Abstract
Childhood adversity increases vulnerability to alcohol use disorders and preclinical models are needed to investigate the underlying neurobiological mechanisms. The present study modeled early-life adversity by rearing male and female C57BL/6J mouse pups in a limited bedding and nesting (LBN) environment, which induces erratic maternal care. As adults, mice were given limited access to two-bottle choice (2BC) alcohol drinking, combined or not with chronic intermittent ethanol (CIE) vapor inhalation to induce alcohol dependence. We tested the hypothesis that LBN rearing might exacerbate or facilitate the emergence of the motivational and affective effects of CIE. Consistent with our hypothesis, although LBN-reared males consumed the same baseline levels of alcohol as controls, they escalated their ethanol intake at an earlier stage of CIE exposure, i.e., after 4 rounds vs. 5 rounds for controls. In contrast, females were insensitive to both LBN rearing and CIE exposure. Males were further subjected to a behavioral test battery. Withdrawal from CIE-2BC increased digging activity and lowered mechanical nociceptive thresholds regardless of early-life conditions. On the other hand, LBN-reared CIE-2BC males showed reduced open arm exploration in the elevated plus maze and increased immobility in the tail suspension test compared to alcohol-naïve counterparts, while no group differences were detected among control-reared males. Finally, LBN rearing and alcohol exposure did not affect grooming in response to a sucrose spray (splash test), novel object recognition, or corticosterone levels. In summary, the LBN experience accelerates the transition from moderate to excessive alcohol drinking and produces additional indices of affective dysfunction during alcohol withdrawal in C57BL/6J male mice.
Keywords: Early-life stress, Resilience, Addiction, Negative reinforcement, Coping
Highlights
-
•
Early-life adversity was generated by rearing C57BL/6J mouse pups in a limited bedding and nesting (LBN) environment.
-
•
Alcohol dependence was induced in adulthood via chronic intermittent ethanol (CIE) inhalation.
-
•
The LBN experience accelerated alcohol intake escalation in males.
-
•
LBN exacerbated affective disturbances upon CIE withdrawal in males.
-
•
Alcohol intake in females was insensitive to both LBN and CIE.
1. Introduction
Vulnerability to alcohol dependence due to early-life adversity is well established in humans (see Enoch, 2011 for review). Exposure to family adversity and stressful events during the first three years of life is linked to externalizing behavior problems in childhood, which in turn predict alcohol use disorder (AUD) in adulthood (Englund et al., 2008; Enoch et al., 2010). Adverse childhood experiences exert a cumulative influence on the risk to develop adult AUD, which suggests that graded neurodevelopmental insults mediate this acquired vulnerability (Felitti et al., 1998). Preclinical models of the facilitatory effect of early-life adversity on problem drinking are needed to investigate the pathophysiological mechanisms at play.
Stressful manipulations during the early postnatal period or adolescence can promote alcohol drinking in rats or mice (see Becker et al., 2011; McDonnell-Dowling and Miczek, 2018 for review). However, most existing preclinical models of alcohol drinking escalation induced by early-life adversity yield sub-intoxicating blood ethanol concentrations (BECs) and do not discriminate between patterns of moderate/controlled vs. excessive/compulsive alcohol drinking. Moreover, limited research has been conducted in C57BL/6J mice, in which a wealth of genetic tools is available for mechanistic investigation (Advani et al., 2007; Lopez et al., 2011; Moriya et al., 2015). This limitation is all the more critical given that maternal separation, an early-life adversity procedure that elicits long-lasting emotional-like disturbances, stress hyperreactivity and alcohol drinking escalation in rats, does not trigger similar changes in mice (Holmes et al., 2005; Millstein and Holmes, 2007; Tan et al., 2017). Accordingly, the present study aimed to develop a novel model of alcohol dependence vulnerability induced by early-life adversity in C57BL/6J mice.
To do so, we combined the limited bedding and nesting (LBN) model of early-life adversity with the chronic intermittent ethanol vapor inhalation (CIE) model of alcohol dependence. The LBN paradigm consists of providing limited resources to dams during the first postnatal week. This environment produces fragmented and unpredictable patterns of maternal care, and a host of molecular, cellular, and behavioral alterations in the adult offspring (see Chen and Baram, 2016; Molet et al., 2014; Walker et al., 2017 for review). Notably, LBN-reared C57BL/6J mice exhibit persistent changes in hypothalamic corticotropin-releasing factor expression and dentate gyrus volume, learning and memory impairments, and enhanced hyponeophagia (Malter Cohen et al., 2013; Naninck et al., 2015; Rice et al., 2008). On the other hand, exposure to CIE increases alcohol self-administration and produces affective disturbances during withdrawal in rodents, which parallels some of the diagnosis criteria of AUD in humans (see Becker and Lopez, 2016; Vendruscolo and Roberts, 2014 for review). Notably, CIE-exposed C57BL/6J males given limited access to alcohol drinking during two-bottle choice (2BC) sessions gradually escalate their voluntary ethanol consumption progressing from sub-intoxicating, yet physiologically relevant, BECs during baseline to BECs clearly exceeding the intoxication threshold (i.e., 80 mg/dL) after CIE exposure (Becker and Lopez, 2004). Furthermore, C57BL/6J mice withdrawn from CIE exhibit affective, cognitive, and sleep alterations (Badanich et al., 2011; Becker and Lopez, 2004; Huitron-Resendiz et al., 2018; Jury et al., 2017; Kroener et al., 2012; Pleil et al., 2015; Rose et al., 2016; Sidhu et al., 2018; Varodayan et al., 2018). The present study tested the hypothesis that LBN rearing might exacerbate or facilitate the emergence of the motivational and affective effects of CIE in C57BL/6J males and females.
2. Materials and methods
2.1. Animals and breeding
Virgin 8-week-old female (n = 16) and male (n = 6) C57BL/6J mice (stock #000664) were obtained from The Jackson Laboratory (Sacramento, CA) and housed in an uncrowded, quiet animal facility room on a 12-h light/dark cycle with free access to lab chow and water. Beginning on postnatal day (P)75, 2–3 females were paired with each male for breeding. Females were examined daily for evidence of a vaginal plug (confirmation of successful mating, considered to be embryonic day [E]0). Pregnant females were separated on E17, prior to parturition. Dams were checked for parturition every 12 h, and the day of birth was considered P0.
All procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committees of University of California - Irvine (UCI) and The Scripps Research Institute (TSRI).
2.2. Chemicals
Ethanol (200 proof) was obtained from PHARMCO-AAPER (Brookfield, CT). 15% (v:v) ethanol was prepared by diluting ethanol in reverse osmosis-purified water. Pyrazole was obtained from Sigma (St Louis, MO). Pyrazole was dissolved in either saline or 20% (v:v) ethanol in saline. Fresh solutions were prepared weekly.
2.3. Experimental timeline
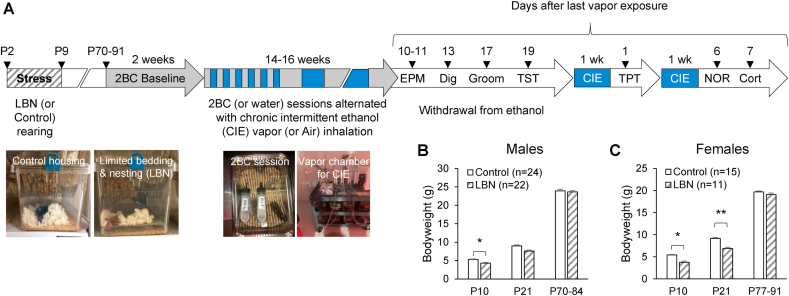
The experimental timeline is summarized in Fig. 1A. C57BL/6J male and female offspring were reared under LBN (n = 22 males and 11 females) or Control (n = 24 males and 15 females) conditions from P2 to P9 at UCI. All mice were transferred to TSRI when they were 3.5–5 weeks old. Six weeks later, they were moved to reverse light cycle rooms (at which point they experienced an 8-h phase advance of the light cycle), habituated for 6 days (males) or 13 days (females), and single-housed 3 days before testing started. C57BL/6J mice need 5.7 ± 0.7 days to re-entrain their circadian rhythm following an 8-h advance of the light-dark cycle, so males had theoretically recovered from the shift by the time testing started (Legates et al., 2009). The LBN paradigm has been shown to produce sex-dependent consequences in several behaviors and brain functions (reviewed in Walker et al., 2017). Based on this knowledge, we decided a priori to analyze data from males and females separately and optimize our experimental design to detect an interaction between early-life history and vapor exposure within each sex. For logistic reasons, it was impossible to test 72 mice in parallel. Accordingly, the male and female cohorts were staggered by one week. Mice remained single-housed in Sani-Chips bedding (Envigo, Placentia, CA) with ad libitum access to reverse osmosis-purified water and sterilized food chow (Teklad LM-485, Envigo) throughout the duration of the experiment. All behavioral testing was conducted during the dark phase under red light illumination (light offset at 10:00 a.m. for males, 9:00 a.m. for females).
Fig. 1.
