Abstract
The 2015 European Society of Cardiology/European Respiratory Society treatment guidelines recommend frequent risk assessment in pulmonary arterial hypertension utilizing risk variables. Our objectives were: (1) to investigate the impact of inhaled treprostinil on risk stratification using the French noninvasive approach and REVEAL 2.0, and (2) to analyze the prognostic utility of both risk stratification methods in the predominantly New York Heart Association/World Health Organization functional class III/IV cohorts of TRIUMPH and BEAT. A post hoc analysis was performed to assess risk at baseline and follow-up at Week 12 in the TRIUMPH cohort (n = 148) and at Week 16, 21, and 30 in the inhaled treprostinil naïve placebo BEAT cohort (n = 73). Overall survival, clinical worsening-free survival, and pulmonary arterial hypertension-related hospitalization-free survival were all assessed in the pooled TRIUMPH and inhaled treprostinil naïve placebo BEAT cohorts based on risk group/strata at Week 12/16 follow-up. Inhaled treprostinil improved REVEAL 2.0 risk stratum (OR: 2.38, 95% CI: 1.09–5.19, p = 0.0298) and REVEAL 2.0 score (p = 0.0008) compared to placebo in the TRIUMPH cohort at Week 12. REVEAL 2.0 risk stratum and the number of low-risk criteria by the French approach improved at Weeks 16, 21, and 30 in the inhaled treprostinil naïve placebo BEAT cohort. Combining cohorts, REVEAL 2.0 risk stratification at follow-up was prognostic for clinical worsening-free, pulmonary arterial hypertension hospitalization-free, and overall survival, whereas the number of low-risk criteria was not. These post-hoc pooled analyses suggest inhaled treprostinil improves risk status and indicates that the REVEAL 2.0 calculator may be more suitable than the French noninvasive method for evaluating short-term clinical change in the New York Heart Association/World Health Organization functional class III/IV population.
Keywords: pulmonary arterial hypertension, treprostinil, risk stratification, Registry to Evaluate Early and Long-term PAH disease management (REVEAL), French noninvasive, TRIUMPH
Introduction
Pulmonary arterial hypertension (PAH) is a rare and progressive disease characterized by increased pulmonary vascular resistance which can lead to right ventricular failure and eventual death.1 Treatment options have expanded greatly in the past 20 years which have substantially improved the prognosis of many PAH patients.2 However, despite the availability of treatments which delay clinical worsening or improve PAH symptoms, many patients continue to develop right ventricular failure.3,4 As such, longitudinal risk assessments in patients with PAH may help predict patient outcomes and inform treatment decisions.5
The 2015 European Society of Cardiology (ESC)/European Respiratory Society (ERS) treatment guidelines6 and the proceedings of the 6th World Symposium on pulmonary hypertension7 recommend risk assessment using various clinical and imaging parameters at regular intervals. Based on a comprehensive risk assessment, patients are categorized as low, intermediate, or high risk based on an estimated one-year mortality of < 5%, 5–10%, and > 10%, respectively. Achieving and maintaining low-risk status is the ideal goal of PAH treatment.8
Three abbreviated versions of the 2015 ESC/ERS risk assessment have been evaluated retrospectively in incident populations of PAH patients in the French, Swedish, and Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension registries.5,8,9 A separate set of stratification methodologies has also been evaluated in the Registry to Evaluate Early and Long-term PAH disease management (REVEAL).10–12 The French noninvasive approach determines the number of low-risk criteria using only the New York Heart Association (NYHA)/World Health Organization (WHO) functional class (FC), brain natriuretic peptide (BNP)/N-Terminal pro-brain natriuretic peptide (NT-proBNP), and six-minute walk distance (6WMD).5 The recently redefined REVEAL 2.0 stratifies patient risk using up to 13 variables, including disease etiology, demographics, all-cause hospitalization within previous six months, echocardiogram, pulmonary function test, right heart catheterization parameters, NYHA/WHO FC, vital signs, 6MWD, BNP/NT-proBNP, and renal insufficiency.12–15 Only 7 of 13 variables are required to maintain the validity of the REVEAL 2.0 calculator.12–14 More recently, two abridged versions have been developed, REVEAL Lite 1 and Lite 2, which require eight and six variables, respectively.13,14 These abridged versions have not yet been validated. Thus far, validations of risk assessments have focused on NYHA/WHO FC II/III. The predictive capabilities of these methods in NYHA/WHO FC III/IV are largely unknown.
Inhaled treprostinil (Tyvaso; Silver Spring, MD) is FDA-approved for the treatment of PAH based on the TRIUMPH study.16 During the 12-week study, inhaled treprostinil significantly improved exercise capacity in 235 patients with PAH and NYHA FC III/IV symptoms on background therapy with bosentan or sildenafil.17 Long-term outcomes for these patients in the TRIUMPH-OLE extension study indicated continued improvement in exercise capacity.18 The event-driven BEAT study was designed to assess the efficacy and safety of oral esuberaprost versus placebo when added to background therapy with inhaled treprostinil in WHO FC III/IV patients with PAH. No improvement was seen in morbidity or mortality when esuberaprost was added to inhaled treprostinil in patients receiving background single or dual therapy with an endothelin receptor antagonist and/or phosphodiesterase type 5 inhibitor/soluble guanylate cyclase stimulator.19
In this post hoc analysis, we focused on evaluating the benefits of inhaled treprostinil therapy in NYHA/WHO FC III/IV patients using risk stratification and long-term outcomes. The cohort of patients who were previously naïve to inhaled treprostinil and received placebo (iTNP) (n = 73) in the BEAT study provides an opportunity to further study the short- and long-term effects of inhaled treprostinil therapy.19 The iTNP cohort closely approximates the TRIUMPH study population, allowing for combined analyses. The TRIUMPH and BEAT studies collected noninvasive risk parameters, such as NYHA/WHO FC, NT-proBNP, and 6MWD, at baseline and follow-up.17,19 Furthermore, the event-driven nature of the BEAT study allows for long-term risk stratification in patients initiated on inhaled treprostinil. In addition to long-term data from the BEAT cohort, long-term outcomes for the TRIUMPH cohort were collected in TRIUMPH-OLE and allowed for assessment of prognostic utility of risk stratification in NYHA/WHO FC III/IV PAH patients.18
The primary objectives of these post hoc analyses were two-fold. We aimed to (1) evaluate the impact of inhaled treprostinil on risk stratification in the TRIUMPH and BEAT iTNP cohorts and (2) determine if risk stratification using the French noninvasive method and/or the REVEAL 2.0 calculator is predictive of long-term morbidity and mortality in cohorts of primarily NYHA/WHO FC III/IV PAH patients. This is the first evaluation of risk strata focusing on a predominantly higher risk PAH population.
