Abstract
Chronic stress represents a vulnerability factor for anxiety and depressive disorders and has been widely used to model aspects of these disorders in rodents. Disinhibition of somatostatin (SST)-positive GABAergic interneurons in mice by deletion of γ2 GABAA receptors selectively from these cells (SSTCre:γ2f/f mice) has been shown to result in behavioral and biochemical changes that mimic the responses to antidepressant doses of ketamine. Here we explored the extent to which SSTCre:γ2f/f mice exhibit resilience to unpredictable chronic mild stress (UCMS). We found that male SSTCre:γ2f/f mice are resilient to UCMS-induced (i) reductions in weight gain, (ii) reductions in SST-immuno-positive cells in medial prefrontal cortex (mPFC), (iii) increases in phosphorylation of eukaryotic elongation factor 2 (eEF2) in mPFC, and (iv) increased anxiety in a novelty suppressed feeding test. Female SSTCre:γ2f/f mice were resilient to UCMS-induced reductions in SST-immuno-positive cells indistinguishably from males. However, in contrast to males, they showed no UCMS effects on weight gain independent of genotype. Moreover, in mPFC of female γ2f/f control mice, UCMS resulted in paradoxically reduced p-EF2 levels without stress effects in the SSTCre:γ2f/f mutants. Lastly, female SSTCre:γ2f/f mice showed increased rather than reduced UCMS induced anxiety compared to γ2f/f controls. Thus, disinhibition of SST interneurons results in behavioral resilience to UCMS selectively in male mice, along with cellular resilience of SST neurons to UCMS independent of sex. Thus, mechanisms underlying vulnerability and resilience to stress are sex specific and map to mPFC rather than hippocampus but appear unrelated to changes in expression of SST as a marker of corresponding interneurons.
Keywords: Antidepressant, eEF2, Somatostatin, Neural inhibition, Excitation-inhibition balance
Abbreviations
- 129
129X1/SvJ
- BL/6J
C57BL/6J
- eEF2
eukaryotic elongation factor 2
- eEF2K
eukaryotic elongation factor 2 kinase
- EPM
elevated plus maze
- GABA
γ-aminobutyric acid
- GABAA receptor
Ionotropic GABA receptor
- ILC
infralimbic cortex
- IP
immuno-positive
- MDD
major depressive disorder
- mPFC
medial prefrontal cortex
- mTOR
mammalian target of rapamycin
- NMDA
N-methyl-D-aspartate
- NS
no stress
- NSFT
novelty suppressed feeding test
- OFT
open field test
- OLM
oriens lacunosum molecular
- PLC
prelimbic cortex
- PV
parvalbumin
- SPT
sucrose preference test
- SSPT
sucrose splash test
- SST
somatostatin
- STPT
serial two-photon tomography
- UCMS
unpredictable chronic mild stress
- VIP
vasoactive intestinal peptide
- WT
wildtype
1. Introduction
Chronic stress is a known risk factor for diverse psychiatric disorders, particularly major depressive disorder (MDD) (Stroud et al., 2011; Treadway et al., 2015). Stress-induced elevations of extracellular glutamate are widely recognized as mediators of the detrimental cellular and behavioral effects of chronic stress and also implicated in the etiology of major depressive disorder (MDD) (Moghaddam and Jackson, 2004; McEwen et al., 2016). Preclinical and clinical evidence points to defects in GABAergic inhibition as mediators of stress vulnerability and causal factors for MDD (Luscher and Fuchs, 2015; Ghosal et al., 2017; Newton et al., 2019). In particular, MDD is associated with reduced expression of somatostatin (SST), a marker representative of about 30% of cortical GABAergic interneurons (Fee et al., 2017). In support of a causative role of SST neuron dysfunction in stress-related neuropsychiatric disorders, rodent models subjected to chronic stress exhibit disproportionate gene expression changes in SST neurons (Lin and Sibille, 2015; Girgenti et al., 2019), while micro-infusion of SST into the brain (Engin et al., 2008), knock out of the SST gene (Lin and Sibille, 2015) and chemogenetic silencing of SST neurons (Soumier and Sibille, 2014) result in altered emotional behavior of mice.
GABAergic interneurons are the principal neurons mediating neural inhibition in the brain. In addition to SST neurons, they include interneurons that express the Ca2+ binding protein parvalbumin (PV, approximately 40%), or the 5-HT3a receptor (30%) as identifying marker proteins (Rudy et al., 2011). SST neurons are structurally and functionally heterogeneous but they include Martinotti cells in the neocortex and Oriens Lacunosum Moleculare (OLM) and bistratified cells in the hippocampus as major subtypes that specifically target the distal apical dendrites of pyramidal cells (Muller and Remy, 2014; Yavorska and Wehr, 2016). Functionally, these cells provide network activity-dependent supra-linear feedforward inhibition to pyramidal cells (Kapfer et al., 2007; Tan et al., 2008). Their activity scales with network activity in the theta frequency, a property that is also regulated by anxiolytic and antidepressant drugs (McNaughton et al., 2007).
Anxiety and depressive disorders are increasingly recognized to involve chronic imbalances of neural excitation and inhibition due to defects in GABAergic inhibition and excesses in glutamatergic excitation (Luscher and Fuchs, 2015). Reduced GABA levels found in MDD patients are restored by antidepressant drug treatment (Sanacora et al., 2002; Bhagwagar et al., 2004; Kucukibrahimoglu et al., 2009). Studies in rodents indicate that relatively modest, genetically-induced defects in GABAergic inhibition can cause anxiety- and depression-related behavioral changes (Crestani et al., 1999; Earnheart et al., 2007; Vollenweider et al., 2011; Kolata et al., 2018) that are reversible by chronic treatment with the tricyclic antidepressant desipramine (Shen et al., 2010) or with the rapid antidepressant ketamine (Ren et al., 2016). The antidepressant behavioral effects of ketamine in GABAA receptor (GABAAR)-mutant mice are associated with restoration and potentiation of GABAergic synaptic inhibition of pyramidal cells (Ren et al., 2016). Similarly, chronic stress-induced downregulation of GABAergic synapses can be reversed by ketamine (Ghosal et al., 2020). Collectively, these data suggest that GABA itself may have antidepressant properties.
To test by genetic means whether GABA has antidepressant properties, we recently examined mice with disinhibited SST neurons, using deletion of γ2 GABAARs selectively from these neurons (SSTCre:γ2f/f mice) (Fuchs et al., 2017). As predicted, SST cells of SSTCre:γ2f/f mice were hyperexcitable, resulting in increased GABAergic inhibition of hippocampal and cortical pyramidal cell targets. Consistent with a shift in synaptic excitation:inhibition balance towards greater inhibition (Heise et al., 2016), the circuit changes of SSTCre:γ2f/f mice were accompanied by reduced activity of the Ca2+ dependent enzyme eukaryotic elongation factor 2 kinase (eEF2K) and reduced phosphorylation of its target, eEF2. Behaviorally, SSTCre:γ2f/f mice reproduced the anxiolytic- and antidepressant-like behavior of mice that have been treated with ketamine. Lastly, SST mRNA and protein levels in SSTCre:γ2f/f mice remained unaffected, suggesting that the behavioral changes of SSTCre:γ2f/f mice can be attributed to increased GABAergic inhibition of pyramidal cells. Collectively, these data indicated that enhancing GABAergic synaptic inhibition of pyramidal cell dendrites through disinhibition of SST neurons reproduces the prolonged synaptic, biochemical and behavioral consequences of ketamine treatment (Fuchs et al., 2017) (reviewed in Luscher et al., 2020). Consistent with a putative neuroprotective mechanism, SST cells have been shown to delimit NMDA receptor mediated Ca2+ entry into dendritic spines of pyramidal cells (Chiu et al., 2013), which may attenuate the detrimental effects of excessive or untimely glutamatergic input to these cells (Lau and Tymianski, 2010). However, whether SSTCre:γ2f/f mice are further protected from the negative consequences of chronic stress has not yet been explored. Here we have extended our studies to test whether SSTCre:γ2f/f male and female mice exhibit resilience to chronic stress, using exposure to unpredictable chronic mild stress (UCMS) as a model.
2. Materials and methods
2.1. Animals
All animal experiments were approved by the Institutional Animal Care and Use Committees (IACUC) of the Pennsylvania State University and conducted in accordance with guidelines of the National Institutes of Health (NIH). SSTCre mice (also known as Ssttm2.1(cre)Zjh/J, Stock #013044), Ai9 mice (B6.Cg-Gt(ROSA)26Sor tm9(CAGtdTomato)Hze/J, Stock No 007909) and C57BL/6J mice (BL/6J, Stock No. 000664) were all obtained from Jackson Laboratory (Bar Harbor, ME, USA). The γ2f/f mouse line (Gabrg2tm2Lusc/J, Stock No: 016830, Jackson Laboratory) containing Gabrg2 alleles flanked by lox P sites was generated in house (Schweizer et al., 2003). All mice were backcrossed to the BL/6J strain for five or more generations. To limit interference of the SSTCre allele with emotional behavior, the SSTCre locus was strictly maintained in the heterozygous state (Lin and Sibille, 2015). The mice compared in experiments were produced as littermates, with the SSTCre allele present as a single copy (hemizygous) in male breeders to prevent germ line recombination. Breeding pairs and non-stressed control mice were maintained on a 12:12 h reverse light–dark cycle (lights off at 8AM) with food and water available ad libitum. The mice were genotyped at the time of weaning using PCR of tail DNA and an AccuStart II PCR Genotyping Kit (Quantabio, Beverly, MA, USA) and primers described on the JAX web site or in Schweizer et al. (2003). Biochemical and behavioral analyses of animals were all done with the experimenter blinded to genotype and treatment.
