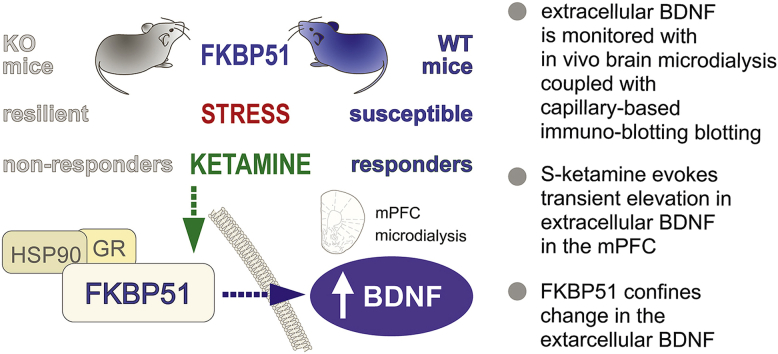
Abstract
We report here the involvement of the stress-responsive glucocorticoid receptor co-chaperone FKBP51 in the mechanism of in vivo secretion of mature BDNF (mBDNF). We used a novel method combining brain microdialysis with a capillary electrophoresis-based immunoassay, to examine mBDNF secretion in the medial prefrontal cortex (mPFC) in vivo in freely moving mice. By combining optogenetic, neurochemical (KCl-evoked depolarization), and transgenic (conditional BDNF knockout mice) means, we have shown that the increase in extracellular mBDNF in vivo is determined by neuronal activity. Withal, mBDNF secretion in the mPFC of mice was stimulated by a systemic administration of S-ketamine (10 or 50 mg/kg) or S-hydroxynorketamine (10 mg/kg).
KCl- and S-ketamine-evoked mBDNF secretion was strongly dependent on the expression of FKBP51. Moreover, the inability of S-ketamine to evoke a transient secretion in mBDNF in the mPFC in FKBP51- knockout mice matched the lack of antidepressant-like effect of S-ketamine in the tail suspension test. Our data reveal a critical role of FKBP51 in mBDNF secretion and suggest the involvement of mBDNF in the realization of immediate stress-coping behavior induced by acute S-ketamine.
Keywords: BDNF, FKBP51, Ketamine, Stress susceptibility, Neuroplasticity, Microdialysis
Graphical abstract
1. Introduction
Brain-derived neurotrophic factor (BDNF) is a secreted neurotrophic protein (Barde et al., 1982; Dieni et al., 2012; Sasi et al., 2017), which is essential for neuronal plasticity both in brain development and maintenance (Monteggia et al., 2007; Park and Poo, 2013). Insufficiency of the BDNF system was proposed as a causality factor of depression (Chen et al., 2001; Dunham et al., 2009; Egan et al., 2003; Hashimoto et al., 2004; Lee and Kim, 2008; Murakami et al., 2005; Notaras et al., 2015; Pandey et al., 2010). In turn, intact or increased BDNF expression in the forebrain contributes to resistance to depression (Monteggia et al., 2007), stress-resilience (Nasrallah et al., 2019; Yang et al., 2016). Recent studies convincingly showed that BDNF signaling in the brain is an ultimate prerequisite for therapeutic activity of selective serotonin reuptake inhibitors and tricyclic antidepressants (Björkholm and Monteggia, 2016; Ma et al., 2016). The specific action of these drugs was in part attributed to increase in BDNF expression and signaling, as well as to fast BDNF-independent effects on tropomyosin receptor kinase B phosphorylation (Rantamäki et al., 2011; Saarelainen et al., 2003). Immediate BDNF signaling is required for the effect of rapidly acting antidepressants ketamine (Yang et al., 2015; Zanos et al., 2016), (2R,6R)-hydroxynorketamine, and GLYX-13 (Fukumoto et al., 2019; Kato et al., 2018). A rapid antidepressant activity of ketamine in the experimental studies involving a test paradigm of stress-coping suggests that components of stress signaling may be implemented in realization of ketamine's effect.
The stress-responsive glucocorticoid receptor co-chaperone FK506 binding protein 51 (FKBP51; encoded by the gene fkbp5) sets the negative feedback sensitivity of the hypothalamic-pituitary-adrenal (HPA) axis, determines chronic stress susceptibility, and is associated with depression and response to antidepressant (Binder, 2009; Hartmann et al., 2012; Hoeijmakers et al., 2014; Touma et al., 2011). The expression of FKBP51 correlates with plasma BDNF levels in depressed patients (Gassen et al., 2015, 2014). FKBP51 is also required for stress-coping induced by paroxetine, amitriptyline, and lithium in stress-coping models in mice (Gassen et al., 2016). We hypothesized that FKBP51 mediates acute antidepressant actions by influencing BDNF signaling. Therefore, we set out to directly monitor the release of BDNF in the medial prefrontal cortex (mPFC) after treatment with the rapidly acting FDA-approved antidepressant S-ketamine to see if the effect of S-ketamine is FKBP51-dependent.
One of the significant constraints in examining the secretion of BDNF and other signaling proteins in vivo is the insufficiency of existing sampling methods and analytic routines. In vivo studies inferring the immediate effects of BDNF signaling on fear extinction were done by either utilizing TrkB antagonist or by using specific BDNF scavenger proteins (Rosas-Vidal et al., 2018, 2014). However, these approaches are accompanied by a decrease in the bioavailability of BDNF or may engage other local effects of TrkB, or even other Trk receptors.
Intracerebral microdialysis is a powerful in vivo method to directly monitor signaling molecules in the extracellular space (Anderzhanova and Wotjak, 2013). However, its conventional applications are fraught with a low recovery of macromolecules that makes proteins and peptides hardly detectable. A recent study introduced an interdigitated microelectrode biosensor that renders the measurement of BDNF in microdialysates (Yoo et al., 2016). However, such a technique requires a relatively high sample volume (9 μl) and does not allow one multiplexing of the analysis.
Here we report a novel approach that combines advanced brain microdialysis with a capillary electrophoresis-based immunoassay (CEIA), thereby providing reliable measurements of extracellular mBDNF in vivo with a 30 min time resolution. Using this method, we show that the acute antidepressant activity of S-ketamine corresponds to the increase in extracellular mBDNF content in the mPFC of mice and that this effect depends on the expression of the stress-susceptibility factor co-chaperone FKBP51.
2. Methods and materials
2.1. Animals
Male C57BL/6 NRjMpi mice (Martinsried, Germany), male BDNFfl/fl–Nex-Cre+/– (BDNF-cKO) (32) and respective wild-type (WT) mice (University Hospital Würzburg, Germany), Ai32(RCL-channelrhodopsin2– (H134R)/EYEP)fl/fl–Nex-Cre+/– mice (ChR2–Nex-Cre+/–) (Martinsried, Germany), and FKBP51–KO (Hartmann et al., 2012) and respective WT mice (Martinsried, Germany; male and female) of 10–14 w/o were housed in cages of 4–5 individuals at 21 °C with a 12:12 h light-dark cycle with food and water ad libitum before experiments. Starting from the day of surgery, mice were single-caged; however, the visual and olfactory contacts were possible. In the experiment where tissue content of BDNF was assessed after S-ketamine administration, mice were also single-caged one week until the treatment/brain harvesting day. Allocation of animals to experimental groups in respect to date of birth and litter was done using a random number generation approach in Excel. All procedures were done in accordance with European Communities Council Directive 2010/63/EU and approved by the Government of Upper Bavaria.
2.2. Drugs
S-Ketamine (Ratiopharm, Germany) and its active metabolite S-hydroxynorketamine (S–HNK) (Tocris, USA) were freshly dissolved in sterile saline and injected intraperitoneally (i.p.) in doses of 10 or 50 mg/kg in a volume of 10 ml/kg.
2.3. Tail suspension test (TST)
Female WT or FKBP51–KO mice were fixed by the tail, 45 cm above the floor, with a distance of 15 cm to the nearest object in a light and sound protected chamber under dim (40 Lux) light. To avoid tail climbing, which is prevalent in mice on the B6 background, we funneled a tail through a tube during the test (plastic tube, id of 4 mm, length of 45 mm). This prevents mice from grasping the tail grasp and, thus, from climbing. Mice were tested in a withing-subject experimental design with single S-ketamine injection and repeated TST at 30 min, 120 min and 24 h after drug administration. Video-recordings for 6 min and subsequent analysis of behavior (ANY-maze, Stoelting, Ireland) were done by a trained observer blinded to genotype and treatment groups. S-ketamine (50 mg/kg, i.p.) or saline were injected once. Struggling time, which served as a measure of active stress-coping and antidepressant-like effect, was examined 30 min, 120 min, and 24 h after the moment of injection in the same mice.