A. Experimental timeline. C57BL/6J male and female pups were reared under limited bedding and nesting (LBN) or control conditions from postnatal days (P) 2–9. Once mice reached adulthood, they were subjected to limited-access two-bottle choice (2BC) ethanol drinking (grey boxes). Once ethanol intake stabilized, some of the mice were exposed to chronic intermittent ethanol (CIE) vapor inhalation (blue boxes). 2BC sessions were alternated with vapor inhalation over the course of 14–16 weeks. After their last week of 2BC, males were subjected to the elevated plus maze (EPM), digging (Dig), splash (Groom), and tail suspension (TST) tests. They were then exposed to an additional week of CIE and tested in the tail pressure test (TPT) 32 h into withdrawal. After a final week of CIE, they were tested for novel object recognition (NOR) and blood was collected for corticosterone measurement (Cort). B–C. Body weights in males (B) and females (C) at the end of the early-life adversity (P10), at weaning (P21) and at the beginning of alcohol drinking (P70-91). * or **, effect of LBN, p < 0.05 or p < 0.01.
Mice were first subjected to eleven sessions of two-bottle choice (2BC) alcohol drinking to stabilize their ethanol intake. Males from each early-life condition were then split into 3 subgroups of equivalent baseline intake: 1. Air-water mice (Control, n = 8; LBN, n = 6), which had access to two bottles of water during 2BC sessions from that point onward and inhaled air only, 2. Air-2BC mice (Control, n = 8; LBN, n = 8), which had access to ethanol and water during 2BC sessions and inhaled air only, and 3. CIE-2BC mice (Control, n = 8; LBN, n = 8), which had access to ethanol and water during 2BC sessions and were exposed to chronic intermittent ethanol (CIE) vapor inhalation. Due to a smaller sample size, females were split in only 2 subgroups: Air-2BC (Control, n = 7; LBN, n = 5) and CIE-2BC (Control, n = 8; LBN, n = 6). Each experimental subgroup contained up to 4 mice from the same litter.
2BC sessions and vapor exposure were then alternated in two phases. During the priming phase, 2BC sessions were followed by one night (16 h) of ethanol vapor (or air) inhalation (5:00 p.m.-9:00 a.m.), for a total of 6 times, with at least 48 h between the end of vapor exposure and the subsequent 2BC session. This priming phase was meant to enable the detection of group differences at an early stage of CIE exposure. Weeks of CIE (or air) inhalation (4 × 16-h intoxication/8-h withdrawal, Mon-Fri) were then alternated with weeks of 2BC (Mon-Fri) for a total of 5 (males) or 6 (females) rounds, as described previously (Becker and Lopez, 2004; Kreifeldt et al., 2013).
After their last week of 2BC, males were subjected to the elevated plus maze, digging, splash, and tail suspension tests (10–11, 13, 17 and 19 days after last vapor exposure, respectively). They were then exposed to an additional week of CIE and tested in the tail pressure assay 32 h into withdrawal. They were exposed to a final week of CIE and tested for novel object recognition (habituation to arena, training, and testing on withdrawal days 3, 5 and 6, respectively) and blood was collected for corticosterone measurement on withdrawal day 7. One LBN male died 2 days into withdrawal from this last vapor exposure.
The small size of the Control and LBN female groups did not allow for the constitution of Air-water subgroups. Furthermore, one LBN Air-2BC female and one LBN CIE-2BC female died during the course of the alcohol drinking experiment. Accordingly, the experimental design would have been suboptimal and underpowered for the analysis of affective, nociceptive, and cognitive phenotypes during withdrawal from CIE-2BC in females.
2.4. Limited bedding and nesting procedure
Early-life adversity was induced P2–P9 via an impoverished environment with limited bedding and nesting (referred to as LBN), as described previously (Rice et al., 2008). Briefly, on the morning of P2, litters were adjusted to no more than 8 pups, yielding litter sizes of 5–8. Control dams and litters (n = 7) were placed in cages with standard amounts of corn husk bedding (~650 mL) and one square piece of cotton-like nesting material measuring 5 cm × 5 cm. This material is shredded by the dam to create a nest area for her pups. LBN dams and litters (n = 6) were placed in cages fitted with a fine-gauge plastic-coated aluminum mesh platform (mesh dimensions 0.4 × 0.9 cm, catalog no. 57398; McNichols Co., Tampa, FL) sitting ~2.5 cm above the cage floor. Bedding was reduced to only sparsely cover the cage floor (~60 mL), and one-half of a square of nesting material was provided. Both groups were completely undisturbed until the morning of P10, at which point all cages were changed to standard cages with ample bedding and nesting material. At P21, pups were weaned and housed with same-sex littermates in groups of 3–5. Body weights were recorded at P10 and P21.
2.5. Ethanol drinking
Voluntary ethanol consumption was assessed in 2-h sessions during which mice had access to a bottle of water and a bottle of 15% (v:v) ethanol in their home cage, as described previously (Becker and Lopez, 2004; Kreifeldt et al., 2013). Sessions started at the beginning of the dark phase and were conducted Mon-Fri. The position of the ethanol and water bottles was alternated between sessions to control for side preference. Ethanol intake was determined by weighing bottles before and after the session, subtracting the weight lost in bottles placed in an empty cage (to control for spill/evaporation) and dividing by the mouse body weight (measured weekly).
2.6. Chronic intermittent ethanol vapor exposure
Ethanol vapor exposure was conducted as described previously (Becker and Lopez, 2004; Kreifeldt et al., 2013). The inhalation chambers were made of sealed standard plastic mouse cages. An electronic metering pump (Iwaki EZB11D1-PC) dripped 95% ethanol into a flask placed on a warming tray at a temperature of 50 °C. Drip rate was adjusted to achieve target BECs of 150–250 mg/dL. An air pump (Hakko HK-80L) conveyed vaporized ethanol from the flask to each individual chamber. The air flow was set at a rate of 15 L/min for each pair of chambers. Each chamber was diagonally divided by a mesh partition to provide single housing for two mice. Mice received a loading injection of ethanol (1.5 g/kg) and pyrazole (68 mg/kg) in saline, intraperitoneally administered in a volume of 0.1 mL/10 g body weight, before each 16-h ethanol vapor inhalation session. Pyrazole, an alcohol dehydrogenase inhibitor, served to maintain stable BECs during the 16-h period. Air-water and Air-2BC mice also received pyrazole. Blood was sampled from the caudal vein once a week in each CIE-2BC mouse, at the end of a 16-h intoxication session. The tip of the tail was nicked with a scalpel blade, blood was collected with a heparinized capillary tube and centrifuged at 13,000 g for 10 min. BECs were measured by gas chromatography and flame ionization detection (Agilent 7820A).
2.7. Elevated plus maze (EPM) test
The EPM apparatus consisted of a 5 cm × 5 cm central square connected to two opposite open arms and two opposite closed arms. Each arm was 30-cm long and 5-cm wide. The closed arms had 15-cm high walls while the open arms had 3-mm high ledges. The runway was placed on top of a 30-cm high stand. The runway floors were made of matte grey acrylic, all other surfaces were made of clear acrylic. The mouse was placed in the central square of the apparatus, facing a closed arm, and allowed to explore freely for 5 min. The apparatus was wiped with 70% ethanol in between mice. The test was recorded by a camera mounted above the EPM and connected to a computer. The distance traveled, number of entries, and time spent in each area of the EPM were calculated by the ANY-maze program (Stoelting Co., Wood Dale, IL). The total distance traveled was used as an index of locomotor activity and the time spent in the open arms was used as an index of anxiety-like behavior, as previously described (Komada et al., 2008; Rodgers and Dalvi, 1997). The apparatus design (open arms with ledges and closed arms with transparent walls) was meant to encourage exploration of the open arms and facilitate the detection of an anxiogenic-like effect of alcohol withdrawal (Dere et al., 2002; Horii and Kawaguchi, 2015).
2.8. Digging test
Digging activity was assessed according to Deacon (2006). The mouse was placed in the middle of a standard acrylic mouse cage (with no lid) filled with a 5-cm depth of Sani-Chip bedding (Envigo). The latency to dig, number of digging bouts and duration of digging were measured using stopwatches and a tally counter for a total duration of 3 min. Bedding was changed between each mouse. Testing was conducted under red light. Digging is a spontaneous, species-typical behavior performed by wild mice foraging for buried food in their natural habitats and exhibited by laboratory mice placed on a thick layer of bedding substrate. Digging behavior is sensitive to multiple psychotropic drugs (see Deacon, 2006 and references therein) and we previously reported that it is robustly increased during withdrawal from CIE (Sidhu et al., 2018).
2.9. Splash test
The splash test was conducted in the home cage, under red light, using the method of Ducottet et al. (2004). A solution of 10% sucrose was sprayed on the dorsal coat of the mouse using a single squirt from a standard gardening spray bottle in mist position. The latency to groom, the number of grooming bouts and duration of grooming were measured using stopwatches and a tally counter for a total duration of 5 min. Grooming activity is used as an index of self-care that is degraded in mouse models of depressive-like behavior (d'Audiffret et al., 2010; Yalcin et al., 2005).
2.10. Tail suspension test (TST)
This test was used to assess the coping strategy used by mice facing an acute, inescapable stress (Cryan et al., 2005; Steru et al., 1985). The tail of the mouse was inserted in a hollow cylinder (3.5-cm length, 1-cm diameter, 1 g) to prevent tail climbing as described by Can et al. (2012). The 2-cm end of a 17-cm piece of tape was adhered to the mouse tail and back to itself, leaving the distal 2–3 mm of the tail protruding out of the tape. The other end of the tape was stuck to a shelf placed 30 cm above the bench, such that the tape hung vertically. The duration of immobility of each mouse was measured for 6 min. Relative levels of immobility were interpreted in terms of active vs. passive stress-coping strategy (Anyan and Amir, 2018; Commons et al., 2017; Molendijk and de Kloet, 2015).
