Abstract
Cocaine use and withdrawal prompt stress system responses. Stress and the negative affective state produced by cocaine withdrawal are major triggers for relapse. FKBP5 is a co-chaperone of the glucocorticoid receptor and regulates HPA axis negative feedback. The role of FKBP5 in cocaine-related behaviors has not been studied. The FKBP5 inhibitor SAFit2 was used to examine the role of FKBP5 in anxiety-like behavior during early cocaine withdrawal and in stress-induced reinstatement following cocaine self-administration in male and female rats. Withdrawal from cocaine self-administration resulted in heightened anxiety-like behavior in female rats, which was significantly attenuated by SAFit2 administration. SAFit2 pretreatment prior to stress-induced reinstatement to cocaine seeking significantly reduced active lever presses of males. In female rats, SAFit2 administration prevented stress-induced reinstatement for rats in metestrus or diestrus, but not proestrus or estrus phases at the time of reinstatement. These data suggest an important role for FKBP5 in stress-related behaviors following cocaine self-administration, particularly in females.
Keywords: Sex differences, FKBP51, Cocaine, Reinstatement, Anxiety
1. Introduction
Cocaine abuse and dependence continue to be major health problems in the US (Kariisa et al., 2019). Abstinence from chronic cocaine use precipitates an intense withdrawal syndrome. Symptoms include cognitive dysfunction, sleep disturbances, depression, anxiety, persistent cocaine cravings, and fatigue (Gawin, 1991; Weddington et al., 1990). The goal of treatment is to prevent relapse to cocaine use during abstinence, and current treatments utilize behavioral and/or psychosocial approaches (Penberthy et al., 2010). Addition of a medication that helps to reduce relapse could increase the success rate for patients in treatment for cocaine use disorder. As of now, there are no FDA-approved medications for the treatment of cocaine use disorder.
Sex differences in cocaine use and relapse to cocaine dependence have been documented in humans (Back et al., 2005; Kosten et al., 1993) and in rodent models of addiction (Becker and Hu, 2008; Lynch, 2018). While males are overall more likely to use cocaine, females escalate from use to dependence more quickly (Brady and Randall, 1999; Griffin et al., 1989), are more sensitive to the subjective effects of cocaine (Lukas et al., 1996; Sofuoglu et al., 1999), and are more vulnerable to relapse (Hudson and Stamp, 2011; Kuhn and Francis, 1997). Females are also more susceptible to stress-related and negative affective disorders such as post-traumatic stress disorder (PTSD), anxiety, and depression (Breslau et al., 1997; McLean et al., 2011). This may contribute to divergent reasons for initial use of cocaine (Fox and Sinha, 2009; McCance-Katz et al., 1999) or relapse to continued use (McKay et al., 1996).
FKBP5 modulates glucocorticoid receptor sensitivity to cortisol (Scammell et al., 2001) by binding to the receptor complex, reducing the affinity of the glucocorticoid receptor for cortisol, and preventing its translocation to the nucleus (Wochnik et al., 2005). Frequent activation of the HPA axis and subsequent cortisol release can lead to increased FKBP5 production, glucocorticoid receptor resistance, and HPA axis dysregulation. Due to this, FKBP5 has been implicated in the pathology of human psychiatric diseases such as anxiety, depression, and PTSD (Binder et al., 2008; Ising et al., 2008; Lekman et al., 2008; Minelli et al., 2013; Pérez-Ortiz et al., 2013). Since cocaine use disorder is associated with HPA axis dysregulation, and FKBP5 mRNA is upregulated in rats following chronic cocaine administration (Connelly and Unterwald, 2019), we hypothesized that FKBP5 may be an important mediator of anxiety produced by chronic cocaine exposure and in stress-induced relapse. Thus, the following studies employed a selective inhibitor of FKBP5, SAFit2, to probe the role of FKBP5 in cocaine withdrawal-induced anxiety, as well as stress-induced reinstatement to cocaine seeking. This set of experiments first compared cocaine self-administration behaviors between male and female rats. Then, the effect of SAFit2 on anxiety-like behaviors during withdrawal was measured using the open field test. Finally, these studies investigated the effect of SAFit2 administration on stress-induced reinstatement to cocaine seeking in male and female rats. Results demonstrate that FKBP5 contributes to anxiety and stress-induced relapse in a sex-dependent manner.
2. Methods
2.1. Animals
Male and female Sprague Dawley rats (Charles River Laboratories, Wilmington, MA) arrived at 7 weeks of age. Rats were housed two per cage in a room on a 12-h reverse light cycle (lights off at 9AM), and had unlimited access to water and standard rat chow. Rats were allowed to acclimate to the facility and the light cycle for at least one week before jugular catheter implantation surgery. Following jugular catheter implantation surgery, rats were individually housed in the same space. All procedures were approved by the Institutional Animal Care and Use Committee of Temple University and were in compliance with the NIH Guide for the Care and Use of Laboratory Animals.
2.2. Drugs
Cocaine hydrochloride (acquired from the NIDA Drug Supply Program) was dissolved in 0.9% saline at a concentration of 2 mg/ml and was administered intravenously (see section 2.3.2). SAFit2 (molecular formula C46H62N2O10; [(1R)-3-(3,4-dimethoxyphenyl)-1-[3-(2-morpholin-4-ylethoxy)phenyl]propyl] (2S)-1-[(2S)-2-cyclohexyl-2-(3,4,5-trimethoxyphenyl)acetyl]piperidine-2-carboxylate) is the first FKBP5 inhibitor with selectivity over FKBP4 (FKBP5 Ki 6 ± 2 nM, FKBP4 Ki > 50,000 nM (Gaali et al., 2015)). SAFit2 was synthesized as described (Gaali et al., 2015) and was solubilized in 8% ethanol, 10% polyethylene glycol 300, and 10% Tween 80 in saline to produce a concentration of 10 mg/ml. SAFit2 was administered by intraperitoneal injection in a volume of 2 ml/kg body weight to achieve a 20 mg/kg dose.
2.3. Cocaine self-administration procedures
2.3.1. Jugular vein catheter implantation surgery and maintenance
Rats were anesthetized with isoflurane (5% induction, 2–3% maintenance) and polyurethane catheters 13 cm long (males) or 11 cm long (females) (Instech Laboratories, Plymouth Meeting, PA) were implanted into the right jugular vein as previously described (Enman et al., 2015). The catheter travelled subcutaneously over the scapula and was affixed to a back-mounted port (Plastics One, Roanoke, VA). On the day of surgery, rats received 5 mg/kg enrofloxacin (Baytril®) antibiotic and 1 mg/kg meloxicam for analgesia. Catheters were flushed with 0.2–0.3 ml heparinized (100USP/ml) saline containing 2.25 mg/ml enrofloxacin for 3 days following surgery. Catheters were flushed with heparinized saline before each self-administration session, and locked with heparinized saline containing 0.225 mg/ml enrofloxacin after each session. Catheters were flushed daily with heparinized saline plus 0.225 mg/ml enrofloxacin otherwise. Cocaine self-administration began 5 days following catheter implantation surgery.
2.3.2. Self-administration apparatus and procedure
Experiments were carried out in standard modular chambers within a sound-attenuating cabinet (Med Associates, Fairfax, VT). Each chamber featured a house light opposite two retractable levers with circular lights positioned above them, and a footshock-generating harness (Med Associates) attached to the bar floor. A swivel-tether system connected to the back-mounted port of the rat delivered a drug infusion via a 10-ml syringe in a computer-controlled syringe pump.
Once the session was initiated, the house light illuminated and the two levers extended. Responses on the active (right) lever triggered the light above it, activated a tone, and delivered an infusion of cocaine. The house light then turned off and a 20 s time out began. During the time out, the house light remained dark and any subsequent presses of the active lever were recorded, but had no effect. Responses on the inactive (left) lever were recorded but had no programmed consequences. When the session concluded, the levers retracted and all lights extinguished.
