC1 has been reported to play an important role in many different tumorigenesis, it is aberrantly expressed in gastric cancer 9, esophageal cancer 30, meningioma 25, liver cancer 11, and glioma 10 and functions as an oncogene 9-11, 30. We found that NKCC1 was highly expressed in a variety of tumors through analyzing The Cancer Genome Atlas project database using GEPIA software (http://gepia.cancer-pku.cn/). NKCC1 was also highly expressed in GC tissues by analyzing the RNA-seq expression data of 408 GC tissue specimens and 211 normal gastric tissues in the database. The subsequent experiments will be carried out for further verification.
Previous studies show that NKCC1 promotes the invasion and migration of meningioma 31, glioma 10, 32, and liver cancer 11 cells and proliferation of esophageal cancer 30 and liver cancer 11 cells. In addition, a previous study reported that blocking NKCC1 in GC cells can inhibit cell cycle progression by disrupting the intracellular Cl- concentration 33. Significantly higher levels of NKCC1 were detected in GC tissues patients compared to the paired para-tumor tissues, which was closely related to the Tumor size, lymph node metastasis and TNM stage, suggesting that upregulated expression of NKCC1 in GC might facilitate the tumor growth and metastatic phenotype. This was consistent with the data from TGCA datasets, further confirming that NKCC1 is highly expressed in GC tissues. These findings underscore a potentially important role of NKCC1 as an underlying biological mechanism in the progression of GC. To clarify the biological function of NKCC1 in regulating GC cell growth, motility and invasiveness, a series of in vitro assays were conducted. The results showed that knocking down NKCC1 in the GC cell lines inhibited EMT and downregulated the related factors, resulting in lower proliferation, invasion and migration in vitro. In in vitro experiments, the expression of NKCC1 in gastric epithelial cells (GES-1) and six gastric cancer cell lines was first detected, which was relatively high in AGS and MKN-28 cells, but relatively low in SGC-7901 and MGC-803 cells. The expression of proteins in different locations of the cell plays a different role. Immunofluorescence experimental results indicate that NKCC1 is mainly expressed in the cytoplasm and cell membrane of gastric cancer cells as a plasma membrane protein. Next, we changed the expression of NKCC1 in gastric cancer cells. In contrast, NKCC1 overexpression promoted EMT and increased the proliferation, invasion and migration of GC cells. In addition, it is reported that the matrix metalloproteinases (MMPs) enhanced tumor cell motility and invasion by degrading the extracellular matrix 34-35. Silencing NKCC1 decreased MMP 2/9 levels, while its overexpression produced the opposite effects. The above data suggested that NKCC1 may be a new indicator for the growth and metastasis of gastric cancer, and may act as an oncogene in the progression of gastric cancer as well as plays an important role in the proliferation, invasion and migration of GC cells.
EMT plays an important role in the infiltration, invasion, and distant metastasis of various tumors. EMT not only initiates tumor invasion and migration, but is also an indicator of the distant metastasis ability of tumor cells 36-37. Studies show that EMT promotes GC progression via the MAPK-JNK signaling pathway, and activating this pathway induces tumor EMT 38-40. Consistent with this, knockdown of NKCC1 inhibited EMT-related proteins including Snail, vimentin, MMP2 and MMP9 but increased E-cadherin, while NKCC1 overexpression had the opposite effects. Several studies have shown that JNK promoted EMT and enhanced the invasion and migration of GC cells 24, 38-40, which was consistent with our findings. Furthermore, NKCC1 silencing also inhibited JNK activation without affecting P-38 and ERK. Not surprisingly, therefore, targeted inhibition of JNK in the GC cells overexpressing NKCC1 reversed EMT. Immunofluorescence staining demonstrated that E-cadherin expression was up-regulated and vimentin expression was down-regulated after GC cells overexpressing NKCC1 was treated with the JNK inhibitor SP600125. Hence, NKCC1 may regulate the occurrence of EMT through MAPK-JNK signal pathway. After SP600125, a JNK inhibitor was added to gastric cancer cells with overexpressed NKCC1, the EMT-related proteins were changed. The inactivation of MAPK-JNK reversed the overexpression of NKCC1 and induced EMT. The invasion and migration ability of GC cells with overexpressed NKCC1 was inhibited, indicating that NKCC1 could mediate EMT by activating the MAPK-JNK signal pathway. As a result, it was concluded that MAPK-JNK signal pathway played an important role in the progression of GC mediated by NKCC1.