2.4. Brain microdialysis and optogenetics
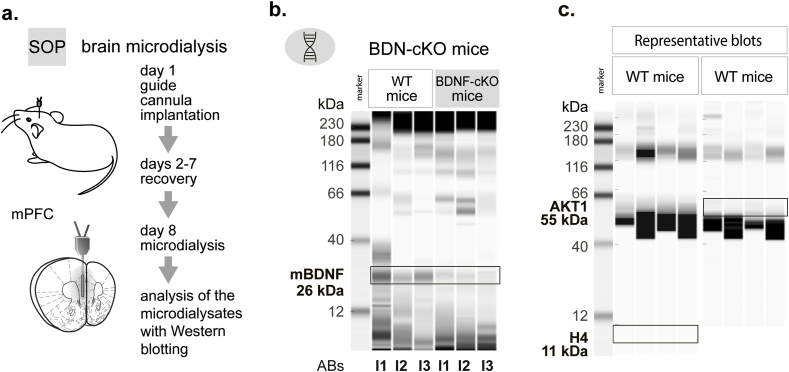
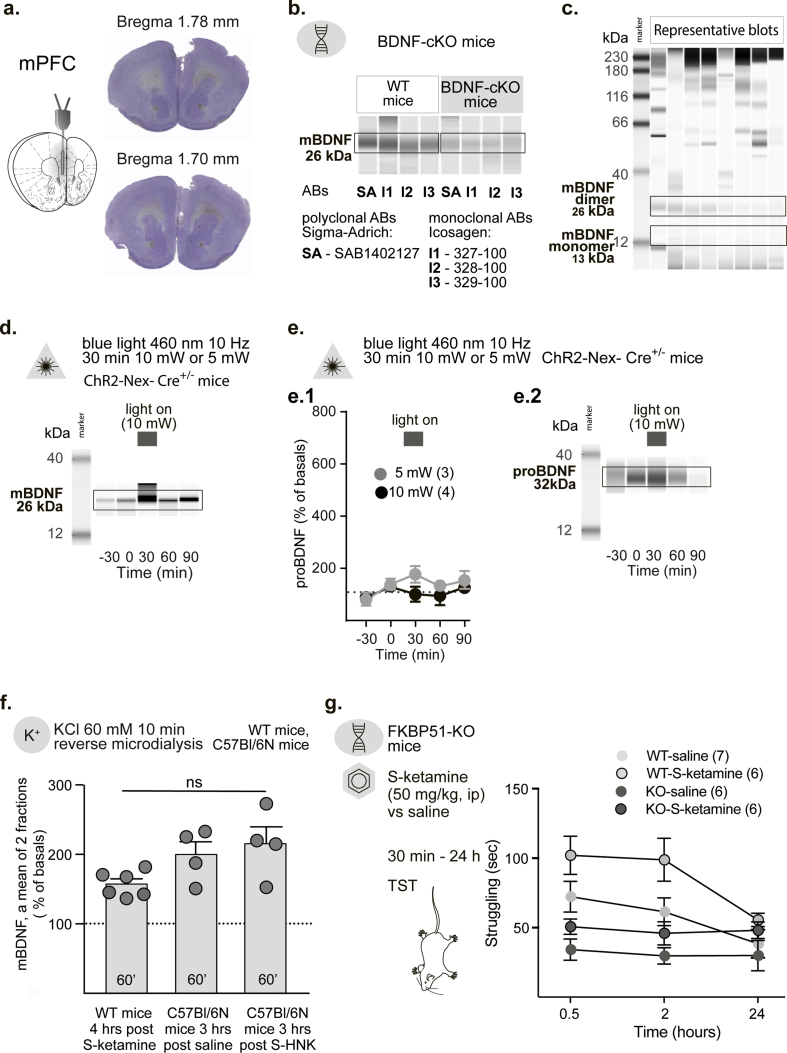
The general workflow of the experiments is depicted in Fig. 1a. Surgeries were performed as described before (Yen et al., 2015). Coordinates for guide cannula/probe with an optic fiber were AP 2.00 mm, ML 0.35 mm, and DV–1.50/3.50 mm, from bregma (Paxinos and Franklin, 2013). Animals were allowed to recover from surgery for 7 days in individual microdialysis cages (16 × 16 × 32 cm3). A microdialysis probe was inserted into the implanted guide cannula under short isoflurane anesthesia (2% in air) 18 h before the basal samples collection. The optogenetic stimulation experiment was performed in anesthetized animals (isoflurane 2% in oxygen) on the day of surgery/probe implantation. Microdialysis probes CMA12 HighCO Metal Free, 100-kDa cut off, 2 mm membrane (CMA Microdialysis, Sweden, Cat.N. 8011222) were used in all experiments.
Fig. 1.
mBDNF in microdialysates of freely moving mice.
a. General workflow of a microdialysis experiment.
b. mBDNF is detectable in medial prefrontal cortex (mPFC) lysates in wild type (WT) mice, but not in BDNF conditional KO (BDNF-cKO) mice. Abs – monoclonal antibodies. I1 – Icosagen Cat.N. 100–327; I2 – Icosagen Cat.N. 100–327; I3 – Icosagen Cat.N. 100–329.
c. Neither cytosolic nor nuclear proteins appear in microdialysates of mPFC. AKT1 - RAC-alpha serine/threonine-protein kinase, H4 - histone H4.
The perfusion line comprised of FET tubing (0.15 mm ID, Microbiotech Se, Sweden), a 15 cm-PVC inset tubing (0.19 mm ID, Elemental Scientific, USA), and a dual-channel liquid swivel (Microbiotech Se, Sweden). Before the experiment, lines were perfused for 8 h with 5% polyethylenimine (Sigma-Aldrich Cat.N. 408727) in sterile water to reduce unspecific binding and then washed with sterile water for 24 h. The perfusion medium was sterile RNase free Ringer's solution (BooScientific, USA) containing 1% BSA (Sigma-Aldrich, Cat.N. A9418). The perfusion medium was delivered to the probe at a flow rate of 0.38 μl/min with a syringe pump (Harvard Apparatus, USA) and withdrawn at a flow rate of 0.40 μl/min with a peristaltic pump MP2 (Elemental Scientific, USA), thus ensuring drainage of the perfused area.
Basal samples were collected before a stimulation phase that allowed us to express changes in the extracellular mBDNF content of each sample as a percent to averaged initial values. KCl (60 mM, in Ringer's solution, equilibrated in the total osmolality by reducing the NaCl concentration) was delivered locally to the probe in a 10 min pulse mode using a liquid switch (Univentor, Ltd, Malta). Drugs (S–HNK or S-ketamine) were injected acutely i.p.
For optical stimulation of ChR2, pulsed (15 ms at 10 Hz, Master-8, A.M.P.I., Israel) laser light (Omicron-Laserage, Rodgau-Dudenhofen, Germany, LightHUB-4) of 460 nm was applied. The laser output was set to measure 5 mW or 10 mW at the single fiber tip (CFML12L20, 200 μm diameter, cut flat to 7 mm length, Thorlabs, USA). The fiber implants were placed close to the microdialysis probe within the perfused area.
Thirty minutes microdialysis fractions were collected on ice into 1.5 ml protein LoBind tubes (Eppendorf, Germany) preloaded with 0.5 μl of a cocktail of protease inhibitors 1:50 (Roche, Cat.N. 4693159001), immediately frozen on dry ice and further kept at −80 °C. After the experiments mice were killed in isoflurane overdose, probes were removed, brains collected and frozen for verification of the probe's placement. Representative cryosections (25 μm, stained with cresyl violet) of the target region within the mPFC are shown in Fig. s1a.
2.5. Tissue sampling
To examine the tissue levels of mBDNF in the mPFC of WT and FKBP51–KO mice were decapitated 90 min after S-ketamine (10 mg/kg, i.p.) or saline injections. Brains were collected and stored at −80 °C. mPFC dissection was done with a 0.5 mm ID stainless steel micro-puncher (FST, Germany) during brain cryosectioning (Yen et al., 2015).
2.6. Capillary electrophoresis-based immunoassay (CEIA)
The advanced CEIA utilizes the separation of protein in a small diameter glass capillary. The capillaries are impermeable for proteins; all molecules subjected to the analysis retain inside and are immobilized to the capillary wall through a chemical UV-activated capture. This approach increases the sensitivity of protein blotting; therefore, the original method developed by ProteinSimple is a method of choice when an analysis is limited by a small amount of a biological sample or by low expression levels of target proteins (ProteinSimple, 2019). Microdialysates contain a low quantity of molecules of interest due to their lower recovery during filtration via a semipermeable membrane. Therefore, CEIA was chosen to analyze the mBDNF content in our experiments.