2.2. UCMS protocol
SSTCre:γ2f/f and γ2f/f littermate mice were separated by genotype and sex at the time of weaning. At 8–10 week of age, they were further divided into no stress (NS) and UCMS groups with balancing for sucrose preference and body weight. UCMS mice were singly housed and subjected to six weeks of UCMS consisting of 1–3 mild stressors/day for varied durations on a random schedule as described (Elizalde et al., 2008). Stressors included removal of bedding (4–24 h), wet cage floor (30 min), rotating mice among cages, cage tilted at a 45° angle (12–18 h), food or water deprivation (12–18 h), change in light-dark cycle, transfer to 4 °C chilled cage, and shaking home cage on a rotating platform (60 RPM, radius: 0.78 cm, 1 min). All stressors were applied during both light and dark phases. NS mice were housed in groups of 2–4 in a separate room. UCMS was continued throughout behavioral analyses with single housing as the only stressor for the 12 h preceding testing.
2.3. Immunohistochemistry and serial two-photon tomography
Mice were anesthetized with Avertin or isoflurane inhalation and perfused with ice-cold phosphate buffered saline (PBS) followed by 4% paraformaldehyde (PFA) in PBS (pH = 7.4). Brains were postfixed for 6 h or overnight in the same solution at 4 °C. For immunohistochemistry, floating sections (50 μm) were immunostained using rat anti-somatostatin (1:250, MAB354, Millipore, Burlington, MA, USA), rabbit anti-parvalbumin (PV, 1:500, ab11427, Abcam, Cambridge, MA, USA), and guinea pig anti-NeuN (1:1000, ABN90, Millipore) and developed using secondary goat antibodies conjugated to Alexa 647 or 488 (Jackson Immuno Research, West Grove, PA, USA) or Cy3 (Molecular Probes, Eugene, OR, USA). All samples from a given sex were processed in parallel. Single optical sections were imaged using a Zeiss LSM Pascal confocal microscope with a 20x objective with at least 3 images quantified per area of interest and brain using Image J (https://imagej.nih.gov/ij/). The densities of SST- and PV-IP cells were normalized to the density of NeuN-IP cells. For serial two-photon tomography (STPT), the mice were perfused as above and the brains postfixed over-night and then embedded in oxidized agarose and cross-linked in sodium borohydrate buffer solution overnight at 4 °C. Using a TissueCyte 1000, imaging system (Tissuevision), series of images of tdTomato-(tdT)-fluorescent cells were acquired in 12 × 16 XY tiles (X and Y resolution = 1 μm) across 280 serial 50 μm z-sections of each mouse. A custom built algorithm was used to perform stitching, signal detection, image registration, and automatic cell counting in defined anatomical regions throughout the brain as described (Kim et al., 2015, 2017).
2.4. Western blotting
Tissue extracts in 50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 2 mM EDTA, 0.1% sodium dodecyl sulfate, 1% Triton X-100, 1 mM NaVO3, 5 mM NaF and 1X protease inhibitor cocktail (Roche, Basel, Switzerland) were analyzed on 4–12% sodium dodecyl sulfate-polyacrylamide gels, transferred to polyvinylidene difluoride membranes and probed with mouse anti β-tubulin (1:10 000, no. T8328, Sigma-Aldrich, St Louis, MO, USA), rabbit anti-phospho-eEF2 (Thr56) (1:500, no. 2331), and rabbit anti-eEF2 (1:500, no. 2332, Cell Signaling Technology, Danvers, MA, USA). Immunoblots were developed using IRDye secondary antibodies and the protein bands imaged and quantitated using an Odyssey CLx infrared imager (LI-COR, Lincoln, NE, USA).
2.5. Behavioral analyses
NS mice were group housed and subjected to only the minimal handling necessary and subjected to behavioral testing along with UCMS exposed mice. Behavioral tests were conducted by an experimenter blind to genotype, during the first 5 h of the dark phase using two tests per week. All behavioral experiments were performed under red light (except for the Open Field Test (OFT)). The animals were tested two at a time with the sequence and pairing of animals changed randomly between tests. Testing was initiated with an Open Field Test (OFT), followed by NSFT, home cage feeding, Sucrose Preference test (SPT), Elevated Plus maze (EPM), and sucrose splash test (SSPT). For the OFT (Shen et al., 2012; Fuchs et al., 2017), the mice were transferred from the holding room (dark phase) and immediately exposed to an odor-saturated 50 × 50 × 20 cm opaque Plexiglass arena and standard room illumination (75 lx) for 10 min. The total distance traveled over 10 min and the % time spent in the 30 × 30 cm center square during the first 5 min were quantitated using an EthoVision XT video tracking system (Noldus Information Technologies, Leesburg, VA, USA). All other tests were conducted under red light. For the NSFT (Shen et al., 2010; Fuchs et al., 2017), the mice were singly housed for 24 h and food deprived for 18 h before testing. They were transferred to the corner of a Plexiglass arena (50 × 50 × 20 cm) containing three cm of saw dust bedding, with a pellet of rodent chow placed on a white cotton nesting square (6 × 6 × .5 cm) in the center of the arena. The latency to feed was hand-scored with feeding defined as the mouse biting into the chow while resting on its hind paws. Trials were stopped after 10 min, even if no feeding occurred. To assess appetite, three days following the NSFT the mice were singly housed with saw dust bedding and food deprived for 18 h as above and then were given a pre-weighed rodent chow pellet in their home cage during the first 4 h of the dark phase. The chow was weighed again 10 min later and the amount consumed was calculated. For the SPT, the mice were housed in standard open lid cages and trained to drink from 25 mL plastic pipettes (with sealed tops) for 12 h with a choice of water and 0.5% sucrose. The pipette positions were switched at the start and 12 h time point of the total 24 h measuring period. For any given training or testing session, half of the mice had the sucrose pipettes positioned to the left of the water-containing pipettes and for the other half the positions were inverted. For the EPM (Lister, 1987), the mice were placed into the center square of an elevated (40 cm) crossbar with two open and two closed arms (30 × 5 cm), facing a closed arm. The closed arms were surrounded by 20 cm walls of clear Plexiglas. The edges of open arms were raised by 2 mm to minimize accidental falling. The behavior was video recorded for 5 min using an EthoVision XT video tracking system. Mice that fell off the EPM were excluded from analysis. For the SSPT (Isingrini et al., 2010), the mice were singly housed for 24 h, transferred to an empty cage and sprayed on their backs twice with a fixed volume (0.7 mL) of 10% sucrose solution using a 250 mL spray bottle, to stimulate grooming behavior. The mice were immediately returned to their home cage and the grooming frequency and duration recorded manually over 5 min.
2.6. Statistics
Statistical testing was performed using Prism 7 software (Graphpad, La Jolla, CA) or, for 3-way repeated measure ANOVAs, SAS (SAS Institute, Cary, NC 27513). For all data sets, outliers identified using the ROUT method (Prism 7) were discarded. Normal distribution of data was verified using the D'Agostino-Pearson omnibus normality test and group means were compared by 2-way or 3-way ANOVA followed by post hoc Fisher's LSD or Tukey test as indicated in Figure legends.