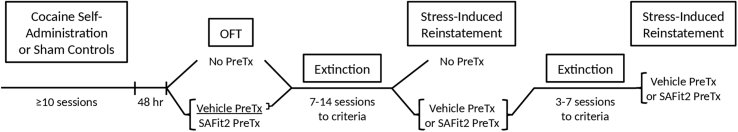
A timeline of experimental procedures can be found in Fig. 1. Rats were allowed to self-administer cocaine during daily 2-h sessions on a fixed-ratio 1 (FR1) schedule of reinforcement. Each infusion delivered a 0.5 mg/kg dose of cocaine. Rats were required to achieve a minimum of 10 infusions for at least 10 consecutive days before proceeding to the next phase of the experiment. The total number of self-administration sessions was dependent on how fast each individual rat achieved 10 or more infusions. The average number of sessions was 12.3 for females and 16.9 for males. In order to provide proper experimental controls for anxiety testing, after catheter surgeries were complete a sub-group of rats from each sex were designated “shams”. During the daily self-administration session these rats were exposed to the chamber with only the house light illuminated (no cocaine was available); they were treated identically otherwise.
Fig. 1.
Experimental timeline.Fig. 1 shows a timeline of experimental procedures. Male and female rats underwent IV cocaine self-administration. Forty-eight hours following the last session, rats were tested for anxiety-like behavior in the open field test (OFT) with no pretreatment. A subset of female rats underwent OFT testing following vehicle or SAFit2 pretreatment. Male and female rats that did not receive SAFit2 began extinction training. Once criteria were met, rats were tested for stress-induced reinstatement in a counterbalanced manner following vehicle and SAFit2 administration.
2.4. Open field testing
Forty-eight hours following the last self-administration session, rats were tested for anxiety-like behavior in the open field test. Rats were injected with 20 mg/kg SAFit2 or vehicle 4 h before testing. Each rat was tested only once. Rats that received vehicle went on to extinction training. The open field apparatus consisted of a 45 cm square box with a black floor grid enclosing a 15 cm square center area, gray walls and an open top. Room lighting was adjusted to approximately 65 lux in the center of the field, and approximately 35 lux in the corners. Each rat was placed in the same corner to initiate the test and allowed to explore the open field for 10 min. Open field tests were video recorded from above. Behaviors were scored from the videos by two experimenters, one blind to treatment condition. These scores were averaged to attain the final data. The time a rat spent in the center grid of the open field as well at the number of entries a rat made into the center area were measured. Movement of the head, upper torso, and both front paws into the center was considered an entry and time was counted.
2.5. Extinction and stress-induced reinstatement procedures
During extinction training, rats were placed in the chamber, and presses on either lever had no effect. Rats underwent daily extinction sessions until, for two consecutive sessions, responding decreased to less than 30% of their active lever presses during the final three self-administration sessions. When rats reached extinction criteria, they underwent stress-induced reinstatement testing the following day. The stressor consisted of 15 min of variably-timed mild footshocks (10s–70s, randomly generated, mean 35s) delivered in the self-administration chamber. Footshocks lasted for 0.5 s at 0.5 mA. Rats were monitored for signs of disproportionate distress or excessive vocalizing (which did not occur). Immediately following the footshock stress, a reinstatement session (identical to previous self-administration conditions, but without cocaine) lasted for 2 h, during which active and inactive lever presses were recorded. Using a within-subjects design, rats were tested with SAFit2 or vehicle, i.e. each rat underwent extinction and reinstatement twice in a counterbalanced manner. Rats received 20 mg/kg SAFit2 or vehicle 4 h prior to footshock.
2.6. Estrous cycle phase determination
In order to assess the effect of ovarian hormones on reinstatement behavior, vaginal cytology samples were collected and estrous phase was determined following previously described protocols (Cora et al., 2015; Marcondes et al., 2002). Immediately following the reinstatement session, the rat was gently restrained by gripping the base of the tail and lifting the hind legs. A sterile cotton swab dipped in sterile saline was briefly inserted into the vagina and gently twisted. The cotton swab was then rolled/pressed onto a microscope slide (Fisherbrand Superfrost Plus Slides, Waltham, MA) for later analysis. Slides were stained with 0.1% crystal violet (Fisher Scientific, Waltham, MA), then washed twice with diH2O and allowed to dry. Slides were assessed using a simple light microscope. Estrous cycle phase was determined by examining cell number, type (epithelial cell, leukocyte), and morphology (round, nucleated epithelial cell vs cornified epithelial cell).
2.7. Statistical analyses
Statistical analyses were carried out using GraphPad Prism v8.0 software. All data are displayed as mean ± SEM. Significant differences between two groups were evaluated using unpaired t-tests (or unpaired t-tests with Welch's correction when variances differed significantly). Significant differences between three or more groups were assessed by ordinary two-way ANOVA, repeated measures two-way ANOVA, or repeated measures three-way ANOVA where appropriate, followed by Sidak post hoc tests. A p value of <0.05 was considered significant.
3. Results
3.1. Sex differences in cocaine self-administration behaviors
3.1.1. Female rats acquired cocaine self-administration faster and obtained more cocaine infusions compared to male rats
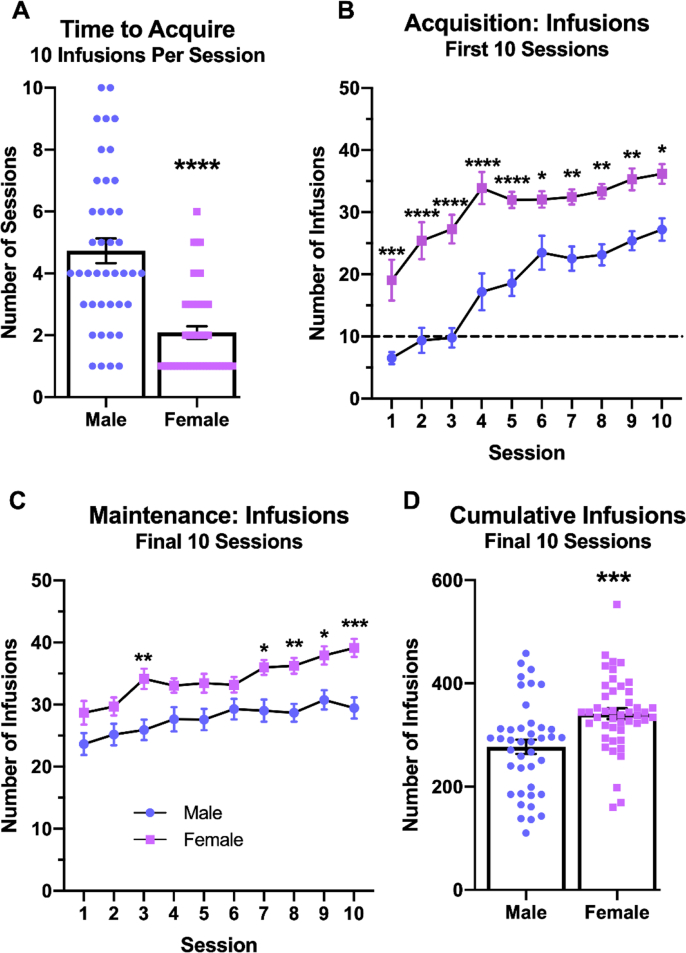
In order to compare acquisition rate and cocaine intake between male and female rats, data were compiled from all rats studied. Acquisition rate was investigated by comparing the number of sessions until successful acquisition (i.e., ≥10 infusions per session) between male and females (Fig. 2A). Female rats acquired cocaine self-administration after significantly fewer sessions (2.1 ± 1.4) compared to male rats (4.7 ± 2.6) (Welch-corrected t(59.83) = 5.897 p < 0.0001). To further quantify acquisition, the mean number of infusions from each of the first 10 self-administration sessions was compared (Fig. 2B). Two-way repeated measures ANOVA found significant main effects of session (F(9,765) = 26.20 p < 0.0001) and sex (F(1,85) = 48.12 p < 0.0001), as well as a significant interaction (F(9,765) = 1.946 p = 0.0429). Post hoc tests revealed that female rats achieved more infusions than males at each session, particularly during session 2 through 5 (p < 0.0001 for each individual session).
Fig. 2.
Female rats acquired cocaine self-administration faster and earned more cocaine infusions than male rats. Rats were allowed to self-administer cocaine, 0.5 mg/kg/infusion, under an FR1 schedule in daily 2-h sessions. The number of sessions until acquisition of self-administration is compared between male and female rats (A). The numbers of infusions earned are shown during the first ten (B) and the last ten (C) self-administration sessions in male and female rats. Additionally, the cumulative number of infusions for each rat during the last 10 sessions is shown by sex (D). Post hoc and t tests: * = p < 0.05, ** = p < 0.01, *** = p < 0.001, **** = p < 0.0001. N = 41 male rats, 46 female rats.