To assess the mBDNF content in brain samples, tissues were lysed in 50 mM Tris, 150 NaCl, 1 mM Na2-EDTA buffer containing a cocktail of protease inhibitors (Sigma-Aldrich, USA), sonicated and heated at 95 °C for 10 min. Microdialysis samples were treated in accordance to the protocol of gel-free CEIA (ProteinSimple, USA). Five μl of biological samples were used for the analysis (of total 12 μl collected plus 0.5 μl cocktail of protease inhibitors). Separation and immunodetection of proteins were done using the WES Simple immuno-blotting system (ProteinSimple, 2019). The primary antibodies were: anti-mBDNF (1:50–1:500), polyclonal Sigma-Aldrich, Cat.N. SAB1405514, monoclonal Icosagen, Estonia, Cat.NN 327-100, 328-100, 329-100 (both types were produced by immunization with a full-length recombinant mature human BDNF); anti-proBDNF 32 kDa (1:500) NovusBio, Cat.N. NBP2-36693; anti-H4 (1:1000) NovusBio, Cat.N. NBP2-16848; anti-AKT1 (1:1000) NovusBio, Cat.N. NBP1-51602. Secondary antibodies were anti rabbit polyclonal IgG (Cell Signalling, Cat.N. 7074).
2.7. Dopamine measurement
Dopamine measurement in microdialysates was done with HPLC with electrical detection, as previously described (Yen et al., 2015). The Ultimate3000 system (ThermoFisher scientific was employed for monoamine measurement. Separation was done on a stationary phase column YMC-TriArt C18, 150 mm × 3 mm (YMC, Japan) with a mobile phase containing phosphate-citrate buffer, pH = 3.9 and 6% of ACN. The electrode potential were set at −75 mV, 220 mV and 350 mV (guard cell) with gain of 2 nA. Quantification was performed using external standard calibration (0.1–5 nM). LOD for dopamine was 0.06 nM.
2.8. Statistical analysis
Data (mean ± SEM) were analyzed using the GraphPad Prism 7.0 software. Microdialysis data is expressed as a percent to mean basal values. Total mBDNF tissue levels were normalized to values from saline-treated groups. The sample size calculations were done using GPower 3.1 (Franz Faul, Heinrich-Heine-Universität Düsseldorf). All microdialysis experiment were done based on estimated Cohen's d > 1.5. Taking α = 0.05, and 1-β = 0.8, the initial group size was n = 6. Design of the behavioral experiment was set assuming lower effect size (Cohen's 1.0 < d < 1.5, α = 0.05, and 1-β = 0.8) that resulted in initial group size n = 8. The final groups size varied from n = 3 to 6 (see the figures and figure legend in respect to a specific experiment). Data were checked for outliers using the ROUT protocol, Q = 1% (Motulsky and Brown, 2006). Comparisons of means were done using the two-tailed unpaired Student's t-test. The one-way, two-way or three-way ANOVAs were performed with subsequent posthoc tests (Tukey's or Sidak's) when appropriate to examine the effects of genotype, optogenetic, or pharmacological stimulation. P-values < 0.05 were considered statistically significant.
3. Results
3.1. Capturing mBDNF from microdialysates
CEIA enabled a stable detection of mBDNF protein in mPFC microdialysates. To confirm the signal specificity, we compared blots of mPFC tissue lysates from WT and BDNF-cKO mice using primary monoclonal mBDNF antibodies of three different clones (Icosagen). In WT mice all monoclonal antibodies produced a solid immunoreactivity signal at 26 kDa, corresponding to MW of the dimerized mBDNF molecule (Fig. 1b). We rarely observed quantifiable signal in the range of 12–13 kDa corresponding to the molecular weight of the BDNF monomer (Leibrock et al., 1989) (Fig. 1b, Fig. s1c). In contrast, both in tissue lysates (Fig. 1b) and in microdiaysates (Fig. 2c, blots are not shown) of BDNF-cKO mice, mBDNF specific signal at 26 kDa was largely reduced. Detection of BDNF with primary polyclonal mBDNF antibodies (Sigma-Aldrich) revealed the same finding (Fig. s1 b).
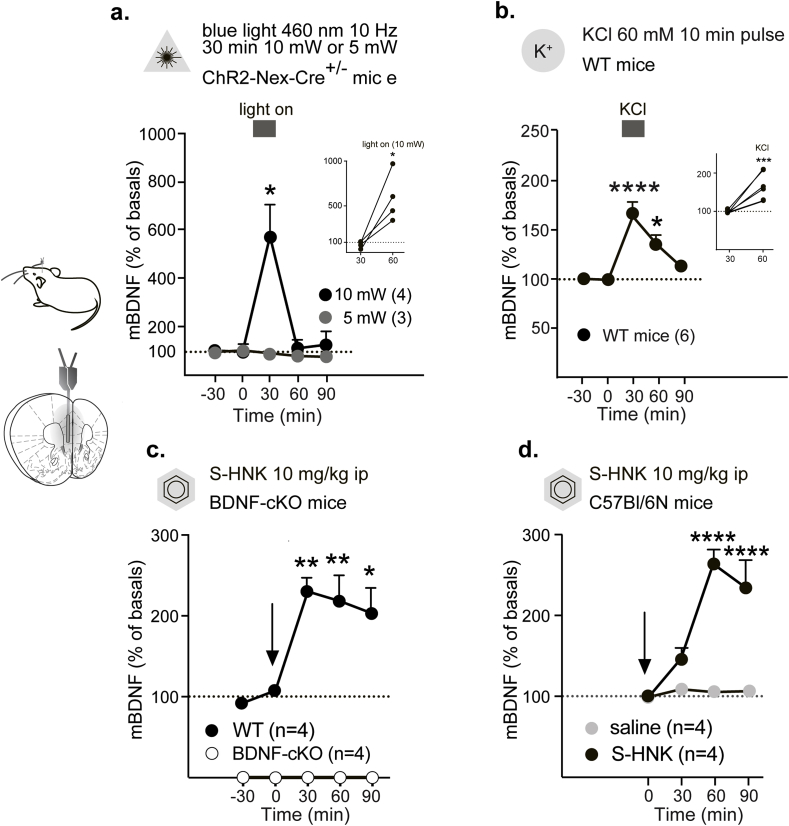
Fig. 2.
mBDNF secretion in response to local depolarization and systemic pharmacological intervention.
Infographics show the location of the microdialysis probe.
a. Optogenetic stimulation with blue light of 10 mW (n = 4), but not with 5 mW (3) results in a sharp transient increase in extracellular mBDNF levels in the mPFC. Bar indicates the period of stimulation. * - p < 0.05, when compared between two groups in a Tukey's multiple comparison test or, when compared with baseline samples, in an unpaired Student's test.
b. KCl (60 mM)-evoked depolarization in the mPFC is accompanied by a moderate transient elevation of extracellular mBDNF levels. Bar indicates the period, during which the pulse stimulation was applied. n = 6; **** - p < 0.0001, ** - p < 0.01, when compared with baseline samples in a Tukey's multiple comparisons test.
c. S-hydroxynorketamine (10 mg/kg) evoked an increase in extracellular mBDNF content in WT (n = 4) but not in BDNF-cKO mice (n = 4). ** - p < 0.01, * - p < 0.05, when compared with baseline samples in a Tukey's multiple comparisons test.
d. S-hydroxynorketamine (10 mg/kg, n = 4) but not saline (n = 4) evoked an increase in extracellular BDNF content in C57Bl/6N mice. **** - p < 0.01, * - p < 0.05, when compared between two groups in a Tukey's multiple comparison test.
Since it could not be excluded that mBDNF detected in the microdialysates may spillover into the extracellular space from destroyed cells, we looked for proteins that are indicative for impaired cell integrity, such as the cytosolic protein marker kinase AKT1 (55 kDa) and the nuclear protein histone H4 (11 kDa). Both proteins were undetectable in microdialysates (Fig. 1c). The examination of sequential baseline samples indicated the durability of the relative extracellular mBDNF levels at basal conditions. Thus, the analysis of the data present in Fig. 3b1 proved no difference across four basal samples in each experimental group (one-way ANOVA: dfs = {(3,20. (3,16)}, Fs < 1.451, ps > 0.258).