3. Results
3.1. SST immuno-positive neurons in the mPFC of SSTCre:γ2f/f male and female mice are resilient to the detrimental effects of chronic mild stress
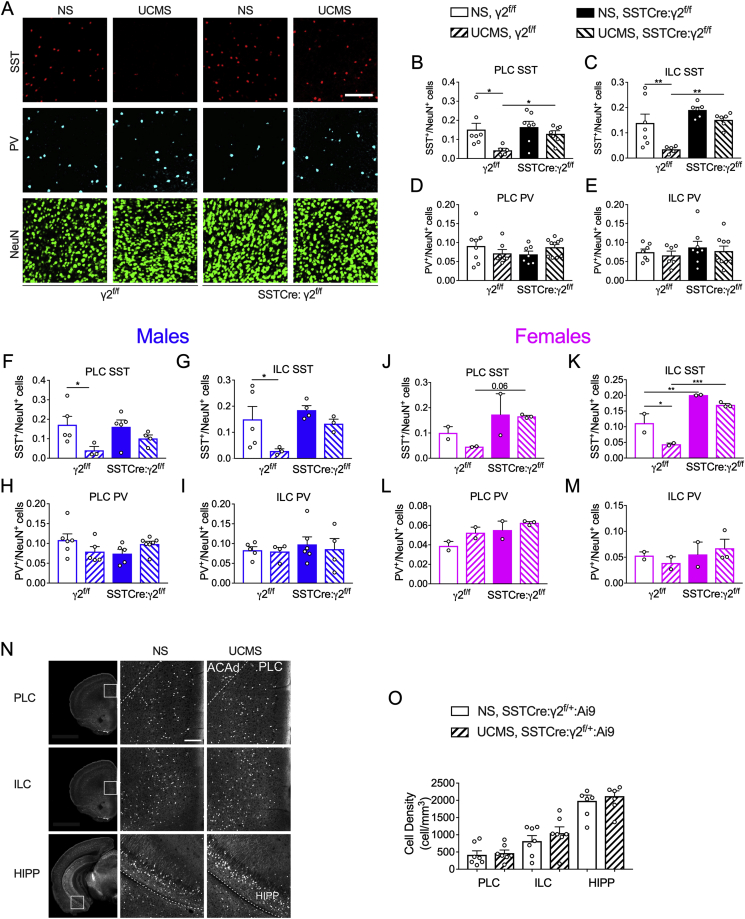
UCMS exposure of mice results in marked neocortical reductions of SST mRNA and protein expression (Lin and Sibille, 2015; Banasr et al., 2017) and corresponding reductions in the density of SST immuno-positive (IP) neurons in hippocampus of UCMS exposed rats (Czeh et al., 2015). To begin to assess whether the anxiolytic- and antidepressant-like phenotype of SSTCre:γ2f/f mice includes resilience to stress-induced loss of SST-IP neurons, we subjected eight to ten week old SSTCre:γ2f/f mice and γ2f/f littermate controls to six weeks of UCMS, followed by quantitation of the density of SST-IP interneurons. NS control mice of both genotypes were analyzed in parallel. Notably and in contrast to previous experiments done with mice on the 129 genetic background (Fuchs et al., 2017), all experiments in the current study were performed with mice backcrossed to the BL/6J strain, which is more widely used for chronic stress studies of mice. We focused on the mPFC because markers of GABAergic inhibition in this brain area are prominently affected by UCMS (Veeraiah et al., 2014; Czeh et al., 2015; Lin and Sibille, 2015; Ma et al., 2016; Banasr et al., 2017) and strongly implicated in stress-associated affective disorders (Treadway et al., 2015). We performed separate quantitation of cell densities in the prelimbic (PLC) and infralimbic cortex (ILC) to account for differences in SST cell densities (Kim et al., 2017) and neural connectivity (He et al., 2016; Liu and Carter, 2018) of these brain regions. Parvalbumin immuno-positive (PV-IP) interneurons were analyzed in the same sections as an additional reference, and we controlled for variations in total neuron density by normalizing the interneuron density to the density of cells expressing the pan-neuronal marker NeuN. Interestingly, exposure of γ2f/f mice (both sexes combined) to UCMS resulted in the predicted reduction in the density of SST-immuno-positive (IP) in both PLC and ILC and neither of these effects were observed in SSTCre:γ2f/f littermates (Fig. 1A–C). We observed no effects of UCMS on the density of PV-IP neurons, regardless of genotype (Fig. 1D, E). We observed similar effects when we segregated the data by sex. In males, UCMS resulted in significant downregulation of SST+ cells in PLC and ILC of γ2f/f mice but not SSTCre:γ2f/f littermates (Fig. 1F,G). Female mice showed an overall pattern similar to males. That is, UCMS-exposed vs. NS γ2f/f female mice showed a reduced density of SST-IP neurons in mPFC (PLC + ILC combined, genotype effect: F1,14 = 35.96, p < 0.001, stress effect: F1,14 = 5.59, p = 0.03, ANOVA; p = 0.03, n = 4, Fisher's LSD), and no such effect was evident in SSTCre:γ2ff/mice (p = 0.41, n = 4–6, Fisher's LSD, data not shown). When the PLC and ILC were analyzed separately, the UCMS effects on SST+ cell density in γ2f/f mice were significant in the ILC (Fig. 1K) while in the PLC the stress effect did not reach significance due to low sample numbers (Fig. 1L). As in males, UCMS exposure had no effects on SST+ cell densities in SSTCre:γ2f/f littermates (Fig. 1J,K) nor on the density of PV+ neurons, independent of genotype (Fig. 1L,M).
Fig. 1.
SSTCre:γ2f/fmice are resilient to chronic stress-induced downregulation of SST. A–E) Quantification of SST- and PV-IP interneurons in NS and UCMS-exposed SSTCre:γ2f/f and γ2f/f mice. A) Representative micrographs of PLC sections immuno-stained for SST (red), PV (cyan) and NeuN (green). Scale bar, 150 μm. B-E) Summary statistics of interneuron densities normalized to NeuN-positive neurons. B) UCMS reduced the density of SST-IP cells in the PLC of γ2f/f mice (F1, 22 = 7.435, p = 0.012, ANOVA, p = 0.01, Fisher's test) but not SSTCre:γ2f/f mice (p = 0.33). The density of SST-IP cells was greater in UCMS SSTCre:γ2f/f than UCMS γ2f/f mice (p = 0.004). C) In the ILC, UCMS reduced the density of SST-IP cells in γ2f/f (F1,20 = 9.923, p = 0.005, ANOVA; p = 0.004, Fisher's test) but not SSTCre:γ2f/f mice (p = 0.24). The density of SST-IP cells was greater in UCMS SSTCre:γ2f/f than UCMS γ2f/f mice (p = 0.002). D,E) The density of PV-IP cells in the PLC (D) and ILC (E) was unaffected by UCMS and genotype. F) In the PLC of males, UCMS reduced the density of SST-IP cells in γ2f/f mice (F1,13 = 7.03, p = 0.020, ANOVA; p = 0.028, Fisher's test) but not SSTCre:γ2f/f mice (p = 0.24). G) Similarly, in the ILC, UCMS reduced the density of SST-IP cells in γ2f/f (F1,11 = 5.44, p = 0.040, ANOVA; p = 0.038, Fisher's LSD) but not SSTCre:γ2f/f mice (p = 0.35). The density of SST-IP cells trended higher in UCMS SSTCre:γ2f/f vs UCMS γ2f/f mice (p = 0.096). H,I) The density of PV-IP cells in the PLC (H) and ILC (I) of males was unaffected by UCMS and genotype. J,K) The density of SST-IP cells of female mice was increased in SSTCre:γ2f/f vs. γ2f/f mice in both PLC (J) (F1,5 = 6.85, p = 0.047) and ILC (K) (F1,5 = 65.44, p = 0.0005) with a trend for increased density of SST-IP cells in UCMS SSTCre:γ2f/f vs. UCMS γ2f/f mice (p = 0.06). In the ILC, the density of SST-IP cells was reduced by UCMS (F1,5 = 13.86, p = 0.014) and increased in NS SSTCre:γ2f/f vs. NS γ2f/f mice (p = 0.006) and increased in UCMS SSTCre:γ2f/f vs. UCMS γ2f/f mice (p = 0.0009). UCMS selectively reduced the density of SST-IP cells in γ2f/f (p = 0.018) but not SSTCre:γ2f/f mice. L,M) The density of PV-IP cells in the PLC (L) and ILC (M) was unaffected by stress (PLC: F1,5 = 4.21, p = 0.10; ILC: F1,5 = 0.0042, p = 0.95) but increased in PLC of SSTCre:γ2f/f vs. γ2f/f mice. No such genotype effect was evident in the ILC (F1,5 = 0.77, p = 0.42). The density of PV-IP cells in the PLC was increased in SSTCre:γ2f/f vs. γ2f/f mice independent of stress (F1,5 = 6.67, p = 0.049). N) STPT of NS and UCMS SSTCre:γ2f/+:Ai9 mice, with micrographs illustrating tdT-positive cells from the PLC, ILC and ventral hippocampus (HIPP). Square insets in the first column of images highlight areas enlarged in the second and third column. Dotted lines demarcate the anterior cingulate cortex (ACAd) from the PLC and the postpiriform transition area from the hippocampus (HIPP), respectively. O) Summary data comparing densities of tdT-positive cells in NS vs. UCMS treated SSTCre:γ2f/+:Ai9 mice. Scale bar, 200 μm. Data represent 2-way ANOVA and Fisher's test. Graphs represent means ± SE. *p < 0.05, **p < 0.01, ***p < 0.001. For a complete list of ANOVAs and posthoc tests see Supplement 2. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.2. UCMS-induced reductions in SST-IP cells represent loss of SST expression rather than loss of cells
We next asked whether UCMS-induced reductions in the density of SST-IP cells indicates loss of these cells or merely reduced expression of SST. We genetically labeled SST neurons by crossing SSTCre mice with the Cre reporter Ai9, which enables quantitation of these cells independent of altered SST protein expression in adulthood. We previously showed that deletion of one γ2 allele in SSTCre:γ2f/+:Ai9 mice does not measurably affect the function of SST neurons, nor does it affect anxiety- and depression-related behavior (Fuchs et al., 2017). Thus, for practical reasons we used SSTCre:γ2f/+:Ai9 mice for this experiment. Comparison of UCMS exposed and NS SSTCre:γ2f/+:Ai9 mice by STPT using tdT fluorescence as a marker for SST cells revealed unaltered densities of cells in PLC, ILC as well as ventral hippocampus (Fig. 1N,O). We also found no differences in the density of these cells between UCMS exposed SSTCre:γ2f/+:Ai9 and UCMS SSTCre:γ2f/f:Ai9 mice (not shown). Therefore, UCMS-induced reductions in SST-IP cells reflect reduced expression of SST rather than loss of SST+ neurons.
