Abstract
MicroRNAs (miRNAs) are noncoding RNAs that participate in the pathophysiology of depression by targeting many functional genes. As shown in our previous study, chronic stress up-regulates miR-34a in the hippocampus. However, little is known about the mechanism by which miR-34a regulates the process of depression or its functions as an antidepressant by regulating its targets. In the present study, the dynamic alterations in miR-34a expression and the mechanism underlying miR-34a regulation were assessed after the administration of the antidepressant fluoxetine to mice exposed to chronic stress. In addition, the effects of miR-34a inhibition on mice were directly evaluated. Both lipopolysaccharide (LPS) and corticosterone treatment caused depression-like symptoms and increased miR-34a expression. Additionally, the expression of miR-34a, which was regulated by tropomyosin receptor kinase B (TrkB)/MEK1/ERK signaling, was consistent with the onset of action of fluoxetine. A luciferase reporter assay identified synaptotagmin-1 and Bcl-2 as the targets of miR-34a. Moreover, a miR-34a antagomir exerted antidepressant-like effects, activated TrkB/MEK1/ERK signaling and improved spine morphology in the hippocampus. In conclusion, hippocampal miR-34a overexpression was a typical feature in depression-like animals, and miR-34a downregulation exerts antidepressant-like effects by restoring the spine morphology through its target synaptotagmin-1.
Keywords: miR-34a, Depression, Synaptotagmin-1, Spine morphology
Graphical abstract
Highlights
-
•
LPS and corticosterone cause depression and miR-34a overexpression.
-
•
Fluoxetine affects miR-34a in a dynamic alteration in chronic stress.
-
•
Inhibition of TrkB and ERK signaling upregulates the expression of miR-34a.
-
•
Synaptotagmin-1 and Bcl-2 are the targets of miR-34a.
-
•
Inhibition of miR-34a improves spinal morphology.
List of abbreviations
- ANOVA
analysis of variance
- BDNF
brain-derived neurotrophic factor
- LPS
lipopolysaccharide
- miRNA
microRNA
- mut
mutant
- NMDA
N-methyl-D-aspartic acid receptor
- PBS
phosphate-buffered saline
- qPCR
real-time quantitative polymerase chain reaction
- TrkB
tropomyosin receptor kinase B
- wt
wild-type
1. Introduction
Depression is a common mood disorder that is caused by a variety of factors, with prominent and persistent mood depression as the main clinical features. In severe cases, patients with depression may experience suicidal thoughts and behaviors. The etiology and pathogenesis of depression currently remain unclear, and no obvious signs and abnormal laboratory indicators have been identified. In summary, depression is considered to result from the interactions of biological, psychological and social factors.
MicroRNAs (miRNAs) are a class of non-coding single-stranded small RNAs of approximately 22 nucleotides in length that are widely involved in the mechanisms regulating neural development, cell proliferation and differentiation, apoptosis, and other life processes by acting on mRNAs of the corresponding target genes. Based on the accumulating evidence from clinical investigations and experimental studies, the expression of miRNAs is altered in patients with depression or in animals with depression-like symptoms (Gururajan et al., 2016; Maffioletti et al., 2016; O'Connor et al., 2013; Zhou et al., 2018), indicating that miRNAs participate in the pathophysiology of depression. For example, some miRNAs detected in the peripheral blood of patients with mood disorders were altered in a recent study, and thus are considered as peripheral biomarkers for diagnosis and the treatment response (Lopez et al., 2018). In contrast, antidepressants regulate the expression of miRNAs, such as miR-146b-5p, miR-24-3p and miR-425-3p, in peripheral blood samples from patients with depression or mice with depressive-like symptoms (Lopez et al., 2017). In addition, according to a clinical trial, miR-1202 regulates the expression of metabotropic glutamate receptor-4 and predicts the antidepressant response at baseline (Lopez et al., 2014). These observations not only reflect the findings described above but also suggest that the pharmacological mechanisms of antidepressants may be at least partially mediated by the regulation of miRNAs. Therefore, the ability to define the changes in the miRNA spectrum in depression and reveal the corresponding target genes/proteins regulated by miRNAs will be helpful to further elucidate the complicated pathophysiology of depression (Ferrua et al., 2019).
Interestingly, using an miRNA array and verifying the data using real-time quantitative polymerase chain reaction (qPCR), our previous study observed increased miR-34a-5p expression in the hippocampus of mice exposed to chronic stress (Liu et al., 2015). In addition to our recent finding, miR-34a levels are increased in mice with a cognitive impairment that had been exposed to total abdominal irradiation (Cui et al., 2017). Cognitive impairment of mice correlated with the elevated miR-34a levels in the hippocampus and prefrontal cortex (Sarkar et al., 2019). In contrast, the downregulation of miR-34a has been shown to improve spatial cognitive function, protect neurogenesis (Zhang et al., 2018), and reverse anesthesia-induced learning and memory dysfunctions by suppressing cell apoptosis (Li et al., 2018). The increased expression of miR-34a in mice with depressive-like symptoms and the role of downregulating miR-34a in protecting hippocampal neurogenesis implied that miR-34a might be associated with the pathophysiology of depression. However, researchers have not determined whether various depression-like models cause the same changes in miR-34a expression and whether clinical antidepressants act in parallel to changes in miR-34a expression. The present study initially assessed the involvement of miR-34a in lipopolysaccharide (LPS)- and corticosterone-induced depression-like animals, as the hyperactivation of neuroinflammation and neuroendocrine are involved in the pathophysiology of depression (Remus and Dantzer, 2016; Rincon-Cortes et al., 2019), to address these issues. Then, the dynamic alterations in miR-34a expression in mice exposed to chronic stress and treated with the antidepressant fluoxetine were detected, as the chronic stress model mimics the symptoms of depression and has been widely adopted to evaluate the neurobiological mechanisms of depression or antidepressants (Willner, 2017). Subsequently, we verified that tropomyosin receptor kinase B (TrkB)/MEK/ERK/miR-34a signaling is required for the effects of fluoxetine. Moreover, a bioinformatics analysis and luciferase reporter assay were performed to identify the putative binding targets of miR-34a and to evaluate how miR-34a regulates the silencing of genes involved in the pathophysiology of depression in mice. Finally, the mechanism underlying the effects of miR-34a on behaviors and spine morphology were also assessed in the hippocampus of mice exposed to chronic stress.
2. Methods
2.1. Animals
Male C57BL/6 mice (24 ± 2 g; 7–8 weeks old) were purchased from Shanghai Slac Animal Center, PR China. Animals were housed in groups of four per cage (320 × 180 × 160 mm) under a normal light/dark schedule for every 12 h. The animals were acclimated to the housing environment for 1 week before the experiments. The ambient temperature and relative humidity were set to 22 ± 2 °C and at 55 ± 5%, respectively. Food and water were freely available throughout the study, except for the illustration during chronic stress. All procedures were approved by the college and performed in accordance with the National Regulations for the Administration of Affairs Concerning Experimental Animals in China (Regulations for the Administration of Affairs Concerning Experimental Animals, approved by the State Council on 31 October, 1988 and promulgated by Decree No. 2 of the State Science and Technology Commission on 14 November, 1988).
2.2. Reagents
LPS (Escherichia coli 055:B5) (L2880), fluoxetine (PHR1394) and the anti-β-actin antibody (A3854) conjugated to HRP was purchased from Sigma (St. Louis, USA). Corticosterone (C0388) was purchased from TCI (Tokyo, Japan). K252a was purchased from Alomone (Jerusalem, Israel). U0126 (sc-222395) was purchased from Santa Cruz (Santa Cruz, USA). The miR-34a-5p mimic (miR10000542-1), miR-34a-5p mimic control (miR1N0000002-1), miR-34a-5p agomir (miR40000542-4), agomir control (miR4N0000002-4), miR-34a-5p antagomir (miR30000542-4) and antagomir control (miR3N0000002-4) were purchased from Ribobio Co., Ltd. (Guangzhou, China). The dual-luciferase reporter assay kit (E1910) was purchased from Promega (Madison, USA). The primary antibody against pTrkB (CY5598) was purchased from Abways (Shanghai, China). The primary antibodies against TrkB (4603), pMEK1 (9127), MEK1 (2352), pERK (4370) and ERK (4695) were purchased from Cell Signaling (Cambridge, USA). The primary antibodies against Bcl-2 (ab692) and synaptotagmin-1 (ab13259) was purchased from Abcam (Cambridge, USA).