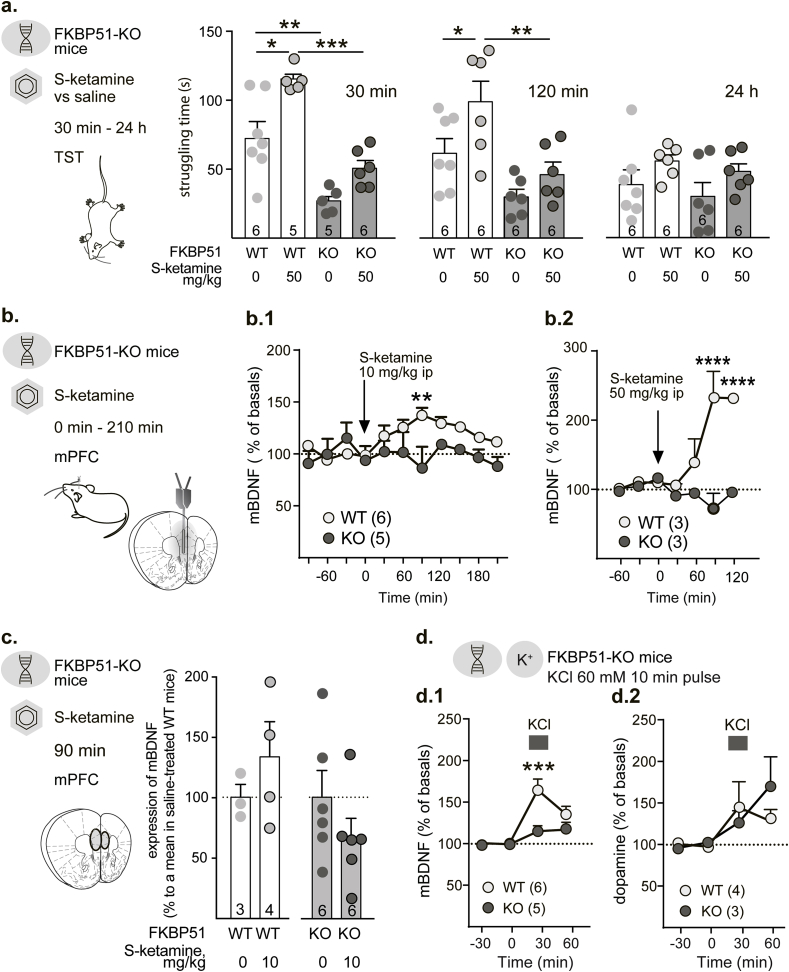
Fig. 3.
FKBP51-determined effect of ketamine on mBDNF secretion in the mPFC of mice.
a. Infographics describe the design of the experiment. Struggling time of WT and FKBP51–KO (KO) mice shown in a serial TST (performed 30 min, 120 min or 24 h after S-ketamine (50 mg/kg, i.p.) injection. *** - p < 0.001, ** - p < 0.01, and * - p < 0.05, when compared in a Tukey's multiple comparisons test. Number on the bars represent the group size (apparent difference is due to exclusion of two outlier values).
b. Infographics describe the design of the experiment and schematically show a probe placement. mBDNF secretion in the mPFC peaks 90 min after S-ketamine was injected in doses of 10 mg/kg, i.p., (b.1, n = 6/5) and 50 mg/kg, i.p (b.2, n = 3/3). Arrows indicate the time of drug injection. **** - p < 0.0001, ** - p < 0.01, when compared between WT and FKBP51–KO (KO) mice in a Sidak's multiple comparison test.
c. Infographics describe the design of the experiment and show the area or micro-punch dissection of the mPFC. Relative changes in the tissue content of BDNF in the prefrontal cortex after S-ketamine administration (10 mg/kg, ip). WT - wild type mice (n = 3/4), FKBP51–KO (KO) (n = 6/6) mice. . Number on the bars represent the group size.
d. FKBP51 expression specifically affect mBDNF secretion (d.1, n = 6/5), but not dopamine release (d.2, n = 4/3) evoked by KCl-stimulated depolarization in the mPFC. Bar indicates the period, during which the pulse stimulation was applied. **** - p < 0.0001, ** - p < 0.05, when compared between WT and FKBP51–KO (KO) mice in a Sidak's multiple comparison test.
3.2. The origin and responsiveness of measured extracellular mBDNF levels
In order to test if principal cortical neurons are, indeed, a major source of mBDNF measured in mPFC microdialysates, we applied optogenetics in combination with microdialysis. Neuronal depolarization evoked by stimulation of ChR2 with 460 nm light of 10 mW, but not of 5 mW, elicited a 6-fold increase in the mBDNF levels in the mPFC of ChR2–Nex-Cre+/– mice (Fig. 2a, Fig. s1c). Two-way ANOVA revealed significant effects of time (F (4,20) = 7.25, p = 0.001)and light intensity (F (4,20) = 6.93, p = 0.001), as well as a time x light intensity interaction (F (1,5) = 9.95, p = 0.025). Every subject in the 10 mW group showed an increase in the extracellular mBDNF (unpaired Student's t-test, t = 3.62, df = 6, p = 0.01, Fig. 2a inset). The observed difference in the effect of stimulation with 10 mW and 5 mW light may be due to a relatively moderate ChR2 expression in neurons in these mice. Optogenetic stimulation with the light of both intensities did not result in any changes in proBDNF levels, which were also measured in this experiment. Two-way ANOVA revealed no significance for any of the factors or factorial interactions: dfs = {16,4,1}, Fs < 2.57, ps > 0.18 (Fig. s1e.1, 1e.2).
In the next experiment, cell type-unspecific depolarization with a local 10 min pulse of 60 mM KCl resulted in a moderate transient elevation of extracellular mBDNF levels in the mPFC of freely moving WT mice. This effect also points at a neuronal origin of a stimulation-evoked increase in the extracellular mBDNF levels (one-way ANOVA, F (5,20) = 14.23, p < 0.0001, Fig. 2b; unpaired Student's t-test, t = 6.17, df = 10, p = 0.0001, Fig. 2b inset).
We subjected BDNF-cKO and WT mice to systemic treatment with S–HNK (10 mg/kg, i.p.). In BDNF-cKO mice, mBDNF was not detectable in the microdialysates. In WT mice the treatment resulted in a rapid 2.5-fold elevation of extracellular mBDNF in the mPFC (one-way ANOVA, F (4,15) = 7.93, p = 0.001), (Fig. 2c). We proved that the effect was specific to S–HNK and did not result from a possible injection stress (Naplekova et al., 2019). S–HNK (10 mg/kg, i.p.) evoked a 2.5-fold increase in extracellular mBDNF in the mPFC of C57Bl/6 mice, while a saline injection did not result in any changes (two-way ANOVA (time and treatment): time: (F (3,18) = 13.33, p < 0.001) treatment: (F (1,6) = 71.72, p = 0.001), interaction: (F (3,18) = 11.98, p = 0.002) Fig. 2d).
3.3. Role of FKBP51 in S-ketamine evoked antidepressant action and BDNF secretion
An immediate NMDA receptor-independent antidepressant effect of S–NHK has recently been reported in mice (Zanos et al., 2016). The strong potency of S–HNK to increase extracellular mBDNF levels suggests that stress-coping, which is facilitated by the drug, may depend on acute changes in BDNF signaling. Following this idea, we examined the behavioral and neurochemical effects of S-ketamine in FKBP51–KO mice with an increased capacity to cope with stress (Hartmann et al., 2012; Hoeijmakers et al., 2014; Touma et al., 2011).
The antidepressant activity of S-ketamine (50 mg/kg, i.p) in FKBP51–KO and respective WT female mice was evaluated in a series of TSTs by measuring struggling time. Tests were performed 30 min, 120 min, and 24 h after injection. Three-way mixed effect ANOVA revealed the significant effects of time (F(2,63) = 5.33, p = 0.007), genotype (F(1,63) = 31.47, p < 0.0001), and treatment (F(1,63) = 15.90, p = 0.0002 with no factorial interaction (F(2,63) = 0.331, p = 0.719) (Fig. s1g). Subsequent two-way ANOVA proved that S-ketamine showed an antidepressant-like activity 30 min (treatment F (1,18) = 15.99, p = 0.0008, Fig. 3a left panel) and 120 min (two-way ANOVA, treatment F (1,20) = 7.787, p = 0.011, Fig. 3a middle panel) after injection in WT mice. The genotype difference was also confirmed for observation were made at 30 and 120 min after injection (F(1,18) = 47.45, p < 0.001 and F(1,20) = 14.30, p = 0.001, respectively). FKBP51–KO mice showed reduced struggling from the very beginning with no significant potentiation by S-ketamine. The difference between genotype disappeared 24 h after injection (two-way ANOVA, genotype F (1,20) = 1.417, p = 0.247 and the effect of treatment showed only a tendency of significance (F(1,20) = 3.232, p = 0.087 Fig. 3b right panel, also, Fig. s1g). No significant effect of main factors’ interaction was found across all time points dfs = {(1,18), (1,20), (1,20)}, (Fs < 1.583, ps > 0.222).