2.11. Tail pressure test
Mechanical nociceptive thresholds were assessed by applying pressure on the tail using a digital Randall-Selitto apparatus (Harvard Apparatus, Holliston, MA), as previously described by Elhabazi et al. (2014). The mice were first habituated to enter a restrainer pouch made of woven wire (stainless steel 304L 200 mesh, Shanghai YiKai) over three days. On testing days, the mouse was gently introduced into the restrainer and the distal portion of the tail was positioned under the conic tip of the apparatus. The foot switch was then depressed to apply uniformly increasing pressure onto the tail until the first nociceptive response (struggling or squeaking) occurred. The force (in g) eliciting the nociceptive response was recorded. A cutoff force of 600 g was enforced to prevent tissue damage. The measure was repeated on the medial and proximal parts of the tail of the same mouse, with at least 30 s between each measure. The average of the three measures (distal, medial, proximal) was used for statistical analysis.
2.12. Novel object recognition
Long-term object memory was assessed as described by Lueptow (2017). Testing was conducted under white light (350 lux). Mice were individually habituated during 10 min to a 50 cm × 50 cm enclosure with 38-cm high walls made of beige ABS plastic (San Diego Instruments 7001-0067) and lined with bedding. Twenty-four hours later, two identical objects (20-mL glass vial filled with water) were taped to the floor of the arena, 11 cm away from two adjacent corners and 22 cm away from each other, and the mouse was given 10 min to freely explore them. On the following day, one of the two objects was replaced with another object (medium-sized paper clip) and the mouse was again given 10 min to explore. The side on which the object was replaced with a novel object was counterbalanced across mice. The arena and objects were wiped with ethanol (70% v:v) between mice to remove odor cues. The mouse head position was video-tracked and circular areas extending 2 cm away from each object were defined in ANY-maze. The discrimination index was calculated as the ratio of time spent in the novel object circle over time spent in the familiar object circle. One Control CIE-2BC mouse was identified as an outlier using the Grubbs’ test (Grubbs, 1969) and was therefore excluded.
2.13. Corticosterone assay
Mice were euthanized by cervical dislocation immediately followed by decapitation, 2–5 h into the dark phase. Trunk blood was collected into EDTA-coated tubes. Plasma was separated by centrifugation at 1600 g for 10 min at 4 °C and stored at −80 °C. Corticosterone levels were measured in duplicates using the Corticosterone Enzyme Immunoassay Kit (Arbor Assays, Ann Arbor, MI) according to the manufacturer's instructions.
2.14. Statistical analysis
Data analysis was performed in Statistica 13.3 (TIBCO Software Inc.). Data from males and females were analyzed separately. Linear mixed-model analyses were used to evaluate litter effects (Zorrilla, 1997) and determine the effect of LBN while accounting for intralitter likeness, as per Golub and Sobin (2020). Early-life history was defined as a fixed factor and litter as a random factor nested under early-life history. Expected mean squares were estimated using the over-parameterized model and degrees of freedom were computed using Satterthwaite's method of denominator synthesis. To determine the effect of CIE and the interaction of LBN with CIE, data were analyzed by two-way analysis of variance (ANOVA) with early-life history (Control, LBN) and alcohol exposure (Air-water [when relevant], Air-2BC, CIE-2BC) as between-subject variables. Tukey posthoc tests were conducted when appropriate. Repeated measures ANOVA (RM-ANOVA, with time as within-subject variable) was used to analyze ethanol drinking acquisition and escalation. Planned comparisons were conducted using two-tailed unpaired t-tests to test our hypothesis that LBN facilitates the effect of CIE on ethanol intake and negative affect. To further examine the trajectory of ethanol intake escalation in each group, the proportion of mice whose weekly average intake exceeded 4 g/kg was subjected to Kaplan-Meier survival analysis. We selected 4 g/kg as a criterion because a consumption of 4 g/kg in 2 h yields BECs ≈150 mg/dL (Becker and Lopez, 2004; Contet et al., 2014), which corresponds to twice the BEC threshold for ethanol intoxication, as well as to the lower end of the BEC range targeted during ethanol vapor inhalation. Data are shown as mean ± s.e.m. in the graphs.
3. Results
3.1. The effect of early-life adversity on body weight dissipates during development
A significant effect of litter was detected on body weights at P10 (males: F11,33 = 14.8, p < 0.0001; females: F7,17 = 17.8, p < 0.0001) and P21 (males: F11,33 = 15.4, p < 0.0001; females: F7,17 = 9.8, p < 0.0001) but faded at adult age (males: F11,33 = 2.0, p = 0.061; females: F7,17 = 1.5, p = 0.22). Accounting for litter effects, the body weights of LBN males were smaller than those of Control males at P10 (F1,11.5 = 5.4, p = 0.039), a trend was detected at P21 (F1,11.5 = 3.68, p = 0.080), and there was no significant difference by the time mice started self-administering alcohol (F1,14.7 = 0.03, p = 0.87) (Fig. 1B). The body weights of LBN females were significantly smaller than those of Control females at both P10 (F1,7.1 = 10.8, p = 0.013) and P21 (F1,7.3 = 16.4, p = 0.005), and a trend persisted at adult age (F1,8.7 = 3.8, p = 0.084) (Fig. 1C).
3.2. Early-life adversity accelerates alcohol intake escalation in males
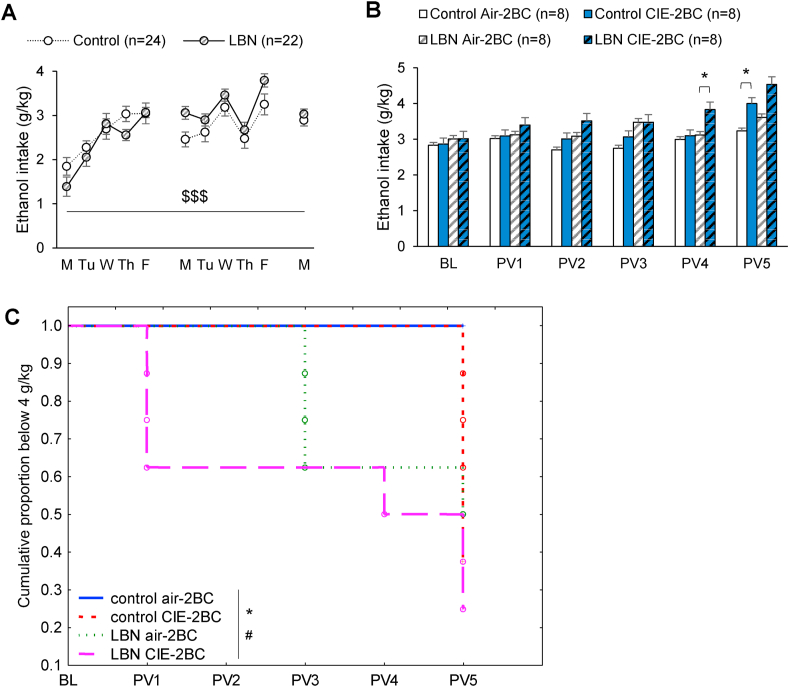
In males, no litter effects were detected on ethanol intake during acquisition, either during the first week (F11,33 = 1.5, p = 0.18), second week (F11,33 = 1.6, p = 0.14), or on the last day of baseline (F11,33 = 1.4, p = 0.21). There was a significant interaction between LBN rearing and days, whereby LBN males drunk less ethanol than Control males at the beginning of acquisition, but more later on (Fig. 2A, F10,440 = 2.9, p = 0.001). Accordingly, RM-ANOVA of weekly intakes revealed a significant effect of time in LBN males (F1,21 = 33.9, p < 0.0001), but not in Control males (F1,23 = 3.06, p = 0.09). By the end of acquisition, Control and LBN males consumed the same amount of alcohol on the last day of baseline (F1,16.3 = 1.3, p = 0.27) and had a similar cumulative ethanol intake (29.8 ± 1.6 vs. 30.8 ± 0.9 g/kg, F1,14.8 = 3*10−5, p = 0.996).
Fig. 2.
Ethanol intake in C57BL/6J males raised under control or LBN conditions and subjected to chronic 2BC drinking combined with Air or CIE inhalation. A. Daily ethanol intake during baseline. $$$, LBN × Days interaction, p < 0.001. B. Weekly ethanol intake during CIE exposure. *, t-test, Air-2BC vs. CIE-2BC, p < 0.05. C. Kaplan-Meier survival curve showing the proportion of mice that have not yet escalated their ethanol intake above 4 g/kg *, effect of CIE, p < 0.05; #, effect of LBN, p < 0.05.
