Abstract
目的
系统评价牙周基础治疗对慢性肾病伴牙周炎患者炎症因子的影响。
方法
计算机检索中国学术期刊全文数据库(CNKI)、万方数据库、中国生物医学文献数据库 (CBM)、PubMed、EMbase以及Cochrane Library等数据库,检索时限为从建库截止到2019年12月。由2名研究者收集所有关于牙周基础治疗(牙周非手术治疗)对于慢性肾病伴牙周炎患者炎症因子[C反应蛋白(CRP)、白细胞介素(IL)-6、肿瘤坏死因子(TNF)-α]影响的文献,并且根据纳入排除标准对文献进行筛选,对研究的质量进行严格评价和资料提取,用Revman 5.3软件对符合标准的随机对照试验进行Meta分析。
结果
最终纳入了6项研究分析,Meta分析结果显示,与对照组相比,牙周基础治疗能显著降低慢性肾病伴牙周炎患者CRP水平[MD=-0.58,95%CI(-1.13,-0.02),P=0.04]和IL-6水平[MD=-2.76,95%CI(-5.15,-0.37),P=0.02],但TNF-α水平[MD=-3.87,95%CI(-8.79,1.05),P=0.12]没有得到明显改善。
结论
慢性肾病伴牙周炎的患者在规律治疗肾病的同时行牙周基础治疗,不仅能够缓解其牙周炎症状况,还可在一定程度上改善全身的部分炎症因子的状态,有利于慢性肾病和牙周炎的控制和治疗。
Keywords: 慢性肾病, 牙周炎, 牙周基础治疗, C反应蛋白, 白细胞介素-6, 肿瘤坏死因子-α
Abstract
Objective
A study was conducted to systematically evaluate the clinical efficacy of inflammatory factors in patients with chronic kidney disease and periodontitis after non-surgical periodontal therapy.
Methods
We searched the databases of CNKI, Wanfang, CBM, PubMed, Embase, and Cochrane Library from inception to December 2019. Two reviewers independently collected all literature related to inflammatory factors in patients with chronic kidney disease and periodontitis after non-surgical periodontal therapy. These factors include C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α). The literature was screened according to the inclusion and exclusion criteria. The quality of the studies was strictly evaluated, and the data were extracted. The literature of randomized controlled trials in accordance with the standards was Meta-analyzed with Revman 5.3 software.
Results
Six randomized controlled trials were included. Compared with the control groups, the results of meta-analysis showed that non-surgical periodontal therapy significantly reduced the levels of CRP [MD=-0.58, 95%CI (-1.13, -0.02), P=0.04] and IL-6 [MD=-2.76, 95%CI (-5.15, -0.37), P=0.02] in these patients but not that of TNF-α [MD=-3.87, 95%CI (-8.79, 1.05), P=0.12].
Conclusion
Simultaneous regular renal treatment and non-surgical periodontal therapy can help relieve the periodontal damage on patients with chronic kidney disease and periodontitis. Moreover, it can improve the status of some inflammatory factors. This finding is conducive to the control and treatment of chronic kidney disease and periodontitis and needs to be a focus of research and in clinical operation.
Keywords: chronic kidney disease, periodontitis, periodontal therapy, C-reactive protein, interleukin-6, tumor necrosis factor-α
慢性肾病(chronic kidney disease,CKD)是一个全球化的公共健康问题,CKD的主要表现是肾小球和肾小管的滤过率逐渐降低,肾单位便出现了渐进性不可逆转的恶化表现[1]。美国肾脏基金会指南[2]根据肾小球滤过率将CKD划分为5个期,伴随着病程的发生和进展,逐渐出现肾功能降低和解剖结构改变,甚至是尿沉淀物等异常情况,它的治疗包括从Ⅰ期的高危因素控制等到Ⅴ期的腹膜透析、血液透析,甚至是移植等[3]。