We performed microdialysis measurements of extracellular mBDNF in the mPFC of FKBP51–KO and WT male mice during acute S-ketamine treatment (10 or 50 mg/kg, i.p.) (Fig. 3b). S-ketamine with a 90 min delay dose-dependently increased the extracellular mBDNF levels in WT mice. Surprisingly, at both doses, S-ketamine did not change the extracellular content of mBDNF in FKBP51–KO mice. Two-way ANOVA of the S-ketamine 10 mg/kg effect showed a significant genotype (F (1,99) = 15.67, p = 0.0001), but no time (F (10,99) = 1.42, p = 0.183) or interaction effect (F (10,99) = 1.64, p = 0.103), Fig. 3b1). Treatment with S-ketamine 50 mg/kg caused an even more pronounced increase in mBDNF levels in WT mice, again without any effect in FKBP51–KO mice. Two-way ANOVA revealed a significant genotype (F (1,25) = 26.63, p < 0.001) and a genotype × time interaction effects (F (5,25) = 6.94, p < 0.001), but no significant time effect F (6,25) = 2.52, p = 0.095 (Fig. 3b2).
We enquired if the transient increase in the extracellular mBDNF is mirrored in changes in total mBDNF levels in the mPFC upon S-ketamine treatment. In an independent experiment, we showed that S-ketamine (10 mg/kg i.p.) did not evoke any significant changes in mPFC tissue of total mBDNF (normalized to saline-treated group) both in WT and FKBP51–KO male mice 90 min after injection (unpaired Student's t-test in WT mice: ts < 0.942, dfs = {5,10}, ps > 0.273, Fig. 3c).
The essential role of FKBP51 controlling extracellular BDNF levels was also observed for depolarization-evoked release. Local perfusion with 60 mM KCl in the mPFC increased the extracellular mBDNF content in WT, but not in FKBP51–KO mice. Two-way ANOVA (time, genotype) showed a significant time (F (3,27) = 17.28, p < 0.001) and genotype effect (F (1,27) = 7.22, p = 0.024) as well as time × genotype interaction(F (3,27) = 6.01, p = 0.003), Fig. 3d1). Moreover, the genotype difference was an effective factor for mBDNF, specifically, since 60 mM KCl evoked dopamine release similarly in FKBP51–KO and WT mice. Two-way ANOVA showed a significant time effect (F (3,20) = 3.87, p = 0.025), but no genotype (F (1,20) = 0.19, p = 0.668) or interaction effect (F (3,20) = 0.95, p = 0.435), Fig. 3d2).
4. Discussion
The present study established a new experimental approach, which allows monitoring of BDNF secretion in the brain in freely moving mice. With its help, we characterized cortical glutamatergic neurons as the main source of BDNF release in vivo within the mPFC and demonstrated that the rapidly acting antidepressant S-ketamine triggers the accumulation of BDNF in the extracellular space. This effect was absent in FKBP51 deficient mice.
The in vivo BDNF measurements became feasible upon the modification of a standard brain microdialysis protocol (Anderzhanova and Wotjak, 2013; Yen et al., 2015). A new method is exploiting a probe with 100-kDa membrane cut off being perfused at a low flow rate in a push-pull mode and combining the improved sampling with CEIA for proteins analysis. Using this approach only 5 μl of microdialysate was required to measure mBDNF release in vivo with a 30 min time resolution.
The mBDNF molecule primarily occurred in microdialysates as a homodimer. The specificity of analysis was proven by the observed harsh decrease in mBDNF content in microdialysates, as well as in lysates from mPFC of BDNF-cKO mice with selective depletion of BDNF from forebrain glutamatergic neurons(Rauskolb et al., 2010). At the same time, a faint signal was seen in these mice proteins indicating a multicellular source of released BDNF in the brain, therefore, putting the question on the origin of the residual mBDNF to a further quest.
However, most of BDNF is expressed in neurons (Barde et al., 1982; Dieni et al., 2012; Hofer et al., 1990) and the increase in BDNF secretion in vitro upon KCl-induced depolarization (Balkowiec and Katz, 2002; Fukumoto et al., 2019; Goodman et al., 1996) strongly indicates the neuronal origin of released BDNF molecule. As shown in the present study, KCl-stimulated secretion appears as a characteristic of the releasable pool of neuronal mBDNF also in vivo: A comparable increase was observed in mice with different genetic backgrounds after various preceding interventions (saline vs. S-ketamine vs. S–HNK). Moreover, our results of the optogenetic experiment in ChR2–Nex-Cre+/– mice further proved that the principal neurons in the mPFC are the source of mBDNF in the extracellular space at condition of neuronal activity stimulation. Strikingly, upon optogenetic stimulations, we did not observe any increase in the extracellular content of mBDNF precursor proBDNF, which is packed together with cleaved mBDNF into dense-core vesicles. This may indicate the mechanisms of the neuronal activity-dependent increase in the extracellular mBDNF levels other than that are engaging the devastation of dense-core vesicles (Dieni et al., 2012).
To scrutinize our hypothesis on the interaction of FKBP51-dependent cell signaling and the mechanism of S-ketamine action, we firstly confirmed an antidepressant-like behavioral effect of S-ketamine in WT mice. Given the rather high concentration of S-ketamine (50 mg/kg) we cannot rule out that the increase in struggling observed in WT mice relates, at least in part, to a possible, but not obligatory, appearance of psychostimulant effects of the compound (Cruz et al., 2009). The stimulant effect is, indeed, not a bound feature of the selected dose of ketamine (50 mg/kg). Thus, the antidepressant activity of ketamine injected in the dose 50 mg/kg in the EPM test (an increase in the number of open arm entries) did not coincide with a measure of locomotor activity (increase in a total number of entries) examined 30 min after injection (Engin et al., 2009). The same paper states that in the open filed test the observed increase in the number of rearings (an indicator of explorative activity in this test) and the increase in the time spent in the central quadrant (a measure of anxiolytic effect) was not accompanied by changes in the number of line crossing (locomotor activity) (Engin et al., 2009). However, even a possibility of psychostimulant effect in the given experimental conditions renders the lack of significant “antidepressant” effects of S-ketamine in FKBP51–KO the more remarkable. In contrast, S-ketamine neither facilitated active coping nor stimulated mBDNF secretion in FKBP51–KO mice. Our results are in line with recent data revealing that FKBK51 reduces methylation of bdnf gene's promoter in cell culture and showing a positive correlation between FKBP51 expression levels and plasma BDNF in humans (Gassen et al., 2015). Moreover, our data suggest that an increase in extracellular mBDNF appears as a link between FKBP51 and the immediate antidepressant action of S-ketamine.
A comparison of behavioral and neurochemical changes reveals a mismatch between early onset of antidepressant-like activity of S-ketamine (30 min) and the delayed peak in the extracellular mBDNF content (90 min). This might speak against a direct involvement of acutely released mBDNF in antidepressant action of S-ketamine. Our study does not address the question on the effect of S-ketamine in animal's depression models, and all experiments were performed in naïve animals with the expected characteristic difference in stress-coping. Such a follow-up study on depression model would be helpful to prove BDNF as a link between FKBP51 and antidepressant action of S-ketamine.
The delay in peaking of mBDNF in dialysates upon S-ketamine action may be related to objective factors, both technical and biological. Firstly, a possible initial absorption of BDNF molecule in the perfusion line during increase of its amount would retain it inside the system for some time. Secondly, delay in the increase of BDNF expression, which occurs after single injection of ketamine (Xue et al., 2016), may contribute to increase in releasable fraction of BDNF. Thirdly, glia, as a mediator of mBDNF recycling, also may damper apparent increase in mBDNF accumulation (Vignoli et al., 2016).