CIE exposure is known to gradually increase voluntary ethanol intake in C57BL/6J males (Becker and Lopez, 2004; Kreifeldt et al., 2013). We hypothesized that a history of early-life adversity would sensitize mice to the effect of CIE. Consistent with this hypothesis, planned comparisons indicated that, by post-vapor week 4 (PV4), CIE-exposed LBN males had escalated their weekly intake compared to Air-exposed counterparts (t14 = -2.2, p = 0.048), while Air- and CIE-exposed Control mice still consumed equivalent amounts (t14 = -0.4, p = 0.69, Fig. 2B). Escalation became significant in Control mice on PV5 (t14 = -2.3, p = 0.036). Analyzing the proportion of mice whose weekly average intake exceeded 4 g/kg further demonstrated that the trajectory of ethanol intake escalation was significantly accelerated by LBN (Fig. 2C, Z = 2.4, p = 0.018), as well as by CIE (Z = 2.1, p = 0.038). Accordingly, RM-ANOVA over the entire experiment detected a trend for an effect of early-life adversity (F1,28 = 3.6, p = 0.067), with LBN males tending to consume more alcohol than their Control counterparts overall (Fig. 2B and Suppl. Fig. 1A). There was also a significant CIE × Week interaction, reflecting CIE-induced escalation of ethanol intake in both Control and LBN males (F5,140 = 2.6, p = 0.025). The LBN x CIE × Week interaction was not significant (F5,140 = 0.7, p = 0.61), owing to LBN accelerating intake escalation only by one week.
Blood ethanol concentrations achieved during CIE exposure were similar between Control and LBN males (Suppl. Fig. 2A, main effect of LBN: F1,14 = 0.3, p = 0.60; accounting for litter effects on individual CIE weeks, p's > 0.14).
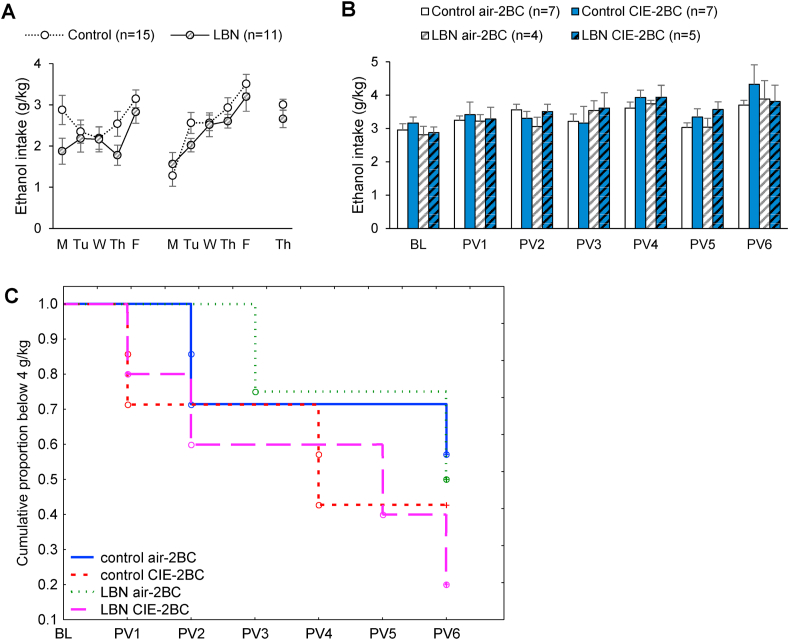
3.3. Early-life adversity does not impact alcohol intake in females
In females, litter effects on ethanol intake were not detected during the first week of acquisition (F7,15 = 1.2, p = 0.39), but they emerged during the second week (F7,15 = 3.7, p = 0.016) and were still significant on the last day of baseline (F7,15 = 3.0, p = 0.034). RM-ANOVA indicated that LBN had no significant influence on the acquisition of ethanol drinking (Fig. 3A, LBN × days interaction: F10,220 = 1.2, p = 0.29). Accounting for litter effects, Control and LBN females consumed equivalent amounts of alcohol on the first week (F1,8.9 = 1.3, p = 0.28), second week (F1,7.6 = 0.001, p = 0.98), and last day of baseline (F1,7.7 = 0.04, p = 0.85), and they had a similar cumulative ethanol intake (28.8 ± 1.8 vs. 25.2 ± 1.7 g/kg, F1,7.8 = 0.3, p = 0.57).
Fig. 3.
Ethanol intake in C57BL/6J females raised under control or LBN conditions and subjected to chronic 2BC drinking combined with Air or CIE inhalation. A. Daily ethanol intake during baseline. B. Weekly ethanol intake during CIE exposure. C. Kaplan-Meier survival curve showing the proportion of mice that have not yet escalated their ethanol intake above 4 g/kg.
Litter effects were no longer detected following vapor exposure (p's > 0.19 on individual PV weeks). In contrast to males, CIE exposure did not significantly affect ethanol intake in females (Fig. 3B and Suppl. Fig. 1B, main effect of CIE: F1,19 = 0.5, p = 0.49; CIE × week interaction: F6,114 = 0.5, p = 0.83) and there was no influence of early-life adversity on ethanol intake following CIE (LBN × CIE interaction: F1,19 = 0.02, p = 0.89). Analyzing the proportion of mice whose weekly average intake exceeded 4 g/kg indicated that a subset of mice within each subgroup escalated their intake above this threshold over time (Fig. 3C) and this trajectory was not significantly altered by LBN (Z = 0.28, p = 0.78) or CIE (Z = 1.3, p = 0.20).
Blood ethanol concentrations achieved during CIE exposure were similar between Control and LBN females (Suppl. Fig. 2B, main effect of LBN, F1,10 = 2.2, p = 0.17). Accounting for litter effects on individual CIE weeks, there was a significant difference between groups on CIE1 (F1,7.5 = 8.6, p = 0.02), but not on any subsequent week (p's > 0.31).
3.4. Early-life adversity exacerbates negative affect during alcohol withdrawal in males
To identify effects of early-life adversity on withdrawal phenotypes relevant to alcohol dependence vulnerability, Control and LBN males were tested in assays probing their affective, nociceptive, cognitive, and hormonal states. Ethanol-naïve (Air-water) counterparts were tested in parallel to evaluate the effect of chronic alcohol drinking independent of the effect of CIE. We hypothesized that a history of early-life adversity would facilitate the emergence of negative affect in mice withdrawn from CIE. Accordingly, we predicted that, in some assays, CIE-2BC mice would differ from Air-water (or Air-2BC) mice only, or to a greater extent, if they had been exposed to LBN. Planned comparisons confirmed this prediction in two assays: EPM and TST (details below). Results from linear mixed model analyses are presented in Table 1 and results from two-way ANOVAs are presented in Table 2.
Table 1.
Linear mixed model analyses were used to evaluate litter effects and determine the effect of early-life adversity while accounting for the variance contributed by litter. Data were obtained in C57BL/6J males raised under Control or LBN conditions, exposed to chronic alcohol drinking combined or not with chronic intermittent ethanol vapor inhalation (Air-water, Air-2BC, CIE-2BC), and withdrawn from alcohol.
| Test | Withdrawal timepoint (after last vapor) | Measure | Effect of litter | Effect of LBN |
|---|---|---|---|---|
| Elevated plus-maze | 10–11 days | % time on open arms (Fig. 3A) | F11,33 = 0.7, p = 0.75 | F1,22.3 = 0.06, p = 0.81 |
| Total distance traveled (Fig. 3B) | F11,33 = 0.7, p = 0.74 | F1,22.1 = 1.6, p = 0.21 | ||
| Digging | 13 days | Digging duration (Fig. 3C) | F11,33 = 4.3, p = 0.0005 | F1,12.7 = 1.1, p = 0.31 |
| Splash | 17 days | Number of grooming bouts (Fig. 3D) | F11,33 = 3.0, p = 0.007 | F1,13.4 = 0.8, p = 0.40 |
| Tail suspension | 19 days | Immobility duration (Fig. 3E) | F11,33 = 1.5, p = 0.18 | F1,16.0 = 0.3, p = 0.57 |
| Tail pressure | 32 h | Nociceptive threshold (Fig. 3F) | F11,33 = 1.4, p = 0.21 | F1,16.3 = 1.1, p = 0.31 |
| Novel object recognition | 6 days | Discrimination index (Fig. 3G) | F11,31 = 1.5, p = 0.16 | F1,17.3 = 1.4, p = 0.25 |
| Corticosterone | 7 days | Plasma levels (Fig. 3H) | F11,32 = 2.1, p = 0.055 | F1,15.9 = 0.1, p = 0.72 |
Table 2.