炎症作为危险因素可使CKD发展成终末期肾脏病(end stage renal disease,ESRD),引发“尿毒症难题”,并涉及氧化应激和全身炎症,这些指数也和CKD并发心血管疾病(cardiovascular diseases,CVD)所息息相关[4]–[5]。CKD、ESRD所产生的炎症反应还是导致因CVD致死的关键因素,因此,现已有学者提出了“减少生化炎症标志物”作为该人群的治疗目标结果[6]。
牙周炎是一种微生物相关、宿主介导并导致牙周附着丧失的炎症[7],是成人牙缺失的主要原因。2018年6月,世界联合研讨会正式确定了关于牙周病的新分类方法[8],将1999年分类法[9]中的慢性和侵袭性现均归为牙周炎,并重新划分为4期、3级。研究[10]发现,牙周健康者早期可因个别牙位发生轻度炎症而发展为牙龈炎,再进展成牙周炎,在临床上可见探诊出血、附着丧失,严重者[11]由于牙槽骨的破坏,往往还伴有根分叉病变、牙髓病变、牙松动甚至脱落等。其病程发展缓慢,受多种风险因素的影响,包括年龄、教育水平、吸烟、压力以及影响宿主免疫炎症反应等多种疾病[12]。经过长期的临床实践发现,在牙周炎易感个体中,部分炎症因子可以避开局部防御、侵入循环系统、引起全身炎症反应[13]。
牙周炎和其他全身性疾病(如CVD或代谢综合征)之间的联系在过去已有报道[14]–[15],而CKD和牙周炎之间的确切关系还在探索中。牙周炎或构成了CKD的一种促进或危险因素,这不只是源于临床研究[16],还包括动物模型[17]–[18]上的探索,结果显示牙周炎可促进肾功能恶化并造成肾脏的病理损伤,通过炎性机制的这种方式来加重肾脏损害。有研究发现,一方面CKD患者自身牙周炎症负担较重[19],存在持续的炎症状态,表现为C反应蛋白(C-reactive protein,CRP)、白细胞介素(interleukin,IL)-6、肿瘤坏死因子(tumor necrosis factor,TNF)-α等炎症因子的不同程度的升高[20],另一方面,牙周炎患者在炎症活跃期局部可以产生相同的细胞因子加速CKD的进展[21]。那么对于CKD伴牙周炎患者,就会考虑是否能通过牙周基础治疗的方式,在减缓牙周炎进展的同时,还在一定程度上改善CKD患者的全身炎症因子状况,防止微炎症状态的进一步扩张[22]。为进一步评价牙周基础治疗关于炎症因子的影响,本研究拟对国内外的随机对照试验进行Meta分析,为CKD的协同治疗提供循证医学的依据。
1. 材料和方法
1.1. 文献检索
在中文数据库中国期刊全文数据库(CNKI)、万方数据库(Wan Fang Data)和中国生物医学文献数据库(CBM)中检索“慢性肾病”、“透析”、“牙周炎”、“C反应蛋白”、“白细胞介素-6”、“肿瘤坏死因子-α”、“炎症因子”、“牙周治疗”等关键词。在英文数据库PubMed、EMbase以及Cochrane Library数据库中检索“chronic kidney disease”、“kidney”、“renal”、“dialysis”、“periodontitis”、“C-reactive protein”、“interleukin-6”、“tumor necrosis factor-α”、“inflammatory factors”、“periodontal therapy”等关键词。检索日期限定为建库至2019年12月31日所有公开发表的文献,语言限定是英文和中文。
1.2. 文献纳入与排除标准
1)研究类型:中、英文里是随机对照试验的文献,无论盲法与否。
2)研究对象:需要是同时被确定成CKD以及牙周炎的患者。符合CKD定义的患者标准为肾损伤或者不明原因的肾小球滤过率(glomerular filtration rate,GFR)<60 mL·min−1,并持续达3个月以上[23]。因牙周炎新旧分类的过渡,筛选患者时根据牙周炎的临床情况,只需符合以下具体口内条件:口内存留牙数≥12颗,口腔内不同象限至少有2个位点牙周探诊深度(probing depth,PD)>4 mm,临床附着丧失(clinical attachment loss,CAL)≥3 mm。分别满足以上2个关于CKD和牙周炎的选择标准后,并且,同时需要排除的患者有3个月内做过牙周治疗、精神病患者、恶性肿瘤、严重系统性疾病、妊娠及哺乳期妇女。
3)干预措施:试验组和对照组均给予规律且相同的肾病治疗,试验组在此基础上另给予牙周基础治疗。
4)结局指标:对牙周基础治疗前后的CRP、IL-6、TNF-α水平含量有所报道。
5)排除标准:①无原始临床数据;②非随机对照试验;③只有方案尚未定论的;④综述、回顾性研究、病例报告、动物试验和摘要;⑤总样本量<30,随访时间<4周。
1.3. 检索策略及资料提取
由2名评价员独立根据纳入与排除标准筛选文献、提取文章的数据并交叉核对资料,如遇到分歧,则通过讨论或者咨询第三方协助判断。筛选文献时先阅读文题和摘要, 排除了明显不相关的文献后,再阅读全文,通过制定完善的数据表对文献信息进行汇总,并确定最终是否纳入。提取的信息包括:1)第一作者;2)发表年份;3)年龄;4)研究时间;5)试验组与对照组样本量;6)样本性别;7)时间点;8)针对CKD的治疗方式;9)结局指标。
1.4. 质量评价
筛选文献时应将满足条件的都纳入,再根据“Cochrane偏倚风险评估工具”通过高质量纳入,低质量排除的方法进行质量把控,由2名评价员评价纳入研究的偏倚风险,且须按照Cochrane手册针对随机对照试验的偏倚风险评估工具进行。分别将对以下7个方面进行质量评价:1)随机序列的产生;2)分配隐藏;3)参与者盲法;4)结局评估中的盲法;5)失访或退出报告;6)选择性报告结果;7)其他偏倚。
1.5. 统计学分析
使用Review Manager(Revman)统计分析软件(5.3版本)来进行统计学的分析。本分析的结局指标属于计量资料,故采用均数差(mean difference,MD)与95%可信区间(confidence interval,CI)来作为其效应指标,并设定P<0.05为有统计学意义。并且需要对各个纳入研究结果之间的异质性进行χ2检验,若P<0.1考虑各试验间存在明显的异质性。以I2值代表异质性的大小,当I2值高于50%,采用随机效应模型合并效应量,并探索异质性的来源;反之使用固定效应模式。
2. 结果
2.1. 文献的检索和纳入研究的一般信息
最初检索文献3 315篇,阅读题目,排除无关研究2 698篇,另有重复文献46篇。阅读摘要后再排除文献488篇,进一步阅读全文后排除文献77篇,最终6项随机对照试验被纳入[24]–[29],包含中、英文各3篇,具体文献的筛选过程见图1。
图 1. 文献筛选过程.