In summary, NKCC1 acts as an oncogene in GC and promotes invasion and migration through activating the MAPK-JNK/EMT pathway. This study proves NKCC1 to be a potential therapeutic target in GC. NKCC1 was highly expressed in human GC samples, and may be a marker for regulating the prognosis of gastric cancer. NKCC1 could regulate the proliferation, invasion and migration of gastric cancer cells in vitro. It was further confirmed that NKCC1 could induce EMT to promote the invasion and migration of GC cells through MAPK-JNK pathway. Our study showed that NKCC1 played an important role in the proliferation and metastasis of gastric cancer and may become a candidate marker for clinical diagnosis and treatment of GC.
Acknowledgments
The authors thank the Key Research and Development program of Science and Technology Department of Guangxi (grant no. 2017AB45153), the Scientific Research and Technology-department Program of Guangxi (grant no. 1598011-4) and Innovation Project of Guangxi Graduate Education (grant no. YCBZ2019043).
Authors' Contributions
All authors are the guarantors of integrity of the entire study. Jun-fu Wang and Kun Zhao contributed to the study, collected the data, analyzed the data and wrote this manuscript. Ye-yang Chen, Yue Qiu and Jin-hui Zhu contributed to the Immunohistochemistry experiment. Conduted the western blot analyses. Bo-pei Li and Zheng Wang contributed to the Real-time PCR. Jun-qiang Chen contributed to designed and check this manuscript.
Ethical Statement
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Author biography
Dr. Jun-qiang Chen is the director of the department of the First Affiliated Hospital of Guangxi Medical University, director of Gastrointestinal Surgery, doctor of Clinical Medicine, Professor, chief physician, and a doctoral tutor. He has profound knowledge in basic research and clinical work of gastric cancer, has a solid clinical foundation and rich scientific research experience, is proficient in cell biology, molecular biology and animal experiment technology, and has been published more than 50 articles related to basic and clinical research of Gastric cancer as the first or corresponding author, and has undertaken and completed a number of research projects related to Gastric cancer. Among them, “Clinical and Basic Research Related to Gastric Cancer Progress” won the second prize of Guangxi Science and Technology Progress Award in 2018, “Synthesis, Evaluation and Promotion of Medical Evidence for Oncology Surgery and Related Diseases” won the third prize of Guangxi Science and Technology Progress Award in 2017.
Funding
The present study was supported by the Key Research and Development program of Science and Technology Department of Guangxi (grant no. 2017AB45153), the Scientific Research and Technology-department Program of Guangxi (grant no. 1598011-4) and Innovation Project of Guangxi Graduate Education (grant no. YCBZ2019043)).
Abbreviations
- NKCC1
Na+/K+/2CI- cotransporter 1
- GC
gastric cancer
- EMT
Epithelial-Mesenchymal Transition
- qRT-PCR
real-time quantitative polymerase chain reaction
References
- 1.Plummer M, Franceschi S, Vignat J, Forman D, de Martel C. Global burden of gastric cancer attributable to Helicobacter pylori. Int J Cancer. 2015;136:487–490. doi: 10.1002/ijc.28999. [DOI] [PubMed] [Google Scholar]
- 2.Cai Z, Liu Q. Understanding the Global Cancer Statistics 2018: implications for cancer control. Sci China Life Sci. 2019. 10.1007/s11427-019-9816-1. [DOI] [PubMed]
- 3.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 4.Goh LY, Leow AH, Goh KL. Observations on the epidemiology of gastrointestinal and liver cancers in the Asia-Pacific region. J Dig Dis. 2014;15:463–468. doi: 10.1111/1751-2980.12164. [DOI] [PubMed] [Google Scholar]