Two-way ANOVAs were used to determine the effect of alcohol exposure and its interaction with early-life adversity. Data were obtained in C57BL/6J males raised under Control or LBN conditions, exposed to chronic alcohol drinking combined or not with chronic intermittent ethanol vapor inhalation (Air-water, Air-2BC, CIE-2BC), and withdrawn from alcohol.
| Test | Withdrawal timepoint (after last vapor) | Measure | Effect of alcohol | Alcohol × LBN interaction |
|---|---|---|---|---|
| Elevated plus-maze | 10–11 days | % time on open arms (Fig. 3A) | F2,40 = 3.3, p = 0.048 | F2,40 = 2.7, p = 0.080 |
| Total distance traveled (Fig. 3B) | F2,40 = 1.1, p = 0.36 | F2,40 = 1.0, p = 0.37 | ||
| Digging | 13 days | Digging duration (Fig. 3C) | F2,40 = 5.5, p = 0.008 | F2,40 = 0.1, p = 0.91 |
| Splash | 17 days | Number of grooming bouts (Fig. 3D) | F2,40 = 1.3, p = 0.28 | F2,40 = 3.7, p = 0.033 |
| Tail suspension | 19 days | Immobility duration (Fig. 3E) | F2,40 = 4.18, p = 0.023 | F2,40 = 3.1, p = 0.056 |
| Tail pressure | 32 h | Nociceptive threshold (Fig. 3F) | F2,40 = 4.3, p = 0.02 | F2,40 = 0.2, p = 0.83 |
| Novel object recognition | 6 days | Discrimination index (Fig. 3G) | F2,38 = 0.04, p = 0.96 | F2,38 = 0.6, p = 0.57 |
| Corticosterone | 7 days | Plasma levels (Fig. 3H) | F2,39 = 0.6, p = 0.55 | F2,39 = 1.4, p = 0.26 |
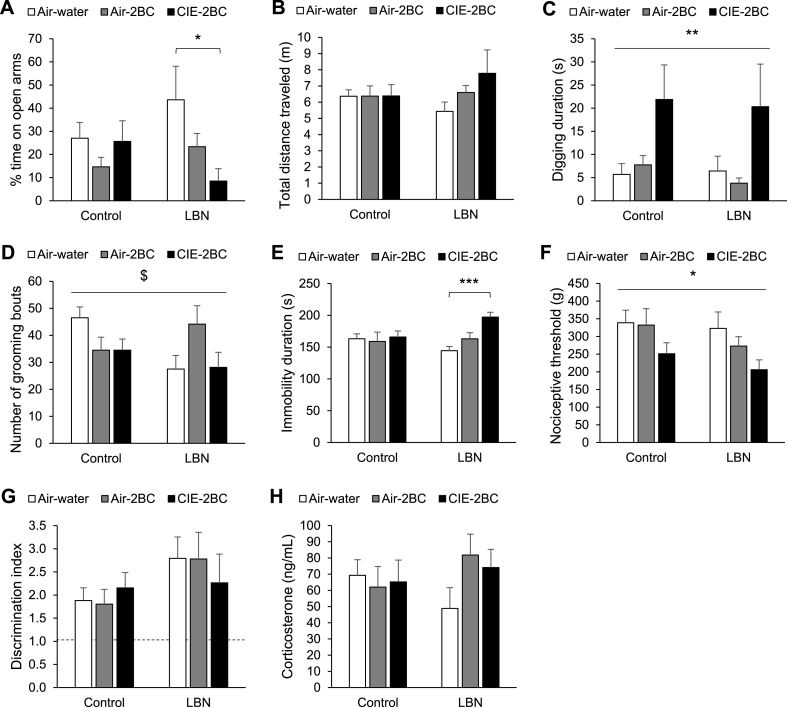
In the EPM, the time spent by Control males on open arms was insensitive to alcohol withdrawal; however, in LBN males, alcohol drinking and inhalation had a cumulative influence on open arm exploration, with CIE-2BC mice spending significantly less time on the open arms than Air-water mice (t12 = 2.5, p = 0.026), and Air-2BC mice exhibiting an intermediate phenotype (Fig. 4A). Accordingly, the ANOVA detected a trend for an LBN × Alcohol interaction. There was no impact of early-life history or alcohol exposure on the total distance traveled (Fig. 4B). In the digging test, males withdrawn from CIE showed a robust increase in digging activity regardless of early-life history (Fig. 4C). In the splash test, there was a significant LBN × Alcohol interaction in the grooming activity elicited by sucrose spraying. Alcohol-withdrawn Control mice tended to groom less than their alcohol-naïve counterparts and to the same extent as alcohol-naïve LBN mice, but posthoc comparisons did not identify significant pairwise differences between groups (Fig. 4D). In the TST, consistent with our prediction, alcohol withdrawal did not affect Control males, while it significantly increased immobility duration in CIE-2BC LBN males compared to their alcohol-naïve counterparts (t12 = -5.0, p = 0.0003); Air-2BC LBN males exhibited an intermediate phenotype (Fig. 4E). Accordingly, the ANOVA detected a trend for an LBN × Alcohol interaction. In the tail pressure test, mice withdrawn from CIE had lower mechanical nociceptive thresholds (i.e., hyperalgesia) regardless of early-life history (Fig. 4F). In the NOR test, all groups exhibited significant discrimination of the novel object (single-sample t-test against 1, p < 0.05) and there was no influence of LBN rearing or alcohol exposure on NOR performance (Fig. 4G). Point-sampled corticosterone levels were not affected by early-life history nor chronic alcohol exposure (Fig. 4H).
Fig. 4.
Affective, nociceptive, cognitive, and hormonal phenotypes associated with withdrawal from moderate and excessive alcohol drinking in C57BL/6J males raised under control or LBN conditions. A-B. Elevated plus maze: % time spent on the open arms (A) and total distance traveled (B). *, t-test, LBN Air-water vs LBN CIE-2BC, p < 0.05. C. Digging test: digging duration. **, main effect of CIE, p < 0.01. D. Splash test: number of grooming bouts. $, LBN × Alcohol interaction, p < 0.05. E. Tail suspension test: immobility duration. ***, t-test, LBN Air-water vs LBN CIE-2BC, p < 0.001. F. Tail pressure test: nociceptive threshold. *, main effect of CIE, p < 0.05. G. Novel objection recognition: discrimination index. H. Plasma corticosterone levels.
4. Discussion
Our results show that LBN rearing accelerates the escalation of voluntary ethanol intake by one round of CIE exposure and produces behavioral indices of negative affect during withdrawal from CIE, as manifested by reduced open space exploration and passive stress coping strategy in LBN CIE-2BC males compared with alcohol-naïve counterparts. In contrast, neither LBN nor CIE affected ethanol intake in females. These findings support the notion that combining the LBN paradigm of early-life adversity with the CIE-2BC paradigm of alcohol dependence in C57BL/6J males provides a valuable mouse model to examine the neurobiological mechanisms mediating vulnerability to AUD induced by childhood adversity.
4.1. The impact of early-life adversity on alcohol consumption is sex-dependent
The abnormal maternal behavior of LBN dams provokes chronic stress in the pups, as manifested by elevated plasma corticosterone levels and reduced body weights (Arp et al., 2016; Naninck et al., 2015; Rice et al., 2008). Body weights typically normalize in adulthood, while the long-term impact on corticosterone can be variable. Consistent with these previous reports, we observed reduced body weights in both males and females at P10 and P21, but the difference was no longer significant by the time the mice started 2BC sessions, thereby eliminating a potential confound in the calculation of ethanol intake.
During alcohol drinking acquisition, prior to vapor exposure, LBN-reared males, but not females, increased their ethanol intake at a faster rate than their control-reared counterparts. This observation aligns to some extent with the results of an earlier study that had used early chronic social isolation (from weaning onwards) as a model of developmental stress in C57BL/6J mice receiving limited-access 2BC drinking sessions in adulthood (Lopez et al., 2011). Although isolated mice of both sexes consumed more alcohol than their group-housed counterparts, the effect was more pronounced in males than females, as in our study (Lopez et al., 2011). However, in contrast to the robust and sustained effect of social isolation during adolescence, LBN rearing had a short-lasting effect on non-dependent alcohol drinking, as LBN and Control males were indistinguishable by the last baseline day.
To the best of our knowledge, the present study is the first to examine the effects of early-life adversity on alcohol consumption in dependent animals. CIE exposure increased voluntary ethanol intake in both control- and LBN-reared males, but the escalation happened at an earlier stage of CIE exposure in LBN-reared males. We propose that accelerated intake escalation serves as an experimental index of higher vulnerability to alcohol dependence induced by LBN rearing.
In contrast to the male phenotype, CIE did not induce ethanol intake escalation in control-reared females. Combining LBN rearing with CIE exposure did not alter ethanol intake in females either. These negative results may reflect a ceiling effect in the capacity of females to increase their alcohol consumption under the present experimental conditions. Alternatively, the lack of effect of LBN rearing in CIE-exposed females may represent a resilient phenotype, contrasting with the vulnerable phenotype of males. Such sexual dimorphism would be in line with the existing literature showing a relative insensitivity of female rodents to the effects of early-life adversity, including LBN rearing, on behavioral outcomes in adulthood (reviewed in Loi et al., 2017; Walker et al., 2017). One must keep in mind, however, that the resilience of female rodents may not reflect the clinical situation (Enoch, 2011). The discordance between the influence of sex on neurobehavioral outcomes in animals vs. humans is a general limitation of preclinical models and should not be construed as a failure of the LBN or CIE-2BC models in particular (Eliot and Richardson, 2016).
4.2. Early-life adversity exacerbates behavioral indices of negative affect during alcohol withdrawal
In addition to an accelerated ethanol intake escalation, LBN-reared males withdrawn from CIE exhibited affective disturbances that were not detected in control-reared counterparts. Specifically, the combination of LBN with CIE-2BC enhanced aversion for open space exploration (EPM) and passive coping behavior in the face of an acute, inescapable stressor (TST), which may reflect anxiety-like and depressive-like behavior, respectively. We did not detect a main effect of LBN on these measures, consistent with a previous report showing that LBN rearing does not alter the behavior of adult C57BL/6J males tested in the EPM and forced swim test (FST) (Naninck et al., 2015). We also did not detect a main effect of alcohol exposure (chronic 2BC alone or combined with CIE) in these assays, which is again in accordance with the literature. Previous studies examining CIE-withdrawn C57BL/6J males in the EPM found either a lack of effect of CIE (Daut et al., 2015) or were confounded by reduced locomotion (Kash et al., 2009). Likewise, C57BL/6J males withdrawn for 1 week from CIE-2BC (i.e., 2 weeks after last vapor exposure) did not differ from Air-exposed counterparts in the FST (Bray et al., 2017), while reduced immobility was observed 72 h into withdrawal from CIE alone (Maldonado-Devincci et al., 2016).