2.3. Drug administration
LPS was used to induce depression-like symptoms. Mice were randomly divided into Control and LPS groups. Control animals received saline, and LPS animals received LPS (0.83 mg/kg) dissolved in saline via an intraperitoneal injection. The sucrose preference test was performed after the LPS injection, and the forced swimming test was performed immediately after the sucrose preference test.
Then, corticosterone was used to induce depression-like symptoms as well. Mice were randomly divided into Control and Corticosterone groups. Control animals were subcutaneously injected with saline containing 0.1% DMSO and 0.1% Tween-80 once daily. Corticosterone (20 mg/kg) was dissolved in saline containing 0.1% DMSO and 0.1% Tween-80 and injected subcutaneously once daily. The injections were repeated once daily for 21 consecutive days. The sucrose preference test was performed after the last corticosterone injection, and the forced swimming test was performed immediately after the sucrose preference test.
2.4. Cannula implantation
The stereotaxic guide cannula implantation was performed according to our previous study (Yi et al., 2018). In detail, animals were anesthetized using 4% chloral hydrate (5 mL/kg), and guide cannula were implanted into the lateral ventricle (0.6 mm caudal to the bregma, 1.5 mm from the midline and 1.5 mm below the dural surface) using a stereotaxic apparatus via fixation using a tooth bar and insertion of blunt ear bars. The guide cannula was fixed using dental cement and closed with a cannula dummy cap. The animals recovered for one week prior to the experiment.
2.5. Chronic stress procedure
The chronic stress procedure was performed as previously described (Liu et al., 2015). Briefly, the stressors consisted of water deprivation, exposure to an empty bottle, exposure to a soiled cage, successive alternating light/dark sequences every 2 h, a reduction in space, a 45° cage tilt, overnight illumination and exposure to predator sounds. All stressors were applied individually and continuously throughout the 8-week experimental cycle. Animals were firstly subjected to four weeks of chronic stress prior to drug administration. All the animals were deprived of water for 12 h before the sucrose training and formal sucrose preference tests, but otherwise both water and food were freely available to the animals.
2.6. Dynamic evaluation of miR-34a expression in depression
Mice were randomly divided into three groups (n = 28): Control-vehicle group, Control-fluoxetine group, Chronic stress-vehicle group and Chronic stress-fluoxetine group. Fluoxetine (20 mg/kg) was suspended in saline, and thus saline was used as the vehicle. Both fluoxetine and vehicle were orally administered for 1, 2, 3 or 4 weeks, and mice were subjected to chronic stress. The sucrose preference test was performed after last fluoxetine administration, and the forced swimming test was performed immediately after the sucrose preference test.
2.7. Involvement of miR-34a in TrkB/MEK1/ERK signaling
Mice were randomly divided into six groups (n = 8): Control-vehicle group, Chronic stress-vehicle group, Chronic stress-fluoxetine group, Chronic stress-fluoxetine-K252a group, Chronic stress-fluoxetine-U0126 group, and Chronic stress-fluoxetine-miR-34a agomir group. Fluoxetine (20 mg/kg) was suspended in saline. K252a (25 μg/kg) dissolved in saline containing 0.1% DMSO was injected intraperitoneally 30 min prior to fluoxetine administration. U0126 (5 μg, dissolved in artificial cerebrospinal fluid containing 1% DMSO) and miR-34a agomir (2 nmol, dissolved in artificial cerebrospinal fluid) were infused via the cannula 60 min prior to fluoxetine administration. Both inhibitors and fluoxetine were treated once a day for 4 weeks. The sucrose preference test was performed after the administration of the last fluoxetine treatment, and the forced swimming test was performed immediately after the sucrose preference test.
2.8. Luciferase reporter assay
Firstly, the 3′-UTRs were constructed according to the potential binding sites of miR-34a identified in the Targetscan database. Because synaptotagmin-1 and Bcl-2 genes have a long 3′-UTR (~2800 and ~5200 bp), only 120 bp wild-type fragments containing the potential binding target sites (wt) and mutant fragments without the potential binding sites (mut) were cloned to construct reporter vector. Then, the reporter vectors containing the wt or mut synaptotagmin-1/Bcl-2 3′-UTR were transfected with the miR-34a mimic or mimic control into HEK 293 cells. Forty-eight hours after transfection, the luciferase assay was performed using the dual luciferase reporter assay kit according to the manufacturer's protocol.
2.9. Experiment using a miR-34a antagomir injection in vivo
Mice were randomly divided into the following four groups to detect the effects of miR-34a on the putative targets in vivo (n = 12): Control-vehicle group, Control-miR-34a antagomir group, Chronic stress-vehicle group and Chronic stress-miR-34a antagomir group. A control antagomir served as the vehicle. The miR-34a antagomir and antagomir control (2 nmol) were dissolved in artificial cerebrospinal fluid (4 μl administered at an injection rate of 0.5 μl/min) and intracerebroventricularly infused via the cannula once per week during the last 4 weeks of chronic stress. The sucrose preference test was performed after the antagomir injection, and the novelty-suppressed feeding test was performed 24 h after the sucrose preference test.
2.10. Sucrose preference test
Briefly, the mice were initially trained to adapt to the sucrose solution before the formal test (Day 1, two bottles of sucrose solution; Day 2, one bottle of sucrose solution and one bottle of water). After the adaptation period, the mice were deprived of water for 12 h. The test was conducted when the mice were housed in individual cages and had free access to two bottles containing the sucrose solution and water, respectively. After 24 h, the weights of the consumed sucrose solution and water were recorded. Sucrose preference was calculated using the formula sucrose preference = sucrose consumption/(water and sucrose consumption) × 100%.
2.11. Forced swimming test
The mice were placed in a glass cylinder containing water. The glass cylinder had a height of 25 cm and 12 cm diameter, and a water depth of 15 cm. The water temperature was maintained at 23 ± 2 °C. All animals were forced to swim for 6 min and the total immobility time was recorded during the last 4 min of the test. The mouse was judged to be immobile when it floated on the surface without struggling, and only made the necessary actions to keep its head out of the water. Using the recorded video, an observer blinded to the treatments scored the immobility time.
2.12. Novelty-suppressed feeding test
The novelty-suppressed feeding test was performed during an 8 min period. Briefly, the testing apparatus consisted of a plastic box (50 × 50 × 20 cm). Animals were deprived of food for 24 h before the test. At the beginning of the test, a single pellet of food was placed in the center of the box. A mouse was placed in a corner of the maze box. The time from the release of the mouse until it ate the food was recorded. The food consumed in the home cage within 15 min was measured immediately after the test as a control value to exclude the appetite factor.
2.13. qPCR
Hippocampal tissues were dissected and stored at −80 °C. qPCR was performed using the Sangon qPCR miRNA detection kit. Briefly, hippocampal miRNAs were first reverse-transcribed with the RT primer. Then, cDNAs were subjected to 40 cycles of amplification with the corresponding PCR primers, in which SYBR Green I was used as the fluorescence signal and ROX as the control fluorescence signal. All samples were processed at the same time to avoid inter-experiment variances. Three sample replicates from each group were included in the qPCR miRNA validation experiment. The expression of U6 was used as an internal reference for miR-34a.
2.14. Western blot
Tissues were homogenized in RIPA buffer containing the necessary inhibitors. The homogenates were centrifuged at 12000×g for 15 min at 4 °C, and the supernatants were collected. The protein concentrations were determined using a standard BCA assay. Twenty to 50 μg of protein were separated on SDS-PAGE gels and transferred to a PVDF membrane. Then, the membrane was blocked with 5% BSA/TBST buffer for 1 h at room temperature, followed by an incubation with 5% BSA/TBST buffer containing the primary antibodies at 4 °C overnight (anti-TrkB: 1:1000, anti-pTrkB: 1:1000, anti-MEK1: 1:1000, anti-pMEK1: 1:1000, anti-ERK: 1:1000, anti-pERK: 1:1000, anti-synaptotagmin-1: 1:500, anti-Bcl-2: 1:1000, and anti-β-actin: 1:5000). The membranes were subsequently incubated with an HRP-labeled secondary antibody (1:2000 or 1:4000). Finally, the bands were detected using the enhanced chemiluminescence method. The levels of β-actin served as an internal reference for the levels of other proteins.
