Abstract
Background:
A recently published validated classification system divides all orofacial cleft (OFC) subphenotypes into groups based on underlying developmental mechanisms, that is, fusion and differentiation, and their timing, that is, early and late periods, in embryogenesis of the primary and secondary palates.
Aims:
The aim of our study was to define gender differences in prevalence for all subphenotypes in newborns with OFC in the Netherlands.
Methods:
This was a retrospective cross-sectional study on children with OFC born from 2006 to 2016. Clefts were classified in early (E-), late (L-), and early/late (EL-) embryonic periods, in primary (P-), secondary (S-), and primary/secondary (PS-) palates, and further divided into fusion (F-), differentiation (D-), and fusion/differentiation (FD-) defects, respectively.
Results:
A total of 2089 OFC children were analyzed (1311 males and 778 females). Orofacial cleft subphenotypes in females occurred significantly more frequent in the L-period compared to males (66% vs 55%, P = .000), whereas clefts in males occurred significantly more in the EL-periods (40% vs 27%, P = .000). Females had significantly more S-palatal clefts (42% vs 23%, P = .000), while males had significantly more PS-palatal clefts (44% vs 30%, P = .000). Furthermore, the clefts in females were significantly more frequent the result of an F-defect (60% vs 52%, P = .000).
Conclusions:
Orofacial cleft in females mainly occur in the L-period are mostly S-palatal clefts, and are usually the result of an F-defect. Orofacial cleft in males more commonly occur in the EL-periods, are therefore more often combined PS-palatal clefts, and are more frequent D- and FD-defects.
Keywords: embryology, classification, orofacial clefts, subphenotypes, gender
Introduction
Worldwide, 1 in 700 children are born with a nonsyndromic cleft lip and/or palate (Mossey & Modell, 2012). It has been known for years that the prevalence of cleft lip and palate among males is approximately twice that of females, whereas the prevalence for isolated cleft palate is about two-thirds that of females (Shaw et al., 1991; Freitas et al., 2004; Hashmi et al., 2005; Dixon et al., 2011; Mossey & Modell, 2012). Traditionally, orofacial clefting (OFC) is divided into 2 categories: cleft lip with or without cleft palate (CL±P) and cleft palate only (CP) or into 3 categories: CL with or without cleft alveolus (CL±A), cleft lip, alveolus, and palate (CLAP), and CP (Luijsterburg et al., 2014; Watkins et al., 2014; Vermeij-Keers et al., 2018). In general, these classifications and the International Classification of Diseases-10 (World Health Organization) are extremely useful for epidemiological analysis of OFC but are not sufficiently accurate in grouping OFC into subphenotypes for etiological and clinical studies (Luijsterburg et al., 2014; McBride et al., 2016; Vermeij-Keers et al., 2018). However, more detailed registry systems are known that distinguish the different anatomical structures (eg, Kernahan, 1971; Schwartz et al., 1993; Hammond & Stassen, 1999; Kubon et al., 2007) as well as the morphological features (eg, Kriens, 1989; Smith et al., 1998; Ortiz-Posadas et al., 2001; Koul, 2007; Liu et al., 2007; Allori et al., 2017).
The LAHSHAL documentation system (representing Lip, Alveolus, Hard palate, and Soft palate) precisely registers the side, localization, and extent of a cleft or combination of clefts, with complete and incomplete clefts recorded in capital and small letters, respectively. Additionally, the subcutaneous clefts of the hard and/or soft palate can be scored in lowercase and the microforms of lip, alveolus, and palate—bifid uvula and bilateral microform hard palate cleft—can be registered using asterisks in the 7 locations provided by the formula (Kriens, 1989; Härtel et al., 1991). According to McBride et al. (2016), the Royal College of Surgeons of England simplified this palindrome by excluding the second “H” in 1993. However, this precludes the possibility of recording bilateral clefts of the hard palate (CRANE database, Form 1). Embryologically, this is a fallacy because the left and right palatal shelves fuse with each other in the median and each separately with the nasal septum in fronto-occipital direction (Luijsterburg et al., 2014; Vermeij-Keers et al., 2018). As a result, for example, a left-sided complete CLAP combined with a right-sided incomplete cleft hard palate cannot be properly recorded. Although the LAHS(H)AL system facilitates the recording of many OFC in a concise and accurate form, it does not include hypoplasia of the anatomical structures mentioned above. Besides, the underlying developmental mechanisms and timing in embryogenesis of OFC subphenotypes are not taken into account.