3.3. UCMS-exposed SSTCre:γ2f/f male but not female mice show reduced eEF2 phosphorylation in mPFC
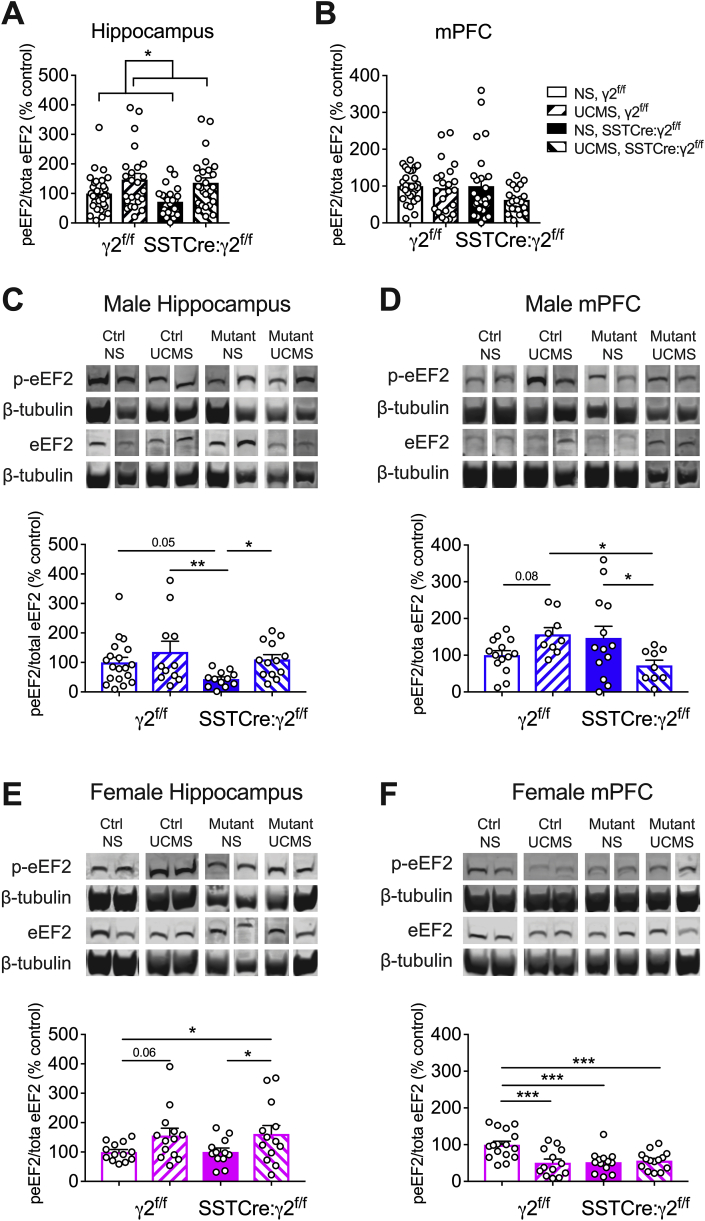
Phosphorylation of eEF2T56 by eEF2 kinase has been shown to be increased by chronic mild stress selectively in the PFC but not hippocampus of male rats (Gronli et al., 2012). Conversely, p-eEF2 is reduced (indicative of increased eEF2 activity) by both conventional and rapid-acting antidepressants (Autry et al., 2011; Opal et al., 2014; Zanos et al., 2016) and required for antidepressant behavioral effects of ketamine (Nosyreva et al., 2013). Together these data suggest that p-eEF2T56 serves as a biochemical marker of chronic stress sensitivity and antidepressant drug action. We previously showed that basal p-eEF2 levels were reduced in stress-naïve SSTCre:γ2f/f mice (129 strain) in both mPFC and hippocampus (Fuchs et al., 2017), which is consistent with the antidepressant-like behavioral phenotype of these mice. Here, we compared the p-eEF2T56 levels of NS and UCMS-exposed SSTCre:γ2f/f mice and γ2f/f controls (BL/6J strain) (Fig. 2). In the hippocampus (pooled sexes), p-eEF2 levels were increased by stress independent of genotype (Fig. 2A). However, no such overall effect was evident in the mPFC (Fig. 2B). The reduced p-eEF2 levels of SSTCre:γ2f/f mice previously reported for the 129 strain were replicated here for the BL/6J strain but only in hippocampus of males and mPFC of females (Fig. 2B,F) and not in mPFC of males nor hippocampus of females (Fig. 2D,E). A likely explanation for this is provided in the Discussion.
Fig. 2.
Male SSTCre:γ2f/fmice are resilient to chronic stress-induced increases in eEF2 phosphorylation in mPFC. A,B) UCMS increased p-eEF2T56/eEF2 independent of sex and genotype in the ventral hippocampus (A) (F1, 102 = 6.22, p = 0.014) but not mPFC (B) (F1, 97 = 2.81, p = 0.10). C,D) Males: Representative western blots of male mice and summary statistics of p-eEF2T56/eEF2 in ventral hippocampus and mPFC. In hippocampus (C), p-eEF2T56/eEF2 showed a stress effect (F1, 51 = 5.93, p = 0.018). In mPFC (D), p-eEF2T56/eEF2 showed an interaction of stress and genotype (F1,41 = 8.63, p = 0.005). UCMS tended to increase p-eEF2T56/eEF2 in γ2f/f mice (p = 0.08, n = 9–14) but reduced this ratio in SSTCre:γ2f/f mice (p = 0.02, n = 9–13). The p-eEF2T56 level of UCMS γ2f/f mice was increased compared to that of UCMS SSTCre:γ2f/f mice (p = 0.02, n = 9).E,F) Females: In hippocampus (E), p-eEF2T56/eEF2 showed an overall stress effect (F1,47 = 7.90, p = 0.007) that was almost significant in γ2f/f (p = 0.06) and significant in SSTCre:γ2f/f mice (p = 0.046, n = 12–13). In mPFC (F), the p-eEF2T56/eEF2 ratio showed stress (F1,52 = 6.50, p = 0.014) and genotype effects (F1,52 = 5.77, p = 0.020) and a stress × genotype interaction (F1,52 = 9.16, p = 0.004). NS SSTCre:γ2f/f mice vs. NS γ2f/f controls showed a paradoxical reduction of p-eEF2T56/eEF2 (p = 0.0003, n = 13–16). 2-way ANOVAs and Fisher's test. Graphs represent means ± SE. *p < 0.05, **p < 0.01, ***p < 0.001.
To test for possible sexually dimorphic effects of stress, we further analyzed the data segregated by sex (Fig. 2C–F). In hippocampus of males, p-eEF2 levels showed a significant stress effect independent of genotype and without stress × genotype interaction (Fig. 2C) indicating lack of resilience to stress in the hippocampus of male SSTCre:γ2f/f mice. By contrast, in the mPFC of males p-eEF2 levels showed a significant interaction of stress and genotype. UCMS tended to increase the p-eEF2 level selectively in γ2f/f mice, while lowering this parameter in SSTCre:γ2f/f littermates. Moreover, p-eEF2 levels were reduced in UCMS SSTCre:y2f/f vs. UCMS y2f/f males. Thus, male SSTCre:γ2f/f mice are resilient to chronic stress-induced increases in p-eEF2 in mPFC but not hippocampus.
As in males, the hippocampus of female γ2f/f and SSTCre:γ2f/f mice showed an overall stress effect independent of genotype and no genotype X stress interaction, indicating lack of resilience to stress in the hippocampus of SSTCre:γ2f/f mice (Fig. 2E). Curiously, the mPFC of female mice showed the expected genotype effect combined with a paradoxical stress effect and a stress × genotype interaction (Fig. 2F). More explicitly, UCMS resulted in a paradoxical reduction of p-eEF2 levels in female γ2f/f mice that is opposite to stress effects reported in the literature for males (Gronli et al., 2012). However, the reduced p-eEF2 levels in stress-naïve SSTCre:γ2f/f vs γ2f/f mice (Fig. 2F) confirms the genotype effect previously reported for the 129 strain of these mice albeit only in females and not in males or when the sexes were combined (Fig. 2B,D). A possible explanation for incomplete replication of data across strain backgrounds is provided in the Discussion.
3.4. SSTCre:γ2f/f male mice exhibit resilience to UCMS-induced increases in anxiety
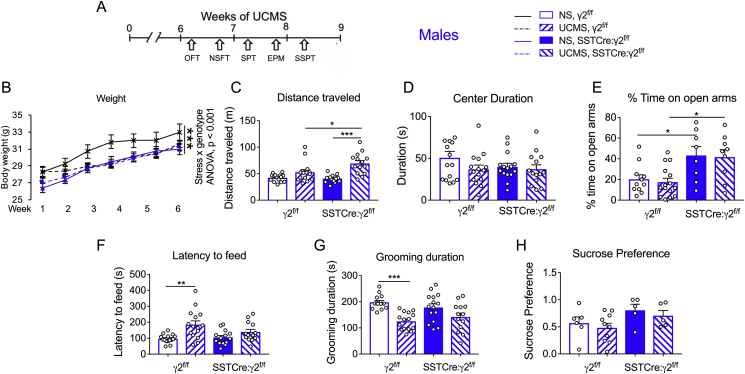
Next, we examined whether apparent stress resilience of male SSTCre:γ2f/f mice was also evident at the level of age related body weight gain and behavior. Weekly body weights of the mice during UCMS exposure (starting at 8–10 weeks of age) revealed a stress × genotype interaction of weight gain, with UCMS exposed γ2f/f mice showing reduced weight gain compared to NS γ2f/f controls. By contrast, the weight gain of SSTCre:γ2f/f mice remained unaffected by UCMS (Fig. 3B), suggesting that these mice are resilient to the chronic stress effects on weight gain. Importantly, a separate cohort of UCMS and NS WT and SSTCre control mice that was tested analogously revealed reduced weight gains of both UCMS SSTCre and UCMS WT mice compared to their respective NS control groups (Fig. S1A). Thus, unspecific Cre-recombination did not contribute to resilience of SSTCre:γ2f/f mice with respect to UCMS-induced reductions in weight gain.
Fig. 3.