2.15. Golgi staining
The Golgi-Cox procedure was described in detail in our previous study (Wang et al., 2017). Whole brains were immersed in the Golgi-Cox solution for 14 days. Then, the Golgi-Cox solution was replaced with a 30% sucrose solution and tissues were incubated for an additional 3 days. Thin sections (50 μm) were obtained using a vibratome. Sections were then treated with ammonium hydroxide for 30 min, followed by a 30 min incubation in Kodak Film Fixer and finally rinsed with distilled water, dehydrated and mounted with resinous medium. During morphological analysis, three pyramidal neurons in CA3 subregion of each hemisphere were selected on three slices at A/P levels (Bregma −2.06 mm, −2.46 mm, −2.80 mm approximately) according to the brain diagram of mouse. The number of spines in secondary dendrites (about 30–100 μm from soma) was quantified tracing. The dendritic spine density in the CA3 subregion of the hippocampus was expressed as the number of spines per 10 μm of dendritic length. The quantitative evaluation was performed by an observer blind to treatment.
2.16. Statistical analysis
All data are reported as means ± SEM. The normal distribution of the data was first verified using the Kolmogorov-Smirnov test, and then analyzed using a one-way or two-way ANOVA followed by the Tukey post hoc test. A value of p < 0.05 was considered statistically significant in the analysis.
3. Results
3.1. miR-34a expression is abnormally elevated in mice with depressive-like symptoms receiving LPS or corticosterone
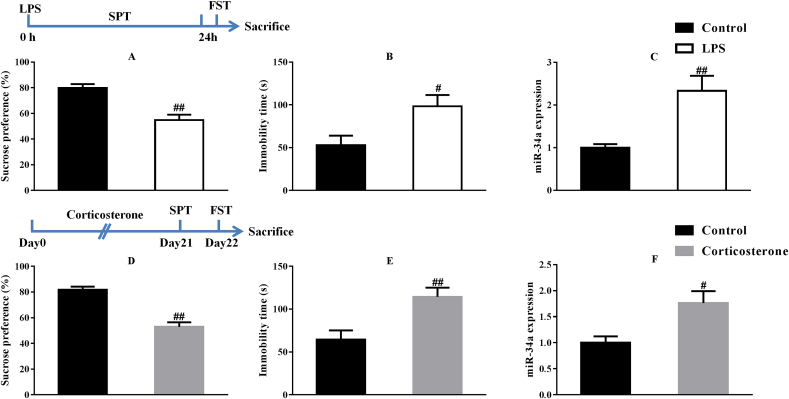
As shown in the behavioral data presented in Fig. 1, mice treated with LPS or corticosterone exhibited a decreased sucrose preference (F(1,14) = 23.50, p < 0.01; F(1,14) = 42.56, p < 0.01, respectively) and increased immobility time (F(1,14) = 6.84, p < 0.05; F(1,14) = 10.84, p < 0.01, respectively), indicating the establishment of depression-like symptoms in mice. Then, the PCR assay revealed increased expression of miR-34a in the hippocampus of mice treated with LPS and corticosterone (F(1,14) = 13.48, p < 0.01; F(1,14) = 8.68, p < 0.05, respectively).
Fig. 1.
LPS and corticosterone injections caused depressive-like behaviors and increased miR-34a expression in the hippocampus (n = 8). #p < 0.05 and ##p < 0.01 compared with the Control-vehicle group.
3.2. Dynamic alterations in the behaviors and miR-34a levels after fluoxetine treatment in mice exposed to chronic stress
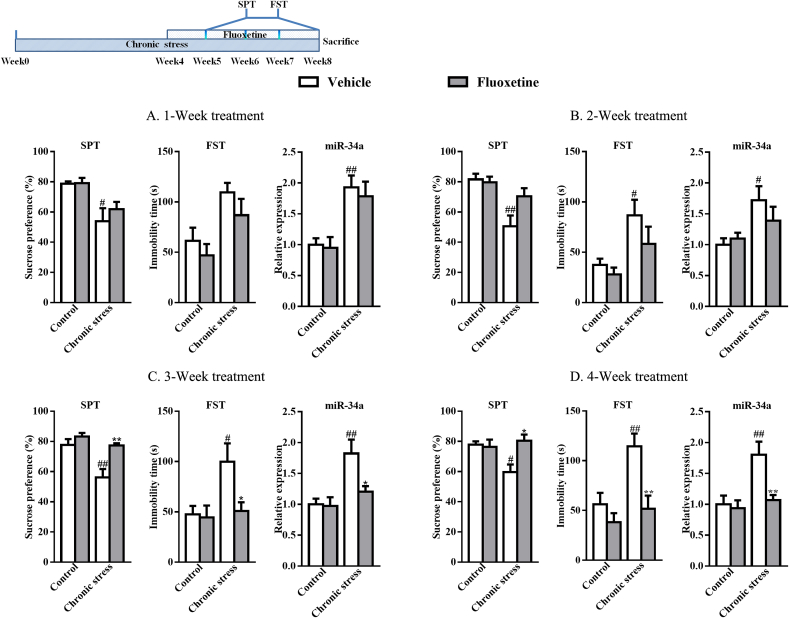
Fig. 2 illustrates the sucrose preference, immobility time and miR-34a levels of mice receiving fluoxetine after 1 (A), 2 (B), 3 (C) or 4 weeks (D). The onset of action occurred within three weeks after fluoxetine treatment, as the reduction in the sucrose preference and the increase in the immobility time were completely reversed by fluoxetine (p < 0.01, p < 0.05, respectively). Similarly, the increase in miR-34a expression induced by chronic stress was attenuated by week 3 of fluoxetine administration (p < 0.05). Similarly, administration with fluoxetine for 4 weeks also reversed the abnormalities in sucrose preference (p < 0.05), immobility time (p < 0.01) and miR-34a expression (p < 0.01) in chronic stress-induced mice.
Fig. 2.
Dynamic evaluation (A: 1 week; B: 2 weeks; C: 3 weeks; and D: 4 weeks) of behaviors and miR-34a expression in mice receiving fluoxetine (n = 7). #p < 0.05 and ##p < 0.01 compared with the Control-vehicle group. *p < 0.05 and **p < 0.01 compared with the Chronic stress-vehicle group. Two-way ANOVA results of (A) SPT: Stress F(1,24) = 15.70, p < 0.01; Treatment F(1,24) = 0.62, p > 0.05; Interaction F(1,24) = 0.51, p > 0.05; FST: Stress F(1,24) = 11.97, p < 0.01; Treatment F(1,24) = 2.12, p > 0.05; Interaction F(1,24) = 0.11, p > 0.05; miR-34a: Stress F(1,24) = 22.96, p < 0.01; Treatment F(1,24) = 0.27, p > 0.05; Interaction F(1,24) = 0.06, p > 0.05; (B) SPT: Stress F(1,24) = 15.20, p < 0.01; Treatment F(1,24) = 3.02, p > 0.05; Interaction F(1,24) = 4.47, p < 0.05; FST: Stress F(1,24) = 10.35, p < 0.01; Treatment F(1,24) = 2.34, p > 0.05; Interaction F(1,24) = 0.57, p > 0.05; miR-34a: Stress F(1,24) = 8.31, p < 0.01; Treatment F(1,24) = 0.43, p > 0.05; Interaction F(1,24) = 1.48, p > 0.05; (C) SPT: Stress F(1,24) = 14.10, p < 0.01; Treatment F(1,24) = 13.38, p < 0.01; Interaction F(1,24) = 4.48, p < 0.05; FST: Stress F(1,24) = 5.56, p < 0.05; Treatment F(1,24) = 4.38, p < 0.05; Interaction F(1,24) = 3.43, p > 0.05; miR-34a: Stress F(1,24) = 12.78, p < 0.01; Treatment F(1,24) = 4.80, p < 0.05; Interaction F(1,24) = 4.08, p > 0.05; (D) SPT: Stress F(1,24) = 2.73, p > 0.05; Treatment F(1,24) = 5.14, p < 0.05; Interaction F(1,24) = 6.89, p < 0.05; FST: Stress F(1,24) = 9.22, p < 0.01; Treatment F(1,24) = 11.73, p < 0.01; Interaction F(1,24) = 3.66, p > 0.05; miR-34a: Stress F(1,24) = 9.85, p < 0.01; Treatment F(1,24) = 7.16, p < 0.05; Interaction F(1,24) = 5.10, p < 0.05.