Interestingly, protracted abstinence from chronic free-choice alcohol drinking in C57BL/6J females increases immobility in the FST (Holleran et al., 2016; Pang et al., 2013), which contrasts with our observation that 2 weeks of withdrawal from chronic intermittent limited-access 2BC does not impact TST behavior in control-reared males. This discrepancy suggests that alterations in stress coping strategies in the FST/TST may be sex-specific or require continuous alcohol access prior to withdrawal.
Altogether, the detection of affective phenotypes specific to mice that experienced both LBN and CIE-2BC is relevant to the high prevalence of co-morbid psychopathologies in the AUD population, and particularly in the subset of subjects that experienced childhood adversity (Enoch, 2011; Green et al., 2010; Helle et al., 2020). This finding also supports the notion that LBN-reared males may escalate their ethanol intake faster than control-reared males to mitigate their higher sensitivity to withdrawal-associated negative affect, as a result of negative reinforcement (Koob, 2013).
4.3. Measures not affected by early-life adversity
We had previously described that digging activity is robustly enhanced in C57BL/6J males withdrawn from CIE for 10 days (Sidhu et al., 2018), in accordance with previous reports of increased marble burying (Jury et al., 2017; Pleil et al., 2015; Rose et al., 2016) and akin to the psychomotor agitation characterizing alcohol withdrawal in humans (American Psychiatric Association, 2013). Here, we extend this observation to mice exposed to both CIE and 2BC (CIE-2BC) and show that mice exposed to chronic 2BC only (Air-2BC) display the same level of digging activity as alcohol-naïve mice (Air-water). These outcomes were not modulated by early-life adversity, although we cannot exclude that a ceiling effect prevented us from detecting further increases in digging activity in mice exposed to both LBN and CIE.
Grooming behavior in response to a sucrose spray (splash test) is reduced in mice subjected to unpredictable chronic mild stress (UCMS) and restored by antidepressant treatment (Frisbee et al., 2015; Yalcin et al., 2005), indicating possible relevance for depressive-like states. In our study, the grooming behavior of C57BL/6J males was not impacted by a history of LBN rearing, chronic alcohol exposure, or their combination.
Acute withdrawal from chronic alcohol produces hyperalgesia in both humans and rats (Gatch, 2009; Gatch and Lal, 1999; Jochum et al., 2010) and the acute analgesic effect of alcohol is thought to promote alcohol drinking in dependent subjects (Egli et al., 2012). Consistent with this literature, mice withdrawn from CIE for 32 h had lower mechanical nociceptive thresholds than their Air-exposed counterparts. We also found that LBN rearing alone did not influence mechanical nociception in adulthood, which corroborates a previous report showing that LBN-reared rats have reduced muscular, but not cutaneous, mechanical nociceptive thresholds (Green et al., 2011). Furthermore, LBN did not exacerbate withdrawal-induced mechanical hyperalgesia.
In the present study, LBN and CIE, alone or in combination, did not impact NOR, as all groups were similarly able to discriminate the novel object from the familiar object. This finding is at odds with the effects of CIE and LBN previously reported in the literature (Bath et al., 2017; Brunson et al., 2005; Naninck et al., 2015; Pradhan et al., 2018; Rice et al., 2008). The unexpectedly good performance of LBN-reared males in our study may stem from their extensive handling throughout adulthood, which could have mitigated the consequences of early-life adversity on cognitive outcomes.
We also found that a history of early-life adversity or chronic alcohol exposure did not impact point-sampled plasma corticosterone levels in adulthood. As mentioned above, LBN rearing consistently elevates plasma corticosterone levels in pups, but the long-term impact in adult mice is variable, with some studies reporting elevated levels in males, reduced levels in females, or no change in either sex (Arp et al., 2016; Bath et al., 2016; Naninck et al., 2015; Rice et al., 2008). We did not detect an effect of LBN on corticosterone levels measured 2–5 h in the dark phase, a circadian period during which corticosterone levels in adult C57BL/6J males are relatively stable (Dalm et al., 2005). The lack of LBN effect is consistent with previous reports (Bath et al., 2016; Naninck et al., 2015) but we cannot rule out that differences between groups would have been observed if levels had been measured at a different circadian timepoint, as in (Rice et al., 2008), or following a stressor challenge. Likewise, protracted abstinence from CIE is known to elevate peak, but not nadir, corticosterone levels in rats (Somkuwar et al., 2017), so it is possible that we would have detected differences between groups if we had collected blood at a different circadian timepoint. Finally, we collected blood 7 days after the last vapor exposure, which, in accordance with previous findings in CIE-withdrawn rats and mice, may be too early for corticosterone levels to rise (Maldonado-Devincci et al., 2016; Somkuwar et al., 2017).
4.4. Conclusion
In conclusion, combining LBN with CIE in C57BL/6J males can serve as a preclinical model for the accelerated transition to AUD and higher sensitivity to the negative emotional state associated with alcohol withdrawal in individuals who suffer from problem drinking as a consequence of childhood adversity. Given the high number of genetically engineered mice that are currently available on a C57BL/6J background, this model can facilitate the investigation of the molecular and circuit mechanisms mediating the long-lasting effects of early-life adversity on AUD vulnerability.
While the effects of LBN on CIE-induced phenotypes were qualitatively in line with the consequences of early-life adversity on alcohol use in the human population, their amplitude and statistical significance were modest. The procedure described here could be altered to amplify the effects of LBN on alcohol intake escalation and withdrawal-induced negative affect. An interesting avenue would be to initiate 2BC sessions during adolescence, as childhood adversity is strongly associated with an earlier onset of alcohol use, which in turn predicts the subsequent development of AUD in adulthood (see Enoch, 2011 for review).
CRediT authorship contribution statement
Agbonlahor Okhuarobo: Investigation, Formal analysis, Writing - original draft. Jessica L. Bolton: Methodology, Investigation. Ighodaro Igbe: Supervision. Eric P. Zorrilla: Methodology, Formal analysis, Writing - review & editing. Tallie Z. Baram: Conceptualization, Funding acquisition. Candice Contet: Conceptualization, Funding acquisition, Supervision, Formal analysis, Writing - review & editing.
Declaration of competing interest
The authors have no conflicts of interest to disclose.
Acknowledgments
We wish to thank Max Kreifeldt for his assistance with alcohol vapor inhalation and behavioral testing. We are also grateful for the support of TSRI Alcohol Research Center Animal Models Core, which conducted blood ethanol concentration analysis for this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2020.100269.
Funding
This work was supported by National Institutes of Health grants AA026685 (CC), AA027636 (CC), AA006420 (CC, EZ), AA027372 (CC, TZB), AA027700 (EZ), DA046865 (EZ), as well as stipends from University of Benin, Benin City, Nigeria (AO), Kingsefe Pharmacy, Benin City, Nigeria (AO), the Hewitt Foundation for Medical Research (JLB), and the Brain & Behavior Research Foundation (JLB). These funding sources were not involved in study design, data collection, analysis or interpretation, nor decision to publish.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Daily ethanol intake in C57BL/6J males (A) and females (B) raised under control or LBN conditions and subjected to chronic 2BC drinking combined with Air or CIE inhalation.
Blood ethanol concentrations during CIE exposure in males (A) and females (B) raised under control or LBN conditions. *, effect of LBN, p < 0.05.