Differences in cocaine intake once rats had acquired self-administration behavior (i.e. earned 10 or more infusions) were measured in two ways. First, the mean number of infusions for each of the final 10 sessions was compared between males and females (Fig. 2C). Two-way repeated measures ANOVA indicated significant main effects of session (F(9,765) = 13.16 p < 0.0001) and sex (F(1,85) = 13.91 p = 0.0003), but no significant interaction (F(9,765) = 1.496 p = 0.1448). Planned comparison post hoc tests revealed that females earned significantly more infusions than males during several sessions, in particular the final session (p = 0.0001). Second, the total number of infusions from each rat were quantified, cumulative over the final 10 sessions (Fig. 2D). Female rats achieved a greater total number of infusions (341.5 ± 10.5) compared to males (277.2 ± 13.9) (t(85) = 3.730 p = 0.0003). Not only did female rats acquire the self-administration behavior faster than male rats, female rats earned more cocaine infusions overall.
3.1.2. Female rats reinstated cocaine seeking to a greater extent compared to male rats
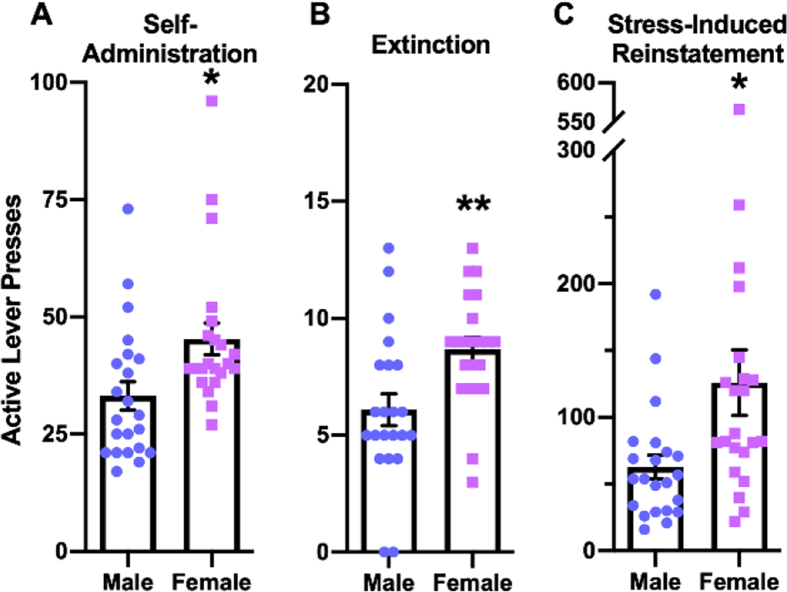
To analyze sex differences in extinction and reinstatement behavior, data were compiled from rats administered vehicle prior to stress-induced reinstatement and compared between males and females. During the final self-administration session (Fig. 3A), females pressed the active lever significantly more than males (45.3 ± 3.4 vs 33.1 ± 3.0 respectively; t(42) = 2.672, p = 0.0107), which agrees with the preceding intake data on infusions earned (shown in Fig. 2C). Correspondingly, female rats pressed the active lever more than male rats during the final extinction session (Fig. 3B) (8.7 ± 0.5 vs 6.1 ± 0.7 respectively; t(42) = 3.054, p = 0.0039), however it should be noted that the difference in extinction responding could be a function of the difference in the threshold of lever presses required to meet extinction criteria (ie, <30% of responses made during the final self-administration sessions). Females showed greater stress-induced reinstatement than males; the active lever pressing of female rats significantly exceeded that of males (125.9 ± 24.5 vs 62.8 ± 9.0; Welch-corrected t(26.56) = 2.423, p = 0.0225) during reinstatement sessions (Fig. 3C).
Fig. 3.
Female rats pressed the active lever more than male rats during self-administration, extinction, and in response to footshock stress-induced reinstatement. The number of active lever presses are shown for male and female rats during the final self-administration session (A), the final extinction training (B), and stress-induced reinstatement (C). t tests: * = p < 0.05, ** = p < 0.01. N = 22 male rats, 22 female rats.
3.2. Anxiety following cocaine self-administration
3.2.1. Female, but not male rats displayed anxiety-like behavior during early abstinence from cocaine self-administration
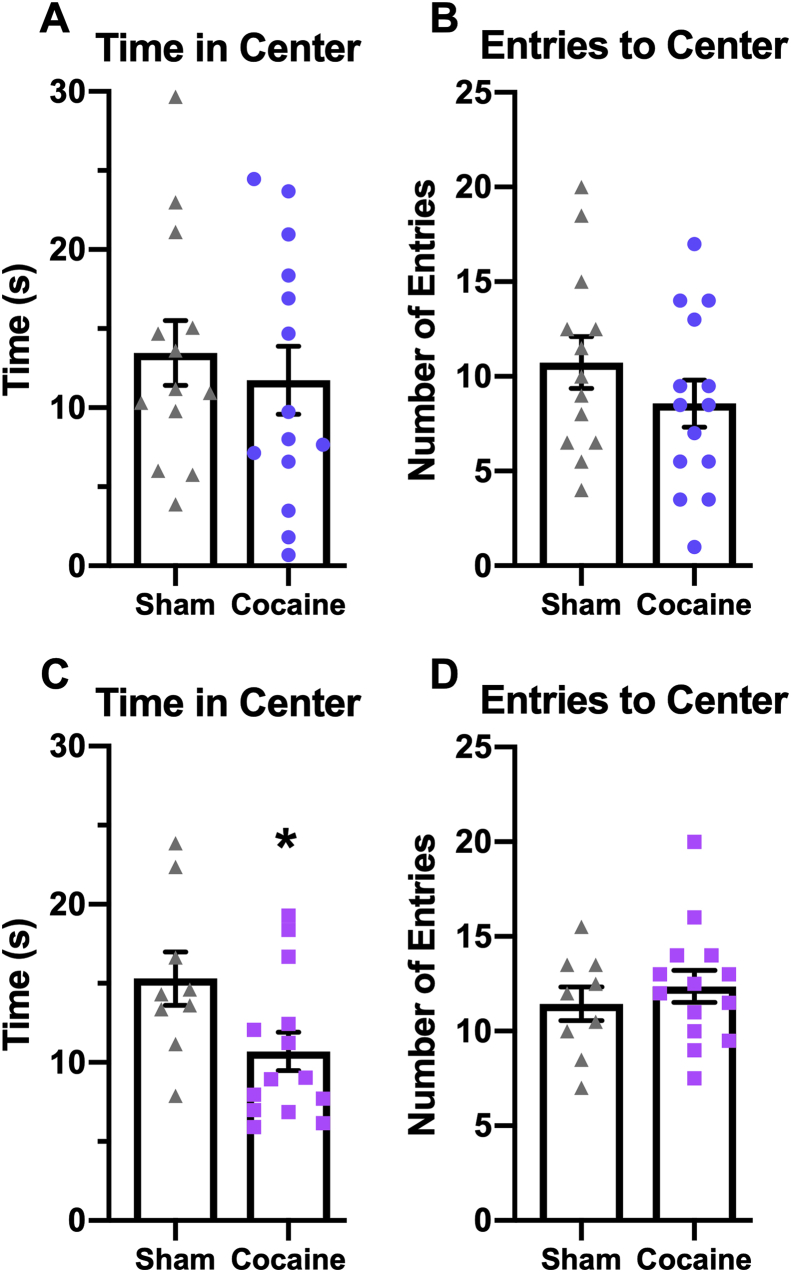
Forty-eight hours following the last self-administration session, rats were tested in the open field for anxiety-like behavior. Male rats abstinent from cocaine for 48 h did not spend a significantly different amount of time in the center area (Fig. 4A) compared to sham controls (t(25) = 0.5816 p = 0.5661). Likewise, male rats in withdrawal from cocaine did not differ from sham controls in the amount of entries into the center area (Fig. 4B) (t(25) = 1.172 p = 0.2523). Compared to sham controls, female rats abstinent from cocaine spent significantly less time in the center area (Fig. 4C) (t(21) = 2.278 p = 0.0333), indicating anxiety-like behavior. Female cocaine-experienced and sham rats did not differ in the number of entries into the center area (Fig. 4D) (t(21) = 0.7160 p = 0.4819). Given that males did not display anxiety-like behavior, they were excluded from further open field testing with SAFit2.