3.3. TrkB/MEK1/ERK/miR-34a signaling is required for the effects of fluoxetine
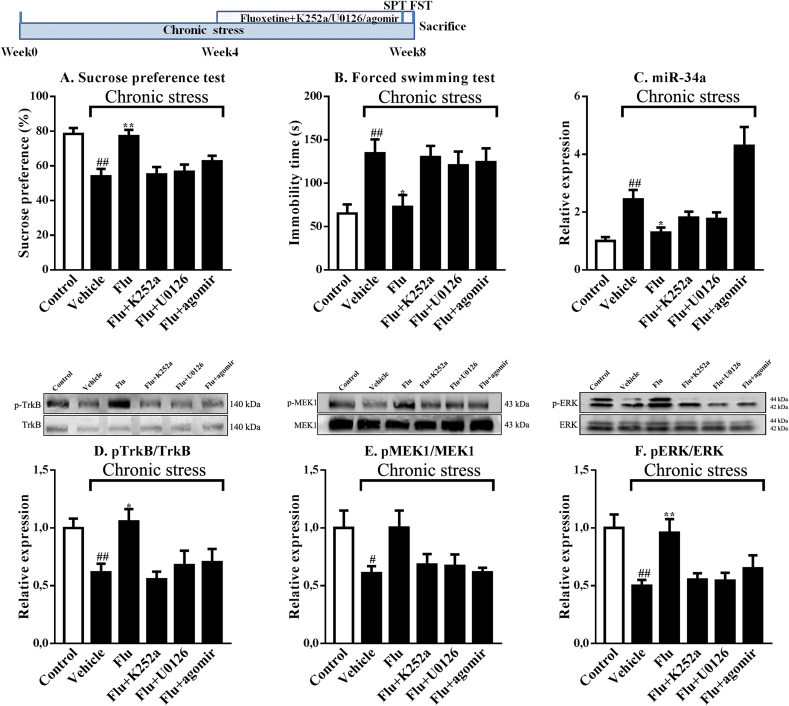
Next, mice were pretreated with a selective antagonist of TrkB (K252a), an inhibitor of MEK/ERK (U0126) or an agonist of miR-34a (miR-34a agomir) prior to fluoxetine administration. As shown in Fig. 3A and B, the antidepressant-like effects of fluoxetine (p < 0.01, p < 0.05, respectively) on sucrose preference and immobility time were significantly blocked by K252a, U0126 and miR-34a agomir. Consistent with the behavioral results, fluoxetine failed to decrease hippocampal miR-34a levels in mice exposed chronic stress that were pretreated with K252a, U0126 or miR-34a agomir (Fig. 3C). In a parallel experiment, western blotting results indicated that fluoxetine did not restore the decreases in the hippocampal pTrkB/TrkB, pMEK1/MEK1 and pERK/ERK ratios when administered in combination with K252a, U0126 or miR-34a agomir (Fig. 3D–F).
Fig. 3.
Pharmacological inhibition of TrkB, MEK/ERK or miR-34a signaling blocks the antidepressant-like effects of fluoxetine on mice exposed to chronic stress (n = 6–8). K252a, U0126, and miR-34a antagomir blocked the improvements in the sucrose preference (A) and immobility time (B), as well as the increases in miR-34a expression (C), pTrkB/TrkB ratio (D), pMEK1/MEK1 ratio (E) and pERK/ERK ratio (F) induced by fluoxetine in mice exposed to chronic stress. #p < 0.05 and ##p < 0.01 compared with the Control-vehicle group. *p < 0.05 and **p < 0.01 compared with the Chronic stress-vehicle group. One-way ANOVA results of (A) F(5,42) = 8.16, p < 0.01; (B) F(5,42) = 4.60, p < 0.01; (C) F(5,42) = 12.39, p < 0.01; (D) F(5,30) = 4.63, p < 0.01; (E) F(5,30) = 3.06, p < 0.05; (F) F(5,30) = 5.82, p < 0.01.
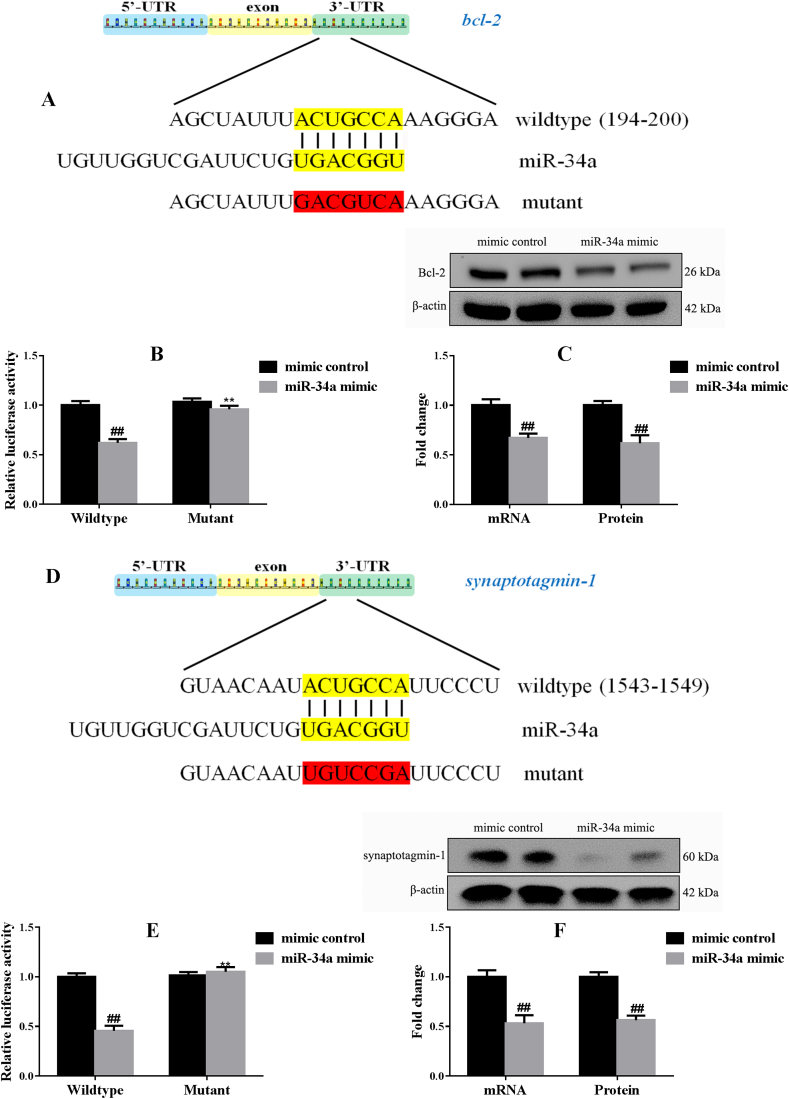
3.4. Synaptotagmin-1 and Bcl-2 are functional targets of miR-34a
According to the bioinformatics analysis, synaptotagmin-1 and Bcl-2 are potential targets of miR-34a. Luciferase reporter constructs carrying the 3′-UTRs of synaptotagmin-1/Bcl-2 containing the potential miR-34a binding sites were constructed and co-transfected with the miR-34a mimic into HEK 293 cells (Fig. 4A and D). As shown in Fig. 4B and E, the miR-34a mimic significantly decreased the luciferase activity of the reporter containing the wildtype 3′-UTRs of synaptotagmin-1/Bcl-2, but not their mutants (p < 0.01 and p < 0.01, respectively). Consistently, the mRNA (F(1,10) = 19.28, p < 0.01; F(1,10) = 19.96, p < 0.01, respectively) and protein (F(1,10) = 17.08, p < 0.01; F(1,10) = 13.92, p < 0.01, respectively) levels of synaptotagmin-1 and Bcl-2 were also decreased by the miR-34a mimic (Fig. 4C and F). Based on these results, miR-34a binds to the synaptotagmin-1 and Bcl-2 3′-UTRs and inhibits their expression in HEK 293 cells.
Fig. 4.