Methods
Clinical studies
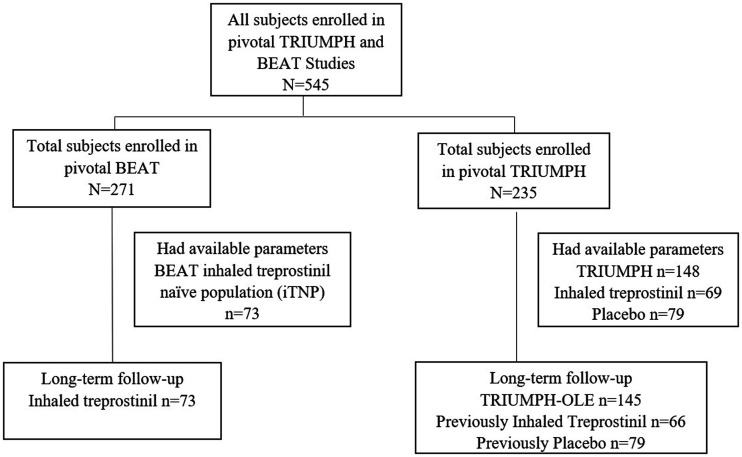
TRIUMPH (NCT00147199) was a 12-week, phase 3, double-blind study in NYHA FC III and IV patients with PAH who received a stable dose of bosentan or sildenafil for at least three months prior to study entry and were randomly assigned to placebo or inhaled treprostinil.17 At Week 12 in the TRIUMPH study, the active group had achieved a median of nine breaths per session. Patients completing TRIUMPH were able to enter the open-label extension study, TRIUMPH-OLE, in which all patients received inhaled treprostinil and were followed up to 4.3 years. In TRIUMPH-OLE, 6MWD and NYHA/WHO FC were collected every three months and patients were followed for clinical worsening events.18 Baseline values were assessed at the initiation of the TRIUMPH study. Of the TRIUMPH subjects, 148 had available risk parameters for analyses. The inhaled treprostinil group included 69 patients and the placebo group included 79 patients. Long-term follow-up in TRIUMPH-OLE was available for 145 of these patients, 66 of whom were initially assigned inhaled treprostinil (Fig. 1).
Fig. 1.
Subject disposition. Of the 545 patients enrolled in TRIUMPH and BEAT iTNP subgroup, 148 patients from TRIUMPH and 73 patients from the BEAT iTNP subgroup had available parameters prior to initiation of inhaled treprostinil and at Week 12/16.
BEAT (NCT01908699) was an event-driven, phase 3, double-blind study in WHO FC III and IV patients with PAH on no, single, or dual oral non-prostacyclin class PAH background therapy, who were randomly assigned to esuberaprost or placebo in addition to inhaled treprostinil.19 Randomization to esuberaprost or placebo was stratified by inhaled treprostinil exposure at screening, allowing for analysis of four distinct cohorts (see supplement for additional study details). The present analysis included the BEAT cohort which was previously naïve to inhaled treprostinil and received placebo during the study (n = 73), subsequently referred to as the BEAT iTNP cohort (Fig. 1). The BEAT iTNP cohort was required to complete a ≥90-day run-in with inhaled treprostinil prior to the baseline timepoint in BEAT, which occurred at a median of 16.4 (IQR: 15.9–17.1) weeks from inhaled treprostinil initiation. Baseline values used for these analyses were measured at study intake, prior to initiation of inhaled treprostinil. The BEAT study assessments at Weeks 4 and 12 occurred at a median of 20.6 (IQR: 20.0–21.4) and 29.9 (IQR: 28.4–30.7) weeks, respectively, from inhaled treprostinil initiation.19 For the purposes of this analysis, the adjusted follow-up timepoints are referred to as Week 16, 21, and 30 from inhaled treprostinil initiation to better align with treprostinil exposure from the TRIUMPH study. The median dose of inhaled treprostinil in this cohort at each of these time points was nine breaths per session, which matched the TRIUMPH study. Double-blind treatment continued until the occurrence of a primary endpoint event, discontinuation of double-blind treatment, or the end of the study. Details of this trial are included in the supplementary material.19
The TRIUMPH and BEAT studies were conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. The institutional review board at each participating center approved the study and written consent was collected from all patients.
Patient population
TRIUMPH and BEAT included adult patients with a confirmed diagnosis of idiopathic or familial PAH or PAH associated with collagen vascular disease, human immunodeficiency virus infection, repaired congenital systemic-to-pulmonary shunts (BEAT only), or drug or toxin exposure. Patients were required to have a right heart catheterization with findings consistent with PAH (mean pulmonary artery pressure ≥25 mmHg, pulmonary capillary wedge pressure ≤15 mmHg, and pulmonary vascular resistance > 3 Wood units) and NYHA/WHO FC III or IV at screening with declining or unsatisfactory clinical response to current PAH treatment. For the present analyses, we included the cohort of patients who entered BEAT and were naïve to inhaled treprostinil and received placebo (BEAT iTNP cohort) along with the TRIUMPH cohort which included both a placebo and inhaled treprostinil group in the pivotal study. For long-term outcomes in TRIUMPH-OLE, both patient groups were placed on inhaled treprostinil.