- 5.Bang CS, Baik GH, Shin IS, Kim JB, Suk KT, Yoon JH. et al. Helicobacter pylori Eradication for Prevention of Metachronous Recurrence after Endoscopic Resection of Early Gastric Cancer. J Korean Med Sci. 2015;30:749–756. doi: 10.3346/jkms.2015.30.6.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson WF, Camargo MC, Fraumeni JF Jr, Correa P, Rosenberg PS, Rabkin CS. Age-specific Trends in Incidence of Noncardia Gastric Cancer in US Adults. JAMA. 2010;303:1723–1728. doi: 10.1001/jama.2010.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu JC, Lytle C, Zhu TT, Payne JA, Benz E Jr, Forbush B 3rd. Molecular Cloning and Functional Expression of the Bumetanide-Sensitive Na-K-Cl cotransporter. Proc Natl Acad Sci U S A. 1994;91:2201–2205. doi: 10.1073/pnas.91.6.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park JH, Saier MH Jr. Phylogenetic, Structural and Functional Characteristics of the Na-K-Cl cotransporter family. J Membr Biol. 1996;149:161–168. doi: 10.1007/s002329900016. [DOI] [PubMed] [Google Scholar]
- 9.Shiozaki A, Miyazaki H, Niisato N, Nakahari T, Iwasaki Y, Itoi H. et al. Furosemide, a blocker of Na+/K+/2Cl- cotransporter, diminishes proliferation of poorly differentiated human gastric cancer cells by affecting G0/G1 state. J Physiol Sci. 2006;56:401–406. doi: 10.2170/physiolsci.RP010806. [DOI] [PubMed] [Google Scholar]
- 10.Zhu W, Begum G, Pointer K. et al. WNK1-OSR1 Kinase-Mediated Phospho-Activation of Na+-K+-2Cl- Cotransporter Facilitates Glioma Migration. Mol Cancer. 2014;13:31. doi: 10.1186/1476-4598-13-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Y, Sun W, Chen N. et al. Discovery of NKCC1 as a Potential Therapeutic Target to Inhibit Hepatocellular Carcinoma Cell Growth and Metastasis. Oncotarget. 2017;8:66328–66342. doi: 10.18632/oncotarget.20240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iser IC, Pereira MB, Lenz G, Wink MR. The Epithelial-to-Mesenchymal Transition-Like Process in Glioblastoma: An Updated Systematic Review and In silico Investigation. Med Res Rev. 2017;37:271–313. doi: 10.1002/med.21408. [DOI] [PubMed] [Google Scholar]
- 13.Tzanakakis G, Kavasi RM, Voudouri K. et al. Role of the Extracellular Matrix in Cancer-Associated Epithelial to Mesenchymal Transition Phenomenon. Dev Dyn. 2018;247:368–381. doi: 10.1002/dvdy.24557. [DOI] [PubMed] [Google Scholar]
- 14.Tam WL, Weinberg RA. The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat Med. 2013;19:1438–1449. doi: 10.1038/nm.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prieto-Garcia E, Diaz-Garcia CV, Garcia-Ruiz I, Agullo-Ortuno MT. Epithelial-to-mesenchymal transition in tumor progression. Med Oncol. 2017;34:122. doi: 10.1007/s12032-017-0980-8. [DOI] [PubMed] [Google Scholar]
- 16.Yamamichi F, Shigemura K, Behnsawy HM. et al. Sonic hedgehog and androgen signaling in tumor and stromal compartments drives epithelial-mesenchymal transition in prostate cancer. Scand J Urol. 2014;48:523–532. doi: 10.3109/21681805.2014.898336. [DOI] [PubMed] [Google Scholar]
- 17.Savagner P. The epithelial-mesenchymal transition (EMT) phenomenon. Ann Oncol. 2010;21(Suppl 7):vii89–vii92. doi: 10.1093/annonc/mdq292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng Z, Wang CX, Fang EH, Wang GB, Tong Q. Role of epithelial-mesenchymal transition in gastric cancer initiation and progression. World J Gastroenterol. 2014;20:5403–5410. doi: 10.3748/wjg.v20.i18.5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maier J, Traenkle B, Rothbauer U. Visualizing Epithelial-Mesenchymal Transition Using the Chromobody Technology. Cancer Res. 2016;76:5592–5596. doi: 10.1158/0008-5472.CAN-15-3419. [DOI] [PubMed] [Google Scholar]
- 20.Schiapparelli P, Guerrero-Cazares H, Magaña-Maldonado R. et al. NKCC1 Regulates Migration Ability of Glioblastoma Cells by Modulation of Actin Dynamics and Interacting with Cofilin. EBioMedicine. 2017;21:94–103. doi: 10.1016/j.ebiom.2017.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma H, Li T, Tao Z, Hai L, Tong L, Yi L. et al. NKCC1 promotes EMT-like process in GBM via RhoA and Rac1 signaling pathways. J Cell Physiol. 2019;234:1630–1642. doi: 10.1002/jcp.27033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu J, Zheng Y, Zhang H, Liu Y, Sun H, Zhang P. Galectin-1 Induces Metastasis and Epithelial-Mesenchymal Transition (EMT) in Human Ovarian Cancer Cells via Activation of the MAPK JNK/p38 Signalling Pathway. Am J Transl Res. 2019;11:3862–3878. [PMC free article] [PubMed] [Google Scholar]