Fig. 4.
Female, but not male, rats display cocaine withdrawal-induced anxiety-like behavior. Rats were tested for anxiety-like behavior 48 h following the last cocaine self-administration session. The amount of time rats spent in the center of the open field (A, C) as well as the number of entries into the center area (B, D) are shown for male (A, B) and female (C, D) rats. N = 13–14 male rats/group, 9–14 female rats/group.
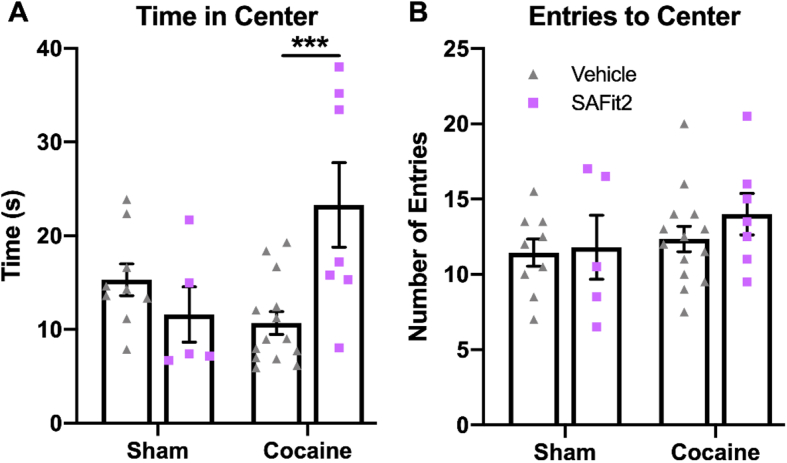
3.2.2. SAFit2 reduced anxiety-like behavior in female rats during early abstinence from cocaine self-administration
To determine the effect of FKBP5 inhibition on anxiety-like behavior in female rats during withdrawal from cocaine self-administration, groups of cocaine-experienced and sham control rats were treated with 20 mg/kg SAFit2 or vehicle 4 h prior to open field testing. When analyzing the amount of time spent in the center of the open field (Fig. 5A), two-way ANOVA showed a significant interaction (F(1,31) = 10.49 p = 0.0029) between main effects of cocaine (F(1,31) = 1.976 p = 0.1698) and SAFit2 (F(1,31) = 3.110 p = 0.0877). Post hoc tests revealed that rats in withdrawal from cocaine and treated with SAFit2 spent significantly more time in the center area compared to rats in cocaine withdrawal injected with vehicle (p = 0.0009). Two-way ANOVA did not identify a significant interaction (F(1,31) = 0.2749 p = 0.6038) nor main effects of cocaine (F(1,31) = 1.607 p = 0.2144) or SAFit2 (F(1,31) = 0.6624 p = 0.4219) on the number of entries to the center area of the open field (Fig. 5B). These results indicate anxiolytic effects of the FKBP5 inhibitor, SAFit2, during withdrawal from cocaine in female rats.
Fig. 5.
SAFit2 administration attenuated anxiety-like behavior in female rats during early abstinence from cocaine self-administration. Rats were tested for anxiety-like behavior 48 h following the last cocaine self-administration session. The amount of time rats spent in the center of the open field (A) as well as the number of entries into the center area (B) following 20 mg/kg SAFit2 or vehicle administration is shown. Post hoc tests: *** = p < 0.001. N = 5–13 rats/treatment/group.
3.3. Stress-induced reinstatement to cocaine seeking
3.3.1. SAFit2 attenuated stress-induced reinstatement to cocaine seeking in male rats
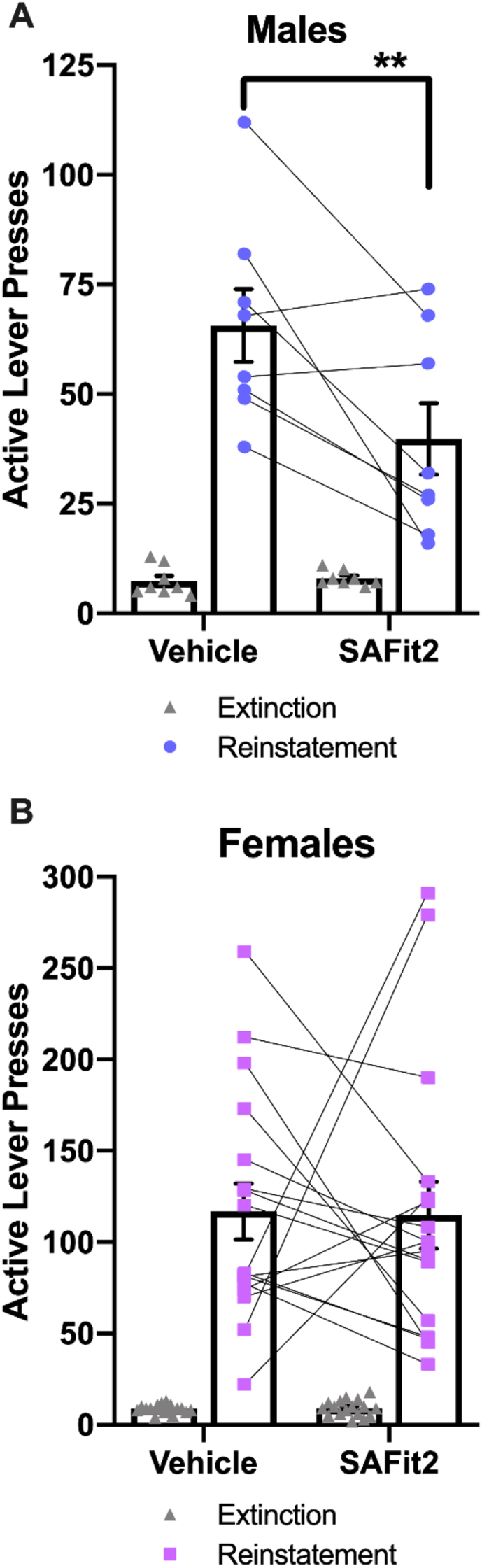
Male rats were injected with 20 mg/kg SAFit2 or vehicle 4 h prior to footshock stress-induced reinstatement, and active lever presses were recorded. Two-way repeated measures ANOVA detected significant main effects of session (F(1,7) = 49.65 p = 0.0002) and SAFit2 (F(1,7) = 7.956 p = 0.0257) as well as a significant interaction (F(1,7) = 10.69 p = 0.0137). Post hoc tests determined that, compared to vehicle controls, rats that received SAFit2 produced fewer active lever presses during reinstatement (p = 0.0055), demonstrating that inhibiting FKBP5 can limit stress-induced reinstatement in male rats (Fig. 6A).
Fig. 6.
SAFit2 administration attenuated stress-induced reinstatement to cocaine seeking in male, but not female rats. The number of active lever presses during extinction and stress-induced reinstatement following vehicle or 20 mg/kg SAFit2 administration is shown for males (A) and females (B). Post hoc tests: ** = p < 0.01. N = 8 males; 18 females.