Fig 1 Literature screening process
2.2. 纳入研究的一般特征
6篇纳入文献的一般信息见表1。在这6项研究中,2项[24],[27]进行腹膜透析治疗,3项[25],[28]–[29]进行血液透析治疗,1项[26]提及使用常规肾病治疗。另外,4项[25]–[26],[28]–[29]均报告了CRP、IL-6、TNF-α水平,1项[24]只报告了IL-6水平,1项[27]只报告了CRP水平。共计总样本量为427例,其中试验组216例,对照组211例,患者的年龄范围约为34~64岁,男女比例约为1.2︰1,随访时间为1~6月不等。本分析采集各项研究时间≤3个月时间截点的数据进行分析。
表 1. 纳入研究的一般特征.
Tab 1 General characteristics of included studies
| 纳入研究 | 年份 | 年龄/岁 | 样本量(T/C) | 性别(男/女) | 时间点 | 治疗方式 | 测量指标 |
| Wehmeyer等[24] | 2013 | 53.4±9.8 | 23/23 | 33/18 | 3个月、6个月 | 腹膜透析 | IL-6、白蛋白 |
| Fang等[25] | 2015 | 54.62±6.32 | 48/49 | 42/55 | 6周、3个月、6个月 | 血液透析 | TNF-α、IL-6、CRP、铁蛋白等 |
| Guo等[26] | 2017 | 57.5±8.5 | 26/27 | 24/29 | 6周 | 常规肾病治疗 | TNF-α、IL-6、CRP |
| 张晋玮等[27] | 2017 | 48.61±4.60 | 31/30 | 30/31 | 1个月 | 腹膜透析 | CRP |
| 李浩萍等[28] | 2018 | 40.28±6.30 | 36/36 | 39/33 | 8周 | 血液透析 | TNF-α、IL-6、CRP、血清肌酐、24 h尿蛋白 |
| 马欣等[29] | 2018 | 39.2±5.2 | 52/46 | 56/42 | 6周 | 血液透析 | TNF-α、IL-6、CRP、血清肌酐、24 h尿蛋白 |
注:T,干预组;C,对照组。
2.3. 方法学质量评价
纳入研究的偏倚风险评价结果见图2、3:以上6篇文章的研究采用“Cochrane偏倚风险评估工具”来进行质量评价,其中3篇[24]–[25],[27]提及使用简单随机数字;2篇[24]–[25]提及研究员盲法;2篇[24]–[25]提及分配隐藏;2篇[24]–[25]提及8例失访,失访率为1.9%,其余4项研究没有失访及退出。4项[26]–[29]研究被认为具有中等偏倚风险,2项[24]–[25]研究被认为具有低等偏倚风险。
图 2. 纳入研究的偏倚风险评价.
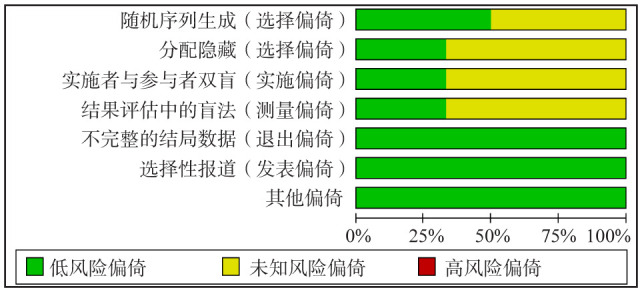
Fig 2 Bias risk assessment for included studies
图 3. 偏倚风险总结.

Fig 3 Summary of bias risk
2.4. Meta分析
2.4.1. CRP水平
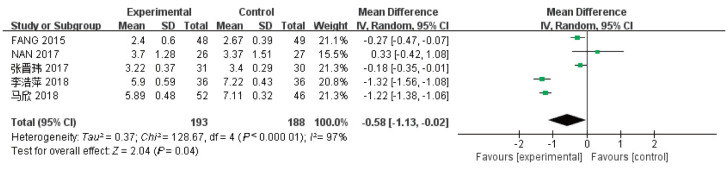
有5项研究[25]–[29]报道了3个月时间点内(≤3个月)的CRP水平值,其中试验组193例,对照组188例,各项研究间存在有异质性(I2=97%,P<0.05),因此使用随机效应模型。Meta分析结果显示,相比于对照组,牙周基础治疗能减少CKD伴牙周炎患者血清中的CRP水平,差异具有统计学意义[MD=-0.58,95%CI(-1.13,-0.02),P=0.04] (图4)。
图 4. 试验组与对照组患者的CRP值的Meta分析森林图.
Fig 4 Meta-analysis forest map of CRP values in trial and control groups
2.4.2. IL-6水平
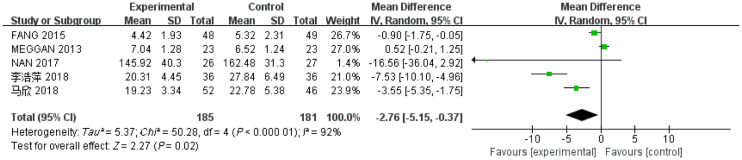
有5项研究[24]–[26],[28]–[29]报道了3个月时间点内(≤3个月)的IL-6水平值,其中试验组185例,对照组181例,各项研究间存在有异质性(I2=92%,P<0.05),使用随机效应模型。Meta分析结果显示,相比于对照组,牙周基础治疗能减少CKD伴牙周炎患者IL-6水平,差异有统计学意义[MD=-2.76,95%CI(-5.15,-0.37),P=0.02](图5)。
图 5. 试验组与对照组患者的IL-6值的Meta分析森林图.