It is worth mentioning that, in contrast to S-ketamine, S–HNK evoked rapid changes in the extracellular BDNF levels. This speaks in favor of its specific involvement in BDNF secretion. Its antidepressant activity in acute stress model was reported by Zanos et al. (2019, 2016). These data support the role of acute changes in BDNF in the realization of the antidepressant activity of ketamine. On the other hand, the psychophysiological context could modify the treatment outcome, even assuming that the neurochemical effect is the same. Remarkably, chronic social defeat stress was a prerequisite to cancel the antidepressant effect of the active metabolite of ketamine R–HNK (Xiong et al., 2019; Zhang et al., 2018). This would meet expectations, assuming a decrease in capacity of BDNF system (in terms of both BDNF availability and TrkB receptor expression) due to chronic stress. However, acute physiological stress evoked by lipopolysaccharide administration also abolished the antidepressant effect of R–HNK in the forced swim test (Yamaguchi et al., 2018). In this case, an omitted therapeutic activity of R–NHK may be due to engagement or/and saturation of mechanisms responsible for protein secretion (including BDNF) at conditions of increased inflammatory signaling (Bussi et al., 2017; Kimura et al., 2017).
The FKBP51-dependent increase in extracellular mBDNF after S-ketamine may seem counterintuitive in perspective of the well-established function of FKPB51 in the development of susceptibility to chronic stress (Binder, 2009; Hartmann et al., 2012; Hoeijmakers et al., 2014) and the role of the intact BDNF system in stress resilience and responsiveness to antidepressant treatment (Castrén and Kojima, 2017; Hashimoto, 2016; Monteggia et al., 2007; Notaras et al., 2015; Pandey et al., 2010). However, this incongruity could be explained, assuming that FKBP51 acts as a ubiquitous factor orchestrating gene-environment interaction and permitting and/or gating the activation of intracellular signaling (Touma et al., 2011). Therefore, FKBP51 plays a dual role in a cell and mediates responses to stimuli bearing positive (antidepressants) and negative (stress) features. In this context, the dissociation between the release of BDNF and dopamine in respect to FKBP51 expression is of particular relevance. It would be of interest also to explore how S-ketamine-induced mBDNF release is changed in FKBP51–KOs and WT mice after chronic stress when an increase in stress-coping can be seen in KOs in contrast to WT mice (Hartmann et al., 2012; Touma et al., 2011). This would address a question on the molecular processes underlying a loss in neuronal plasticity in stress-associated psychopathologies.
This study does not dispatch the query of the exact mechanism of FKBP51-mediated regulation of extracellular mBDNF levels. We can only speculate, for instance, on the involvement of FKBP51 and NMDARs interaction (Blair et al., 2019; Qiu et al., 2019) in setting neuronal activity and, therefore, in controlling mBDNF release (Hashimoto, 2016; Zorumski et al., 2016). This would, however, not fully explain the observed increase in mBDNF either after KCl or after S–HNK.
To sum, the present study introduced a novel method to study the dynamics of mBDNF secretion. The method can be used for the simultaneous monitoring of a set of molecules, for instance, mBDNF and proBDNF. In general, the multiplexing of analysis is only limited by the availability of specific antibodies; therefore, the approach can be potentially widely applied. Using the novel approach, the study revealed that the stress-responsive co-chaperone FKBP51 confines the extracellular accumulation of mBDNF in the mPFC, which is increased by the antidepressant S-ketamine. Therefore, the present data suggest a direction to explore mechanisms of responsiveness to antidepressant therapy and to develop novel antidepressant strategies.
Authors’ contribution
EA designed experiments, performed studies and analysis, and wrote and revised the manuscript; KH performed studies; AJG designed the optogenetic experiment; SA performed behavioral studies and data analysis; PLM performed breeding and genotyping of FKBP51–KOs; MVS designed behavioral experiments, RB designed experiments with BNDF-cKOs and revised the manuscript; CTW designed experiments and revised the manuscript; NCG designed experiments, analyzed data, and revised the manuscript.
CRediT authorship contribution statement
Elmira Anderzhanova: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing. Kathrin Hafner: Formal analysis, Investigation. Andreas J. Genewsky: Investigation, Methodology, Resources. Azza Soliman: Formal analysis, Investigation. Max L. Pöhlmann: Formal analysis, Resources. Mathias V. Schmidt: Methodology, Resources, Writing - review & editing. Robert Blum: Methodology, Resources, Writing - review & editing. Carsten T. Wotjak: Conceptualization, Supervision, Validation, Methodology, Resources, Writing - review & editing. Nils C. Gassen: Project administration, Supervision, Validation, Methodology, Resources, Writing - review & editing.
Declaration of competing interest
The authors report no conflict of interest.
Acknowledgments
The authors thank Mr. Markus Nussbaumer for his help with behavioral experiments and Dr. Claudia Kühne for assistance with histological analyses.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2020.100239.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
figs1.
All pannels: Infographics describe the design of the experiment. a. Cresyl violet-stained 25 μm coronal brain sections with the tract of the microdialysis probes. b. Comparison of the mBDNF band obtained with polyclonal Sigma-Aldrich) or monoclonal (Icosagen) antibodies. c. Representative blots depicting bands/areas corresponding to MW of ranges 25-26 kDa (dimeric mBDNF) and 12-13 kDa (monomeric mBDNF). d. Representative mBDNF blots of microdialysates obtained in the microdialysis optogenetic stimulation experiment. The light bars above correspond to stand-by baseline and recovery 30 min periods; dark bar indicates the period of stimulation. e. e1. Optogenetic stimulation with blue light of 10 mW (n=4) and 5 mW (n=3) did not result in changes in extracellular proBDNF levels in the mPFC. Bar indicates the period of stimulation. e2. Representative proBDNF blots of microdialysates obtained in the microdialysis optogenetic stimulation experiment. The light bars above correspond to stand-by baseline and recovery 30 min periods; dark bar indicates the period of stimulation. f. Cumulative (60 min) relative secretion of mBDNF under KCl-induced (60 mM, 10 min) depolarization in the mPFC (n= 4–6). One-way ANOVA, F (2,11) = 3.18, p < 0.058, ns – non-significant difference, when compared in a Tukey’s multiple comparison test. g. Dynamics in performance of WT and FKBP51-KO mice in the TST. Three-way mixed effect ANOVA revealed a significance of time (F(2,63) = 5.33, p = 0.007), genotype (F(1,63) = 31.47, p < 0.0001), and treatment (F(1,63) = 15.90, p = 0.0002 , Two-way ANOVA (time x group, all four groups there considered) showed significance of time (F (2,63) = 5.33, p = 0.007) and group (F (3,63) = 15.54, p < 0.001)), but no interaction of the two factors (F (3,63) = 1.62, p = 0.157. Tukey’s multiple comparison test showed a difference between S-ketamine-treated WT (n=6) and S-ketamine-treated KO (n=6) mice 0.5 h and 2 h after drug administration (p = 0.025 and p = 0.018, respectively), a difference between performance of S-ketamine-treated WT mice measured 2 h and 24 h after drug injection (p = 0.028) and a tendency to differ when compared 0.5 and 24 h time-points (p = 0.063); did not show any difference between saline-treated WT (n=7) and saline-treated KO (n= 6) mice at any time point (p = 0.203, p = 0.458, and p > 0.999, respectively.