Male SSTCre:γ2f/fmice are resilient to chronic stress-induced emotional behavior. A) Experimental design. B) Male SSTCre:γ2f/f mice were resilient to UCMS-induced reductions in weight gain as evidenced by a stress × genotype interaction (F1,385 = 11.23, p < 0.001, 3-way repeated measurement ANOVA). C,D) In the OFT, there was an interaction of stress x genotype in the total distance traveled (C) (F1,55 = 5.969, p = 0.018). UCMS increased locomotion of SSTCre:γ2f/f (p < 0.001, n = 14–15) and UCMS-exposed SSTCre:γ2f/f showed greater locomotion than UCMS-exposed γ2f/f controls (p = 0.015, n = 14–15). The center duration (D) was unaffected by stress and genotype. E) In the EPM, there was an anxiolytic-like genotype effect on % time spent on open arms (F1,40 = 17.04, p < 0.001), but no stress effect (F1,40 = 0.14, p = 0.71). Post hoc tests confirmed the anxiolytic phenotype of NS SSTCre:γ2f/f mice vs. NS γ2f/f controls (p = 0.046, n = 8–11) and UCMS SSTCre:γ2f/f mice vs. UCMS γ2f/f controls (p = 0.016, n = 9–15). F) In the NSFT, there was a stress effect on latency to feed (F1,53 = 14.44, p < 0.001) and a trend for a stress × genotype interaction (F1,53 = 2.841, p = 0.098). UCMS increased the latency to feed of γ2f/f mice (p = 0.0012, n = 15) but not SSTCre:γ2f/f mice (p = 0.47, n = 13–14). G) In the SSPT, there was an overall stress effect (F1,53 = 22.09, p < 0.001). UCMS reduced the grooming duration of γ2f/f (p < 0.001, n = 14–15) but not SSTCre:γ2f/f mice (p = 0.14, n = 13–15). H) The SPT showed a genotype effect, with a greater sucrose preference in SSTCre:γ2f/f compared to γ2f/f mice (F1,22 = 4.76, p = 0.04). However, post hoc comparisons of NS γ2f/f vs. NS SSTCre:γ2f/f (p = 0.45), and UCMS γ2f/f vs. UCMS SSTCre:γ2f/f (p = 0.41, n = 5–10) were not significant, and there was no UCMS effect (F1,22 = 0.78, p = 0.39), nor a UCMS X genotype interaction (F1,22 = 0.0042, p = 0.95). Data were analyzed by 2-way ANOVA and Tukey test. Bar graphs represent means ± SE. *p < 0.05, **p < 0.01, ***p < 0.001.
When we analyzed γ2f/f and SSTCre:γ2f/f mice in an OFT, we observed an interaction of genotype and stress on distance traveled, with an UCMS-induced increase in locomotion of SSTCre:γ2f/f mice but not γ2f/f mice (Fig. 3C). The time spent in the center of the OFT remained unchanged (Fig. 3D). The stress-induced hyperactivity of male SSTCre:γ2f/f mice then led us to focus the subsequent behavioral analyses on largely locomotion-insensitive behavioral parameters. In the EPM, we observed a genotype effect on percent time spent on open arms, with an anxiolytic-like increase in open arm time in SSTCre:γ2f/f vs. γ2f/f mice independent of stress (Fig. 3E). This confirms the anxiolytic-like phenotype previously reported for the 129 strain of SSTCre:γ2f/f mice (Fuchs et al., 2017). Notably, similar to the OFT the EPM failed to show anxiogenic-like consequences of UCMS in γ2f/f mice (Fig. 3D,E); however, we observed anxiogenic-like effects of UCMS in wildtype (WT) and SSTCre control groups in both of these tests (Figs. S1C and D, Supplement 1). The indistinguishable behavior of WT and SSTCre mice in the OFT and EPM confirmed that the anxiolytic-like phenotype of SSTCre:γ2f/f vs. γ2f/f mice required Cre-mediated recombination of the γ2 locus.
In the NSFT, the latency to feed of γ2f/f and SSTCre:γ2f/f mice showed an overall stress effect and a trend for a stress × genotype interaction (Fig. 3F). Post hoc tests showed an anxiogenic-like increase in latency to feed in UCMS γ2f/f vs. NS γ2f/f mice, but not in UCMS SSTCre:γ2f/f mice vs. NS SSTCre:γ2f/f mice, suggesting resilience of SSTCre:γ2f/f mice to the anxiogenic effects of chronic stress. This phenotype was dependent on Cre-mediated recombination as UCMS increased the latency to feed of SSTCre mice in a manner indistinguishable from WT mice (Fig. S1). Home cage feeding of mice that were food deprived and refed in their home cage remained unaffected by genotype, and UCMS exposed SSTCre:γ2f/f mice ate less rather than more than NS SSTCre:γ2f/f mice (Fig. S2A). Thus, the stress resilient phenotype of SSTCre:γ2f/f mice in the NSFT was not explained by increased appetite.
In the SSPT, we observed an overall stress effect on grooming duration, with UCMS γ2f/f mice spending less time grooming than NS γ2f/f mice. By contrast, grooming behavior of SSTCre:γ2f/f remained unaffected by UCMS (Fig. 4G). However, the grooming duration was unaffected by UCMS also in SSTCre mice (Fig. S1F), suggesting that resilience of SSTCre:γ2f/f mice in this test was independent of Gabrg2 locus inactivation and the test therefore not informative. Similarly, the SPT showed a small but significant overall stress effect on sucrose preference but post hoc comparisons were not significant and the test therefore not informative (Fig. 3H). Taken together, the data indicate that male SSTCre:γ2f/f are resilient to UCMS-induced reductions in weight gain and UCMS-induced increases in anxiety in the NSFT. Stress resilience could not be assessed in other tests (OFT, EPM, SSPT, SPT) because corresponding behavioral parameters were insensitive to UCMS or because of aberrant behavior of SSTCre control mice.
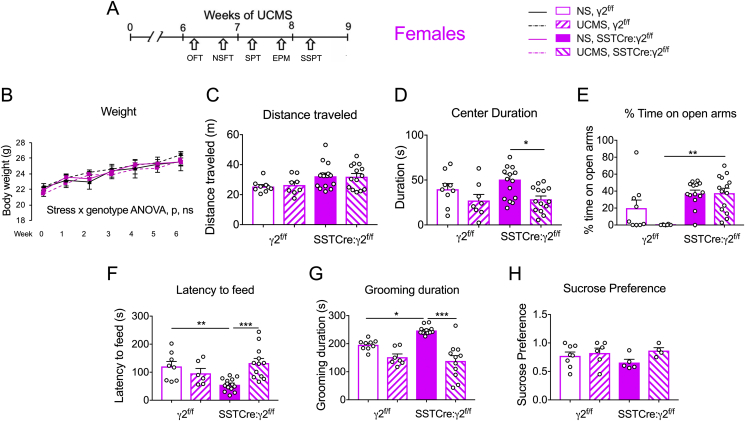
Fig. 4.
Female SSTCre:γ2f/fmice do not exhibit behavioral resilience to UCMS. Behavioral analyses of female SSTCre:γ2f/f mice and γ2f/f controls under NS and UCMS conditions. A) Experimental design. B) Weight gain of female mice was unaffected by UCMS. and genotype (stress x genotype F1,293 = 2.53, p = 0.11, 3-way repeated measurement ANOVA). C,D) The distance traveled in the OFT was increased in SSTCre:γ2f/f vs. γ2f/f mice (C) (F1,42 = 7.76, p = 0.008) independent of stress. The center duration (D) showed an anxiogenic effect of UCMS (F1,42 = 8.50, p = 0.0057). UCMS decreased the time spent in the center of SSTCre:γ2f/f (p = 0.02, n = 14–15) but not γ2f/f mice (p = 0.55, n = 8–9). E) In the EPM, SSTCre:γ2f/f vs. γ2f/f mice spent an increased percentage of time on open arms (F1,39 = 14.53, p = 0.0005) independent of UCMS. F) The latency to feed in the NSFT showed a UCMS effect (F1,40 = 4.613, p = 0.038) and a stress × genotype interaction (F1,40 = 12.43, p = 0.0011) with the expected reduction in latency to feed in NS SSTCre:γ2f/f vs. NS γ2f/f mice (p = 0.009, n = 9–15) and a reduced feeding latency in UCMS vs. NS SSTCre:γ2f/f mice (p < 0.001, n = 13–15) but not UCMS vs. NS γ2f/f mice (p = 0.73, n = 8–9). G) The SSPT showed a UCMS-induced overall reduction in grooming duration (F1,36 = 39.23, p < 0.0001) and an interaction of stress x genotype effects (F1,36 = 7.053, p = 0.0117). NS SSTCre:γ2f/f mice spent more time grooming than NS γ2f/f mice (p = 0.017, n = 9–13), and UCMS reduced the grooming duration in SSTCre:γ2f/f (p < 0.001, n = 11–13) but not γ2f/f mice (p = 0.11, n = 8–9). H) The SPT showed no effects of UCMS and genotype. Data were analyzed by 2-way ANOVA and Tukey test. Bar graphs represent means ± SE. *p < 0.05, **p < 0.01, ***p < 0.001.