Fig 5 Meta-analysis forest map of IL-6 values in trial and control groups
2.4.3. TNF-α水平
有4项研究[25]–[26],[28]–[29]报道了3个月时间点内(≤3个月)的TNF-α水平值,其中试验组162例,对照组158例,各项研究间存在有异质性(I2=97%,P>0.05),使用随机效应模型。Meta分析结果显示,相比于对照组,牙周基础治疗不能明显减少CKD伴牙周炎患者TNF-α水平,两组间差异无统计学意义[MD=-3.87,95%CI(-8.79,1.05),P=0.12](图6)。
图 6. 试验组与对照组患者的TNF-α值的Meta分析森林图.
Fig 6 Meta-analysis forest map of TNF-α values in trial and control groups
2.5. 异质性分析
本研究的异质性评估中,I2>50%,考虑异质性较大,考虑原因可能是因为部分纳入研究的样本量较少、各个研究的基线水平、随访时间以及检测使用的仪器不同等不一致从而出现临床异质性。此时,采用随机效应模型来作Meta分析可校正其异质性,也使结果更加可信。
2.6. 敏感性分析
对结局指标在进行分析的时候,依次排除每一个研究后,再次作Meta分析,发现尽管每次少纳入一个研究指标却对结局的影响没有明显的变化,这说明本研究的系统评价稳定,结果可靠。由于此次研究最终只纳入了6篇研究文献,尚不能够进行漏斗图分析,故无法判断是否存在发表偏倚。
3. 讨论
牙周炎作为慢性炎症的重要来源,以牙龈卟啉单胞菌、福赛坦菌、具核梭杆菌等为主的牙周致病菌能耐受吞噬作用,逃避免疫攻击[30],它们释放的内毒素可引起局部及外周血中产生某些细胞炎症因子,引发局部牙周组织的慢性炎症,存在促进全身性的炎症发展的可能[31]。也因此有研究[32]认为牙周致病菌是肾功能损害的非传统因素之一。当对CKD患者的口内微生物环境进行研究[33]后发现,牙周致病菌含量较高,可释放和聚集炎症细胞因子。牙周病原体还有黏附、侵入的能力,可使冠状动脉内皮细胞增殖、加速动脉粥样硬化形成、引起血小板聚集形成血栓而进一步造成肾病患者的肾血流障碍,或致慢性肾衰竭[34]。
在本研究纳入的6篇随机对照试验中,各只有1项研究显示IL-6[24]和TNF-α[25]为阴性结局,经过Meta分析后显示,和对照组相比,试验组的CRP、IL-6减少具有统计学意义(P<0.05),而TNF-α的减少的差异无统计学意义(P>0.05)。经过牙周干预后,CRP、IL-6下降的机制尚不明确,可能为牙周致病菌的脂多糖和其他细菌成分可以激活一系列炎性因子,或诱发肝细胞产生急性期反应,牙周基础治疗能够清除牙周致病菌,缩小炎症范围,进入血液的炎症介质降低,使其水平明显降低[35]。而TNF-α参与炎症和组织破坏有关,它的下降却表现不明显,原因可能是本分析中其样本含量最少引起偏差,还有可能是选取的时间点为3个月内,牙周炎症消退尚不充分,加之TNF-α本身敏感性不够高[22]。有纵向干预研究[35]认为,牙周基础治疗的最佳效果时间为从基线起后的6个月。
在肝脏合成的CRP是最重要的急性期蛋白,被视为炎症和内皮功能障碍的系统标志物,也是CVD的预测因子,其敏感性好,半衰期长,因此作为评价微炎症的可靠性标志[36]。横断面调查[20]显示,牙周破坏程度与CRP水平呈正相关,其表达尤其受到IL-6的调控。一方面,IL-6在牙周炎中主要参与了牙槽骨的吸收,随着炎症加重而逐渐活跃;另一方面,IL-6在CKD患者中可由肾脏系膜细胞持续分泌产生,加剧肾小球的病理改变[37]。有大量文献表明,CKD伴牙周炎患者的炎症程度和CRP、IL-6密切相关[38],牙周基础治疗的效果评价除减轻CRP[39]–[40]和IL-6[41]等标志物外,还有部分指标,如变化不明显的TNF-α[42]、肌酐[43]以及改善显著的24 h尿蛋白含量、GFR等[35],[44]都具有临床指导意义。在牙周炎患者和CKD患者的血清中炎症因子水平都有增加,通过炎症反应它们之间呈现出了双向关系[45],CRP、IL-6和TNF-α等炎症介质可以通过牙周袋溃疡进入血液循环,扩散至其他远隔器官,增加炎症负荷[46]。通过分析表明,牙周基础治疗的方法尽管使得不同的炎症因子消除情况表现不一,但不妨碍人们探讨牙周炎与CKD在炎症问题上具有叠加作用[47],该干预方法对于CKD伴牙周炎患者不仅仅改善了口内的牙周临床指标,还适量减轻了微环境中的炎症负担。这和Graziani等[48]认为的牙周基础治疗除了对全身炎症标志物有积极作用,还对GFR有一定正面影响的研究结果也是一致的,其中GFR作为评价肾排泄功能的最佳综合指标,提示了牙周基础治疗的重要性,它在一定程度上可降低CKD患者进展至ESRD的风险。
对大众而言,应当早期认识和预防CKD的发展,不断有研究试图通过牙周炎与CKD呈正相关的信息[49]来探讨CKD的另一面,自2005年Kshirsagar等[50]首次提出了牙周炎是CKD的危险因素,相继有队列研究[51]–[52]、系统评价[53]–[55]报道了它们之间的关系。对于CKD患者而言,细菌感染在临床上很常见,随之而来的免疫功能的抑制,糖尿病发病率的增加,营养状况的恶化,使它成为了死亡的一个主要原因[56]–[58]。控制感染便成为了研究的重点。
本研究的局限性有:1)本研究中出现临床异质性难以消除;2)文献纳入数量相对不足,只检索了中英文献,忽视了其他语种的文献研究,另有4篇[6],[59]–[61]方案虽符合纳入标准,却尚处于研究阶段;3)部分文献未报告盲法,可能导致偏倚。
综上所述,目前证据显示牙周基础治疗对于CKD伴牙周炎患者是有效的,它有利于缓解局部和全身的炎症状态,改善肾功能的一些相关炎症指标,并且有可能降低CKD患者并发CVD的风险,对于临床疗效是具有积极作用的。本研究因受到纳入文献数量和质量的限制,结果分析存在一定的不足,因此,更多的高质量、大样本和长期随访的随机对照试验结果是有必要展开来佐证研究分析。
Funding Statement
[基金项目] 重庆市卫计委重点资助课题(2015ZDXM018);重庆高校创新团队建设计划资助课题(CXTDG201602006)
Supported by: Key Funding Projects of Chongqing Municipal Health Planning Commission (2015ZDXM018); Project of Innovation Team Construction in Chongqing University (CXTDG201602006).
Footnotes
利益冲突声明:作者声明本文无利益冲突。
References
- 1.Baioni CS, de Souza CM, Ribeiro Braosi AP, et al. Analysis of the association of polymorphism in the osteoprotegerin gene with susceptibility to chronic kidney disease and periodontitis[J] J Periodont Res. 2008;43(5):578–584. doi: 10.1111/j.1600-0765.2008.01098.x. [DOI] [PubMed] [Google Scholar]
- 2.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification[J] Am J Kidney Dis. 2002;39(2 Suppl 1):S1–S266. [PubMed] [Google Scholar]
- 3.殷 苏燕, 郑 法雷. 慢性肾病分期及评估指标的探讨[J] 世界医学杂志. 2003(21):57–59. [Google Scholar]; Yin SY, Zheng FL. Study on stage and evaluation index of chronic kidney disease[J] Int J Med. 2003(21):57–59. [Google Scholar]