The 3′-UTRs of synaptotagmin-1 and Bcl-2 are direct targets of miR-34a-5p in HEK 293 cells (n = 6). One of the predicted miR-34a binding sites in the 3′-UTRs of synaptotagmin-1 (A) and Bcl-2 (D) mRNA are shown. The 3′-UTR reporter assay was performed in HEK 293 cells 48 h after transfection. The reporter assay confirmed that the miR-34a mimic was capable of significantly inhibiting luciferase expression in cells expressing the wild-type 3′-UTRs of synaptotagmin-1 (B) and Bcl-2 (E). Levels of the synaptotagmin-1 (C) and Bcl-2 (F) mRNAs and proteins were reduced in HEK 293 cells treated with the miR-34a mimic. ##p < 0.01 compared with the wildtype mimic control group and **p < 0.01 compared with the wildtype miR-34a mimic group. Two-way ANOVA results of (B) Genotype F(1,20) = 24.77, p < 0.01; Treatment F(1,20) = 36.55, p < 0.01; Interaction F(1,20) = 16.64, p < 0.01; (E) Genotype F(1,20) = 49.56, p < 0.01; Treatment F(1,20) = 34.30, p < 0.01; Interaction F(1,20) = 45.49, p < 0.01.
3.5. The miR-34a antagomir attenuates the depressive-like behaviors in mice exposed to chronic stress
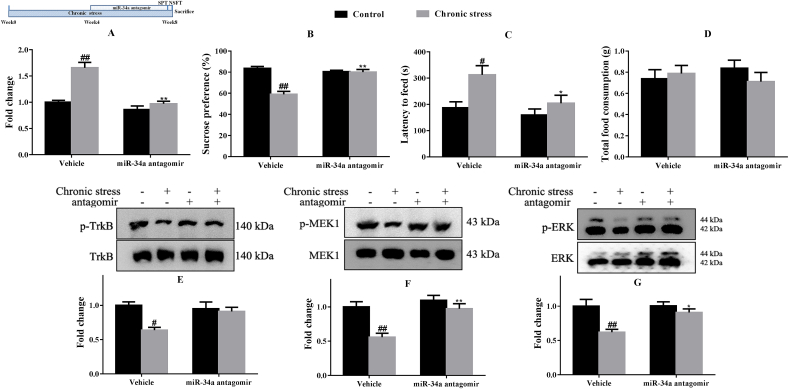
Next, we assessed the effects of the miR-34a inhibitor on the behaviors of mice exposed to chronic stress. As shown in Fig. 5A, the miR-34a antagomir intervention (p < 0.01) significantly inhibited the expression of miR-34a in the hippocampus of mice exposed to chronic stress (p < 0.01).
Fig. 5.
The miR-34a antagomir inhibited miR-34a expression (A), increased the sucrose preference (B), and decreased the latency to feed (C) but did not alter total food consumption (D) in mice subjected to chronic stress (n = 6 or 12). Western blots showing increases in the pTrkB/TrkB ratio (E), pMEK1/MEK1 ratio (F) and pERK/ERK ratio (G) in the hippocampus of mice treated with the miR-34a antagomir (n = 4). #p < 0.05 and ##p < 0.01 compared with the Control-vehicle group. *p < 0.05 and **p < 0.01 compared with the Chronic stress-vehicle group. Two-way ANOVA results of (A) Stress F(1,20) = 30.60, p < 0.01; Treatment F(1,20) = 35.35, p < 0.01; Interaction F(1,20) = 15.34, p < 0.01; (B) Stress F(1,44) = 31.60, p < 0.01; Treatment F(1,44) = 16.11, p < 0.01; Interaction F(1,44) = 30.08, p < 0.01; (C) Stress F(1,44) = 9.02, p < 0.01; Treatment F(1,44) = 5.79, p < 0.05; Interaction F(1,44) = 2.04, p > 0.05; (D) Stress F(1,44) = 0.23, p > 0.05; Treatment F(1,44) = 0.02, p > 0.05; Interaction F(1,44) = 1.18, p > 0.05; (E) Stress F(1,12) = 9.01, p < 0.05; Treatment F(1,12) = 2.77, p > 0.05; Interaction F(1,12) = 5.80, p < 0.05; (F) Stress F(1,12) = 16.36, p < 0.01; Treatment F(1,12) = 13.40, p < 0.01; Interaction F(1,12) = 5.19, p < 0.05; (G) Stress F(1,12) = 12.90, p < 0.01; Treatment F(1,12) = 4.86, p < 0.05; Interaction F(1,12) = 4.38, p > 0.05.
The sucrose preference test revealed a decrease in the sucrose preference in response to chronic stress (p < 0.01) that was progressively increased after the miR-34a antagomir injection (p < 0.01) (Fig. 5B). In addition, the latency to feeding, which was increased by chronic stress exposure (p < 0.05), was ameliorated by the injection of miR-34a antagomir as well (p < 0.05) (Fig. 5C). At the same time, no apparent difference in total food consumption was observed among these four groups (Fig. 5D).
In addition, chronic stress decreased the ratios of pTrkB/TrkB (p < 0.05), pMEK1/MEK1 (p < 0.01) and pERK/ERK (p < 0.01) in the hippocampus, while the miR-34a antagomir normalized the ratios of pMEK1/MEK1 (p < 0.01) and pERK/ERK (p < 0.05) (Fig. 5E–G). In addition, miR-34a antagomir tended to increase the ratio of pTrkB/TrkB (p = 0.0581).
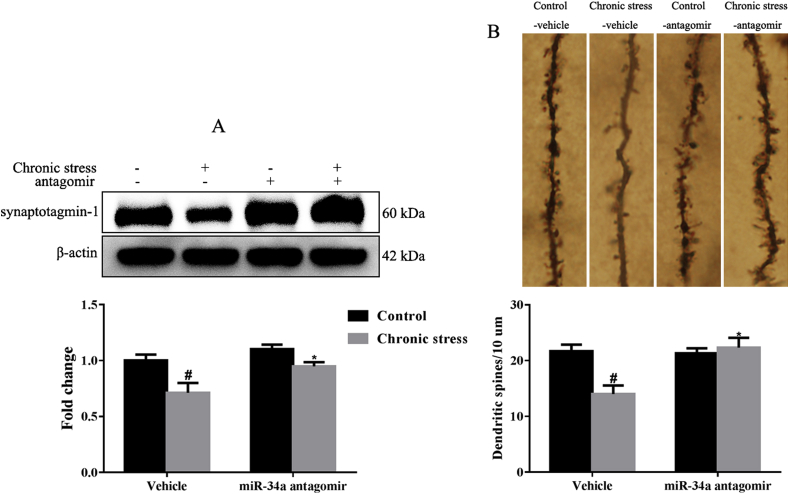
3.6. The miR-34a antagomir alleviates the altered spine morphology in mice exposed to chronic stress
As the luciferase reporter assay confirmed that synaptotagmin-1 was the functional targets of miR-34a, the levels of the synaptotagmin-1 were evaluated in the mouse hippocampus (Fig. 6). Chronic stress reduced synaptotagmin-1 levels (p < 0.05), while the miR-34a antagomir restored its levels (p < 0.05) (Fig. 6A). In addition, as shown in Fig. 6B, the decrease in the density of dendritic spines, induced by chronic stress (p < 0.05) was markedly reversed by the miR-34a antagomir treatment (p < 0.05).
Fig. 6.
The miR-34a antagomir increased the levels of its targets synaptotagmin-1 and improved spine morphology (n = 3–5). (A) Representative photomicrograph of synaptotagmin-1 and a summary of data obtained from the western blots. (B) Representative photomicrograph and summary data for Golgi staining. #p < 0.05 compared with the Control-vehicle group. *p < 0.05 compared with the Chronic stress-vehicle group. Two-way ANOVA results of (A) Stress F(1,16) = 14.38, p < 0.01; Treatment F(1,16) = 8.47, p < 0.05; Interaction F(1,16) = 1.38, p > 0.05; (B) Stress F(1,8) = 5.80, p < 0.05; Treatment F(1,8) = 8.35, p < 0.05; Interaction F(1,8) = 9.80, p < 0.05.