3.3.2. The effect of SAFit2 on stress-induced reinstatement in female rats May depend on ovarian hormones
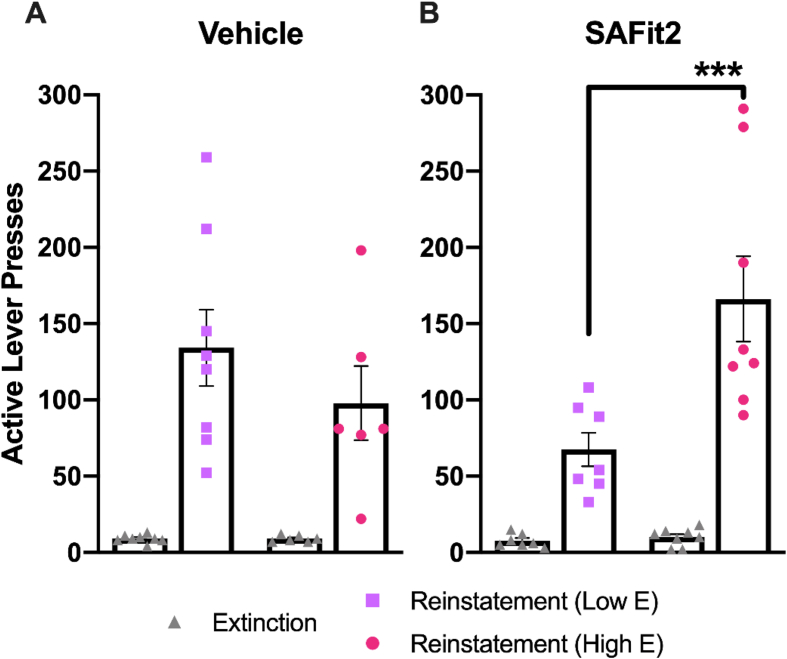
Four hours prior to footshock stress-induced reinstatement, female rats were injected with 20 mg/kg SAFit2 or vehicle, and active lever presses were counted. Two-way repeated measures ANOVA showed a significant main effect of session (F(1,17) = 64.33 p < 0.0001) but no effect of SAFit2 (F(1,17) = 0.6586 p = 0.4283) and no significant interaction (F(1,17) = 0.6964 p = 0.4156). A significant effect of session indicates active lever presses were significantly lower on extinction days versus reinstatement days, which is to be expected (Fig. 6B). Given that FKBP5 also complexes with other steroid hormone receptors (Zannas et al., 2016), female rats were separated by estrous phase into two groups: 1. proestrus and estrus (postulated relatively high estrogen level) and 2. metestrus and diestrus (postulated relatively low estrogen level). There was no difference in reinstatement responding between rats in proestrus/estrus and rats in metestrus/diestrus following vehicle administration (Fig. 7A). Two-way repeated measures ANOVA found a significant effect of session (F(1,12) = 36.81 p < 0.0001), but not estrous phase (F(1,12) = 0.9939 p = 0.3385) nor an interaction between the two (F(1,12) = 1.059 p = 0.3237). However, two-way repeated measures ANOVA revealed a significant interaction (F(1,13) = 9.878 p = 0.0078) between the main effects of session (F(1,13) = 49.51 p < 0.0001) and estrous cycle stage (F(1,13) = 9.335 p = 0.0092) following SAFit2 administration (Fig. 7B). Post hoc tests showed that rats injected with SAFit2 in proestrus/estrus pressed the active lever significantly more than rats injected with SAFit2 in metestrus/diestrus (p = 0.0003; Fig. 7B). Given these results, the effect of SAFit2 on stress-induced reinstatement in females may depend on estrous phase and ovarian hormone levels.
Fig. 7.
The effect of SAFit2 administration on stress-induced reinstatement in female rats may depend on estrous cycle phase and ovarian hormones. The number of active lever presses during extinction and stress-induced reinstatement following vehicle (A) or 20 mg/kg SAFit2 administration (B) is shown for female rats in metestrus/diestrus (presumed Low E) and proestrus/estrus (presumed High E). Post hoc tests: *** = p < 0.001. N = 15 females total.
4. Discussion
First and foremost, the studies presented here replicate documented sex differences in the acquisition rate, intake, and reinstatement of cocaine self-administration. Specifically, female rats acquire the self-administration behavior faster than males, earn more cocaine infusions than males, and respond to a greater extent than males during reinstatement (Becker and Hu, 2008; Bobzean et al., 2014; Davis et al., 2008; Lynch and Carroll, 1999; Swalve et al., 2016). These studies determined that these sex differences are driven by the effect of estrogen on motivated behaviors. In the current study, female rats achieved substantially more infusions than males during the first several sessions of acquisition, but this gap narrowed during maintenance. The reason for this is unclear, but may be due to estrogen-mediated acceleration of associative cue learning in females. Indeed, a sophisticated set of experiments from Calipari and colleagues found a complex interaction between cocaine-related cues and estrous cycle phase; drug seeking, usually enhanced during estrus, was no different across the estrous cycle in the absence of cues (Johnson et al., 2019).
A sex difference we did not anticipate was a withdrawal-induced anxiety-like phenotype in female rats, but not in male rats. Previous studies from our own lab and others demonstrated anxiety-like behavior in both males and females following non-contingent chronic cocaine administration (Ambrose-Lanci et al., 2010; Perrine et al., 2008; Sarnyai et al., 1995), and several other groups have shown anxiety-like behavior following cocaine self-administration (Aujla et al., 2008; Buffalari et al., 2012; Nunes et al., 2019). One explanation for this discrepancy is the schedule and timing of cocaine administration. In our hands using short-access cocaine self-administration (ie, 2-h sessions), both male and female rats acquired and maintained self-administration of cocaine, successfully extinguished this behavior, strongly reinstated lever pressing in response to mild footshock, and females displayed withdrawal-induced anxiety-like behavior. Since male rats displayed anxiety-like behavior in the defensive burying test following chronic 6-h access to cocaine (Aujla et al., 2008), it is possible we would have seen an anxiety-like phenotype using the open field test in the male rats had we extended the session time to 6 h. Other explanations could be that anxiety needs to be activated by a stressor to distinguish a withdrawal phenotype, or that the time of maximal anxiety experienced during withdrawal may differ between males and females. Alternatively, the reason for this sex difference in withdrawal-induced anxiety may be straightforward: female rats self-administered more cocaine than male rats in the present study leading to greater withdrawal symptoms. To the authors’ knowledge this is the first study to directly compare anxiety-like behavior following cocaine self-administration in male and female rats. The few studies that have measured anxiety following cocaine self-administration tested only male rats (Aujla et al., 2008; Buffalari et al., 2012; Mantsch et al., 2008; Nunes et al., 2019). Likewise, studies that found no sex differences in drug-taking during maintenance (Anker and Carroll, 2010; Cosgrove et al., 2002; Jackson et al., 2006) did not measure subsequent anxiety-like behavior. Because cocaine intake has been positively correlated with severity of anxiety-like behavior in males (Buffalari et al., 2012), this leaves open the possibility that, at least in the current described experiments, female rats showed an anxiety-like phenotype while males did not because females consumed more cocaine. The effects of the FKBP5 inhibitor SAFit2 on cocaine withdrawal-induced anxiety was investigated in females only since females showed the anxiogenic phenotype. SAFit2 increased the time female rats spent in the center of the open field, indicating that its administration reduced anxiety-like behavior during early cocaine withdrawal.
Although evidence of the anxiolytic effect of SAFit2 in rodents exists (Hartmann et al., 2015), SAFit2 had not yet been examined in the context of cocaine-related behaviors. We hypothesized that administration of SAFit2 would also attenuate stress-induced reinstatement to cocaine seeking by enhancing HPA axis negative feedback. Indeed, SAFit2 administration significantly reduced the number of active lever presses produced by male rats during reinstatement. The results from female rats were not as definitive. There was no significant difference in lever pressing following SAFit2 administration as compared with vehicle when data from all females were analyzed together. When data were assessed by postulated high and low estrous levels, an effect of SAFit2 became apparent. Estrous cycle phase was recorded immediately following the reinstatement session. We collapsed the four estrous phases (proestrus, estrus, metestrus, diestrus) into two groups based on the relative levels of estrogen and progesterone present during each phase (Butcher et al., 1974) due to the relatively small sample size of our study. Rats in metestrus or diestrus phases were combined into one group as hormone levels are relatively low during these phases; conversely, rats in proestrus or estrus phases were combined into another group as hormone levels during these phases are relatively high (Butcher et al., 1974). Ultimately, we found that SAFit2 attenuated stress-induced reinstatement of rats in metestrus/diestrus at the time of reinstatement. Furthermore, rats in metestrus/diestrus pressed the active lever significantly less than rats in proestrus/estrus following SAFit2 administration prior to reinstatement.