Outcomes
Long-term outcomes were observed for the TRIUMPH-OLE cohort and for all patients in the BEAT iTNP cohort. Clinical worsening for the TRIUMPH cohort was defined post hoc as death, study discontinuation due to disease progression, PAH hospitalization (unadjudicated or investigator reported), or addition of a new PAH therapy and a decrease of ≥15% from baseline 6MWD. Clinical worsening (adjudicated) for the BEAT iTNP cohort was defined per protocol as death, PAH hospitalization, initiation of parenteral prostacyclin therapy related to worsening PAH, disease progression (decrease from baseline 6MWD ≥15% and worsening NYHA/WHO FC and/or symptoms of right heart failure), or unsatisfactory long-term clinical response (decrease from baseline 6MWD ≥15% and sustained NYHA/WHO FC III or IV symptoms for 24 weeks).
Risk assessment
Patients were stratified according to three risk assessment methods: REVEAL 2.0,12 REVEAL Lite 2.0,13,14 and the French noninvasive method.5 REVEAL 2.0 scores ≤6 were classified as low risk, scores of 7–8 were intermediate, and scores of > 9 were classified as high risk.12 REVEAL Lite 2.0 scores ≤5 were classified as low risk, scores of 6–7 were intermediate, and scores of > 8 were classified as high risk.13,14 The French noninvasive method classified risk by number of noninvasive low-risk criteria (NYHA/WHO FC I/II, 6MWD > 440 m, and NT-proBNP < 300 ng/L).5 Eight parameters (etiology, demographics (age and gender), renal insufficiency, NYHA/WHO FC, vital signs (heart rate and systolic blood pressure), 6MWD, NT-proBNP) of the 13 included in the REVEAL 2.0 risk calculator were available and used for calculation. The REVEAL Lite 2.0 score includes six parameters (renal insufficiency, NYHA/WHO FC, vital signs (heart rate and systolic blood pressure), 6MWD, NT-proBNP). In the calculation of the REVEAL risk score, missing heart rate, systolic blood pressure, or estimated glomerular filtration rate (eGFR) were imputed as worst case. Subject eGFR was calculated without consideration of patient race since these data were not available for patients enrolled in TRIUMPH. Idiopathic and familial PAH were combined during data collection in the respective studies; therefore, patients were assumed to be idiopathic for calculation of the REVEAL 2.0 score in the present analysis. A sensitivity analysis was conducted with the assumption of familial PAH for the purpose of the REVEAL 2.0 score (Supplement Figure S1).
Statistical analyses
All assessments were summarized by descriptive statistics, as appropriate. Comparisons for categorical assessments were by chi-square tests and comparisons for continuous assessments were by analyses of variance or non-parametric alternatives, as appropriate. Time to event for long-term outcomes (survival, PAH-related hospitalization, and clinical worsening) over two years was analyzed via Kaplan–Meier curves for all risk score groups. Concordance indexes (c-indexes) were used to evaluate discrimination of the risk assessment tools by evaluating concordance between predicted versus actual survival time (0.5 = random concordance; 1 = perfect concordance). Restricted mean survival time was calculated as the area under the Kaplan–Meier curve up to a specified time. Odds ratios and their associated 95% confidence intervals were derived from logistic regression models. Wald scores and their associated p-values were derived from logistic regression models to assess the key drivers of score improvement, a dichotomized variable indicating those that were improved vs. not improved. Hazard ratios and their associated 95% confidence intervals were derived from Cox proportional hazards models. p-Values ≤0.05 were considered significant and no adjustments were made for multiplicity.
Results
Risk stratification in the TRIUMPH cohort
Of the 235 PAH patients included in TRIUMPH, 148 had all three French noninvasive parameters collected at baseline and Weeks 12 and 145 had long-term follow-up data in the open-label extension study. In this cohort of TRIUMPH subjects, the majority were female (83%) with idiopathic or familial PAH (53%). Ninety-seven percent were NYHA/WHO FC III and the median (IQR) 6WMD and NT-proBNP at baseline were 352 (298, 404) m and 636 (215, 1483) pg/mL, respectively. At baseline, the mean REVEAL 2.0 risk score (RRS) was 7.36 ± 2.2 with 35%, 31%, and 35% stratified as low, intermediate, or high risk, respectively. The majority of patients were higher risk and had 0 (66%) or 1 (32%) low-risk criteria by the French noninvasive method (Table 1).
Table 1.
Baseline characteristics.