- 4.Fox CS, Matsushita K, Woodward M, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a Meta-analysis[J] Lancet. 2012;380(9854):1662–1673. doi: 10.1016/S0140-6736(12)61350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stenvinkel P, Carrero JJ, Axelsson J, et al. Emerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: how do new pieces fit into the uremic puzzle[J] Clin J Am Soc Nephrol. 2008;3(2):505–521. doi: 10.2215/CJN.03670807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trivedi R, Fares G, Nunez VB, et al. Novel PAradigm to improve inflammatory burden in end stage renal disease (rePAIR): study protocol for a randomized controlled trial[J] Trials. 2018;19(1):370. doi: 10.1186/s13063-018-2760-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darveau RP, Tanner A, Page RC. The microbial challenge in periodontitis[J] Periodontol 2000. 1997;14(1):12–32. doi: 10.1111/j.1600-0757.1997.tb00190.x. [DOI] [PubMed] [Google Scholar]
- 8.Caton JG, Armitage G, Berglundh T, et al. A new classification scheme for periodontal and peri-implant diseases and conditions—introduction and key changes from the 1999 classification[J] J Clin Periodontol. 2018;45(Suppl 20):S1–S8. doi: 10.1111/jcpe.12935. [DOI] [PubMed] [Google Scholar]
- 9.Armitage GC. Development of a classification system for periodontal diseases and conditions[J] Ann Periodontol. 1999;4(1):1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 10.郭 淑娟, 刘 倩, 丁 一. 牙周病和植体周病国际新分类简介[J] 国际口腔医学杂志. 2019;46(2):125–134. [Google Scholar]; Guo SJ, Liu Q, Ding Y. A brief introduction of the new classification scheme for periodontal and peri-implant diseases and conditions[J] Int J Stomatol. 2019;46(2):125–134. [Google Scholar]
- 11.Raes M, D'hondt R, Teughels W, et al. A 5-year randomized clinical trial comparing minimally with moderately rough implants in patients with severe periodontitis[J] J Clin Periodontol. 2018;45(6):711–720. doi: 10.1111/jcpe.12901. [DOI] [PubMed] [Google Scholar]
- 12.Genco RJ, Borgnakke WS. Risk factors for periodontal disease[J] Periodontol 2000. 2013;62(1):59–94. doi: 10.1111/j.1600-0757.2012.00457.x. [DOI] [PubMed] [Google Scholar]
- 13.Slade GD, Offenbacher S, Beck JD, et al. Acute-phase inflammatory response to periodontal disease in the US population[J] J Dent Res. 2000;79(1):49–57. doi: 10.1177/00220345000790010701. [DOI] [PubMed] [Google Scholar]
- 14.Linden GJ, Lyons A, Scannapieco FA. Periodontal systemic associations: review of the evidence[J] J Periodontol. 2013;84(4 Suppl):S8–S19. doi: 10.1902/jop.2013.1340010. [DOI] [PubMed] [Google Scholar]
- 15.Nibali L, Tatarakis N, Needleman I, et al. Clinical review: association between metabolic syndrome and periodontitis: a systematic review and Meta-analysis[J] J Clin Endocrinol Metab. 2013;98(3):913–920. doi: 10.1210/jc.2012-3552. [DOI] [PubMed] [Google Scholar]
- 16.Chambrone L, Foz AM, Guglielmetti MR, et al. Periodontitis and chronic kidney disease: a systematic review of the association of diseases and the effect of periodontal treatment on estimated glomerular filtration rate[J] J Clin Periodontol. 2013;40(5):443–456. doi: 10.1111/jcpe.12067. [DOI] [PubMed] [Google Scholar]
- 17.Pontes Andersen CC, Holmstrup P, Buschard K, et al. Renal alterations in prediabetic rats with periodontitis[J] J Periodontol. 2008;79(4):684–690. doi: 10.1902/jop.2008.070433. [DOI] [PubMed] [Google Scholar]
- 18.Lee MM, Chu EY, El-Abbadi MM, et al. Characterization of mandibular bone in a mouse model of chronic kidney disease[J] J Periodontol. 2010;81(2):300–309. doi: 10.1902/jop.2009.090379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grubbs V, Plantinga LC, Crews DC, et al. Vulnerable populations and the association between periodontal and chronic kidney disease[J] Clin J Am Soc Nephrol. 2011;6(4):711–717. doi: 10.2215/CJN.08270910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu KJ, Liu QH, Chen W, et al. Prevalence and risk factors of CKD in Chinese patients with periodontal disease[J] PLoS One. 2013;8(8):e70767. doi: 10.1371/journal.pone.0070767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kapellas K, Singh A, Bertotti M, et al. Periodontal and chronic kidney disease association: a systematic review and Meta-analysis[J] Nephrology (Carlton) 2019;24(2):202–212. doi: 10.1111/nep.13225. [DOI] [PubMed] [Google Scholar]