3.5. SSTCre:γ2f/f female mice differ from males in that they remain sensitive to UCMS-induced anxiety
Female SSTCre:γ2f/f mice showed resilience to UCMS-induced reductions in the density of SST-IP cells, similar to males. However, they differed from males in that they showed a paradoxical UCMS-induced reduction of p-eEF2T56 in the mPFC. Female mice (produced as littermates of males tested in Fig. 3) underwent the same UCMS protocol and behavioral tests as the males (Fig. 4A) but were tested on different days within the same week. As for males, we repeated these experiments in a separate cohort of SSTCre and WT littermates, to assess the possible contribution of unspecific Cre effects (Fig. S3). In contrast to males, UCMS did not alter the body weight gain of female mice, independent of genotype (Fig. 4B). In the OFT, female mice showed a genotype effect on locomotion (Fig. 4C). Measurements of center duration revealed an overall anxiogenic effect of UCMS. UCMS significantly decreased the center duration of SSTCre:γ2f/f but not γ2f/f mice, suggesting a chronic stress-induced increase in anxiety selectively in SSTCre:γ2f/f mice (Fig. 4D). Notably, a similar UCMS-induced reduction in center duration was evident also in SSTCre and WT control animals, confirming that SSTCre:γ2f/f mice showed normal sensitivity to stress (Fig. S3C). In the EPM, there was a genotype effect on percent time spent on open arms (Fig. 4E). NS SSTCre:γ2f/f vs. NS γ2f/f mice showed the expected anxiolytic-like phenotype reported previously for the 129 strain of these mice (Fuchs et al., 2017). UCMS-exposed SSTCre:γ2f/f mice vs. UCMS γ2f/f mice showed an anxiolytic-like increase in the time on open arms. This result may indicate stress resilience of female SSTCre:γ2f/f mice but is compromised by absence of significant UCMS effects in γ2f/f, WT and SSTCre controls (Fig. 4E and Fig. S3D). In the NSFT, the latency to feed showed a significant effect of stress and a genotype × stress interaction (Fig. 4F). NS SSTCre:γ2f/f mice showed a reduced latency to feed vs. NS γ2f/f mice, as previously seen with the 129 strain of these mice. However, UCMS increased the latency to feed selectively in SSTCre:γ2f/f mice but not γ2f/f mice (Fig. 4F), which indicates enhanced rather than reduced stress sensitivity of the mutants and is opposite to the stress resilient phenotype described in this test for males (Fig. 3F). In the SSPT, there was a stress effect and stress × genotype interaction on grooming duration (Fig. 4G). NS SSTCre:γ2f/f mice showed an increased grooming duration compared to NS γ2f/f mice, suggesting reduced emotionality at baseline as expected based on the same phenotype previously seen with the 129 strain of these mice. UCMS-exposed SSTCre:γ2f/f mice showed reduced grooming duration compared to their NS controls with a similar trend apparent also in UCMS γ2f/f mice vs. NS γ2f/f mice. Thus, SSTCre:γ2f/f female mice remained sensitive to the detrimental effects of UCMS in this test (Fig. 4G). The SPT did not reveal any genotype or stress effects in female mice and therefore was not suitable to assess genotype dependent changes in stress sensitivity (Fig. 4H).
Taken together, SSTCre:γ2f/f female mice differed from males in that they remained susceptible to UCMS in the OFT, SSPT and NSFT. Sex differences were most striking in the NSFT, where male SSTCre:γ2f/f mice were resilient and female SSTCre:γ2f/f mice showed increased susceptible to UCMS. Results for the EPM were ambiguous due to the absence of clear stress effects in γ2f/f controls.
4. Discussion
We here have shown that genetic disinhibition of SST cells renders male mice resilient to UCMS across four different levels of analyses. Systemically, SSTCre:γ2f/f male mice showed resilience to UCMS-induced reductions in weight gain. At the cell and tissue level of the mPFC, male SSTCre:γ2f/f mice showed resilience to UCMS-induced reductions in SST expression and UCMS-induced increases in p-eEF2. Lastly, at the behavioral level, SSTCre:γ2f/f males were resilient to UCMS-induced increases in anxiety in the NSFT. Notably, behavioral sensitivity to UCMS of γ2f/f controls and resilience to UCMS of SSTCre:γ2f/f mice correlated with bidirectional changes in eEF2 phosphorylation specifically in mPFC but not in hippocampus. In contrast to males, female SSTCre:γ2f/f mice appeared more sensitive to the behavioral effects of UCMS than their γ2f/f controls and they lacked any signs of resilience to UCMS induced changes in eEF2 phosphorylation. Nevertheless, female SSTCre:γ2f/f mice were resilient to UCMS induced reductions in SST-IP cells. Collectively, the data indicate that sex-specific mechanisms underlying vulnerability and resilience to stress map to mPFC rather than hippocampus. Moreover, maintaining the density of SST-IP cells (a measure of SST expression) is insufficient to confer stress resilience to female mice. This is noteworthy, given that SST is consistently downregulated both in chronic stress exposed rodents and postmortem brain of MDD patients (Fee et al., 2020).
We previously showed that stress naïve SSTCre:γ2f/f mice exhibit normal SST mRNA and protein levels, suggesting that the anxiolytic and antidepressant-like phenotype of these mice did not involve altered SST levels (Fuchs et al., 2017). Nevertheless, micro-infusion of SST into brain of mice is known to have anxiolytic and antidepressant-like consequences (Engin et al., 2008). Here we showed that behavioral resilience to UCMS of male SSTCre:γ2f/f mice correlated with stabilized SST expression, which raises the question whether stabilized SST levels or activity-dependent increases in the release of SST contribute to behavioral stress resilience of male SSTCre:γ2f/f mice. Two lines of evidence suggest that this was not the case. First, disinhibition of SST neurons and stabilizing the expression of SST was insufficient to confer behavioral stress resilience to female mice. Second, SST heterozygous mice with correspondingly reduced expression of SST show normal baseline emotional behavior and unaltered UCMS-induced emotional reactivity (Lin and Sibille, 2015), These data suggest that the emotional behavior of mice is insensitive to moderate changes in SST expression and that downregulation of SST in response to stress in mice and in MDD serves merely as a marker of SST neuron dysfunction. Consistent with this interpretation, UCMS results in largely nonoverlapping sex-specific transcriptome changes in SST neurons (Ghosal et al., 2020). Given that SSTCre:γ2f/f mice mimic ketamine-induced potentiation of GABAergic inhibition of pyramidal cells (Ren et al., 2016; Fuchs et al., 2017) and prefrontal reductions in eEF2 phosphorylation (Fuchs et al., 2017), it seems likely that the stress-resilient phenotype of male SSTCre:γ2f/f mice similarly involves enhanced GABAergic inhibition of pyramidal cells.
Our results revealed marked sex-specific effects of SST neuron disinhibition on eEF2 phosphorylation. Increased eEF2 phosphorylation is known to inhibit translation while reduced p-eEF2 is associated with increased dendritic translation (Sutton et al., 2007; Taha et al., 2013). UCMS-induced increases in p-eEF2 are consistent with similar findings described by others in rats (Gronli et al., 2012). Conversely, reduced p-eEF2 levels in male SSTCre:γ2f/f mice are consistent with reduced phosphorylation of eEF2 observed in ketamine treated male mice (Autry et al., 2011; Gideons et al., 2014). Ketamine-induced antidepressant effects are further associated with prominent enhancement of GABAergic synaptic inhibition (Ren et al., 2016; Ghosal et al., 2020). Therefore, our data from male mice suggest that bidirectional changes in synaptic excitation:inhibition ratios resulting from UCMS or SST neuron disinhibition are linked to emotion-related behavioral outcomes by Ca2+-mediated changes in mPFC p-eEF2 levels. No such relationship was evident for female mice.
While tissue measures of p-eEP2 point to sex specific UCMS effects on translation at the tissue level (thought be representative mainly of pyramidal cell dendrites), recent analyses of cell specific transcriptomes by RNAseq has revealed male- and SST neuron-specific activation of the Integrated Stress Response (ISR) pathway, evidenced by prominent downregulation of elongation initiation factor 2 and other ribosomal transcripts representative of this pathway (Lin and Sibille, 2015; Girgenti et al., 2019). Remarkably, the ISR pathway was unaffected in SST neurons of female mice, although they showed overall more numerous gene expression changes than males (Girgenti et al., 2019). Our finding of similar downregulation of SST-IP cells in UCMS-exposed male and female γ2f/f mice therefore suggests that the SST gene is not a target of the ISR pathway.
Stress-induced defects in GABAergic inhibition may be corroborated by altered density or function of PV interneurons, although we found no stress or genotype effects on the density of these neurons. Consistent with our findings, PV neurons show much fewer sex-specific transcript changes that SST neurons (Girgenti et al., 2019) and there is no consensus regarding the contribution of PV neurons to stress vulnerability, with the majority of preclinical studies showing unaltered or downregulated PV neuron densities and one group reporting upregulation by chronic stress (reviewed in Fogaca and Duman, 2019). Similarly, compared to SST neurons, there are few reports pointing to altered PV neuron density in MDD (Sibille et al., 2011).
We noted in the Result section that stress-naive SSTCre:γ2f/f mice of the BL/6J strain only partially replicated the anxiolytic and antidepressant phenotype previously reported for the 129 strain. This may be explained by the recently discovered BL/6J strain-specific hypomorphic allele for the Gabra2 gene that results in a ~50% reduced expression of α2 GABAARs (Mulligan et al., 2019). Hemizygous knockout of this gene in the 129 strain of mice results in heightened anxiety in the NSFT and increased immobility in forced swim and tail suspension tests, respectively (Vollenweider et al., 2011), thereby strongly suggesting that reduced expression of α2 GABAARs in BL/6J vs 129 mice contributes to altered baseline emotional behavior of these mice. Moreover, because α2 and γ2 subunits are part of the same GABAAR complex and essential for inhibitory synapse function (Essrich et al., 1998), deletion of the γ2 subunit from SST cells in SSTCre:γ2f/f mice is predicted to result in a lesser degree of disinhibition of these neurons in the BL/6J vs. 129 strain of mice, which explains the less robust anxiolytic and antidepressant phenotype of the BL/6J compared to 129 strain of SSTCre:γ2f/f mice.