In addition to glucocorticoid receptors, FKBP5 interacts with other steroid hormone receptors, importantly the estrogen and progesterone receptors (Hubler et al., 2003; Shrestha et al., 2015). The relationship between FKBP5 and progesterone receptors is similar to that of FKBP5 and glucocorticoid receptors. FKBP5 and progesterone receptors also form a short feedback loop, such that FKBP5 decreases progesterone receptor sensitivity, and progesterone receptor activation results in induction of FKBP5 mRNA (Hubler et al., 2003). Some evidence indicates that FKBP5 associates with estrogen receptors and promotes their activity (Shrestha et al., 2015). In a cell model, estradiol (the predominant estrogen) potentiates the increase in FKBP5 mRNA produced by corticosterone and subsequent sequestration of glucocorticoid receptors in the cytosolic compartment (Malviya et al., 2013). Although the FKBP5 gene does not have an estrogen response element (Hubler and Scammell, 2004), estradiol may regulate FKBP5 gene expression indirectly. Indeed, estradiol enhances HPA axis activity and disrupts HPA axis negative feedback (Heck and Handa, 2019) in which FKBP5 plays a major role; progesterone generally opposes these effects.
Inhibiting FKBP5 with SAFit2 would then not only enhance HPA axis negative feedback regulated by glucocorticoid receptors, but also increase progesterone receptor sensitivity and may disturb the effects of estradiol. We found SAFit2 administration to be effective at reducing cocaine seeking in female rats that were in an estrous phase with relatively low levels of estrogen (metestrus/diestrus), but not relatively high levels of estrogen (proestrus/estrus). This could be due to many factors. Perhaps the dose of SAFit2 was insufficient to overcome the potentiating effect of estrogen on the HPA axis following footshock stress. Alternatively, the time of testing after SAFit2 administration may have been too soon, since one study found a decrease in anxiety-like behavior 16 h, but not 1 h, following SAFit2 administration (Hartmann et al., 2015). Full dose-effect and time-course studies are needed to resolve this issue.
In addition to enhancing HPA axis activation, estrogens facilitate cocaine self-administration (Jackson et al., 2006), potentiate dopamine signaling in the nucleus accumbens following cocaine administration (Becker and Hu, 2008), and increase cocaine seeking during reinstatement (Doncheck et al., 2018; Hudson and Stamp, 2011). Estradiol exerts these effects through three known receptors: ERα, ERβ, and the G-protein-coupled estrogen receptor (GPER) (Almey et al., 2015). Sex differences in responses to cocaine are most likely mediated by estrogen receptors found in the mesolimbic dopamine pathway (Calipari et al., 2017; Tonn Eisinger et al., 2018). Estradiol directly and indirectly increases dopamine release in the nucleus accumbens of male and female rodents (Cummings et al., 2014; Thompson and Moss, 1994) most likely by activating the fast-acting GPER (Yoest et al., 2018). Furthermore, estradiol enhances the activity as well as sensitivity to dopamine of ventral tegmental area dopaminergic neurons (Calipari et al., 2017; Vandegrift et al., 2017; Zhang et al., 2008), although the estrogen receptor subtype responsible for this has yet to be elucidated. Evidence indicates that behavioral responses to drugs of abuse in female rodents require ERα, ERβ, or both (Hilderbrand and Lasek, 2018; Larson and Carroll, 2007; Satta et al., 2018; Van Swearingen et al., 2013). Determining the role of estrogen receptor type and location in the effects of cocaine will provide valuable information for understanding the sex differences in cocaine addiction, as well as the role of FKBP5 in these differences.
It should be noted that the effect of SAFit2 administration on rat vaginal cell type, number, or morphology has not been studied. SAFit2 was administered 4 h prior to footshock stress and subsequent 2-h reinstatement session, meaning collection of vaginal cytology samples occurred more than 6 h following SAFit2 administration. The entirety of metestrus phase can last only 6 h in the rat (Cora et al., 2015). If it is possible for a rat to experience an entire cycle phase in the time between SAFit2 administration and vaginal smear, the accuracy of assignment to estrous phase at time of SAFit2 treatment is suspect. Future studies should aim to analyze estrous cycle phase in a timely manner without disrupting the primary cocaine-related behavior experiments. Additional experiments examining the effect of SAFit2 on estrous cycle and FKBP5 interaction with the estrogen and progesterone receptors are vital to developing FKBP5 inhibitors for therapeutic use in females.
5. Conclusions
The studies reported here support previously reported sex differences in the acquisition, maintenance, and reinstatement of cocaine self-administration behaviors, and expand those results to include divergence in the expression of withdrawal-induced anxiety-like behavior as well as responses to stress-induced reinstatement. These experiments identify the FKBP5 inhibitor SAFit2 as a possible therapeutic for prevention of relapse during cocaine withdrawal in males. In females, use of FKBP5 inhibitors may need to be correctly timed in relation to hormonal cycle to be most effective.
Declarations of interest
KLC, CCW, JLB, MB, FH, EMU: none.
Funding
This work was supported by the National Institutes of Health grants P30 DA013429, T32 DA007237, R01 DA018326.
CRediT authorship contribution statement
Krista L. Connelly: Conceptualization, Methodology, Formal analysis, Investigation, Writing - original draft, Visualization. Cassandra C. Wolsh: Investigation. Jeffrey L. Barr: Investigation. Michael Bauder: Investigation, Resources. Felix Hausch: Resources, Project administration, Writing - review & editing. Ellen M. Unterwald: Conceptualization, Methodology, Resources, Writing - review & editing, Supervision, Project administration, Funding acquisition.
Acknowledgements
The authors would like to thank Emma Fitzsimmons for her technical assistance.
References
- Almey A., Milner T.A., Brake W.G. Estrogen receptors in the central nervous system and their implication for dopamine-dependent cognition in females. Horm. Behav. 2015;74:125–138. doi: 10.1016/J.YHBEH.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrose-Lanci L.M., Sterling R.C., Van Bockstaele E.J. Cocaine withdrawal-induced anxiety in females: impact of circulating estrogen and potential use of delta-opioid receptor agonists for treatment. J. Neurosci. Res. 2010;88:816–824. doi: 10.1002/jnr.22259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker J.J., Carroll M.E. Sex differences in the effects of allopregnanolone on yohimbine-induced reinstatement of cocaine seeking in rats. Drug Alcohol Depend. 2010;107:264–267. doi: 10.1016/j.drugalcdep.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aujla H., Martin-Fardon R., Weiss F. Rats with extended access to cocaine exhibit increased stress reactivity and sensitivity to the anxiolytic-like effects of the mGluR 2/3 agonist LY379268 during abstinence. Neuropsychopharmacology. 2008;33:1818–1826. doi: 10.1038/sj.npp.1301588. [DOI] [PubMed] [Google Scholar]
- Back S.E., Brady K.T., Jackson J.L., Salstrom S., Zinzow H. Gender differences in stress reactivity among cocaine-dependent individuals. Psychopharmacology (Berlin) 2005;180:169–176. doi: 10.1007/s00213-004-2129-7. [DOI] [PubMed] [Google Scholar]
- Becker J.B., Hu M. Sex differences in drug abuse. Front. Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder E.B., Bradley R.G., Liu W., Epstein M.P., Deveau T.C., Mercer K.B., Tang Y., Gillespie C.F., Heim C.M., Nemeroff C.B., Schwartz A.C., Cubells J.F., Ressler K.J. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. J. Am. Med. Assoc. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobzean S.A.M., DeNobrega A.K., Perrotti L.I. Sex differences in the neurobiology of drug addiction. Exp. Neurol. 2014;259:64–74. doi: 10.1016/j.expneurol.2014.01.022. [DOI] [PubMed] [Google Scholar]