- 24.Pang B, Wu N, Guan R, Pang L, Li X, Li S. et al. Overexpression of RCC2 Enhances Cell Motility and Promotes Tumor Metastasis in Lung Adenocarcinoma by Inducing Epithelial-Mesenchymal Transition. Clin Cancer Res. 2017;23:5598–5610. doi: 10.1158/1078-0432.CCR-16-2909. [DOI] [PubMed] [Google Scholar]
- 25.Zhao K, Wang Z, Li X, Liu J-l, Tian L, Chen J-q. Exosome-mediated transfer of CLIC1 contributes to the vincristine-resistance in gastric cancer. Mol Cell Biochem. 2019;462:97–105. doi: 10.1007/s11010-019-03613-9. [DOI] [PubMed] [Google Scholar]
- 26.Yue X, Cui Y, You Q, Lu Y, Zhang J. MicroRNA124 negatively regulates chloride intracellular channel 1 to suppress the migration and invasion of liver cancer cells. Oncol Rep. 2019;42:1380–1390. doi: 10.3892/or.2019.7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lastraioli E, Guasti L, Crociani O. et al. herg1 Gene and HERG1 Protein Are Overexpressed in Colorectal Cancers and Regulate Cell Invasion of Tumor Cells. Cancer Res. 2004;64:606–611. doi: 10.1158/0008-5472.can-03-2360. [DOI] [PubMed] [Google Scholar]
- 28.Wang X-T, Nagaba Y, Cross HS, Wrba F, Zhang L, Guggino SE. The mRNA of L-Type Calcium Channel Elevated in Colon Cancer. Am J Pathol. 2000;157:1549–1562. doi: 10.1016/S0002-9440(10)64792-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Panet R, Marcus M, Atlan H. Overexpression of the Na(+)/K(+)/Cl(-) Cotransporter Gene Induces Cell Proliferation and Phenotypic Transformation in Mouse Fibroblasts. J Cell Physiol. 2000;182:109–118. doi: 10.1002/(SICI)1097-4652(200001)182:1<109::AID-JCP12>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 30.Shiozaki A, Nako Y, Ichikawa D, Konishi H, Komatsu S, Kubota T. et al. Role of the Na (+)/K (+)/2Cl(-) cotransporter NKCC1 in cell cycle progression in human esophageal squamous cell carcinoma. World J Gastroenterol. 2014;20:6844–6859. doi: 10.3748/wjg.v20.i22.6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sontheimer H. An unexpected role for ion channels in brain tumor metastasis. Exp Biol Med (Maywood) 2008;233:779–791. doi: 10.3181/0711-MR-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson MD, O'Connell M. Na-K-2Cl cotransporter and aquaporin 1 in arachnoid granulations, meningiomas, and meningiomas invading dura. Hum Pathol. 2013;44:1118–1124. doi: 10.1016/j.humpath.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 33.Ohsawa R, Miyazaki H, Niisato N, Shiozaki A, Iwasaki Y, Otsuji E. et al. Intracellular chloride regulates cell proliferation through the activation of stress-activated protein kinases in MKN28 human gastric cancer cells. J Cell Physiol. 2010;223:764–770. doi: 10.1002/jcp.22088. [DOI] [PubMed] [Google Scholar]
- 34.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oh J, Takahashi R, Kondo S. et al. The Membrane-Anchored MMP Inhibitor RECK Is a Key Regulator of Extracellular Matrix Integrity and Angiogenesis. Cell. 2001;107:789–800. doi: 10.1016/s0092-8674(01)00597-9. [DOI] [PubMed] [Google Scholar]
- 36.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Min J, Liu L, Li X, Jiang J, Wang J, Zhang B. et al. Absence of DAB2IP promotes cancer stem cell like signatures and indicates poor survival outcome in colorectal cancer. Sci Rep. 2015;5:16578. doi: 10.1038/srep16578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee YS, Kim SY, Song SJ. et al. Crosstalk between CCL7 and CCR3 promotes metastasis of colon cancer cells via ERK-JNK signaling pathways. Oncotarget. 2016;7:36842–36853. doi: 10.18632/oncotarget.9209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng HL, Lin CW, Yang JS, Hsieh MJ, Yang SF, Lu KH. Zoledronate Blocks Geranylgeranylation Not Farnesylation to Suppress Human Osteosarcoma U2OS Cells Metastasis by EMT via Rho A Activation and FAK-inhibited JNK and p38 Pathways. Oncotarget. 2016;7:9742–9758. doi: 10.18632/oncotarget.7138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meng H, Wu J, Huang Q, Yang X, Yang K, Qiu Y. et al. NEDD9 promotes invasion and migration of colorectal cancer cell line HCT116 via JNK/EMT. Oncol Lett. 2019;18:4022–4029. doi: 10.3892/ol.2019.10756. [DOI] [PMC free article] [PubMed] [Google Scholar]