- 22.玛衣努尔 艾赛提, 白尔娜 吾守尔, 陈 晓涛. 肾透析伴有慢性牙周炎患者牙周基础治疗的临床疗效观察[J] 甘肃医药. 2017;36(2):107–108. [Google Scholar]; Mayinuer AST, Baierna WSR, Chen XT. Clinical observation of periodontal treatment in renal dialysis and chronic periodontitis patients[J] Gansu Med J. 2017;36(2):107–108. [Google Scholar]
- 23.Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from kidney disease: improving global outcomes (KDIGO)[J] Kidney Int. 2005;67(6):2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 24.Wehmeyer MM, Kshirsagar AV, Barros SP, et al. A randomized controlled trial of intensive periodontal therapy on metabolic and inflammatory markers in patients with ESRD: results of an exploratory study[J] Am J Kidney Dis. 2013;61(3):450–458. doi: 10.1053/j.ajkd.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fang FC, Wu BL, Qu Q, et al. The clinical response and systemic effects of non-surgical periodontal therapy in end-stage renal disease patients: a 6-month randomized controlled clinical trial[J] J Clin Periodontol. 2015;42(6):537–546. doi: 10.1111/jcpe.12411. [DOI] [PubMed] [Google Scholar]
- 26.Guo N, Lin GB. Effects of nonsurgical periodontal therapy on serum inflammatory factor levels in patients with chronic kidney disease and periodontitis[J] Biomed Res. 2017;28(9):3899–3902. [Google Scholar]
- 27.张 晋玮, 刘 建山, 马 明, et al. 牙周基础治疗对行腹膜透析牙周炎患者的影响[J] 天津医药. 2017;45(3):282–284. [Google Scholar]; Zhang JW, Liu JS, Ma M, et al. Effects of periodontal basic treatment on peritoneal dialysis in patients with periodontitis[J] Tianjin Med J. 2017;45(3):282–284. [Google Scholar]
- 28.李 浩萍, 李 明勇. 慢性肾病伴牙周炎患者牙周基础治疗疗效及对肾功能指标的影响[J] 北华大学学报(自然科学版) 2018;19(3):374–377. [Google Scholar]; Li HP, Li MY. Therapeutic effect of periodontal basic treatment in patients with chronic renal disease and periodontitis and its effect on renal function[J] J Beihua Univ Nat Sci. 2018;19(3):374–377. [Google Scholar]
- 29.马 欣, 李 昊, 丑 海燕, et al. 牙周基础治疗对慢性肾脏病伴牙周炎患者牙周指数及龈沟液炎性因子和肾功能指标的影响[J] 中华实用诊断与治疗杂志. 2018;32(3):255–257. [Google Scholar]; Ma X, Li H, Chou HY, et al. Effect of periodontal therapy on periodontal index, gingival crevicular fluid inflammatory factors and renal indexes in patients with chronic kidney disease and periodontitis[J] J Chin Pract Diagn Ther. 2018;32(3):255–257. [Google Scholar]
- 30.Ximénez-Fyvie LA, Haffajee AD, Socransky SS. Comparison of the microbiota of supra- and subgingival plaque in health and periodontitis[J] J Clin Periodontol. 2000;27(9):648–657. doi: 10.1034/j.1600-051x.2000.027009648.x. [DOI] [PubMed] [Google Scholar]
- 31.Akram Z, Abduljabbar T, Abu Hassan MI, et al. Cytokine profile in chronic periodontitis patients with and without obesity: a systematic review and Meta-analysis[J] Dis Markers. 2016;2016:4801418. doi: 10.1155/2016/4801418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.邹 华伟, 张 士文, 袁 泉. 慢性肾脏病对牙周组织的影响[J] 国际口腔医学杂志. 2014;41(6):730–734. [Google Scholar]; Zou HW, Zhang SW, Yuan Q. Effect of chronic kidney disease on periodontal tissues[J] Int J Stomatol. 2014;41(6):730–734. [Google Scholar]
- 33.Takeuchi Y, Ishikawa H, Inada M, et al. Study of the oral microbial flora in patients with renal disease[J] Nephrology (Carlton) 2007;12(2):182–190. doi: 10.1111/j.1440-1797.2007.00767.x. [DOI] [PubMed] [Google Scholar]