In conclusion, enhancing GABAergic inhibition of pyramidal cell dendrites by disinhibition of SST interneurons confers molecular, cellular and behavioral resilience to detrimental effects of stress selectively in male mice. In contrast to males, disinhibition of SST interneurons protects from UCMS only at the level of SST expression in SST+ interneurons, which appears inconsequential for behavioral outcomes. Future experiments will need to address whether, as predicted by the data presented here, disinhibition of SST neurons delimited to mPFC is sufficient to confer resilience and whether resilience is also seen in chronic stress models that consistently lead to anhedonia. Lastly, it will be important to examine whether UCMS effects on the ISR pathway are attenuated in SSTCre:γ2f/f mice and, if they are, whether any such effects are sex and cell-type specific.
CRediT authorship contribution statement
Sarah J. Jefferson: Conceptualization, Formal analysis, Writing - original draft, Writing - review & editing. Mengyang Feng: Validation, Formal analysis, Writing - original draft, Writing - review & editing. URee Chon: Formal analysis. Yao Guo: Investigation. Yongsoo Kim: Supervision, Formal analysis. Bernhard Luscher: Validation, Conceptualization, Writing - original draft, Writing - review & editing.
Declaration of competing interest
The authors report no study related financial interests or potential conflicts of interest.
Acknowledgments
We thank Fabiola K. Maldonado for technical assistance. We are grateful to Dr. Max Crowley for expert advice on statistical analyses and to Dr. Nicole Crowley for stimulating discussions. This work was supported by the National Institutes of Health (grants MH099851 to B.L., MH116176 to Y.K. and -MH112306 to S.J.J) as well as Tobacco Cure Funds from the Pennsylvania Department of Health to Y.K. Its contents are solely the responsibility of the authors and do not necessarily represent the views of the funding agency.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2020.100238.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- Autry A.E., Adachi M., Nosyreva E., Na E.S., Los M.F., Cheng P.F., Kavalali E.T., Monteggia L.M. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banasr M., Lepack A., Fee C., Duric V., Maldonado-Aviles J., DiLeone R., Sibille E., Duman R.S., Sanacora G. Characterization of GABAergic marker expression in the chronic unpredictable stress model of depression. Chronic Stress. 2017;1:1–13. doi: 10.1177/2470547017720459. Thousand Oaks. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagwagar Z., Wylezinska M., Taylor M., Jezzard P., Matthews P.M., Cowen P.J. Increased brain GABA concentrations following acute administration of a selective serotonin reuptake inhibitor. Am. J. Psychiatr. 2004;161:368–370. doi: 10.1176/appi.ajp.161.2.368. [DOI] [PubMed] [Google Scholar]
- Chiu C.Q., Lur G., Morse T.M., Carnevale N.T., Ellis-Davies G.C., Higley M.J. Compartmentalization of GABAergic inhibition by dendritic spines. Science. 2013;340:759–762. doi: 10.1126/science.1234274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani F., Lorez M., Baer K., Essrich C., Benke D., Laurent J.P., Belzung C., Fritschy J.M., Luscher B., Mohler H. Decreased GABAA-receptor clustering results in enhanced anxiety and a bias for threat cues. Nat. Neurosci. 1999;2:833–839. doi: 10.1038/12207. [DOI] [PubMed] [Google Scholar]
- Czeh B., Varga Z.K., Henningsen K., Kovacs G.L., Miseta A., Wiborg O. Chronic stress reduces the number of GABAergic interneurons in the adult rat hippocampus, dorsal-ventral and region-specific differences. Hippocampus. 2015;25:393–405. doi: 10.1002/hipo.22382. [DOI] [PubMed] [Google Scholar]
- Earnheart J.C., Schweizer C., Crestani F., Iwasato T., Itohara S., Mohler H., Luscher B. GABAergic control of adult hippocampal neurogenesis in relation to behavior indicative of trait anxiety and depression states. J. Neurosci. 2007;27:3845–3854. doi: 10.1523/JNEUROSCI.3609-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elizalde N., Gil-Bea F.J., Ramirez M.J., Aisa B., Lasheras B., Del Rio J., Tordera R.M. Long-lasting behavioral effects and recognition memory deficit induced by chronic mild stress in mice: effect of antidepressant treatment. Psychopharmacology. 2008;199:1–14. doi: 10.1007/s00213-007-1035-1. [DOI] [PubMed] [Google Scholar]
- Engin E., Stellbrink J., Treit D., Dickson C.T. Anxiolytic and antidepressant effects of intracerebroventricularly administered somatostatin: behavioral and neurophysiological evidence. Neuroscience. 2008;157:666–676. doi: 10.1016/j.neuroscience.2008.09.037. [DOI] [PubMed] [Google Scholar]
- Essrich C., Lorez M., Benson J.A., Fritschy J.M., Luscher B. Postsynaptic clustering of major GABAA receptor subtypes requires the gamma 2 subunit and gephyrin. Nat. Neurosci. 1998;1:563–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- Fee C., Banasr M., Sibille E. Somatostatin-positive gamma-aminobutyric acid interneuron deficits in depression: cortical microcircuit and therapeutic perspectives. Biol. Psychiatr. 2017;82:549–559. doi: 10.1016/j.biopsych.2017.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fee C, Prevot T, Misquitta K, Banasr M, Sibille E (2020). doi: 10.1101/2020.02.19.956672. [DOI] [PubMed]
- Fogaca M.V., Duman R.S. Cortical GABAergic dysfunction in stress and depression: new insights for therapeutic interventions. Front. Cell. Neurosci. 2019;13:87. doi: 10.3389/fncel.2019.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs T., Jefferson S.J., Hooper A., Yee P.H., Maguire J., Luscher B. Disinhibition of somatostatin-positive GABAergic interneurons results in an anxiolytic and antidepressant-like brain state. Mol. Psychiatr. 2017;22:920–930. doi: 10.1038/mp.2016.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal S., Hare B., Duman R.S. Prefrontal cortex GABAergic deficits and circuit dysfunction in the pathophysiology and treatment of chronic stress and depression. Curr Opin Behav Sci. 2017;14:1–8. doi: 10.1016/j.cobeha.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal S., Duman C.H., Liu R.J., Wu M., Terwilliger R., Girgenti M.J., Wohleb E., Fogaca M.V., Teichman E.M., Hare B., Duman R.S. Ketamine rapidly reverses stress-induced impairments in GABAergic transmission in the prefrontal cortex in male rodents. Neurobiol. Dis. 2020;134:104669. doi: 10.1016/j.nbd.2019.104669. [DOI] [PubMed] [Google Scholar]
- Gideons E.S., Kavalali E.T., Monteggia L.M. Mechanisms underlying differential effectiveness of memantine and ketamine in rapid antidepressant responses. Proc. Natl. Acad. Sci. U. S. A. 2014;111:8649–8654. doi: 10.1073/pnas.1323920111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgenti M.J., Wohleb E.S., Mehta S., Ghosal S., Fogaca M.V., Duman R.S. Prefrontal cortex interneurons display dynamic sex-specific stress-induced transcriptomes. Transl. Psychiatry. 2019;9:292. doi: 10.1038/s41398-019-0642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronli J., Dagestad G., Milde A.M., Murison R., Bramham C.R. Post-transcriptional effects and interactions between chronic mild stress and acute sleep deprivation: regulation of translation factor and cytoplasmic polyadenylation element-binding protein phosphorylation. Behav. Brain Res. 2012;235:251–262. doi: 10.1016/j.bbr.2012.08.008. [DOI] [PubMed] [Google Scholar]
- He M., Tucciarone J., Lee S., Nigro M.J., Kim Y., Levine J.M., Kelly S.M., Krugikov I., Wu P., Chen Y., Gong L., Hou Y., Osten P., Rudy B., Huang Z.J. Strategies and tools for combinatorial targeting of GABAergic neurons in mouse cerebral cortex. Neuron. 2016;91:1228–1243. doi: 10.1016/j.neuron.2016.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heise C. eEF2K/eEF2 pathway controls the excitation/inhibition balance and susceptibility to epileptic seizures. Cerebr. Cortex. 2016 doi: 10.1093/cercor/bhw075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isingrini E., Camus V., Le Guisquet A.M., Pingaud M., Devers S., Belzung C. Association between repeated unpredictable chronic mild stress (UCMS) procedures with a high fat diet: a model of fluoxetine resistance in mice. PloS One. 2010;5 doi: 10.1371/journal.pone.0010404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapfer C., Glickfeld L.L., Atallah B.V., Scanziani M. Supralinear increase of recurrent inhibition during sparse activity in the somatosensory cortex. Nat. Neurosci. 2007;10:743–753. doi: 10.1038/nn1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Venkataraju K.U., Pradhan K., Mende C., Taranda J., Turaga S.C., Arganda-Carreras I., Ng L., Hawrylycz M.J., Rockland K.S., Seung H.S., Osten P. Mapping social behavior-induced brain activation at cellular resolution in the mouse. Cell Rep. 2015;10:292–305. doi: 10.1016/j.celrep.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Yang G.R., Pradhan K., Venkataraju K.U., Bota M., Garcia Del Molino L.C., Fitzgerald G., Ram K., He M., Levine J.M., Mitra P., Huang Z.J., Wang X.J., Osten P. Brain-wide maps reveal stereotyped cell-type-based cortical architecture and subcortical sexual dimorphism. Cell. 2017;171:456–469 e422. doi: 10.1016/j.cell.2017.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolata S.M., Nakao K., Jeevakumar V., Farmer-Alroth E.L., Fujita Y., Bartley A.F., Jiang S.Z., Rompala G.R., Sorge R.E., Jimenez D.V., Martinowich K., Mateo Y., Hashimoto K., Dobrunz L.E., Nakazawa K. Neuropsychiatric phenotypes produced by GABA reduction in mouse cortex and Hippocampus. Neuropsychopharmacology. 2018;43:1445–1456. doi: 10.1038/npp.2017.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucukibrahimoglu E., Saygin M.Z., Caliskan M., Kaplan O.K., Unsal C., Goren M.Z. The change in plasma GABA, glutamine and glutamate levels in fluoxetine- or S-citalopram-treated female patients with major depression. Eur. J. Clin. Pharmacol. 2009;65:571–577. doi: 10.1007/s00228-009-0650-7. [DOI] [PubMed] [Google Scholar]