References
- Anderzhanova E., Wotjak C.T. Brain microdialysis and its applications in experimental neurochemistry. Cell Tissue Res. 2013;354:27–39. doi: 10.1007/s00441-013-1709-4. [DOI] [PubMed] [Google Scholar]
- Balkowiec A., Katz D.M. Cellular mechanisms regulating activity-dependent release of native brain-derived neurotrophic factor from hippocampal neurons. J. Neurosci. Off. J. Soc. Neurosci. 2002;22:10399–10407. doi: 10.1523/JNEUROSCI.22-23-10399.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barde Y.A., Edgar D., Thoenen H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1982;1:549–553. doi: 10.1002/j.1460-2075.1982.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder E.B. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology. 2009;34(Suppl. 1):S186–S195. doi: 10.1016/j.psyneuen.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Björkholm C., Monteggia L.M. Bdnf - a key transducer of antidepressant effects. Neuropharmacology. 2016;102:72–79. doi: 10.1016/j.neuropharm.2015.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair L.J., Criado-Marrero M., Zheng D., Wang X., Kamath S., Nordhues B.A., Weeber E.J., Dickey C.A. The disease-associated chaperone FKBP51 impairs cognitive function by accelerating AMPA receptor recycling. eNeuro. 2019;6 doi: 10.1523/ENEURO.0242-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussi C., Peralta Ramos J.M., Arroyo D.S., Gaviglio E.A., Gallea J.I., Wang J.M., Celej M.S., Iribarren P. Autophagy down regulates pro-inflammatory mediators in BV2 microglial cells and rescues both LPS and alpha-synuclein induced neuronal cell death. Sci. Rep. 2017;7:43153. doi: 10.1038/srep43153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrén E., Kojima M. Brain-derived neurotrophic factor in mood disorders and antidepressant treatments. Neurobiol. Dis. 2017;97:119–126. doi: 10.1016/j.nbd.2016.07.010. [DOI] [PubMed] [Google Scholar]
- Chen B., Dowlatshahi D., MacQueen G.M., Wang J.F., Young L.T. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol. Psychiatr. 2001;50:260–265. doi: 10.1016/s0006-3223(01)01083-6. [DOI] [PubMed] [Google Scholar]
- Cruz S.L., Soberanes-Chávez P., Páez-Martinez N., López-Rubalcava C. Toluene has antidepressant-like actions in two animal models used for the screening of antidepressant drugs. Psychopharmacology. 2009;204:279–286. doi: 10.1007/s00213-009-1462-2. [DOI] [PubMed] [Google Scholar]
- Dieni S., Matsumoto T., Dekkers M., Rauskolb S., Ionescu M.S., Deogracias R., Gundelfinger E.D., Kojima M., Nestel S., Frotscher M., Barde Y.-A. BDNF and its pro-peptide are stored in presynaptic dense core vesicles in brain neurons. J. Cell Biol. 2012;196:775–788. doi: 10.1083/jcb.201201038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham J.S., Deakin J.F.W., Miyajima F., Payton A., Toro C.T. Expression of hippocampal brain-derived neurotrophic factor and its receptors in Stanley consortium brains. J. Psychiatr. Res. 2009;43:1175–1184. doi: 10.1016/j.jpsychires.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Egan M.F., Kojima M., Callicott J.H., Goldberg T.E., Kolachana B.S., Bertolino A., Zaitsev E., Gold B., Goldman D., Dean M., Lu B., Weinberger D.R. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Engin E., Treit D., Dickson C.T. Anxiolytic- and antidepressant-like properties of ketamine in behavioral and neurophysiological animal models. Neuroscience. 2009;161:359–369. doi: 10.1016/j.neuroscience.2009.03.038. [DOI] [PubMed] [Google Scholar]
- Fukumoto K., Fogaça M.V., Liu R.-J., Duman C., Kato T., Li X.-Y., Duman R.S. Activity-dependent brain-derived neurotrophic factor signaling is required for the antidepressant actions of (2R,6R)-hydroxynorketamine. Proc. Natl. Acad. Sci. U. S. A. 2019;116:297–302. doi: 10.1073/pnas.1814709116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassen N.C., Hartmann J., Zschocke J., Stepan J., Hafner K., Zellner A., Kirmeier T., Kollmannsberger L., Wagner K.V., Dedic N., Balsevich G., Deussing J.M., Kloiber S., Lucae S., Holsboer F., Eder M., Uhr M., Ising M., Schmidt M.V., Rein T. Association of FKBP51 with priming of autophagy pathways and mediation of antidepressant treatment response: evidence in cells, mice, and humans. PLoS Med. 2014;11 doi: 10.1371/journal.pmed.1001755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassen N.C., Fries G.R., Zannas A.S., Hartmann J., Zschocke J., Hafner K., Carrillo-Roa T., Steinbacher J., Preißinger S.N., Hoeijmakers L., Knop M., Weber F., Kloiber S., Lucae S., Chrousos G.P., Carell T., Ising M., Binder E.B., Schmidt M.V., Rüegg J., Rein T. Chaperoning epigenetics: FKBP51 decreases the activity of DNMT1 and mediates epigenetic effects of the antidepressant paroxetine. Sci. Signal. 2015;8:ra119. doi: 10.1126/scisignal.aac7695. [DOI] [PubMed] [Google Scholar]
- Gassen N.C., Hartmann J., Zannas A.S., Kretzschmar A., Zschocke J., Maccarrone G., Hafner K., Zellner A., Kollmannsberger L.K., Wagner K.V., Mehta D., Kloiber S., Turck C.W., Lucae S., Chrousos G.P., Holsboer F., Binder E.B., Ising M., Schmidt M.V., Rein T. FKBP51 inhibits GSK3β and augments the effects of distinct psychotropic medications. Mol. Psychiatr. 2016;21:277–289. doi: 10.1038/mp.2015.38. [DOI] [PubMed] [Google Scholar]
- Goodman L.J., Valverde J., Lim F., Geschwind M.D., Federoff H.J., Geller A.I., Hefti F. Regulated release and polarized localization of brain-derived neurotrophic factor in hippocampal neurons. Mol. Cell. Neurosci. 1996;7:222–238. doi: 10.1006/mcne.1996.0017. [DOI] [PubMed] [Google Scholar]
- Hartmann J., Wagner K.V., Liebl C., Scharf S.H., Wang X.-D., Wolf M., Hausch F., Rein T., Schmidt U., Touma C., Cheung-Flynn J., Cox M.B., Smith D.F., Holsboer F., Müller M.B., Schmidt M.V. The involvement of FK506-binding protein 51 (FKBP5) in the behavioral and neuroendocrine effects of chronic social defeat stress. Neuropharmacology. 2012;62:332–339. doi: 10.1016/j.neuropharm.2011.07.041. [DOI] [PubMed] [Google Scholar]
- Hashimoto K. Ketamine's antidepressant action: beyond NMDA receptor inhibition. Expert Opin. Ther. Targets. 2016;20:1389–1392. doi: 10.1080/14728222.2016.1238899. [DOI] [PubMed] [Google Scholar]
- Hashimoto K., Shimizu E., Iyo M. Critical role of brain-derived neurotrophic factor in mood disorders. Brain Res. Brain Res. Rev. 2004;45:104–114. doi: 10.1016/j.brainresrev.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers L., Harbich D., Schmid B., Lucassen P.J., Wagner K.V., Schmidt M.V., Hartmann J. Depletion of FKBP51 in female mice shapes HPA axis activity. PloS One. 2014;9 doi: 10.1371/journal.pone.0095796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer M., Pagliusi S.R., Hohn A., Leibrock J., Barde Y.A. Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. EMBO J. 1990;9:2459–2464. doi: 10.1002/j.1460-2075.1990.tb07423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T., Fogaça M.V., Deyama S., Li X.-Y., Fukumoto K., Duman R.S. BDNF release and signaling are required for the antidepressant actions of GLYX-13. Mol. Psychiatr. 2018;23:2007–2017. doi: 10.1038/mp.2017.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T., Jain A., Choi S.W., Mandell M.A., Johansen T., Deretic V. TRIM-directed selective autophagy regulates immune activation. Autophagy. 2017;13:989–990. doi: 10.1080/15548627.2016.1154254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.-Y., Kim Y.-K. Plasma brain-derived neurotrophic factor as a peripheral marker for the action mechanism of antidepressants. Neuropsychobiology. 2008;57:194–199. doi: 10.1159/000149817. [DOI] [PubMed] [Google Scholar]
- Leibrock J., Lottspeich F., Hohn A., Hofer M., Hengerer B., Masiakowski P., Thoenen H., Barde Y.A. Molecular cloning and expression of brain-derived neurotrophic factor. Nature. 1989;341:149–152. doi: 10.1038/341149a0. [DOI] [PubMed] [Google Scholar]
- Ma M., Ren Q., Yang C., Zhang J.-C., Yao W., Dong C., Ohgi Y., Futamura T., Hashimoto K. Adjunctive treatment of brexpiprazole with fluoxetine shows a rapid antidepressant effect in social defeat stress model: role of BDNF-TrkB signaling. Sci. Rep. 2016;6:39209. doi: 10.1038/srep39209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteggia L.M., Luikart B., Barrot M., Theobold D., Malkovska I., Nef S., Parada L.F., Nestler E.J. Brain-derived neurotrophic factor conditional knockouts show gender differences in depression-related behaviors. Biol. Psychiatr. 2007;61:187–197. doi: 10.1016/j.biopsych.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Motulsky H.J., Brown R.E. Detecting outliers when fitting data with nonlinear regression - a new method based on robust nonlinear regression and the false discovery rate. BMC Bioinf. 2006;7:123. doi: 10.1186/1471-2105-7-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami S., Imbe H., Morikawa Y., Kubo C., Senba E. Chronic stress, as well as acute stress, reduces BDNF mRNA expression in the rat hippocampus but less robustly. Neurosci. Res. 2005;53:129–139. doi: 10.1016/j.neures.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Naplekova P.L., Kudryashov N.V., Kasabov K.A., Kudrin V.S., Anderzhanova E. Structure-specific changes in brain momoamines following acute saline administration. Russ. J. Psysiol. 2019;105 [Google Scholar]