- Brady K.T., Randall C.L. Gender differences in substance use disorders. Psychiatr. Clin. North Am. 1999;22:241–252. doi: 10.1016/S0193-953X(05)70074-5. [DOI] [PubMed] [Google Scholar]
- Breslau N., Davis G.C., Andreski P., Peterson E.L., Schultz L.R. Sex differences in posttraumatic stress disorder. Arch. Gen. Psychiatr. 1997;54:1044. doi: 10.1001/archpsyc.1997.01830230082012. [DOI] [PubMed] [Google Scholar]
- Buffalari D.M., Baldwin C.K., See R.E. Treatment of cocaine withdrawal anxiety with guanfacine: relationships to cocaine intake and reinstatement of cocaine seeking in rats. Psychopharmacology (Berlin) 2012;223:179–190. doi: 10.1007/s00213-012-2705-1. [DOI] [PubMed] [Google Scholar]
- Butcher R.L., Collins W.E., Fugo N.W. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17β throughout the 4-day estrous cycle of the rat. Endocrinology. 1974;94:1704–1708. doi: 10.1210/endo-94-6-1704. [DOI] [PubMed] [Google Scholar]
- Calipari E.S., Juarez B., Morel C., Walker D.M., Cahill M.E., Ribeiro E., Roman-Ortiz C., Ramakrishnan C., Deisseroth K., Han M.-H., Nestler E.J. Dopaminergic dynamics underlying sex-specific cocaine reward. Nat. Commun. 2017;8:13877. doi: 10.1038/ncomms13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly K.L., Unterwald E.M. Chronic cocaine administration upregulates FKBP5 in the extended amygdala of male and female rats. Drug Alcohol Depend. 2019;199:101–105. doi: 10.1016/j.drugalcdep.2019.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cora M.C., Kooistra L., Travlos G. Vaginal cytology of the laboratory rat and mouse. Toxicol. Pathol. 2015;43:776–793. doi: 10.1177/0192623315570339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove K.P., Hunter R.G., Carroll M.E. Wheel-running attenuates intravenous cocaine self-administration in rats: sex differences. Pharmacol. Biochem. Behav. 2002;73:663–671. doi: 10.1016/S0091-3057(02)00853-5. [DOI] [PubMed] [Google Scholar]
- Cummings J.A., Jagannathan L., Jackson L.R., Becker J.B. Sex differences in the effects of estradiol in the nucleus accumbens and striatum on the response to cocaine: neurochemistry and behavior. Drug Alcohol Depend. 2014;135:22–28. doi: 10.1016/j.drugalcdep.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B.A., Clinton S.M., Akil H., Becker J.B. The effects of novelty-seeking phenotypes and sex differences on acquisition of cocaine self-administration in selectively bred High-Responder and Low-Responder rats. Pharmacol. Biochem. Behav. 2008;90:331–338. doi: 10.1016/j.pbb.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doncheck E.M., Urbanik L.A., DeBaker M.C., Barron L.M., Liddiard G.T., Tuscher J.J., Frick K.M., Hillard C.J., Mantsch J.R. 17β-Estradiol potentiates the reinstatement of cocaine seeking in female rats: role of the prelimbic prefrontal cortex and cannabinoid type-1 receptors. Neuropsychopharmacology. 2018;43:781–790. doi: 10.1038/npp.2017.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enman N.M., Arthur K., Ward S.J., Perrine S.A., Unterwald E.M. Anhedonia, reduced cocaine reward, and dopamine dysfunction in a rat model of posttraumatic stress disorder. Biol. Psychiatr. 2015;78:871–879. doi: 10.1016/j.biopsych.2015.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox H.C., Sinha R. Sex differences in drug-related stress-system changes. Harv. Rev. Psychiatr. 2009;17:103–119. doi: 10.1080/10673220902899680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaali S., Kirschner A., Cuboni S., Hartmann J., Kozany C., Balsevich G., Namendorf C., Fernandez-Vizarra P., Sippel C., Zannas A.S., Draenert R., Binder E.B., Almeida O.F.X., Rühter G., Uhr M., Schmidt M.V., Touma C., Bracher A., Hausch F. Selective inhibitors of the FK506-binding protein 51 by induced fit. Nat. Chem. Biol. 2015;11:33–37. doi: 10.1038/nchembio.1699. [DOI] [PubMed] [Google Scholar]
- Gawin F.H. Cocaine addiction: psychology and neurophysiology. Science (80- 1991;251:1580–1586. doi: 10.1126/science.2011738. [DOI] [PubMed] [Google Scholar]
- Griffin M.L., Weiss R.D., Mirin S.M., Lange U. A comparison of male and female cocaine abusers. Arch. Gen. Psychiatr. 1989;46:122. doi: 10.1001/archpsyc.1989.01810020024005. [DOI] [PubMed] [Google Scholar]
- Hartmann J., Wagner K.V., Gaali S., Kirschner A., Kozany C., Rühter G., Dedic N., Häusl A.S., Hoeijmakers L., Westerholz S., Namendorf C., Gerlach T., Uhr M., Chen A., Deussing J.M., Holsboer F., Hausch F., Schmidt M.V. Pharmacological inhibition of the psychiatric risk factor FKBP51 has anxiolytic properties. J. Neurosci. 2015;35 doi: 10.1523/JNEUROSCI.4024-14.2015. 9007–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck A.L., Handa R.J. Sex differences in the hypothalamic–pituitary–adrenal axis' response to stress: an important role for gonadal hormones. Neuropsychopharmacology. 2019;44:45–58. doi: 10.1038/s41386-018-0167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilderbrand E.R., Lasek A.W. Estradiol enhances ethanol reward in female mice through activation of ERα and ERβ. Horm. Behav. 2018;98:159–164. doi: 10.1016/j.yhbeh.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubler T.R., Denny W.B., Valentine D.L., Cheung-Flynn J., Smith D.F., Scammell J.G. The FK506-binding immunophilin FKBP51 is transcriptionally regulated by progestin and attenuates progestin responsiveness. Endocrinology. 2003;144:2380–2387. doi: 10.1210/en.2003-0092. [DOI] [PubMed] [Google Scholar]
- Hubler T.R., Scammell J.G. Intronic hormone response elements mediate regulation of FKBP5 by progestins and glucocorticoids. Cell Stress Chaperones. 2004;9:243. doi: 10.1379/CSC-32R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson A., Stamp J.A. Ovarian hormones and propensity to drug relapse: a review. Neurosci. Biobehav. Rev. 2011;35:427–436. doi: 10.1016/J.NEUBIOREV.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Ising M., Depping A.-M., Siebertz A., Lucae S., Unschuld P.G., Kloiber S., Horstmann S., Uhr M., Mller-Myhsok B., Holsboer F. Polymorphisms in the FKBP5 gene region modulate recovery from psychosocial stress in healthy controls. Eur. J. Neurosci. 2008;28:389–398. doi: 10.1111/j.1460-9568.2008.06332.x. [DOI] [PubMed] [Google Scholar]
- Jackson L.R., Robinson T.E., Becker J.B. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology. 2006;31:129–138. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- Johnson A.R., Thibeault K.C., Lopez A.J., Peck E.G., Sands L.P., Sanders C.M., Kutlu M.G., Calipari E.S. Cues play a critical role in estrous cycle-dependent enhancement of cocaine reinforcement. Neuropsychopharmacology. 2019;44:1189–1197. doi: 10.1038/s41386-019-0320-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariisa M., Scholl L., Wilson N., Seth P., Hoots B. Drug overdose deaths involving cocaine and psychostimulants with abuse potential — United States, 2003–2017. MMWR Morb. Mortal. Wkly. Rep. 2019;68:388–395. doi: 10.15585/mmwr.mm6817a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten T.A., Gawin F.H., Kosten T.R., Rounsaville B.J. Gender differences in cocaine use and treatment response. J. Subst. Abuse Treat. 1993;10:63–66. doi: 10.1016/0740-5472(93)90100-G. [DOI] [PubMed] [Google Scholar]
- Kuhn C., Francis R. Gender difference in cocaine-induced HPA Axis Activation. Neuropsychopharmacology. 1997;16:399–407. doi: 10.1016/S0893-133X(96)00278-3. [DOI] [PubMed] [Google Scholar]
- Larson E.B., Carroll M.E. Estrogen receptor β, but not α, mediates estrogen's effect on cocaine-induced reinstatement of extinguished cocaine-seeking behavior in ovariectomized female rats. Neuropsychopharmacology. 2007;32:1334–1345. doi: 10.1038/sj.npp.1301249. [DOI] [PubMed] [Google Scholar]