| Baseline characteristic | Combined cohorts(n = 221) | TRIUMPH sub-group(n = 148) | BEAT iTNP sub-group(n = 73) |
|---|---|---|---|
| Age, years (mean (range)) | 56 (18–78) | 54 (18–75) | 58 (22–78) |
| Males > 60 years, n (%) | 17 (8) | 8 (5) | 9 (12) |
| Female, % | 79% | 83% | 70% |
| Etiology, n (%) | |||
| Idiopathic or familial PAH | 123 (56) | 78 (53) | 45 (62) |
| Collagen vascular disease | 72 (33) | 51 (35) | 21 (29) |
| Other | 26 (12) | 19 (13) | 7 (10) |
| Background PAH therapy, % | |||
| None | 6% | 0% | 18% |
| ERA | 49% | 68% | 11% |
| PDE5-I | 29% | 32% | 22% |
| ERA + PDE5-I | 15% | 0% | 47% |
| sGC stimulator + ERA | 1% | 0% | 3% |
| 6MWD (m), median (IQR) | 342 (285, 403) | 352 (298, 404) | 326 (244, 386) |
| ≥440, n (%) | 22 (10) | 10 (7) | 12 (16) |
| 320 to < 440, n (%) | 119 (54) | 91 (62) | 28 (38) |
| 165 to <320, n (%) | 72 (33) | 47 (32) | 25 (34) |
| ≤165, n (%) | 8 (4) | 0 (0) | 8 (11) |
| NT-proBNP (pg/mL), median (IQR) | 611 (214–1400) | 636 (215–1483) | 561 (211–1210) |
| <300, n (%) | 73 (33) | 48 (32) | 25 (34) |
| 300 to < 1100, n (%) | 81 (37) | 54 (37) | 27 (37) |
| ≥1100, n (%) | 67 (30) | 46 (31) | 21 (29) |
| NYHA/WHO FC | |||
| Class III, n (%) | 211 (96) | 144 (97) | 67 (92) |
| Class IV, n (%) | 10 (5) | 4 (3) | 6 (8) |
| Heart rate | |||
| >96 beats per minute, n (%) | 20 (9) | 15 (10) | 5 (7) |
| ≤96 beats per minute, n (%) | 201 (91) | 133 (90) | 68 (93) |
| Systolic blood pressure, n (%) | |||
| <110 mmHg | 77 (35) | 58 (39) | 19 (26) |
| ≥110 mmHg | 144 (65) | 90 (61) | 54 (74) |
| Renal insufficiency, n (%) | 44 (20) | 24 (16) | 20 (27) |
| REVEAL 2.0 | |||
| RRS, mean ± SD | 7.41 ± 2.4 | 7.36 ± 2.2 | 7.51 ± 2.4 |
| Risk stratum, % (low/intermediate/high) | 36/30/34 | 35/31/35 | 38/29/33 |
| French noninvasiveNumber of low-risk criteria, % (0/1/2/3) | 64/32/5/0 | 66/32/2/0 | 60/30/10/0 |
6MWD: six-minute walk distance; ERA: endothelin receptor antagonist; PAH: pulmonary arterial hypertension; NT-pro BNP: N-terminal pro-brain natriuretic peptide; NYHA: New York Heart Association; PDE5-I: phosphodiesterase type 5 inhibitor; RRS: REVEAL 2.0 risk score; SD: standard deviation; sGC: soluble guanylate cyclase; WHO FC: World Health Organization Functional Classification; REVEAL: Registry to Evaluate Early and Long-term PAH disease management; IQR: interquartile range.
Note: renal insufficiency was defined as an eGFR < 60 mL/min/1.73 m2.
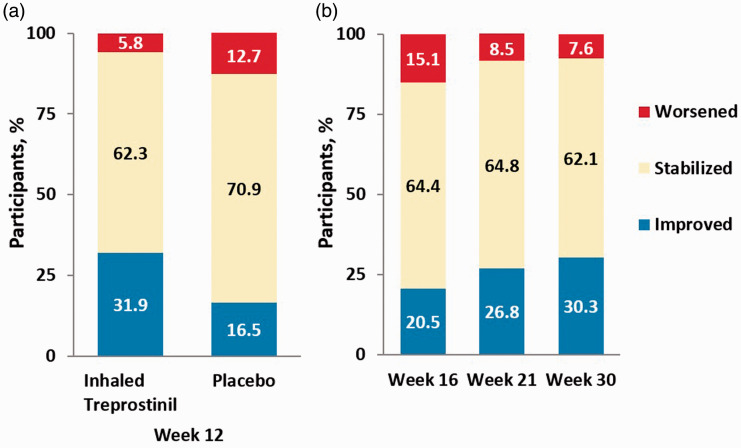
At Week 12, 16.5% of patients receiving placebo and 31.9% of patients receiving inhaled treprostinil improved their REVEAL 2.0 risk stratum from baseline (Fig. 2a, p = 0.028). This means that patients receiving inhaled treprostinil were more than twice as likely to improve their REVEAL 2.0 risk stratum compared to patients receiving placebo (OR: 2.38, 95% CI: 1.09–5.19, p = 0.0298). Forty-five percent of patients on inhaled treprostinil were considered low risk by REVEAL 2.0. At Week 12, a significantly higher proportion of patients receiving inhaled treprostinil had also improved their RRS from baseline compared to placebo (45% vs. 27%, p = 0.02) with a mean RRS change of –0.90 (Table 2, SE: 0.26, p = 0.0008) compared to placebo. Analysis using the abbreviated REVEAL Lite 2.0 calculator yielded similar improvement in RRS (Supplement Figure S2). Improvement in RRS in the inhaled treprostinil group was primarily driven by improvement in systolic blood pressure (Wald = 6.22, p = 0.0127), NT-proBNP (Wald = 5.06, p = 0.0245), and 6MWD (Wald = 2.95, p = 0.0857). Improvement of the number of low-risk criteria (French noninvasive method) from baseline to Week 12 was noted in 34.8% of patients receiving inhaled treprostinil and 29.1% of patients receiving placebo (Supplement Figure S3); however, the difference was not significant. Only 4% of patients using inhaled treprostinil achieved three low-risk criteria at Week 12.
Fig. 2.
Change in REVEAL 2.0 risk stratum from baseline at (a) TRIUMPH Week 12 and (b) BEAT iTNP Weeks 16, 21 and 30. “Improved” indicates a shift from a higher- to lower-risk stratum; “Stabilized” indicates the same stratum; “Worsened” indicates a shift from a lower- to higher-risk stratum.
Table 2.
Change from baseline to Week 12 in REVEAL 2.0 Risk Score in TRIUMPH cohort.
| Study groupa | Baseline |
Week 12 |
|
|---|---|---|---|
| Mean (SD) | Mean (SD) change from baseline | Mean difference (SE), p value | |
| Inhaled TRE (n = 69) | 7.38 (2.21) | –0.81 (1.87) | –0.90 (0.26), p = 0.0008 |
| Placebo (n = 79) | 7.34 (2.12) | 0.09 (1.31) | |
SD: standard deviation; SE: standard error; TRE: treprostinil.
Note: If eGFR was not available, a score of +1 was given for this variable.
ap Values were obtained from a two-sample t-test.
RRS was calculated with the following: demographics, comorbidities (eGFR), NYHA/WHO FC, vital signs, 6MWD, and NT-proBNP. Higher values indicate greater risk for clinical worsening or mortality.