- Nasrallah P., Haidar E.A., Stephan J.S., El Hayek L., Karnib N., Khalifeh M., Barmo N., Jabre V., Houbeika R., Ghanem A., Nasser J., Zeeni N., Bassil M., Sleiman S.F. Branched-chain amino acids mediate resilience to chronic social defeat stress by activating BDNF/TRKB signaling. Neurobiol. Stress. 2019;11:100170. doi: 10.1016/j.ynstr.2019.100170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notaras M., Hill R., van den Buuse M. The BDNF gene Val66Met polymorphism as a modifier of psychiatric disorder susceptibility: progress and controversy. Mol. Psychiatr. 2015;20:916–930. doi: 10.1038/mp.2015.27. [DOI] [PubMed] [Google Scholar]
- Pandey G.N., Dwivedi Y., Rizavi H.S., Ren X., Zhang H., Pavuluri M.N. Brain-derived neurotrophic factor gene and protein expression in pediatric and adult depressed subjects. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2010;34:645–651. doi: 10.1016/j.pnpbp.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Park H., Poo M. Neurotrophin regulation of neural circuit development and function. Nat. Rev. Neurosci. 2013;14:7–23. doi: 10.1038/nrn3379. [DOI] [PubMed] [Google Scholar]
- Paxinos G., Franklin K.B.J. fourth ed. Elsevier, Academic Press; Amsterdam Boston Heidelberg: 2013. Paxinos and Franklin's the Mouse Brain in Stereotaxic Coordinates. [Google Scholar]
- ProteinSimple U. ProteinSimple protocols. 2019. www.proteinsimple.comhttps://www.proteinsimple.com/jess_wes_protocols.html [WWW Document]
- Qiu B., Xu Y., Wang J., Liu M., Dou L., Deng R., Wang C., Williams K.E., Stewart R.B., Xie Z., Ren W., Zhao Z., Shou W., Liang T., Yong W. Loss of FKBP5 affects neuron synaptic plasticity: an electrophysiology insight. Neuroscience. 2019;402:23–36. doi: 10.1016/j.neuroscience.2019.01.021. [DOI] [PubMed] [Google Scholar]
- Rantamäki T., Vesa L., Antila H., Di Lieto A., Tammela P., Schmitt A., Lesch K.-P., Rios M., Castrén E. Antidepressant drugs transactivate TrkB neurotrophin receptors in the adult rodent brain independently of BDNF and monoamine transporter blockade. PloS One. 2011;6 doi: 10.1371/journal.pone.0020567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauskolb S., Zagrebelsky M., Dreznjak A., Deogracias R., Matsumoto T., Wiese S., Erne B., Sendtner M., Schaeren-Wiemers N., Korte M., Barde Y.-A. Global deprivation of brain-derived neurotrophic factor in the CNS reveals an area-specific requirement for dendritic growth. J. Neurosci. Off. J. Soc. Neurosci. 2010;30:1739–1749. doi: 10.1523/JNEUROSCI.5100-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Vidal L.E., Do-Monte F.H., Sotres-Bayon F., Quirk G.J. Hippocampal--prefrontal BDNF and memory for fear extinction. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2014;39:2161–2169. doi: 10.1038/npp.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Vidal L.E., Lozada-Miranda V., Cantres-Rosario Y., Vega-Medina A., Melendez L., Quirk G.J. Alteration of BDNF in the medial prefrontal cortex and the ventral hippocampus impairs extinction of avoidance. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2018;43:2636–2644. doi: 10.1038/s41386-018-0176-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarelainen T., Hendolin P., Lucas G., Koponen E., Sairanen M., MacDonald E., Agerman K., Haapasalo A., Nawa H., Aloyz R., Ernfors P., Castrén E. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J. Neurosci. Off. J. Soc. Neurosci. 2003;23:349–357. doi: 10.1523/JNEUROSCI.23-01-00349.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasi M., Vignoli B., Canossa M., Blum R. Neurobiology of local and intercellular BDNF signaling. Pflügers Archiv. 2017;469:593–610. doi: 10.1007/s00424-017-1964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touma C., Gassen N.C., Herrmann L., Cheung-Flynn J., Büll D.R., Ionescu I.A., Heinzmann J.-M., Knapman A., Siebertz A., Depping A.-M., Hartmann J., Hausch F., Schmidt M.V., Holsboer F., Ising M., Cox M.B., Schmidt U., Rein T. FK506 binding protein 5 shapes stress responsiveness: modulation of neuroendocrine reactivity and coping behavior. Biol. Psychiatr. 2011;70:928–936. doi: 10.1016/j.biopsych.2011.07.023. [DOI] [PubMed] [Google Scholar]
- Vignoli B., Battistini G., Melani R., Blum R., Santi S., Berardi N., Canossa M. Peri-synaptic glia recycles brain-derived neurotrophic factor for LTP stabilization and memory retention. Neuron. 2016;92:873–887. doi: 10.1016/j.neuron.2016.09.031. [DOI] [PubMed] [Google Scholar]
- Xiong Z., Fujita Y., Zhang K., Pu Y., Chang L., Ma M., Chen J., Hashimoto K. Beneficial effects of (R)-ketamine, but not its metabolite (2R,6R)-hydroxynorketamine, in the depression-like phenotype, inflammatory bone markers, and bone mineral density in a chronic social defeat stress model. Behav. Brain Res. 2019;368:111904. doi: 10.1016/j.bbr.2019.111904. [DOI] [PubMed] [Google Scholar]
- Xue W., Wang W., Gong T., Zhang H., Tao W., Xue L., Sun Y., Wang F., Chen G. PKA-CREB-BDNF signaling regulated long lasting antidepressant activities of Yueju but not ketamine. Sci. Rep. 2016;6:26331. doi: 10.1038/srep26331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi J.-I., Toki H., Qu Y., Yang C., Koike H., Hashimoto K., Mizuno-Yasuhira A., Chaki S. (2R,6R)-Hydroxynorketamine is not essential for the antidepressant actions of (R)-ketamine in mice. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2018;43 doi: 10.1038/s41386-018-0084-y. 1900-1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Shirayama Y., Zhang J. -c, Ren Q., Yao W., Ma M., Dong C., Hashimoto K. R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl. Psychiatry. 2015;5:e632. doi: 10.1038/tp.2015.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B., Yang C., Ren Q., Zhang J.-C., Chen Q.-X., Shirayama Y., Hashimoto K. Regional differences in the expression of brain-derived neurotrophic factor (BDNF) pro-peptide, proBDNF and preproBDNF in the brain confer stress resilience. Eur. Arch. Psychiatr. Clin. Neurosci. 2016;266:765–769. doi: 10.1007/s00406-016-0693-6. [DOI] [PubMed] [Google Scholar]
- Yen Y.-C., Gassen N.C., Zellner A., Rein T., Landgraf R., Wotjak C.T., Anderzhanova E. Glycogen synthase kinase-3β inhibition in the medial prefrontal cortex mediates paradoxical amphetamine action in a mouse model of ADHD. Front. Behav. Neurosci. 2015;9:67. doi: 10.3389/fnbeh.2015.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo Y.K., Lee J., Kim Jinsik, Kim G., Kim S., Kim Jeongyeon, Chun H., Lee J.H., Lee C.J., Hwang K.S. Ultra-sensitive detection of brain-derived neurotrophic factor (BDNF) in the brain of freely moving mice using an interdigitated microelectrode (IME) biosensor. Sci. Rep. 2016;6:33694. doi: 10.1038/srep33694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P., Moaddel R., Morris P.J., Georgiou P., Fischell J., Elmer G.I., Alkondon M., Yuan P., Pribut H.J., Singh N.S., Dossou K.S.S., Fang Y., Huang X.-P., Mayo C.L., Wainer I.W., Albuquerque E.X., Thompson S.M., Thomas C.J., Zarate C.A., Gould T.D. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533:481–486. doi: 10.1038/nature17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P., Highland J.N., Liu X., Troppoli T.A., Georgiou P., Lovett J., Morris P.J., Stewart B.W., Thomas C.J., Thompson S.M., Moaddel R., Gould T.D. (R)-Ketamine exerts antidepressant actions partly via conversion to (2R,6R)-hydroxynorketamine, while causing adverse effects at sub-anaesthetic doses. Br. J. Pharmacol. 2019;176:2573–2592. doi: 10.1111/bph.14683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Fujita Y., Hashimoto K. Lack of metabolism in (R)-ketamine’s antidepressant actions in a chronic social defeat stress model. Sci. Rep. 2018;8:4007. doi: 10.1038/s41598-018-22449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorumski C.F., Izumi Y., Mennerick S. Ketamine: NMDA receptors and beyond. J. Neurosci. Off. J. Soc. Neurosci. 2016;36:11158–11164. doi: 10.1523/JNEUROSCI.1547-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]