References
- Advani T., Hensler J.G., Koek W. Effect of early rearing conditions on alcohol drinking and 5-HT1A receptor function in C57BL/6J mice. Int. J. Neuropsychopharmacol. 2007;10(5):595–607. doi: 10.1017/S1461145706007401. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . fifth ed. American Psychiatric Publishing; Arlington, VA: 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Anyan J., Amir S. Too depressed to swim or too afraid to stop? A reinterpretation of the forced swim test as a measure of anxiety-like behavior. Neuropsychopharmacology. 2018;43(5):931–933. doi: 10.1038/npp.2017.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arp J.M., Ter Horst J.P., Loi M., den Blaauwen J., Bangert E., Fernandez G., Joels M., Oitzl M.S., Krugers H.J. Blocking glucocorticoid receptors at adolescent age prevents enhanced freezing between repeated cue-exposures after conditioned fear in adult mice raised under chronic early life stress. Neurobiol. Learn. Mem. 2016;133:30–38. doi: 10.1016/j.nlm.2016.05.009. [DOI] [PubMed] [Google Scholar]
- Badanich K.A., Becker H.C., Woodward J.J. Effects of chronic intermittent ethanol exposure on orbitofrontal and medial prefrontal cortex-dependent behaviors in mice. Behav. Neurosci. 2011;125(6):879–891. doi: 10.1037/a0025922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath K.G., Manzano-Nieves G., Goodwill H. Early life stress accelerates behavioral and neural maturation of the hippocampus in male mice. Horm. Behav. 2016;82:64–71. doi: 10.1016/j.yhbeh.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath K.G., Nitenson A.S., Lichtman E., Lopez C., Chen W., Gallo M., Goodwill H., Manzano-Nieves G. Early life stress leads to developmental and sex selective effects on performance in a novel object placement task. Neurobiol. Stress. 2017;7:57–67. doi: 10.1016/j.ynstr.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker H.C., Lopez M.F. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin. Exp. Res. 2004;28(12):1829–1838. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- Becker H.C., Lopez M.F. An animal model of alcohol dependence to screen medications for treating alcoholism. Int. Rev. Neurobiol. 2016;126:157–177. doi: 10.1016/bs.irn.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker H.C., Lopez M.F., Doremus-Fitzwater T.L. Effects of stress on alcohol drinking: a review of animal studies. Psychopharmacology (Berlin) 2011;218(1):131–156. doi: 10.1007/s00213-011-2443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray J.G., Roberts A.J., Gruol D.L. Transgenic mice with increased astrocyte expression of CCL2 show altered behavioral effects of alcohol. Neuroscience. 2017;354:88–100. doi: 10.1016/j.neuroscience.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunson K.L., Kramar E., Lin B., Chen Y., Colgin L.L., Yanagihara T.K., Lynch G., Baram T.Z. Mechanisms of late-onset cognitive decline after early-life stress. J. Neurosci. 2005;25(41):9328–9338. doi: 10.1523/JNEUROSCI.2281-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Can A., Dao D.T., Terrillion C.E., Piantadosi S.C., Bhat S., Gould T.D. The tail suspension test. J. Vis. Exp. 2012;59 doi: 10.3791/3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Baram T.Z. Toward understanding how early-life stress reprograms cognitive and emotional brain networks. Neuropsychopharmacology. 2016;41(1):197–206. doi: 10.1038/npp.2015.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commons K.G., Cholanians A.B., Babb J.A., Ehlinger D.G. The rodent forced swim test measures stress-coping strategy, not depression-like behavior. ACS Chem. Neurosci. 2017;8(5):955–960. doi: 10.1021/acschemneuro.7b00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contet C., Kim A., Le D., Iyengar S.K., Kotzebue R.W., Yuan C.J., Kieffer B.L., Mandyam C.D. mu-Opioid receptors mediate the effects of chronic ethanol binge drinking on the hippocampal neurogenic niche. Addiction Biol. 2014;19(5):770–780. doi: 10.1111/adb.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan J.F., Mombereau C., Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci. Biobehav. Rev. 2005;29(4–5):571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- d'Audiffret A.C., Frisbee S.J., Stapleton P.A., Goodwill A.G., Isingrini E., Frisbee J.C. Depressive behavior and vascular dysfunction: a link between clinical depression and vascular disease? J. Appl. Physiol. 2010;108(5):1041–1051. doi: 10.1152/japplphysiol.01440.2009. 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalm S., Enthoven L., Meijer O.C., van der Mark M.H., Karssen A.M., de Kloet E.R., Oitzl M.S. Age-related changes in hypothalamic-pituitary-adrenal axis activity of male C57BL/6J mice. Neuroendocrinology. 2005;81(6):372–380. doi: 10.1159/000089555. [DOI] [PubMed] [Google Scholar]
- Daut R.A., Busch E.F., Ihne J., Fisher D., Mishina M., Grant S.G., Camp M., Holmes A. Tolerance to ethanol intoxication after chronic ethanol: role of GluN2A and PSD-95. Addiction Biol. 2015;20(2):259–262. doi: 10.1111/adb.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon R.M. Digging and marble burying in mice: simple methods for in vivo identification of biological impacts. Nat. Protoc. 2006;1(1):122–124. doi: 10.1038/nprot.2006.20. [DOI] [PubMed] [Google Scholar]
- Dere E., Topic B., De Souza Silva M.A., Srejic M., Frisch C., Buddenberg T., Huston J.P. The graded anxiety test: a novel test of murine unconditioned anxiety based on the principles of the elevated plus-maze and light-dark test. J. Neurosci. Methods. 2002;122(1):65–73. doi: 10.1016/s0165-0270(02)00274-1. [DOI] [PubMed] [Google Scholar]
- Ducottet C., Aubert A., Belzung C. Susceptibility to subchronic unpredictable stress is related to individual reactivity to threat stimuli in mice. Behav. Brain Res. 2004;155(2):291–299. doi: 10.1016/j.bbr.2004.04.020. [DOI] [PubMed] [Google Scholar]
- Egli M., Koob G.F., Edwards S. Alcohol dependence as a chronic pain disorder. Neurosci. Biobehav. Rev. 2012;36(10):2179–2192. doi: 10.1016/j.neubiorev.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhabazi K., Ayachi S., Ilien B., Simonin F. Assessment of morphine-induced hyperalgesia and analgesic tolerance in mice using thermal and mechanical nociceptive modalities. J. Vis. Exp. 2014;89 doi: 10.3791/51264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliot L., Richardson S.S. Sex in context: limitations of animal studies for addressing human sex/gender neurobehavioral Health disparities. J. Neurosci. 2016;36(47):11823–11830. doi: 10.1523/JNEUROSCI.1391-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund M.M., Egeland B., Oliva E.M., Collins W.A. Childhood and adolescent predictors of heavy drinking and alcohol use disorders in early adulthood: a longitudinal developmental analysis. Addiction. 2008;103(Suppl 1):23–35. doi: 10.1111/j.1360-0443.2008.02174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch M.A. The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology (Berlin) 2011;214(1):17–31. doi: 10.1007/s00213-010-1916-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch M.A., Steer C.D., Newman T.K., Gibson N., Goldman D. Early life stress, MAOA, and gene-environment interactions predict behavioral disinhibition in children. Gene Brain Behav. 2010;9(1):65–74. doi: 10.1111/j.1601-183X.2009.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti V.J., Anda R.F., Nordenberg D., Williamson D.F., Spitz A.M., Edwards V., Koss M.P., Marks J.S. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am. J. Prev. Med. 1998;14(4):245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Frisbee J.C., Brooks S.D., Stanley S.C., d'Audiffret A.C. An unpredictable chronic mild stress protocol for instigating depressive symptoms, behavioral changes and negative Health outcomes in rodents. J. Vis. Exp. 2015;106 doi: 10.3791/53109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch M.B. Ethanol withdrawal and hyperalgesia. Curr. Drug Abuse Rev. 2009;2(1):41–50. doi: 10.2174/1874473710902010041. [DOI] [PubMed] [Google Scholar]
- Gatch M.B., Lal H. Effects of ethanol and ethanol withdrawal on nociception in rats. Alcohol Clin. Exp. Res. 1999;23(2):328–333. [PubMed] [Google Scholar]
- Golub M.S., Sobin C.A. Statistical modeling with litter as a random effect in mixed models to manage "intralitter likeness. Neurotoxicol. Teratol. 2020;77 doi: 10.1016/j.ntt.2019.106841. [DOI] [PubMed] [Google Scholar]
- Green J.G., McLaughlin K.A., Berglund P.A., Gruber M.J., Sampson N.A., Zaslavsky A.M., Kessler R.C. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: associations with first onset of DSM-IV disorders. Arch. Gen. Psychiatr. 2010;67(2):113–123. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green P.G., Chen X., Alvarez P., Ferrari L.F., Levine J.D. Early-life stress produces muscle hyperalgesia and nociceptor sensitization in the adult rat. Pain. 2011;152(11):2549–2556. doi: 10.1016/j.pain.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubbs F. Procedures for detecting outlying observations in samples. Technometrics. 1969;11(1):1–21. [Google Scholar]
- Helle A.C., Trull T.J., Watts A.L., McDowell Y., Sher K.J. Psychiatric comorbidity as a function of severity: DSM-5 alcohol use disorder and HiTOP classification of mental disorders. Alcohol Clin. Exp. Res. 2020;44(3):632–644. doi: 10.1111/acer.14284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holleran K.M., Wilson H.H., Fetterly T.L., Bluett R.J., Centanni S.W., Gilfarb R.A., Rocco L.E., Patel S., Winder D.G. Ketamine and MAG lipase inhibitor-dependent reversal of evolving depressive-like behavior during forced abstinence from alcohol drinking. Neuropsychopharmacology. 2016;41(8):2062–2071. doi: 10.1038/npp.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A., le Guisquet A.M., Vogel E., Millstein R.A., Leman S., Belzung C. Early life genetic, epigenetic and environmental factors shaping emotionality in rodents. Neurosci. Biobehav. Rev. 2005;29(8):1335–1346. doi: 10.1016/j.neubiorev.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Horii Y., Kawaguchi M. Higher detection sensitivity of anxiolytic effects of diazepam by ledge-free open arm with opaque walled closed arm elevated plus maze in male rats. Behav. Brain Res. 2015;294:131–140. doi: 10.1016/j.bbr.2015.07.059. [DOI] [PubMed] [Google Scholar]