- Lekman M., Laje G., Charney D., Rush A.J., Wilson A.F., Sorant A.J.M., Lipsky R., Wisniewski S.R., Manji H., McMahon F.J., Paddock S. The FKBP5-gene in depression and treatment response—an association study in the sequenced treatment alternatives to relieve depression (STAR*D) cohort. Biol. Psychiatr. 2008;63:1103–1110. doi: 10.1016/j.biopsych.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas S.E., Sholar M., Lundahl L.H., Lamas X., Kouri K., Wines J.D., Kragie L., Mendelson J.H. Sex differences in plasma cocaine levels and subjective effects after acute cocaine administration in human volunteers. Psychopharmacology (Berlin) 1996;125:346–354. doi: 10.1007/BF02246017. [DOI] [PubMed] [Google Scholar]
- Lynch W.J. Modeling the development of drug addiction in male and female animals. Pharmacol. Biochem. Behav. 2018;164:50–61. doi: 10.1016/j.pbb.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch W.J., Carroll M.E. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology (Berlin) 1999;144:77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- Malviya S.A., Kelly S.D., Greenlee M.M., Eaton D.C., Duke B.J., Bourke C.H., Neigh G.N. Estradiol stimulates an anti-translocation expression pattern of glucocorticoid co-regulators in a hippocampal cell model. Physiol. Behav. 2013;122:187–192. doi: 10.1016/J.PHYSBEH.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch J.R., Baker D.A., Francis D.M., Katz E.S., Hoks M.A., Serge J.P. Stressor- and corticotropin releasing factor-induced reinstatement and active stress-related behavioral responses are augmented following long-access cocaine self-administration by rats. Psychopharmacology (Berlin) 2008;195:591–603. doi: 10.1007/s00213-007-0950-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcondes F.K., Bianchi F.J., Tanno A.P. Determination of the estrous cycle phases of rats: some helpful considerations. Braz. J. Biol. 2002;62:609–614. doi: 10.1590/S1519-69842002000400008. [DOI] [PubMed] [Google Scholar]
- McCance-Katz E.F., Carroll K.M., Rounsaville B.J. Gender differences in treatment-seeking cocaine abusers - implications for treatment and prognosis. Am. J. Addict. 1999;8:300–311. doi: 10.1080/105504999305703. [DOI] [PubMed] [Google Scholar]
- McKay J.R., Rutherford M.J., Cacciola J.S., Kabasakalian-McKay R., Alterman A.I. Gender differences in the relapse experiences of cocaine patients. J. Nerv. Ment. Dis. 1996;184:616–622. doi: 10.1097/00005053-199610000-00006. [DOI] [PubMed] [Google Scholar]
- McLean C.P., Asnaani A., Litz B.T., Hofmann S.G. Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. J. Psychiatr. Res. 2011;45:1027–1035. doi: 10.1016/j.jpsychires.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minelli A., Maffioletti E., Cloninger C.R., Magri C., Sartori R., Bortolomasi M., Congiu C., Bignotti S., Segala M., Giacopuzzi M., Gennarelli M. Role of allelic variants of FK506-binding protein 51 (FKBP5) gene in the development of anxiety disorders. Depress. Anxiety. 2013;30:1170–1176. doi: 10.1002/da.22158. [DOI] [PubMed] [Google Scholar]
- Nunes E.J., Bitner L., Hughley S.M., Small K.M., Walton S.N., Rupprecht L.E., Addy N.A. Cholinergic receptor blockade in the VTA attenuates cue-induced cocaine-seeking and reverses the anxiogenic effects of forced abstinence. Neuroscience. 2019;413:252–263. doi: 10.1016/j.neuroscience.2019.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penberthy J.K., Ait-Daoud N., Vaughan M., Fanning T. Review of treatment for cocaine dependence. Curr. Drug Abuse Rev. 2010;3:49–62. doi: 10.2174/1874473711003010049. [DOI] [PubMed] [Google Scholar]
- Pérez-Ortiz J.M., García-Gutiérrez M.S., Navarrete F., Giner S., Manzanares J. Gene and protein alterations of FKBP5 and glucocorticoid receptor in the amygdala of suicide victims. Psychoneuroendocrinology. 2013;38:1251–1258. doi: 10.1016/J.PSYNEUEN.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Perrine S.A., Sheikh I.S., Nwaneshiudu C.A., Schroeder J.A., Unterwald E.M. Withdrawal from chronic administration of cocaine decreases delta opioid receptor signaling and increases anxiety- and depression-like behaviors in the rat. Neuropharmacology. 2008;54:355–364. doi: 10.1016/j.neuropharm.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnyai Z., Bíró É., Gardi J., Vecsernyés M., Julesz J., Telegdy G. Brain corticotropin-releasing factor mediates “anxiety-like” behavior induced by cocaine withdrawal in rats. Brain Res. 1995;675:89–97. doi: 10.1016/0006-8993(95)00043-. [DOI] [PubMed] [Google Scholar]
- Satta R., Certa B., He D., Lasek A.W. Estrogen receptor β in the nucleus accumbens regulates the rewarding properties of cocaine in female mice. Int. J. Neuropsychopharmacol. 2018;21:382–392. doi: 10.1093/ijnp/pyx118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scammell J.G., Denny W.B., Valentine D.L., Smith D.F. Overexpression of the FK506-binding immunophilin FKBP51 is the common cause of glucocorticoid resistance in three New World primates. Gen. Comp. Endocrinol. 2001;124:152–165. doi: 10.1006/gcen.2001.7696. [DOI] [PubMed] [Google Scholar]
- Shrestha S., Sun Y., Lufkin T., Kraus P., Or Y., Garcia Y.A., Guy N., Ramos P., Cox M.B., Tay F., Lin V.C.L. Tetratricopeptide repeat domain 9A negatively regulates estrogen receptor alpha activity. Int. J. Biol. Sci. 2015;11:434–447. doi: 10.7150/ijbs.9311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M., Dudish-Poulsen S., Nelson D., Pentel P.R., Hatsukami D.K. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp. Clin. Psychopharmacol. 1999;7:274–283. doi: 10.1037/1064-1297.7.3.274. [DOI] [PubMed] [Google Scholar]
- Swalve N., Smethells J.R., Zlebnik N.E., Carroll M.E. Sex differences in reinstatement of cocaine-seeking with combination treatments of progesterone and atomoxetine. Pharmacol. Biochem. Behav. 2016;145:17–23. doi: 10.1016/j.pbb.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson T.L., Moss R.L. Estrogen regulation of dopamine release in the nucleus accumbens: genomic‐and nongenomic‐mediated effects. J. Neurochem. 1994;62:1750–1756. doi: 10.1046/j.1471-4159.1994.62051750.x. [DOI] [PubMed] [Google Scholar]
- Tonn Eisinger K.R., Larson E.B., Boulware M.I., Thomas M.J., Mermelstein P.G. Membrane estrogen receptor signaling impacts the reward circuitry of the female brain to influence motivated behaviors. Steroids. 2018;133:53–59. doi: 10.1016/J.STEROIDS.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Swearingen A.E.D., Sanchez C.L., Frisbee S.M., Williams A., Walker Q.D., Korach K.S., Kuhn C.M. Estradiol replacement enhances cocaine-stimulated locomotion in female C57BL/6 mice through estrogen receptor alpha. Neuropharmacology. 2013;72:236–249. doi: 10.1016/j.neuropharm.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandegrift B.J., You C., Satta R., Brodie M.S., Lasek A.W. Estradiol increases the sensitivity of ventral tegmental area dopamine neurons to dopamine and ethanol. PloS One. 2017;12 doi: 10.1371/journal.pone.0187698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weddington W.W., Brown B.S., Haertzen C.A., Cone E.J., Dax E.M., Herning R.I., Michaelson B.S. Changes in mood, craving, and sleep during short-term abstinence reported by male cocaine addicts. Arch. Gen. Psychiatr. 1990;47:861. doi: 10.1001/archpsyc.1990.01810210069010. [DOI] [PubMed] [Google Scholar]
- Wochnik G.M., Rüegg J., Abel G.A., Schmidt U., Holsboer F., Rein T. FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. J. Biol. Chem. 2005;280:4609–4616. doi: 10.1074/jbc.M407498200. [DOI] [PubMed] [Google Scholar]
- Yoest K.E., Quigley J.A., Becker J.B. Rapid effects of ovarian hormones in dorsal striatum and nucleus accumbens. Horm. Behav. 2018;104:119–129. doi: 10.1016/J.YHBEH.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannas A.S., Wiechmann T., Gassen N.C., Binder E.B. Gene-stress-epigenetic regulation of FKBP5: clinical and translational implications. Neuropsychopharmacology. 2016;41:261–274. doi: 10.1038/npp.2015.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Yang S., Yang C., Jin G., Zhen X. Estrogen regulates responses of dopamine neurons in the ventral tegmental area to cocaine. Psychopharmacology (Berlin) 2008;199:625–635. doi: 10.1007/s00213-008-1188-6. [DOI] [PubMed] [Google Scholar]