Using above classifications and/or registry systems may therefore hamper further knowledge on the causes and treatment of OFC. For this reason, a recently published validated classification system, fully based on the pathoembryogenesis of OFC, divides all subphenotypes into groups based on the underlying developmental mechanisms, that is, fusion and differentiation, and their timing, that is, early and late periods, in embryogenesis of the primary and secondary palates (Rozendaal et al., 2012; Luijsterburg et al., 2014; Vermeij-Keers et al., 2018). During the early embryonic period, only the fusion defects of the primary palate (eg, complete CL±A) develop. In the late embryonic period, both the differentiation defects of the primary palate (eg, incomplete CL±A) and the fusion and differentiation defects (eg, (in)complete and submucous CP, respectively) of the secondary palate occur. The aim of our study was to define gender differences in the prevalence for all cleft subphenotypes in newborns with OFC in the Netherlands, using an embryologically based classification.
Method
The study presented here is a retrospective cross-sectional study on children with unilateral or bilateral nonsyndromic OFC born alive from 2006 to 2016. Children with atypical facial clefts, such as midline and oblique facial clefts, an OFC syndrome, sequence or association including isolated and syndromic Robin sequence, children with non-Caucasian or consanguineous parents, and adoptive or foster children were excluded.
Data were provided by the Dutch Association for Cleft Palate and Craniofacial Anomalies. All cleft palate teams in the Netherlands records anonymously cleft subphenotypes and other craniofacial abnormalities through a validated system (Luijsterburg & Vermeij-Keers, 2011; Rozendaal et al., 2012). The following data were noted: date of birth, date of registration, gender, family history of congenital anomalies including OFC, gestational age, any details during pregnancy, birth weight, anatomical description (laterality of the lip, of the premaxilla/maxilla and of the hard palate; soft palate and uvula with clefts only in the median), and morphological description (complete, incomplete, submucous, and hypoplasia) of the cleft, and other head-neck anomalies with respect to soft tissues, tongue, nasal septum, ears, eyes, eyelids, and individual bones of the neurocranium and facial skeleton (Luijsterburg & Vermeij-Keers, 2011). For data collection, the principles outlined in the Declaration of Helsinki were followed.
To categorize the different types of cleft, we used the classification of Vermeij-Keers et al. (2018), making a distinction between early (4-7 weeks postconception) and late (7-12 weeks postconception) embryonic periods (Table 1). In the early period, only fusion defects of the primary palate occur and in the late period those of the secondary palate. Differentiation defects of both the primary and secondary palates only develop in the late period. The primary palate includes the lip, premaxilla/maxilla, that is, alveolus, extending to the incisive foramen. The hard palate, soft palate, and uvula comprise the secondary palate. According to this classification, clefts were classified in early (E-), late (L-), and early/late (EL-) embryonic periods, in primary (P-), secondary (S-), and primary/secondary (PS-) palates, and further divided into fusion (F-), differentiation (D-), and fusion/differentiation (FD-) defects, respectively (Table 2). Symmetrical and asymmetrical bilateral clefts were classified as 1 case, respectively. For example, a left-sided complete and a right-sided incomplete CL±A were classified as EL, P, and FD.
Table 1.
Classification of the Individual Cleft Anomalies of the Primary and Secondary Palate According to Timing and Underlying Fusion and Differentiation Mechanisms in Embryogenesis.a
| Early embryonic period (4-7 weeks postconception) | Late embryonic period (7-12 weeks postconception) | |
|---|---|---|
| Primary palate Fusion defect
|
Primary palate Differentiation defect
|
|
| Secondary palate Fusion defect
|
Secondary palate Differentiation defect
|
Abbreviations: CA, cleft alveolus; CL, cleft lip; CP, cleft palate; CU, cleft uvula.
a Copied from Vermeij-Keers et al. (2018).
b Congenital scar, forme fruste, and subsurface, subcutaneous, or microform cleft lip.
Table 2.
Classification of Orofacial Cleft (OFC) Based on Embryonic and Phenotypic Characteristics.
| Embryonic period | Affected palate | Type of defect |
|---|---|---|
| E (early)a | P (primary palate)b | F (fusion defect) |
| L (late)c | S (secondary palate)d | D (differentiation defect) |
| EL (both) | PS (both) | FD (both) |
a Early embryonic period: 4 to 7 weeks postconception.
b Primary palate: lip, premaxilla/maxilla.
c Late embryonic period: 7 to 12 weeks postconception.
d Secondary palate: hard palate, soft palate, and uvula.
Statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) version 25. The χ2 test was used to identify whether the distribution of males and females with respect to different types of cleft differed significantly. A P value of ≤.05 was used as statistical significance. After determination of a significant difference in distribution, a post hoc test was used to identify the responsible variable, at which the P value for statistical significance was adjusted for the number of comparisons (Bonferroni correction, P = (0.05/6) = .0083) (Beasley & Schumacker, 1995; Garcia-Perez & Nunez-Anton, 2003).
Results
In total, 4119 children were registered from 2006 to 2016 of which 2089 children met the inclusion criteria; 1311 males and 778 females (Table 3). Unilateral and bilateral CL±A and CLAP were recorded in n = 1129 (54%) and n = 323 (16%) patients, respectively. Isolated CP was noted in n = 613 (29%) cases, and additionally, 24 children had an isolated bifid uvula (1%). Family history for OFC was positive in 581 of the children (28%). The mean gestational age was 274.9 days, with a standard deviation of 14.3 days.
Table 3.
Characteristics of the Study Population.a
| Patient characteristics | Males | Females | |
|---|---|---|---|
| Number of patients | 1311 (63) | 778 (37) | 2089 (100) |
| Location of cleft | |||
| Unilateral CL±A and CLAP | 768 (59) | 361 (46) | 1129 (54) |
| Bilateral CL±A and CLAP | 237 (18) | 86 (11) | 323 (16) |
| Isolated CP (incl hypoplastic palate and submucous cleft) | 290 (22) | 323 (42) | 613 (29) |
| Isolated bifid uvula | 16 (1) | 8 (1) | 24 (1) |
| Family history | |||
| Positive for cleft | 581 (28) | ||
| Congenital anomaly other than cleft | 117 (5) | ||
| Negative | 1356 (65) | ||
| Unknown | 35 (2) | ||
| Gestational age (days) | 274.9 ± 14.3 | ||
| Groups | |||
| Embryonic period | |||
| E | 70 (5) | 53 (7) | 123 (6) |
| L | 725 (55) | 517 (66) | 1242 (59) |
| EL | 516 (40) | 208 (27) | 724 (35) |
| Affected palate | |||
| P | 433 (33) | 217 (28) | 650 (31) |
| S | 306 (23) | 331 (42) | 637 (31) |
| PS | 572 (44) | 230 (30) | 802 (38) |
| Subtype of defect | |||
| F | 677 (52) | 467 (60) | 1144 (55) |
| D | 399 (30) | 196 (25) | 595 (28) |
| FD | 235 (18) | 115 (15) | 350 (17) |
Abbreviations: A, alveolus; CL±A, cleft lip with or without cleft alveolus; CLAP, cleft lip, alveolus, and palate; CP, cleft palate only; D, differentiation defect; E, anomaly that arose in the early embryonic period (4-7 weeks postconception); EL, anomaly that arose in both the early and late embryonic periods; F, fusion defect; FD, both fusion and differentiation defects; L, anomaly that arose in the late embryonic period (7-12 weeks postconception); P, primary palate affected; PS, both primary and secondary palates affected; S, secondary palate affected.
a Data presented as proportion n (%), or mean ± standard deviation.