- 34.冯 丹, 林 晓萍. 牙周炎与慢性肾脏病相关性研究现状[J] 口腔医学. 2015;35(1):61–65. [Google Scholar]; Feng D, Lin XP. Research advancement of relationship between chronic kidney disease and periondontitis[J] Stomatology. 2015;35(1):61–65. [Google Scholar]
- 35.房 付春. 牙周炎对终末期肾脏病患者系统炎症、营养状况及脂质代谢水平的影响[D] 广州: 南方医科大学; 2012. [Google Scholar]; Fang FC. The effects of periodontal disease on systemic inflammation, nutritional status and lipid metabolic level in end-stage renal disease patients[D] Guangzhou: Southern Medical University; 2012. [Google Scholar]
- 36.刘 彦, 王 雷, 林 崇韬, et al. 慢性牙周炎伴原发性高血压患者血清hs-CRP水平的检测及其意义[J] 吉林大学学报(医学版) 2013;39(4):795–798. [Google Scholar]; Liu Y, Wang L, Lin CT, et al. Detection of serum levels of hypersensitivity C-reactive protein in patients with essential hypertension and periodontitis and clinical significance[J] J Jilin Univ Med Ed. 2013;39(4):795–798. [Google Scholar]
- 37.苏 焱伦. 慢性肾病患者血清IL-6, SIL-2R, TNF检测的临床意义[J] 放射免疫学杂志. 2004;17(4):299. [Google Scholar]; Su YL. Clinical significance of serum IL-6, SIL-2R, TNF in patients with chronic kidney disease[J] J Radioimmunol. 2004;17(4):299. [Google Scholar]
- 38.艾山 依力哈木, 古丽努尔 阿吾提, 廖 博, et al. 慢性牙周病与慢性肾病相关性研究进展[J] 口腔医学. 2018;38(1):87–91. [Google Scholar]; AiShan Yilihamu, Gulinuer Awuti, Liao B, et al. Research progress of the correlation between chronic periodontal disease and chronic kidney disease (CKD)[J] Stomatology. 2018;38(1):87–91. [Google Scholar]
- 39.Yazdi FK, Karimi N, Rasouli M, et al. Effect of nonsurgical periodontal treatment on C-reactive protein levels in maintenance hemodialysis patients[J] Ren Fail. 2013;35(5):711–717. doi: 10.3109/0886022X.2013.777890. [DOI] [PubMed] [Google Scholar]
- 40.Ismail G, Dumitriu HT, Dumitriu AS, et al. Periodontal disease: a covert source of inflammation in chronic kidney disease patients[J] Int J Nephrol. 2013;2013:515796. doi: 10.1155/2013/515796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cholewa M, Madziarska K, Radwan-Oczko M. The association between periodontal conditions, inflammation, nutritional status and calcium-phosphate metabolism disorders in hemodialysis patients[J] J Appl Oral Sci. 2018;26:e20170495. doi: 10.1590/1678-7757-2017-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blach A, Franek E, Witula A, et al. The influence of chronic periodontitis on serum TNF-alpha, IL-6 and hs-CRP concentrations, and function of graft and survival of kidney transplant recipients[J] Clin Transplant. 2009;23(2):213–219. doi: 10.1111/j.1399-0012.2008.00931.x. [DOI] [PubMed] [Google Scholar]
- 43.童 书青, 王 晓阳, 高 华. 牙周炎与肾功能相关指标的关系研究[J] 临床输血与检验. 2018;20(3):276–279. [Google Scholar]; Tong SQ, Wang XY, Gao H. Study on the relationship between periodontitis and renal function[J] J Clin Transfus Lab Med. 2018;20(3):276–279. [Google Scholar]
- 44.Almeida S, Figueredo CM, Lemos C, et al. Periodontal treatment in patients with chronic kidney disease: a pilot study[J] J Periodont Res. 2017;52(2):262–267. doi: 10.1111/jre.12390. [DOI] [PubMed] [Google Scholar]
- 45.Sun K, 宋 忠臣. 慢性牙周炎与慢性肾病相关性研究进展[J] 口腔医学. 2020;40(1):59–62. [Google Scholar]; Sun K, Song ZC. Research progress on the correlation between chronic periodontitis and chronic kidney disease[J] Stomatology. 2020;40(1):59–62. [Google Scholar]
- 46.Chandy S, Joseph K, Sankaranarayanan A, et al. Evaluation of C-reactive protein and fibrinogen in patients with chronic and aggressive periodontitis: a clinico-biochemical study[J] J Clin Diagn Res. 2017;11(3):ZC41–ZC45. doi: 10.7860/JCDR/2017/23100.9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dannewitz B, Sommerer C, Stölzel P, et al. Status of periodontal health in German patients suffering from chronic kidney disease-data from the GCKD study[J] J Clin Periodontol. 2020;47(1):19–29. doi: 10.1111/jcpe.13208. [DOI] [PubMed] [Google Scholar]