Risk stratification in the BEAT cohort
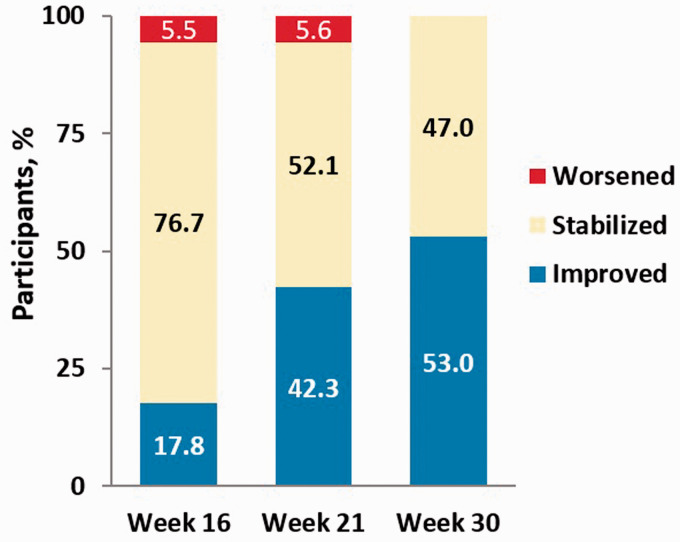
Of 271 patients enrolled in the BEAT study, 73 patients were included in the present analysis (BEAT iTNP). In this cohort, the majority were female (70%) with idiopathic or familial PAH (62%); 83% were receiving FDA-approved PAH background therapy with oral agents; 92% were NYHA/WHO FC III; and the median (IQR) 6WMD and NT-proBNP at baseline were 326 (244–386) m and 561 (211–1210) pg/mL, respectively (Table 1). At baseline, the RRS was 7.51 ± 2.4 with 38, 29, and 33% stratified as low, intermediate, or high risk, respectively. The majority of patients were high risk and had none (60%) or one (30%) low-risk criteria by the French noninvasive method (Table 1). At Week 16, 20.5% of the BEAT iTNP cohort improved their REVEAL risk stratum and 43.8% had improved their RRS compared to baseline. At Weeks 21 and 30, the proportion of subjects improving their REVEAL risk stratum was 26.8% and 30.3%, respectively (Fig. 2b). Similar improvements in RRS were seen over time using the REVEAL Lite 2.0 calculator (Supplement Figure S4). The proportions of patients improving the number of low-risk criteria were 18, 42, and 53% at Weeks 16, 21, and 30, respectively, from inhaled treprostinil initiation (Fig. 3). The number of subjects achieving REVEAL 2.0 low-risk status and 3 low-risk criteria using the French method also increased over time (Table 3). At Week 16, improvements in RRS were driven by 6MWD (Wald = 5.16, p = 0.0231) along with renal function (Wald = 2.58, p = 0.1085) and heart rate (Wald = 1.50, p = 0.2203). Improvements in the number of low-risk criteria by the French noninvasive method were driven by improvement in 6MWD (Wald = 2.10, p = 0.1473).
Fig. 3.
Change in number of low-risk criteria compared to baseline at BEAT iTNP Weeks 16, 21, and 30. “Improved” indicates any increase in the number of low-risk criteria; “Stabilized” indicates the same number of risk criteria; and “Worsened” indicates any decrease in the number of low-risk criteria.
Table 3.
Patients achieving low-risk status over time for the BEAT cohort.
| Week 16 | Week 21 | Week 30 | |
|---|---|---|---|
| REVEAL 2.0 (N) Low-risk, n (%) |
73 31 (42.5) |
70 37 (52.9) |
66 41 (62.1) |
| French noninvasive (N) 3 low-risk criteria, n (%) |
73 0 (0) |
71 4 (5.6) |
66 9 (13.6) |
REVEAL: Registry to Evaluate Early and Long-term PAH disease management.
Notes: REVEAL 2.0 scores of ≤5 were considered “low” risk. Achieving 3 low-risk criteria by the French noninvasive method was considered “low” risk.
REVEAL 2.0 scores of ≤5 were considered “low” risk. Achieving three low-risk criteria by the French Noninvasive method was considered “low” risk.
Risk group/stratum at follow-up and long-term outcomes in the combined cohorts
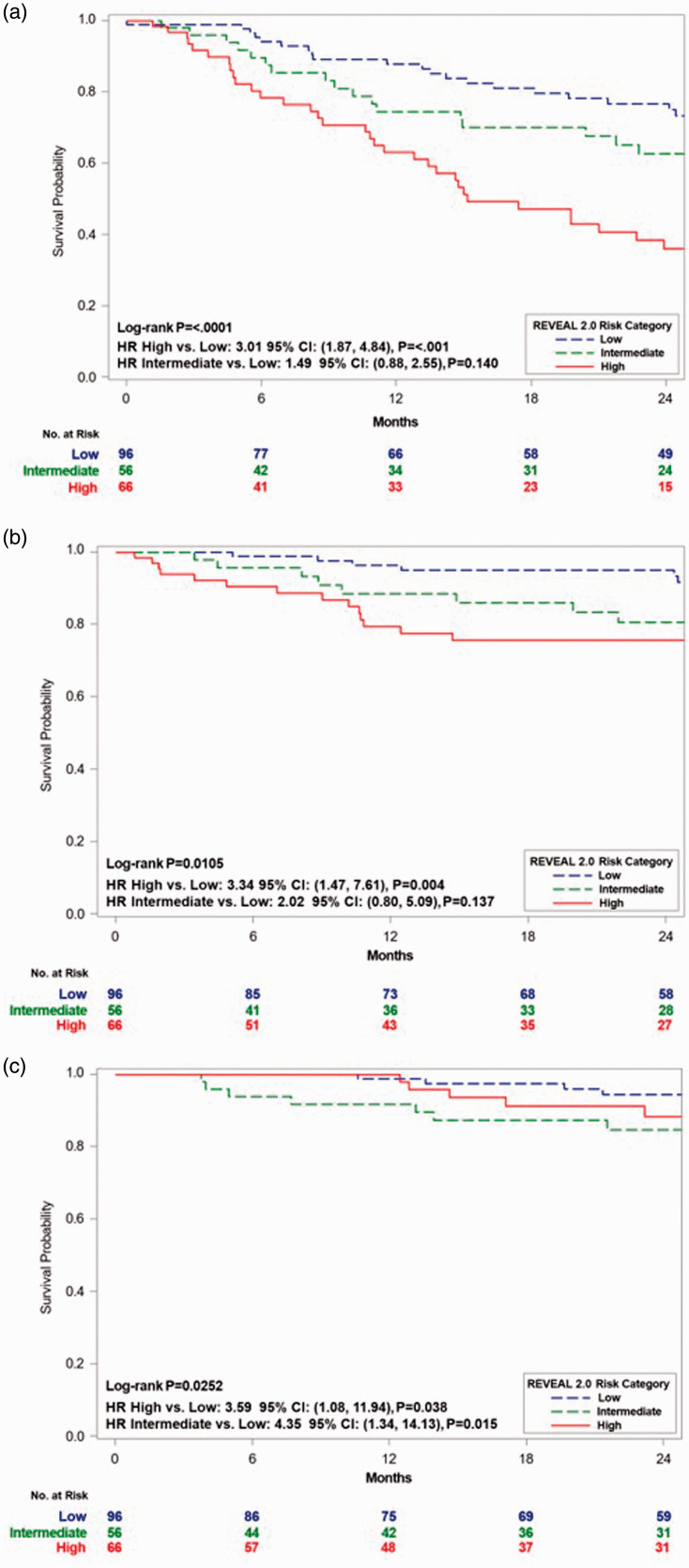