4. Discussion
Notably, miR-34a is a member of the miR-34 family that is located on chromosome 1. Due to the high level of conservation throughout many animal species, miR-34a is one of the most well-studied miRNAs and has attracted the attention of many researchers (Ichimura et al., 2011). As shown in the present study, the hippocampal expression of miR-34a was significantly increased in mice treated with LPS or corticosterone. Subsequently, we evaluated the dynamic alterations in miR-34a expression in response to treatment with the antidepressant fluoxetine from 1 to 4 weeks in mice exposed to chronic stress, another depressive-like animal model that is widely used to mimic the process of depression in humans (Willner, 2017). Consistent with the findings from our previous study (Liu et al., 2015), chronic stress caused an abnormal increase in miR-34a levels in the hippocampus in the present study, indicating that high levels of miR-34a expression was a typical feather in depression-like animals. On the other hand, the dynamic evaluation clearly revealed an attenuation of the increase in miR-34a expression at week 3, when onset of the therapeutic effect of fluoxetine occurred. This finding implies synchronism between miR-34a inhibition and the efficacy of an antidepressant, and provides new insights suggesting that miR-34a regulation is involved in the underlying pathophysiology of depression and the actions of antidepressants.
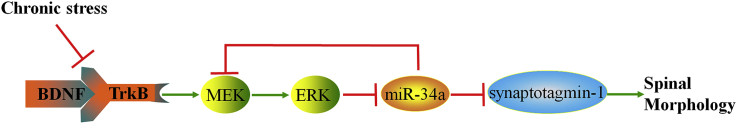
How does depression or an antidepressant modulate the expression of miR-34a? As shown in our previous study, the TrkB/ERK signaling pathway regulates miR-34a expression (Liu et al., 2015). In addition, miR-34a was also reported to regulate ERK signaling, as MEK1, the upstream of ERK, was verified to be a target of miR-34a (Ichimura et al., 2010). Therefore, we next tested whether the antidepressant-like effects of fluoxetine required hippocampal TrkB/MEK1/ERK/miR-34a signaling. Chronic stress significantly decreased the phosphorylation of TrkB, MEK1 and ERK, and increased miR-34a levels in the hippocampus. In contrast, chronic fluoxetine administration completely reversed the inhibition of the TrkB/MEK1/ERK signaling pathway in the hippocampus. However, the effects of fluoxetine were completely prevented by pretreatments with K252a, U0126 and miR-34a agomir. Recently, the primary miR-34a transcript was reported to contain binding motifs for NF-kB, and thus the NF-kB inhibitor Bay 11-7082 decreased miR-34a levels (Sharma et al., 2018). Because NF-κB is inhibited (Cheng et al., 2016; Liu et al., 2018) but ERK is activated (First et al., 2011; Mu et al., 2016) by antidepressants in the hippocampus of rodents exposed to chronic stress, and ERK phosphorylation attenuates NF-κB activation (Chung and Liao, 2016), we speculated that ERK phosphorylation inhibits NF-κB activity, and thus NF-kB is unable to interact with the binding site and inhibit the expression of miR-34a. On the other hand, an increase in miR-34a expression also decreased MEK1 levels, indicating that miR-34a expression is not only regulated by TrkB/MEK1/ERK signaling but also acts as a positive feedback regulator of this signaling cascade. This mechanism might lead to a sustained increase in miR-34a expression during the process of depression or a sustained reduction in response to antidepressants. The finding that co-treatment with fluoxetine and agomir did not further decrease TrkB/MEK/ERK signaling pathway in the hippocampus of stressed mice is interesting. This is partly similar to a previous study showing that TrkB antagonist ANA-12 did not further decrease p-TrkB levels and spine density, but TrkB agonist 7,8-DHF fully reversed the reduction of p-TrkB levels and spine density in the hippocampus of depressive animals (Zhang et al., 2014). Consistently, selective mTOR inhibitor rapamycin or mTOR-shRNA did not decrease p-mTOR levels in chronic stress-induced animals (Xu et al., 2018). We speculate that there is a compensatory mechanism to maintain necessary MEK/ERK levels, which may be required for basic function of neurons.
Using a microarray analysis and qPCR verification, our previous study clearly identified a role for miR-34a in the pathophysiology of depression (Liu et al., 2015). In the present study, based on the conserved sites identified from the Targetscan database, we identified two putative genes, synaptotagmin-1 and Bcl-2, as targets of miR-34a. The database identified several binding sequences for miR-34a in the 3′-UTRs of the synaptotagmin-1 and Bcl-2 mRNAs. In this case, the conserved binding sites between miR-34a and synaptotagmin-1/Bcl-2 mRNAs were verified using a luciferase reporter assay in vitro. The overexpression of miR-34a markedly decreased both synaptotagmin-1 and Bcl-2 levels in wildtype HK293 cells. However, neither synaptotagmin-1 nor Bcl-2 levels were altered in mutant HK293 cells, indicating the consistency between the bioinformatics database and the cell-based data.
A miR-34a antagomir was administered to mice exposed to chronic stress to obtain a reliable and convincing conclusion. The anhedonia caused by chronic stress was attenuated by the injection of the miR-34a antagomir, as the sucrose preference was increased. Consistently, the latency to feed in the novelty-suppressed feeding test was increased by chronic stress but reversed by the miR-34a antagomir. The results of the behavioral tests described above suggested a role for miR-34a inhibition in treating depression. On the other hand, the miR-34a antagomir also increased hippocampal TrkB, MEK1 and ERK phosphorylation, providing evidence to support the hypothesis of the role of miR-34a in regulating MEK1 and TrkB/MEK1/ERK signaling.
Neurotransmitters are involved in the pathophysiology of depression. A recent study indicated that miR-34a was involved in acute regulation of synaptic transmission by its sequence-specific targets in dentate gyrus (Berentsen et al., 2020). Synaptotagmins, which function as calcium sensors to regulate neurotransmitter release, have received widespread attention (Chang et al., 2018; Sudhof, 2013). Synaptotagmin-1 is one of the main proteins located in the synaptic vesicle membrane (Zhou et al., 2017). As shown in the present study, hippocampal synaptotagmin-1 levels were decreased after chronic stress, consistent with a previous report showing that synaptotagmin-1 levels were decreased in the hippocampus after the induction of chronic stress or long-term corticosterone administration (Freitas et al., 2016; Han et al., 2018). In contrast, synaptotagmin-1 levels were increased after fluoxetine treatment compared to vehicle controls (Popova et al., 2017). In the present study, miR-34a inhibition reversed the reduction in synaptotagmin-1 levels in the hippocampus, indicating that the activation or inhibition of miR-34a inversely regulates the expression of synaptotagmin-1 in the hippocampus. Furthermore, the spine morphology is modulated by the synaptic protein synaptotagmin-1 (Agostini et al., 2011b). Thus, Golgi staining was performed to measure the density of dendritic spines and to further verify the effect of the synaptotagmin-1 deficiency on the spine morphology. Chronic stress decreased the dendritic spine density, while the miR-34a antagomir attenuated this reduction, consistent with studies showing that neuronal differentiation, synapse morphology and function are altered in neural stem cells overexpressing miR-34a (Morgado et al., 2015). Taken together, chronic stress alters the spine morphology by increasing miR-34a expression to inhibit synaptotagmin-1 expression.
As shown in the present study, HEK 293 cells transfected with the miR-34a mimic displayed decreased levels of synaptotagmin-1 and Bcl-2. Additionally, two previous studies also reported reduced levels of synaptotagmin-1 in both cortical neurons (Agostini et al., 2011a) and neural stem cells (Morgado et al., 2015) transfected with miR-34a. Based on accumulating evidence, the levels of Bcl-2 are reduced in SH-SY5Y cells (Mao et al., 2015; Wang et al., 2009), cortical neuronal cells (Dai et al., 2017) and other cells transfected with miR-34a. Therefore, combined with the bioinformatics analysis, these studies provided evidence that synaptotagmin-1 and Bcl-2 are targets of miR-34a. However, we do not deny that the use of immunoprecipitated RISC to detect hippocampal miR-34a expression in a mouse model of depression will help to strengthen the conclusions obtained from in vitro experiments. So this is one of the limitations of the present study.