- Lau A., Tymianski M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflügers Archiv. 2010;460:525–542. doi: 10.1007/s00424-010-0809-1. [DOI] [PubMed] [Google Scholar]
- Lin L.C., Sibille E. Somatostatin, neuronal vulnerability and behavioral emotionality. Mol. Psychiatr. 2015;20:377–387. doi: 10.1038/mp.2014.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister R.G. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology. 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- Liu X., Carter A.G. Ventral hippocampal inputs preferentially drive corticocortical neurons in the infralimbic prefrontal cortex. J. Neurosci. 2018;38:7351–7363. doi: 10.1523/JNEUROSCI.0378-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher B., Fuchs T. GABAergic control of depression-related brain states. Adv. Pharmacol. 2015;73:97–144. doi: 10.1016/bs.apha.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher B., Feng M., Jefferson S.J. Antidepressant mechanisms of ketamine: focus on GABAergic inhibition. Adv. Pharmacol. 2020;89:43–78. doi: 10.1016/bs.apha.2020.03.002. [DOI] [PubMed] [Google Scholar]
- Ma K., Xu A., Cui S., Sun M.R., Xue Y.C., Wang J.H. Impaired GABA synthesis, uptake and release are associated with depression-like behaviors induced by chronic mild stress. Transl. Psychiatry. 2016;6 doi: 10.1038/tp.2016.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S., Nasca C., Gray J.D. Stress effects on neuronal structure: Hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology. 2016;41:3–23. doi: 10.1038/npp.2015.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton N., Kocsis B., Hajos M. Elicited hippocampal theta rhythm: a screen for anxiolytic and procognitive drugs through changes in hippocampal function? Behav. Pharmacol. 2007;18:329–346. doi: 10.1097/FBP.0b013e3282ee82e3. [DOI] [PubMed] [Google Scholar]
- Moghaddam B., Jackson M. Effect of stress on prefrontal cortex function. Neurotox. Res. 2004;6:73–78. doi: 10.1007/BF03033299. [DOI] [PubMed] [Google Scholar]
- Muller C., Remy S. Dendritic inhibition mediated by O-LM and bistratified interneurons in the hippocampus. Front. Synaptic Neurosci. 2014;6:23. doi: 10.3389/fnsyn.2014.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan M.K., Abreo T., Neuner S.M., Parks C., Watkins C.E., Houseal M.T., Shapaker T.M., Hook M., Tan H., Wang X., Ingels J., Peng J., Lu L., Kaczorowski C.C., Bryant C.D., Homanics G.E., Williams R.W. Identification of a functional non-coding variant in the GABA A receptor alpha2 subunit of the C57BL/6J mouse reference genome: major implications for neuroscience Research. Front. Genet. 2019;10:188. doi: 10.3389/fgene.2019.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton D.F., Fee C., Nikolova Y.S., Sibille E. Altered GABAergic function, cortical microcircuitry, and information processing in depression. In: Quevedo J., Carvalho A.F., Zarate C.A., editors. Neurobiology of Depression. Academic Press; 2019. pp. 315–329. [Google Scholar]
- Nosyreva E., Szabla K., Autry A.E., Ryazanov A.G., Monteggia L.M., Kavalali E.T. Acute suppression of spontaneous neurotransmission drives synaptic potentiation. J. Neurosci. 2013;33:6990–7002. doi: 10.1523/JNEUROSCI.4998-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opal M.D., Klenotich S.C., Morais M., Bessa J., Winkle J., Doukas D., Kay L.J., Sousa N., Dulawa S.M. Serotonin 2C receptor antagonists induce fast-onset antidepressant effects. Mol. Psychiatr. 2014;19:1106–1114. doi: 10.1038/mp.2013.144. [DOI] [PubMed] [Google Scholar]
- Ren Z., Pribiag H., Jefferson S.J., Shorey M., Fuchs T., Stellwagen D., Luscher B. Bidirectional homeostatic regulation of a depression-related brain state by gamma-aminobutyric acidergic deficits and ketamine treatment. Biol. Psychiatr. 2016;80:457–468. doi: 10.1016/j.biopsych.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B., Fishell G., Lee S., Hjerling-Leffler J. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev Neurobiol. 2011;71:45–61. doi: 10.1002/dneu.20853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G., Mason G.F., Rothman D.L., Krystal J.H. Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. Am. J. Psychiatr. 2002;159:663–665. doi: 10.1176/appi.ajp.159.4.663. [DOI] [PubMed] [Google Scholar]
- Schweizer C., Balsiger S., Bluethmann H., Mansuy I.M., Fritschy J.M., Mohler H., Luscher B. The gamma 2 subunit of GABA(A) receptors is required for maintenance of receptors at mature synapses. Mol. Cell. Neurosci. 2003;24:442–450. doi: 10.1016/s1044-7431(03)00202-1. [DOI] [PubMed] [Google Scholar]
- Shen Q., Fuchs T., Sahir N., Luscher B. GABAergic control of critical developmental periods for anxiety- and depression-related behavior in mice. PloS One. 2012;7 doi: 10.1371/journal.pone.0047441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q., Lal R., Luellen B.A., Earnheart J.C., Andrews A.M., Luscher B. gamma-Aminobutyric acid-type A receptor deficits cause hypothalamic-pituitary-adrenal axis hyperactivity and antidepressant drug sensitivity reminiscent of melancholic forms of depression. Biol. Psychiatr. 2010;68:512–520. doi: 10.1016/j.biopsych.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibille E., Morris H.M., Kota R.S., Lewis D.A. GABA-related transcripts in the dorsolateral prefrontal cortex in mood disorders. Int. J. Neuropsychopharmacol. 2011;14:721–734. doi: 10.1017/S1461145710001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soumier A., Sibille E. Opposing effects of acute versus chronic blockade of frontal cortex somatostatin-positive inhibitory neurons on behavioral emotionality in mice. Neuropsychopharmacology. 2014;39:2252–2262. doi: 10.1038/npp.2014.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud C.B., Davila J., Hammen C., Vrshek-Schallhorn S. Severe and nonsevere events in first onsets versus recurrences of depression: evidence for stress sensitization. J. Abnorm. Psychol. 2011;120:142–154. doi: 10.1037/a0021659. [DOI] [PubMed] [Google Scholar]
- Sutton M.A., Taylor A.M., Ito H.T., Pham A., Schuman E.M. Postsynaptic decoding of neural activity: eEF2 as a biochemical sensor coupling miniature synaptic transmission to local protein synthesis. Neuron. 2007;55:648–661. doi: 10.1016/j.neuron.2007.07.030. [DOI] [PubMed] [Google Scholar]
- Taha E., Gildish I., Gal-Ben-Ari S., Rosenblum K. The role of eEF2 pathway in learning and synaptic plasticity. Neurobiol. Learn. Mem. 2013;105:100–106. doi: 10.1016/j.nlm.2013.04.015. [DOI] [PubMed] [Google Scholar]
- Tan Z., Hu H., Huang Z.J., Agmon A. Robust but delayed thalamocortical activation of dendritic-targeting inhibitory interneurons. Proc. Natl. Acad. Sci. U. S. A. 2008;105:2187–2192. doi: 10.1073/pnas.0710628105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway M.T., Waskom M.L., Dillon D.G., Holmes A.J., Park M.T.M., Chakravarty M.M., Dutra S.J., Polli F.E., Iosifescu D.V., Fava M., Gabrieli J.D.E., Pizzagalli D.A. Illness progression, recent stress, and morphometry of hippocampal subfields and medial prefrontal cortex in major depression. Biol. Psychiatr. 2015;77:285–294. doi: 10.1016/j.biopsych.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeraiah P., Noronha J.M., Maitra S., Bagga P., Khandelwal N., Chakravarty S., Kumar A., Patel A.B. Dysfunctional glutamatergic and gamma-aminobutyric acidergic activities in prefrontal cortex of mice in social defeat model of depression. Biol. Psychiatr. 2014;76:231–238. doi: 10.1016/j.biopsych.2013.09.024. [DOI] [PubMed] [Google Scholar]
- Vollenweider I., Smith K.S., Keist R., Rudolph U. Antidepressant-like properties of alpha2-containing GABA(A) receptors. Behav. Brain Res. 2011;217:77–80. doi: 10.1016/j.bbr.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavorska I., Wehr M. Somatostatin-expressing inhibitory interneurons in cortical circuits. Front. Neural Circ. 2016;10:76. doi: 10.3389/fncir.2016.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P., Moaddel R., Morris P.J., Georgiou P., Fischell J., Elmer G.I., Alkondon M., Yuan P., Pribut H.J., Singh N.S., Dossou K.S., Fang Y., Huang X.P., Mayo C.L., Wainer I.W., Albuquerque E.X., Thompson S.M., Thomas C.J., Zarate C.A., Jr., Gould T.D. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533:481–486. doi: 10.1038/nature17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.