Risk stratification at Week 12/16 and long-term outcomes were assessed in the 218 patients, from the combined TRIUMPH (n = 145) and BEAT iTNP cohorts (n = 73), with sufficient risk parameters available at follow-up. Baseline characteristics for the pooled cohort including 6MWD, NT-proBNP, and WHO FC were similar and are described in Table 1. REVEAL 2.0 risk strata were assessed in the combined cohort and showed a mean RRS of 7.07 ± 2.4 with 44, 26, and 30% stratified as low, intermediate, or high risk. Comparisons of baseline characteristics of patients with improved to REVEAL low-risk category using REVEAL 2.0 and those who worsened or stayed intermediate or high risk are listed in the supplement (Table S3). REVEAL 2.0 risk stratum in the pooled population at Week 12/16 discriminated prognostic groups for clinical worsening-free survival (Fig. 4a, p < 0.0001; C-index: 0.63), PAH-related hospitalization-free survival (Fig. 4b, p = 0.01; C-index: 0.66), and overall survival (Fig. 4c, p = 0.03; C-index: 0.66). Comparing low- and intermediate-risk group stratums to those categorized as high risk at Week 12/16 resulted in a 61% (HR: 0.39; 95% CI: 0.26–0.59, p < 0.001) and 60% (HR: 0.40; 95% CI: 0.20–0.79, p = 0.008) reduced risk of experiencing a clinical worsening event and PAH-related hospitalization, respectively (Fig. 4a and b). Similar results were noted when the abbreviated REVEAL Lite 2.0 calculator was utilized (Supplement Figure S5). There was no significant risk reduction for overall mortality (HR: 0.60; 95% CI: 0.25–1.45, p = 0.255) when comparing the high-risk group to the combined low- and intermediate-risk groups (Fig. 4c). For each one integer drop in RRS at Week 12/16, patients had a 21% (HR: 0.79; 95% CI: 0.65–0.95), 19% (HR: 0.81; 95% CI: 0.74–0.88), and 20% (HR: 0.8; 95% CI: 0.69–0.92) reduction in the risk of death, clinical worsening, and PAH-related hospitalizations, respectively.
Fig. 4.
Kaplan–Meier estimate for (a) clinical worsening-free survival, (b) PAH-related hospitalization-free survival, and (c) overall survival by REVEAL 2.0 risk stratum at Week 12/16 for the pooled TRIUMPH and BEAT iTNP cohorts. A score from <6 was considered low risk, 7 or 8 was considered intermediate risk, and a score for 9 or higher was considered high risk.
HR: hazard ratio; CI: confidence interval; REVEAL: Registry to Evaluate Early and Long-term PAH disease management.
The pooled population was also stratified by number of low-risk criteria (French noninvasive method) achieved at Week 12/16, with 48, 33, 16, and 3% achieving 0, 1, 2, or 3 low-risk criteria, respectively. Risk grouping by number of low-risk criteria achieved did not discriminate between prognostic groups at Week 12/16 (Supplement Figure S6). Achieving any number of low-risk criteria at Week 12/16, however, resulted in a 68% (HR: 0.32; 95% CI: 0.12–0.83, p = 0.019) reduced risk of death within two years compared to those with no low-risk criteria.