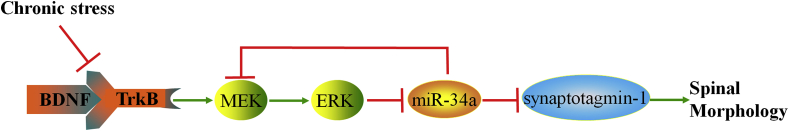
Overall, we proposed a novel mechanism by which chronic stress impaired spine morphology by activating TrkB/MEK1/ERK/miR-34a signaling in the hippocampus. Overexpression of miR-34a decreased synaptotagmin-1 levels by targeting its 3′-UTR sequences and thus damaged the spine morphology (Fig. 7). Based on the matched results obtained from the miRNA sequence database mirbase.org and miRNA target identification tool targetscan.org, both the sequence of miR-34a and target sequences in synaptotagmin-1 are shared by most mammals, including mice, rats and humans, suggesting that the seed sequences of synaptotagmin-1 3′-UTRs targeted by miR-34a are highly conserved.
Fig. 7.
The biological function of miR-34a is mediated by repressing the expression of its targets in a mouse model of depression.
Funding
Funding for this study was provided by the Science Research Foundation of Ministry of Health & United Fujian Provincial Health and Education Project for Tacking the Key Research (2019-WJ-38), Huaqiao University [No. ZQN-PY218], the Outstanding Youth Scientific Research Training Program in Colleges and Universities of Fujian Province [No. JA14015] and the Program for Innovative Research Team in Science and Technology in Fujian Province University.
CRediT authorship contribution statement
Li-Tao Yi: Conceptualization, Investigation, Project administration, Supervision, Writing - original draft, Writing - review & editing. Ji-Xiao Zhu: Conceptualization, Validation. Shu-Qi Dong: Investigation, Methodology. Cheng-Fu Li: Project administration, Formal analysis. Qiu-Ping Zhang: Formal analysis, Validation. Jie Cheng: Investigation, Methodology. Qing Liu: Conceptualization, Methodology.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgements
We would like to thank Dr. Shi-Bin Wang for the help during the experimental design, and thank Instrumental Analysis Center of Huaqiao University for the help of confocal testing.
References
- Agostini M., Tucci P., Killick R., Candi E., Sayan B.S., Rivetti di Val Cervo P., Nicotera P., McKeon F., Knight R.A., Mak T.W., Melino G. Neuronal differentiation by TAp73 is mediated by microRNA-34a regulation of synaptic protein targets. Proc. Natl. Acad. Sci. U.S.A. 2011;108:21093–21098. doi: 10.1073/pnas.1112061109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agostini M., Tucci P., Steinert J.R., Shalom-Feuerstein R., Rouleau M., Aberdam D., Forsythe I.D., Young K.W., Ventura A., Concepcion C.P., Han Y.C., Candi E., Knight R.A., Mak T.W., Melino G. microRNA-34a regulates neurite outgrowth, spinal morphology, and function. Proc. Natl. Acad. Sci. U.S.A. 2011;108:21099–21104. doi: 10.1073/pnas.1112063108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berentsen B., Patil S., Ronnestad K., Goff K.M., Pajak M., Simpson T.I., Wibrand K., Bramham C.R. MicroRNA-34a acutely regulates synaptic efficacy in the adult dentate gyrus in vivo. Mol. Neurobiol. 2020;57:1432–1445. doi: 10.1007/s12035-019-01816-1. [DOI] [PubMed] [Google Scholar]
- Chang S., Trimbuch T., Rosenmund C. Synaptotagmin-1 drives synchronous Ca(2+)-triggered fusion by C2B-domain-mediated synaptic-vesicle-membrane attachment. Nat. Neurosci. 2018;21:33–40. doi: 10.1038/s41593-017-0037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Pardo M., Armini R.S., Martinez A., Mouhsine H., Zagury J.F., Jope R.S., Beurel E. Stress-induced neuroinflammation is mediated by GSK3-dependent TLR4 signaling that promotes susceptibility to depression-like behavior. Brain Behav. Immun. 2016;53:207–222. doi: 10.1016/j.bbi.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung C.Y., Liao F. CXCR3 signaling in glial cells ameliorates experimental autoimmune encephalomyelitis by restraining the generation of a pro-Th17 cytokine milieu and reducing CNS-infiltrating Th17 cells. J. Neuroinflammation. 2016;13:76. doi: 10.1186/s12974-016-0536-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui M., Xiao H., Li Y., Dong J., Luo D., Li H., Feng G., Wang H., Fan S. Total abdominal irradiation exposure impairs cognitive function involving miR-34a-5p/BDNF axis. Biochim. Biophys. Acta. 2017;1863:2333–2341. doi: 10.1016/j.bbadis.2017.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X., Yin Y., Qin L. Valproic acid exposure decreases the mRNA stability of Bcl-2 via up-regulating miR-34a in the cerebellum of rat. Neurosci. Lett. 2017;657:159–165. doi: 10.1016/j.neulet.2017.08.018. [DOI] [PubMed] [Google Scholar]
- Ferrua C.P., Giorgi R., da Rosa L.C., do Amaral C.C., Ghisleni G.C., Pinheiro R.T., Nedel F. MicroRNAs expressed in depression and their associated pathways: a systematic review and a bioinformatics analysis. J. Chem. Neuroanat. 2019;100:101650. doi: 10.1016/j.jchemneu.2019.101650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M., Gil-Ad I., Taler M., Tarasenko I., Novak N., Weizman A. The effects of fluoxetine treatment in a chronic mild stress rat model on depression-related behavior, brain neurotrophins and ERK expression. J. Mol. Neurosci. 2011;45:246–255. doi: 10.1007/s12031-011-9515-5. [DOI] [PubMed] [Google Scholar]
- Freitas A.E., Egea J., Buendia I., Gomez-Rangel V., Parada E., Navarro E., Casas A.I., Wojnicz A., Ortiz J.A., Cuadrado A., Ruiz-Nuno A., Rodrigues A.L.S., Lopez M.G. Agmatine, by improving neuroplasticity markers and inducing Nrf2, prevents corticosterone-induced depressive-like behavior in mice. Mol. Neurobiol. 2016;53:3030–3045. doi: 10.1007/s12035-015-9182-6. [DOI] [PubMed] [Google Scholar]
- Gururajan A., Naughton M.E., Scott K.A., O'Connor R.M., Moloney G., Clarke G., Dowling J., Walsh A., Ismail F., Shorten G., Scott L., McLoughlin D.M., Cryan J.F., Dinan T.G. MicroRNAs as biomarkers for major depression: a role for let-7b and let-7c. Transl. Psychiatry. 2016;6 doi: 10.1038/tp.2016.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y.X., Tao C., Gao X.R., Wang L.L., Jiang F.H., Wang C., Fang K., Chen X.X., Chen Z., Ge J.F. BDNF-related imbalance of copine 6 and synaptic plasticity markers couples with depression-like behavior and immune activation in CUMS rats. Front. Neurosci. 2018;12:731. doi: 10.3389/fnins.2018.00731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura A., Ruike Y., Terasawa K., Shimizu K., Tsujimoto G. MicroRNA-34a inhibits cell proliferation by repressing mitogen-activated protein kinase kinase 1 during megakaryocytic differentiation of K562 cells. Mol. Pharmacol. 2010;77:1016–1024. doi: 10.1124/mol.109.063321. [DOI] [PubMed] [Google Scholar]
- Ichimura A., Ruike Y., Terasawa K., Tsujimoto G. miRNAs and regulation of cell signaling. FEBS J. 2011;278:1610–1618. doi: 10.1111/j.1742-4658.2011.08087.x. [DOI] [PubMed] [Google Scholar]
- Li G.F., Li Z.B., Zhuang S.J., Li G.C. Inhibition of microRNA-34a protects against propofol anesthesia-induced neurotoxicity and cognitive dysfunction via the MAPK/ERK signaling pathway. Neurosci. Lett. 2018;675:152–159. doi: 10.1016/j.neulet.2018.03.052. [DOI] [PubMed] [Google Scholar]
- Liu B.B., Luo L., Liu X.L., Geng D., Liu Q., Yi L.T. 7-Chlorokynurenic acid (7-CTKA) produces rapid antidepressant-like effects: through regulating hippocampal microRNA expressions involved in TrkB-ERK/Akt signaling pathways in mice exposed to chronic unpredictable mild stress. Psychopharmacology (Berl.) 2015;232:541–550. doi: 10.1007/s00213-014-3690-3. [DOI] [PubMed] [Google Scholar]