- Huitron-Resendiz S., Nadav T., Krause S., Cates-Gatto C., Polis I., Roberts A.J. Effects of withdrawal from chronic intermittent ethanol exposure on sleep characteristics of female and male mice. Alcohol Clin. Exp. Res. 2018;42(3):540–550. doi: 10.1111/acer.13584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochum T., Boettger M.K., Burkhardt C., Juckel G., Bar K.J. Increased pain sensitivity in alcohol withdrawal syndrome. Eur. J. Pain. 2010;14(7):713–718. doi: 10.1016/j.ejpain.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Jury N.J., DiBerto J.F., Kash T.L., Holmes A. Sex differences in the behavioral sequelae of chronic ethanol exposure. Alcohol. 2017;58:53–60. doi: 10.1016/j.alcohol.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash T.L., Baucum A.J., 2nd, Conrad K.L., Colbran R.J., Winder D.G. Alcohol exposure alters NMDAR function in the bed nucleus of the stria terminalis. Neuropsychopharmacology. 2009;34(11):2420–2429. doi: 10.1038/npp.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komada M., Takao K., Miyakawa T. Elevated plus maze for mice. J. Vis. Exp. 2008;22 doi: 10.3791/1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G.F. Theoretical frameworks and mechanistic aspects of alcohol addiction: alcohol addiction as a reward deficit disorder. Curr. Top Behav. Neurosci. 2013;13:3–30. doi: 10.1007/7854_2011_129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreifeldt M., Le D., Treistman S.N., Koob G.F., Contet C. BK channel beta1 and beta4 auxiliary subunits exert opposite influences on escalated ethanol drinking in dependent mice. Front. Integr. Neurosci. 2013;7:105. doi: 10.3389/fnint.2013.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroener S., Mulholland P.J., New N.N., Gass J.T., Becker H.C., Chandler L.J. Chronic alcohol exposure alters behavioral and synaptic plasticity of the rodent prefrontal cortex. PLoS One. 2012;7(5) doi: 10.1371/journal.pone.0037541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legates T.A., Dunn D., Weber E.T. Accelerated re-entrainment to advanced light cycles in BALB/cJ mice. Physiol. Behav. 2009;98(4):427–432. doi: 10.1016/j.physbeh.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loi M., Mossink J.C., Meerhoff G.F., Den Blaauwen J.L., Lucassen P.J., Joels M. Effects of early-life stress on cognitive function and hippocampal structure in female rodents. Neuroscience. 2017;342:101–119. doi: 10.1016/j.neuroscience.2015.08.024. [DOI] [PubMed] [Google Scholar]
- Lopez M.F., Doremus-Fitzwater T.L., Becker H.C. Chronic social isolation and chronic variable stress during early development induce later elevated ethanol intake in adult C57BL/6J mice. Alcohol. 2011;45(4):355–364. doi: 10.1016/j.alcohol.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueptow L.M. Novel object recognition test for the investigation of learning and memory in mice. J. Vis. Exp. 2017;126 doi: 10.3791/55718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Devincci A.M., Kampov-Polevoi A., McKinley R.E., Morrow D.H., O'Buckley T.K., Morrow A.L. Chronic intermittent ethanol exposure alters stress effects on (3alpha,5alpha)-3-hydroxy-pregnan-20-one (3alpha,5alpha-THP) immunolabeling of amygdala neurons in C57BL/6J mice. Front. Cell. Neurosci. 2016;10:40. doi: 10.3389/fncel.2016.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malter Cohen M., Jing D., Yang R.R., Tottenham N., Lee F.S., Casey B.J. Early-life stress has persistent effects on amygdala function and development in mice and humans. Proc. Natl. Acad. Sci. U. S. A. 2013;110(45):18274–18278. doi: 10.1073/pnas.1310163110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell-Dowling K., Miczek K.A. Alcohol, psychomotor-stimulants and behaviour: methodological considerations in preclinical models of early-life stress. Psychopharmacology (Berlin) 2018;235(4):909–933. doi: 10.1007/s00213-018-4852-5. [DOI] [PubMed] [Google Scholar]
- Millstein R.A., Holmes A. Effects of repeated maternal separation on anxiety- and depression-related phenotypes in different mouse strains. Neurosci. Biobehav. Rev. 2007;31(1):3–17. doi: 10.1016/j.neubiorev.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Molendijk M.L., de Kloet E.R. Immobility in the forced swim test is adaptive and does not reflect depression. Psychoneuroendocrinology. 2015;62:389–391. doi: 10.1016/j.psyneuen.2015.08.028. [DOI] [PubMed] [Google Scholar]
- Molet J., Maras P.M., Avishai-Eliner S., Baram T.Z. Naturalistic rodent models of chronic early-life stress. Dev. Psychobiol. 2014;56(8):1675–1688. doi: 10.1002/dev.21230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriya Y., Kasahara Y., Hall F.S., Sakakibara Y., Uhl G.R., Tomita H., Sora I. Sex differences in the effects of adolescent social deprivation on alcohol consumption in mu-opioid receptor knockout mice. Psychopharmacology (Berlin) 2015;232(8):1471–1482. doi: 10.1007/s00213-014-3784-y. [DOI] [PubMed] [Google Scholar]
- Naninck E.F., Hoeijmakers L., Kakava-Georgiadou N., Meesters A., Lazic S.E., Lucassen P.J., Korosi A. Chronic early life stress alters developmental and adult neurogenesis and impairs cognitive function in mice. Hippocampus. 2015;25(3):309–328. doi: 10.1002/hipo.22374. [DOI] [PubMed] [Google Scholar]
- Pang T.Y., Renoir T., Du X., Lawrence A.J., Hannan A.J. Depression-related behaviours displayed by female C57BL/6J mice during abstinence from chronic ethanol consumption are rescued by wheel-running. Eur. J. Neurosci. 2013;37(11):1803–1810. doi: 10.1111/ejn.12195. [DOI] [PubMed] [Google Scholar]
- Pleil K.E., Lowery-Gionta E.G., Crowley N.A., Li C., Marcinkiewcz C.A., Rose J.H., McCall N.M., Maldonado-Devincci A.M., Morrow A.L., Jones S.R., Kash T.L. Effects of chronic ethanol exposure on neuronal function in the prefrontal cortex and extended amygdala. Neuropharmacology. 2015;99:735–749. doi: 10.1016/j.neuropharm.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan G., Melugin P.R., Wu F., Fang H.M., Weber R., Kroener S. Calcium chloride mimics the effects of acamprosate on cognitive deficits in chronic alcohol-exposed mice. Psychopharmacology (Berlin) 2018;235(7):2027–2040. doi: 10.1007/s00213-018-4900-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice C.J., Sandman C.A., Lenjavi M.R., Baram T.Z. A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology. 2008;149(10):4892–4900. doi: 10.1210/en.2008-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers R.J., Dalvi A. Anxiety, defence and the elevated plus-maze. Neurosci. Biobehav. Rev. 1997;21(6):801–810. doi: 10.1016/s0149-7634(96)00058-9. [DOI] [PubMed] [Google Scholar]
- Rose J.H., Karkhanis A.N., Chen R., Gioia D., Lopez M.F., Becker H.C., McCool B.A., Jones S.R. Supersensitive Kappa opioid receptors promotes ethanol withdrawal-related behaviors and reduce dopamine signaling in the nucleus accumbens. Int. J. Neuropsychopharmacol. 2016;19(5) doi: 10.1093/ijnp/pyv127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu H., Kreifeldt M., Contet C. Affective disturbances during withdrawal from chronic intermittent ethanol inhalation in C57BL/6J and DBA/2J male mice. Alcohol Clin. Exp. Res. 2018;42(7):1281–1290. doi: 10.1111/acer.13760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somkuwar S.S., Vendruscolo L.F., Fannon M.J., Schmeichel B.E., Nguyen T.B., Guevara J., Sidhu H., Contet C., Zorrilla E.P., Mandyam C.D. Abstinence from prolonged ethanol exposure affects plasma corticosterone, glucocorticoid receptor signaling and stress-related behaviors. Psychoneuroendocrinology. 2017;84:17–31. doi: 10.1016/j.psyneuen.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steru L., Chermat R., Thierry B., Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berlin) 1985;85(3):367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- Tan S., Ho H.S., Song A.Y., Low J., Je H.S. Maternal separation does not produce a significant behavioral change in mice. Exp. Neurobiol. 2017;26(6):390–398. doi: 10.5607/en.2017.26.6.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varodayan F.P., Sidhu H., Kreifeldt M., Roberto M., Contet C. Morphological and functional evidence of increased excitatory signaling in the prelimbic cortex during ethanol withdrawal. Neuropharmacology. 2018;133:470–480. doi: 10.1016/j.neuropharm.2018.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo L.F., Roberts A.J. Operant alcohol self-administration in dependent rats: focus on the vapor model. Alcohol. 2014;48(3):277–286. doi: 10.1016/j.alcohol.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker C.D., Bath K.G., Joels M., Korosi A., Larauche M., Lucassen P.J., Morris M.J., Raineki C., Roth T.L., Sullivan R.M., Tache Y., Baram T.Z. Chronic early life stress induced by limited bedding and nesting (LBN) material in rodents: critical considerations of methodology, outcomes and translational potential. Stress. 2017;20(5):421–448. doi: 10.1080/10253890.2017.1343296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalcin I., Aksu F., Belzung C. Effects of desipramine and tramadol in a chronic mild stress model in mice are altered by yohimbine but not by pindolol. Eur. J. Pharmacol. 2005;514(2–3):165–174. doi: 10.1016/j.ejphar.2005.03.029. [DOI] [PubMed] [Google Scholar]
- Zorrilla E.P. Multiparous species present problems (and possibilities) to developmentalists. Dev. Psychobiol. 1997;30(2):141–150. doi: 10.1002/(sici)1098-2302(199703)30:2<141::aid-dev5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.