Discussion
Regular risk assessments for PAH patients are recommended as part of the standard care, with achieving and maintaining low-risk status as the ideal goal of PAH treatment.6–8 Our post hoc analysis using REVEAL 2.0 demonstrated that patients receiving inhaled treprostinil were able to significantly reduce their RRS and were more than twice as likely to improve their risk stratum compared to placebo during Week 12 of the TRIUMPH study. Sustained improvement in risk stratum was indicated during longer follow-up, as risk stratum continued to improve in the BEAT iTNP cohort at Weeks 16, 21, and 30. These data further support existing evidence demonstrating the benefit of inhaled treprostinil in PAH patients.17–20
The prognostic value of the French noninvasive approach for transplant-free survival was previously shown in post hoc analyses of large-scale clinical trials and registries.5,20–23 The REVEAL calculator, and more recently the REVEAL 2.0 calculator, have been validated in post hoc analyses.11,12,20,22–24 REVEAL Lite 2.0 is an abridged version of the REVEAL 2.0 calculator that has not yet been validated in clinical studies.13,14 Both the REVEAL calculator and the French noninvasive method have been shown to be prognostic of clinical outcomes and assessed the impact of clinical treatment in primarily WHO FC II/III PAH populations of the PATENT-121 and GRIPHON22 clinical studies at Weeks 12 and 24, respectively. Indicatively, evaluation of the French noninvasive and REVEAL 2.0 in the FREEDOM-EV study, which demonstrated the impact of oral treprostinil in a primarily low-risk population, showed significant improvements in the proportion of patients increasing the number of low-risk criteria at Weeks 16–20 and improved RRS at Weeks 12, 24, and 36 compared to placebo.20
Although the French noninvasive method and REVEAL 2.0 have been validated in large, lower risk populations, this study focused on prognostic utility in smaller cohorts of NYHA/WHO FC III/IV PAH patients. Despite 94% of the combined study cohorts receiving at least one PAH therapy prior to treprostinil initiation, most patients had no low-risk criteria and characteristics of intermediate- to high-risk disease according to ESC/ERS guidelines.6 At baseline, 36% of patients were categorized as low risk according to the REVEAL 2.0 calculator with only 5% having 2 or 3 low-risk criteria using the French noninvasive method. REVEAL 2.0 risk stratum at Week 12/16 for the combined TRIUMPH/BEAT studies successfully discriminated outcomes of clinical worsening and PAH-related hospitalizations between low-, intermediate-, and high-risk groups; however, it did not discriminate for overall survival between all groups. This may be due to the limited number of mortality events overall. This analysis supports the utility of RRS to predict long-term outcomes of clinical worsening in patients with PAH who are primarily intermediate to high risk. These data also suggest that stratification using REVEAL 2.0 may be useful in predicting risk of PAH-related hospitalizations alone in addition to overall survival and clinical worsening-free survival. Investigation in a larger dataset of NYHA/WHO FC III/IV PAH patients is needed to confirm the utility of REVEAL 2.0 in predicting overall survival.
In contrast to REVEAL 2.0, the prognostic and discriminatory value of the French noninvasive approach for clinical worsening-free survival, PAH-related hospitalization, and overall survival was not evident in this NYHA/WHO FC III/IV population. Although, patients achieving any number of low-risk criteria at Week 12/16 were found to be at lower risk of death within two years. This is consistent with previous findings using the French noninvasive approach, where differences in transplant-free survival or event-free survival for patients with any low-risk criterion compared to no low-risk criteria are apparent in year 1 or 2, but differences between criteria groups are difficult to distinguish until later timepoints.5,21,22 The inability of the French noninvasive approach to maintain prognostic value in this population may be due to the relatively small proportion (19%) of patients achieving two or three low-risk criteria at Week 12/16. The French noninvasive approach is predominantly designed for distinguishing low risk versus not low risk.5 While no significant differences in the proportion of patients achieving a higher number of low-risk criteria were seen in the TRIUMPH inhaled treprostinil cohort compared to placebo at Week 12, the proportion of patients with a higher number of low-risk criteria in the BEAT iTNP cohort continued to increase throughout Week 30 post-initiation. These data may indicate that in a primarily NYHA/WHO FC III/IV, intermediate- to high-risk population, marked improvement in 6MWD, NT-proBNP, and FC to low-risk status may be challenging during a 12-week follow-up. The small study size may also be a limiting factor. Risk stratification using REVEAL 2.0 may be more likely to indicate improvement in risk status at shorter follow-up and could be used in clinical trial settings and clinical practice in higher risk populations to estimate future prognosis.
Our analysis of various risk assessments was limited by the post hoc nature of our study, including the limitations of previously defined clinical variables and a study design not powered to detect differences. Data were pooled from similar, but not identical, treatment groups to increase the sample size, which may have affected outcomes. Although we cannot definitively state that REVEAL 2.0 is the optimal method for short- and long-term evaluation of risk in the NYHA/WHO FC III/IV population, the prognostic utility of this method is clear.
In conclusion, relatively short courses (3–4 months) of inhaled treprostinil significantly improve REVEAL 2.0 risk status in PAH patients, a majority of whom were already receiving single or dual oral PAH medications. Our post hoc analyses indicate that risk stratification using the REVEAL 2.0 calculator in a small, higher risk population may be more suitable for monitoring change in risk status and have better prognostic utility to predict which patients may have a higher likelihood of clinical worsening than the French noninvasive method. Furthermore, these data indicate that PAH treatment with inhaled treprostinil may improve risk profile as early as Week 12 and continue to have an impact on improvement through at least Week 30 from therapy initiation. These data support guidelines for observing clinical improvements using noninvasive parameters during the first three to six months of therapy, which is consistent with recommendations to re-evaluate patients every three to six months, allowing for early and aggressive intervention or escalation of therapy.6
Supplemental Material
Supplemental material, sj-pdf-1-pul-10.1177_2045894020977025 for Impact of inhaled treprostinil on risk stratification with noninvasive parameters: a post hoc analysis of the TRIUMPH and BEAT studies by Adriano R. Tonelli, Sandeep Sahay, Kathryn W. Gordon, Lisa D. Edwards, Andrew G. Allmon, Meredith Broderick and Andrew C. Nelsen in Pulmonary Circulation
Acknowledgements
The authors would like to thank Natalie Patzlaff, PhD (United Therapeutics Corp.) for editorial support.
Footnotes
Contributorship: All authors contributed to the design of the analysis, interpretation of the data and preparation of the manuscript. L.E. and A.A. performed statistical analyses.
Conflict of interest: A.R.T. has no conflicting interests to declare. S.S. has received personal fees from United Therapeutics Corporation, Actelion and Bayer Pharmaceuticals for consulting and participation in speaker bureaus and advisory boards. K.W.G., L.D.E., M.B., and A.C.N. are employees of United Therapeutics Corporation.
Ethical approval: These original studies were approved by local Institutional Review Boards.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This original studies and publication support for this manuscript were funded by United Therapeutics Corporation.
Guarantor: Dr. Adriano R. Tonelli
ORCID iDs: Adriano R. Tonelli https://orcid.org/0000-0002-0672-1680
Sandeep Sahay https://orcid.org/0000-0002-0672-1680
Lisa D. Edwards https://orcid.org/0000-0002-4867-2151
Supplemental material: Supplemental material for this article is available online.