- 48.Graziani F, Cei S, La Ferla F, et al. Effects of non-surgical periodontal therapy on the glomerular filtration rate of the kidney: an exploratory trial[J] J Clin Periodontol. 2010;37(7):638–643. doi: 10.1111/j.1600-051X.2010.01578.x. [DOI] [PubMed] [Google Scholar]
- 49.Kitamura M, Mochizuki Y, Miyata Y, et al. Pathological characteristics of periodontal disease in patients with chronic kidney disease and kidney transplantation[J] Int J Mol Sci. 2019;20(14):E3413. doi: 10.3390/ijms20143413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kshirsagar AV, Moss KL, Elter JR, et al. Periodontal disease is associated with renal insufficiency in the Atherosclerosis Risk In Communities (ARIC) study[J] Am J Kidney Dis. 2005;45(4):650–657. doi: 10.1053/j.ajkd.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 51.Ricardo AC, Athavale A, Chen JS, et al. Periodontal disease, chronic kidney disease and mortality: results from the third National Health and Nutrition Examination Survey[J] BMC Nephrol. 2015;16:97. doi: 10.1186/s12882-015-0101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sharma P, Dietrich T, Ferro CJ, et al. Association between periodontitis and mortality in stages 3-5 chronic kidney disease: NHANES Ⅲ and linked mortality study[J] J Clin Periodontol. 2016;43(2):104–113. doi: 10.1111/jcpe.12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deschamps-Lenhardt S, Martin-Cabezas R, Hannedouche T, et al. Association between periodontitis and chronic kidney disease: systematic review and Meta-analysis[J] Oral Dis. 2019;25(2):385–402. doi: 10.1111/odi.12834. [DOI] [PubMed] [Google Scholar]
- 54.刘 聪, 王 婷, 张 天夫, et al. 牙周病与慢性肾病相关性的Meta分析[J] 中国实验诊断学. 2016;20(12):2087–2090. [Google Scholar]; Liu C, Wang T, Zhang TF, et al. Meta-analysis of the relationship between periodontal disease and chronic kidney disease[J] Chin J Lab Diagn. 2016;20(12):2087–2090. [Google Scholar]
- 55.Zhang J, Jiang H, Sun M, et al. Association between periodontal disease and mortality in people with CKD: a Meta-analysis of cohort studies[J] BMC Nephrol. 2017;18(1):269. doi: 10.1186/s12882-017-0680-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trimarchi H, Dicugno M, Muryan A, et al. Pro-calcitonin and inflammation in chronic hemodialysis[J] Medicina (B Aires) 2013;73(5):411–416. [PubMed] [Google Scholar]
- 57.Guo M, Chen RY, Xiang FF, et al. Decreased percentage of memory B cells is independently associated with increased susceptibility to infection in patients on maintenance hemodialysis[J] Int Urol Nephrol. 2018;50(11):2081–2090. doi: 10.1007/s11255-018-1977-8. [DOI] [PubMed] [Google Scholar]
- 58.Omari AM, Omari LS, Dagash HH, et al. Assessment of nutritional status in the maintenance of haemodialysis patients: a cross-sectional study from Palestine[J] BMC Nephrol. 2019;20(1):92. doi: 10.1186/s12882-019-1288-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jamieson L, Skilton M, Maple-Brown L, et al. Periodontal disease and chronic kidney disease among Aboriginal adults; an RCT[J] BMC Nephrol. 2015;16:181. doi: 10.1186/s12882-015-0169-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grubbs V, Garcia F, Jue BL, et al. The Kidney and Periodontal Disease (KAPD) study: a pilot randomized controlled trial testing the effect of non-surgical periodontal therapy on chronic kidney disease[J] Contemp Clin Trials. 2017;53:143–150. doi: 10.1016/j.cct.2016.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sharma P, Cockwell P, Dietrich T, et al. Influence of successful periodontal intervention in renal disease (INSPIRED): study protocol for a randomised controlled pilot clinical trial[J] Trials. 2017;18(1):535. doi: 10.1186/s13063-017-2236-5. [DOI] [PMC free article] [PubMed] [Google Scholar]