- Liu F.Y., Cai J., Wang C., Ruan W., Guan G.P., Pan H.Z., Li J.R., Qian C., Chen J.S., Wang L., Chen G. Fluoxetine attenuates neuroinflammation in early brain injury after subarachnoid hemorrhage: a possible role for the regulation of TLR4/MyD88/NF-kappaB signaling pathway. J. Neuroinflammation. 2018;15:347. doi: 10.1186/s12974-018-1388-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez J.P., Fiori L.M., Cruceanu C., Lin R., Labonte B., Cates H.M., Heller E.A., Vialou V., Ku S.M., Gerald C., Han M.H., Foster J., Frey B.N., Soares C.N., Muller D.J., Farzan F., Leri F., MacQueen G.M., Feilotter H., Tyryshkin K., Evans K.R., Giacobbe P., Blier P., Lam R.W., Milev R., Parikh S.V., Rotzinger S., Strother S.C., Lewis C.M., Aitchison K.J., Wittenberg G.M., Mechawar N., Nestler E.J., Uher R., Kennedy S.H., Turecki G. MicroRNAs 146a/b-5 and 425-3p and 24-3p are markers of antidepressant response and regulate MAPK/Wnt-system genes. Nat. Commun. 2017;8:15497. doi: 10.1038/ncomms15497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez J.P., Kos A., Turecki G. Major depression and its treatment: microRNAs as peripheral biomarkers of diagnosis and treatment response. Curr. Opin. Psychiatr. 2018;31:7–16. doi: 10.1097/YCO.0000000000000379. [DOI] [PubMed] [Google Scholar]
- Lopez J.P., Lim R., Cruceanu C., Crapper L., Fasano C., Labonte B., Maussion G., Yang J.P., Yerko V., Vigneault E., El Mestikawy S., Mechawar N., Pavlidis P., Turecki G. miR-1202 is a primate-specific and brain-enriched microRNA involved in major depression and antidepressant treatment. Nat. Med. 2014;20:764–768. doi: 10.1038/nm.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffioletti E., Cattaneo A., Rosso G., Maina G., Maj C., Gennarelli M., Tardito D., Bocchio-Chiavetto L. Peripheral whole blood microRNA alterations in major depression and bipolar disorder. J. Affect. Disord. 2016;200:250–258. doi: 10.1016/j.jad.2016.04.021. [DOI] [PubMed] [Google Scholar]
- Mao S., Sun Q., Xiao H., Zhang C., Li L. Secreted miR-34a in astrocytic shedding vesicles enhanced the vulnerability of dopaminergic neurons to neurotoxins by targeting Bcl-2. Protein & cell. 2015;6:529–540. doi: 10.1007/s13238-015-0168-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgado A.L., Xavier J.M., Dionisio P.A., Ribeiro M.F., Dias R.B., Sebastiao A.M., Sola S., Rodrigues C.M. MicroRNA-34a modulates neural stem cell differentiation by regulating expression of synaptic and autophagic proteins. Mol. Neurobiol. 2015;51:1168–1183. doi: 10.1007/s12035-014-8794-6. [DOI] [PubMed] [Google Scholar]
- Mu R.H., Fang X.Y., Wang S.S., Li C.F., Chen S.M., Chen X.M., Liu Q., Li Y.C., Yi L.T. Antidepressant-like effects of standardized gypenosides: involvement of brain-derived neurotrophic factor signaling in hippocampus. Psychopharmacology (Berl.) 2016;233:3211–3221. doi: 10.1007/s00213-016-4357-z. [DOI] [PubMed] [Google Scholar]
- O'Connor R.M., Grenham S., Dinan T.G., Cryan J.F. microRNAs as novel antidepressant targets: converging effects of ketamine and electroconvulsive shock therapy in the rat hippocampus. Int. J. Neuropsychopharmacol. 2013;16:1885–1892. doi: 10.1017/S1461145713000448. [DOI] [PubMed] [Google Scholar]
- Popova D., Castren E., Taira T. Chronic fluoxetine administration enhances synaptic plasticity and increases functional dynamics in hippocampal CA3-CA1 synapses. Neuropharmacology. 2017;126:250–256. doi: 10.1016/j.neuropharm.2017.09.003. [DOI] [PubMed] [Google Scholar]
- Remus J.L., Dantzer R. Inflammation models of depression in rodents: relevance to psychotropic drug discovery. Int. J. Neuropsychopharmacol. 2016;19 doi: 10.1093/ijnp/pyw028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincon-Cortes M., Herman J.P., Lupien S., Maguire J., Shansky R.M. Stress: influence of sex, reproductive status and gender. Neurobiol. Stress. 2019;10:100155. doi: 10.1016/j.ynstr.2019.100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S., Engler-Chiurazzi E.B., Cavendish J.Z., Povroznik J.M., Russell A.E., Quintana D.D., Mathers P.H., Simpkins J.W. Over-expression of miR-34a induces rapid cognitive impairment and Alzheimer's disease-like pathology. Brain Res. 2019;1721:146327. doi: 10.1016/j.brainres.2019.146327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V.K., Raimondi V., Ruggero K., Pise-Masison C.A., Cavallari I., Silic-Benussi M., Ciminale V., D'Agostino D.M. Expression of miR-34a in T-cells infected by human T-lymphotropic virus 1. Front. Microbiol. 2018;9:832. doi: 10.3389/fmicb.2018.00832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof T.C. A molecular machine for neurotransmitter release: synaptotagmin and beyond. Nat. Med. 2013;19:1227–1231. doi: 10.1038/nm.3338. [DOI] [PubMed] [Google Scholar]
- Wang S.S., Mu R.H., Li C.F., Dong S.Q., Geng D., Liu Q., Yi L.T. microRNA-124 targets glucocorticoid receptor and is involved in depression-like behaviors. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2017;79:417–425. doi: 10.1016/j.pnpbp.2017.07.024. [DOI] [PubMed] [Google Scholar]
- Wang X., Liu P., Zhu H., Xu Y., Ma C., Dai X., Huang L., Liu Y., Zhang L., Qin C. miR-34a, a microRNA up-regulated in a double transgenic mouse model of Alzheimer's disease, inhibits bcl2 translation. Brain Res. Bull. 2009;80:268–273. doi: 10.1016/j.brainresbull.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Willner P. The chronic mild stress (CMS) model of depression: history, evaluation and usage. Neurobiol. Stress. 2017;6:78–93. doi: 10.1016/j.ynstr.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D., Sun Y., Wang C., Wang H., Wang Y., Zhao W., Bao G., Wang F., Cui Z., Jiang B. Hippocampal mTOR signaling is required for the antidepressant effects of paroxetine. Neuropharmacology. 2018;128:181–195. doi: 10.1016/j.neuropharm.2017.10.008. [DOI] [PubMed] [Google Scholar]
- Yi L.T., Mu R.H., Dong S.Q., Wang S.S., Li C.F., Geng D., Liu Q. miR-124 antagonizes the antidepressant-like effects of standardized gypenosides in mice. J. Psychopharmacol. 2018;32:458–468. doi: 10.1177/0269881118758304. [DOI] [PubMed] [Google Scholar]
- Zhang J.C., Wu J., Fujita Y., Yao W., Ren Q., Yang C., Li S.X., Shirayama Y., Hashimoto K. Antidepressant effects of TrkB ligands on depression-like behavior and dendritic changes in mice after inflammation. Int. J. Neuropsychopharmacol. 2014;18 doi: 10.1093/ijnp/pyu077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q.J., Li J., Zhang S.Y. Effects of TRPM7/miR-34a gene silencing on spatial cognitive function and hippocampal neurogenesis in mice with type 1 diabetes mellitus. Mol. Neurobiol. 2018;55:1568–1579. doi: 10.1007/s12035-017-0398-5. [DOI] [PubMed] [Google Scholar]
- Zhou M., Wang M., Wang X., Liu K., Wan Y., Li M., Liu L., Zhang C. Abnormal expression of MicroRNAs induced by chronic unpredictable mild stress in rat hippocampal tissues. Mol. Neurobiol. 2018;55:917–935. doi: 10.1007/s12035-016-0365-6. [DOI] [PubMed] [Google Scholar]
- Zhou Q., Zhou P., Wang A.L., Wu D., Zhao M., Sudhof T.C., Brunger A.T. The primed SNARE-complexin-synaptotagmin complex for neuronal exocytosis. Nature. 2017;548:420–425. doi: 10.1038/nature23484. [DOI] [PMC free article] [PubMed] [Google Scholar]