References
- 1.Tonelli AR, Arelli V, Minai OA, et al. Causes and circumstances of death in pulmonary arterial hypertension. Am J Respir Crit Care Med 2013; 188: 365–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Humbert M, Lau EM, Montani D, et al. Advances in therapeutic interventions for patients with pulmonary arterial hypertension. Circulation 2014; 130: 2189–2208. [DOI] [PubMed] [Google Scholar]
- 3.Van de Veerdonk MC, Kind T, Marcus JT, et al. Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J Am Coll Cardiol 2011; 58: 2511–2519. [DOI] [PubMed] [Google Scholar]
- 4.Van de Veerdonk MC, Marcus JT, Westerhof N, et al. Signs of right ventricular deterioration in clinically stable patients with pulmonary arterial hypertension. Chest 2015; 147: 1063–1071. [DOI] [PubMed] [Google Scholar]
- 5.Boucly A, Weatherald J, Savale L, et al. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur Respir J 2017; 50: 1700889. [DOI] [PubMed] [Google Scholar]
- 6.Galiè N, Humbert M, Vachiery J, et al. ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2015; 46: 903–975. [DOI] [PubMed] [Google Scholar]
- 7.Galiè N, McLaughlin VV, Rubin LJ, et al. An overview of the 6th World Symposium on pulmonary hypertension. Eur Respir J 2019; 53: 1802148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kylhammar D, Kjellstrom B, Hjalmarsson C, et al. A comprehensive risk stratification at early follow-up determines prognosis in pulmonary arterial hypertension. Eur Heart J 2018; 39: 4175–4181. [DOI] [PubMed] [Google Scholar]
- 9.Hoeper MM, Kramer T, Pan Z, et al. Mortality in pulmonary arterial hypertension: predication by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur Respir J 2017; 50: 1700740. [DOI] [PubMed] [Google Scholar]
- 10.Benza RL, Miller DP, Gomberg-Maitland M, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation 2010; 122: 164–172. [DOI] [PubMed] [Google Scholar]
- 11.Benza RL, Gomberg-Maitland M, Miller DP, et al. The REVEAL Registry risk score calculator in patients newly diagnosed with pulmonary arterial hypertension. Chest 2012; 141: 354–362. [DOI] [PubMed] [Google Scholar]
- 12.Benza RL, Gomberg-Maitland M, Elliot CG, et al. Predicting survival in patients with pulmonary arterial hypertension: the REVEAL risk score calculator 2.0 and comparison with ESC/ERS-based risk assessment strategies. Chest 2019; 156: 323–337. [DOI] [PubMed] [Google Scholar]
- 13.Benza RL, Kanwar M, Raina A, et al. Comparison of risk discrimination between the REVEAL 2.0 calculators, the French Pulmonary Registry Algorithm and the Bologna Method in patients with pulmonary arterial hypertension. In: Poster. Presented at the American Thoracic Society international conference, Dallas, TX, USA, 17–22 May 2019.
- 14.Sahay S andRahaghi F.. Real-world experience using combination therapy with riociguat and risk assessment using REVEAL Lite 2.0 in patients with pulmonary arterial hypertension. Pulm Circ 2020; 10: 2045894020910098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson JJ, Lau EM, Lavender M, et al. Retrospective validation of the REVEAL 2.0 risk score with the Australian and New Zealand Pulmonary Hypertension Registry Cohort. Chest 2020; 157: 162–172. [DOI] [PubMed] [Google Scholar]
- 16.United Therapeutics Corp. Tyvaso® (treprostinil) inhalation solution (package insert). Research Triangle Park, NC: United Therapeutics Corp, 2017. [Google Scholar]
- 17.McLaughlin VV, Benza RL, Rubin LJ, et al. Addition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension: a randomized controlled clinical trial. J Am Coll Cardiol 2010; 55: 1915–1922. [DOI] [PubMed] [Google Scholar]
- 18.Benza RL, Seeger W, McLaughlin VV, et al. Long-term effects of inhaled treprostinil in patients with pulmonary arterial hypertension: the Treprostinil Sodium Inhalation Used in the Management of Pulmonary Arterial Hypertension (TRIUMPH) study open-label extension. J Heart Lung Transplant 2011; 30: 1327–1333. [DOI] [PubMed] [Google Scholar]
- 19.Oudiz R, Kramer M, Bartolome S, et al. esuberaprost on morbidity and mortality in world health organization (WHO) functional class III and IV (FC III/IV) patients with pulmonary arterial hypertension: results from the randomized, double-blind, placebo controlled phase 3 trial- beraprost-314D added to Tyvaso (BEAT). Chest 2019; 156: A1707–A1709. [Google Scholar]
- 20.White RJ, Jerjes-Sanchez C, Bohns Meyer GM, et al. Combination therapy with oral treprostinil for pulmonary arterial hypertension. A double-blind placebo-controlled clinical trial. Am J Respir Crit Care Med 2020; 201: 707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Humbert M, Farber HW, Ghofrani HA, et al. Risk assessment in pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Eur Respir J 2019; 53: 1802004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sitbon O, Chin KM, Channick RN, et al. Risk assessment in pulmonary arterial hypertension: insights from the GRIPHON study. J Heart Lung Transplant 2020; 39: 300–309. [DOI] [PubMed] [Google Scholar]
- 23.Sitbon O, Benza RL, Badesch DB, et al. Validation of two predictive models for survival in pulmonary arterial hypertension. Eur Respir J 2015; 46: 152–164. [DOI] [PubMed] [Google Scholar]
- 24.Frost AE, Hoeper MM, Barbera JA, et al. Risk-stratified outcomes with initial combination therapy in pulmonary arterial hypertension: application of the REVEAL risk score. J Heart Lung Transplant 2018; 37: 1410–1417. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-pul-10.1177_2045894020977025 for Impact of inhaled treprostinil on risk stratification with noninvasive parameters: a post hoc analysis of the TRIUMPH and BEAT studies by Adriano R. Tonelli, Sandeep Sahay, Kathryn W. Gordon, Lisa D. Edwards, Andrew G. Allmon, Meredith Broderick and Andrew C. Nelsen in Pulmonary Circulation