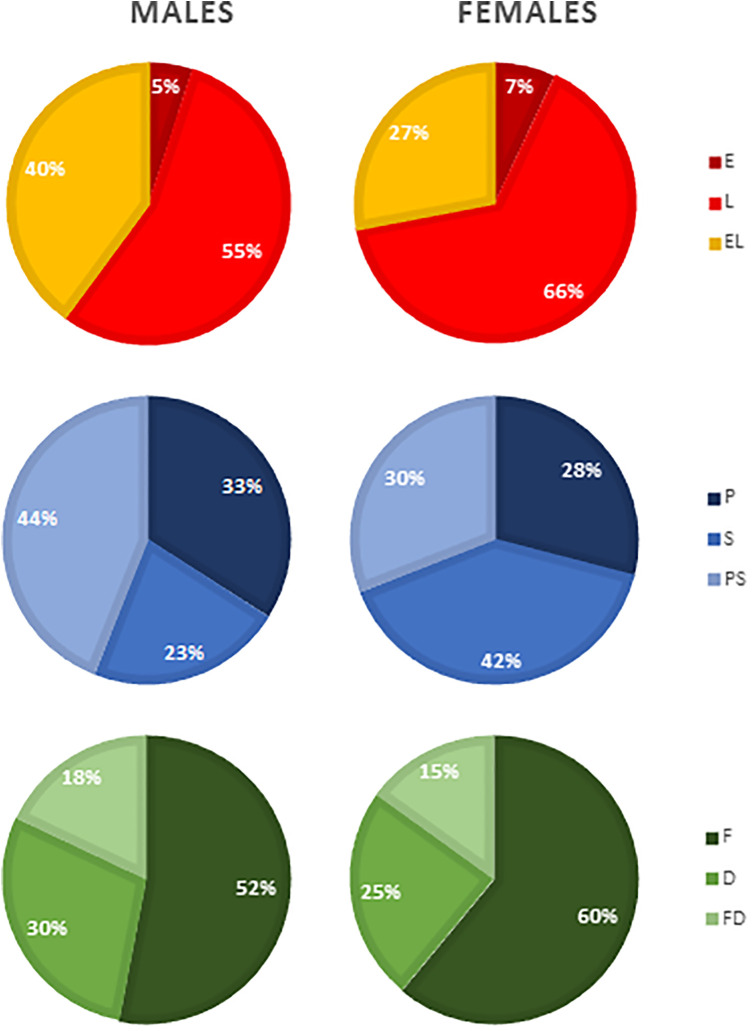
For the embryonic periods, affected palate, and subtype of defect, the distribution of clefts between males and females was significantly different, P = .000, P = .000, and P = .001, respectively (Figure 1). Post hoc testing showed that cleft subphenotypes in females occurred significantly more frequent in the L-period compared to males (66% vs 55%, P = .000), whereas clefts in males significantly more frequent occur in the EL-periods compared to clefts in females (40% vs 27%, P = .000). The frequency of clefts that occurred in the E-period was not significantly different between males and females (5% vs 7%, P = .168). In addition, females had significantly more often S-palatal clefts compared to males (42% vs 23%, P = .000), while males significantly more often had PS-palatal clefts compared to females (44% vs 30%, P = .000). The frequency of P-palatal clefts was not significantly different between males and females (33% vs 28%, P = .014). Furthermore, the clefts in females were significantly more frequent the result of an F-defect compared to males (60% vs 52%, P = .000). However, there was no significant difference between males and females for the frequency of clefts that were the result of a D-defect (30% vs 25%, P = .010) or the result of a FD-defect (18% and 15%, P = .063).
Figure 1.
Gender differences in the distribution of the different types of cleft based on embryonic period, affected palate, and subtype of defect. D indicates differentiation defect; E, anomaly that arose in the early embryonic period (4-7 weeks postconception); EL, anomaly that arose in both the early and late embryonic periods; F, fusion defect; FD, both fusion and differentiation defects; L, anomaly that arose in the late embryonic period (7-12 weeks postconception); P, primary palate affected; PS, both primary and secondary palates affected; S, secondary palate affected.
Discussion
Gender differences in the prevalence of OFC based on the traditional subdivisions into categories are known in the literature. Namely, the prevalence of CL±P among males is approximately twice that of females, whereas the prevalence for isolated CP is about two-thirds that of females (Shaw et al., 1991; Freitas et al., 2004; Hashmi et al., 2005; Dixon et al., 2011; Mossey & Modell, 2012). So far, there is no generally accepted explanation for these gender differences, but these differences may possibly be explained by the differences in the timing of critical stages in craniofacial development between female and male embryos. To date, however, there has been hardly any attention in the literature on gender differences in the timing of these critical embryological stages with regard to the OFC categories in general and the OFC subphenotypes in particular. An exception to this is, for example, the article by Burdi and Silvey (1969) in which the shifting of the female palatal shelves from vertical to horizontal occurs approximately half a week later than in the male embryos increasing the risk for CP and possibly explaining the female preponderance of CP. According to Carlson et al. (2018), genetic variations may contribute to the delay.
Recently, Vermeij-Keers et al. (2018) published a validated classification system that divides all cleft subphenotypes into groups based on the underlying developmental mechanisms, and their timing in embryogenesis. Using this comprehensive classification system, we might reveal new information about gender differences in the prevalence of the subphenotypes of OFC. Our highly significant results (all P = .000) show that OFC in females more often occurs in the L-period are more frequent clefts of the S-palate, and are more often the result of an F-defect compared to clefts in males. Translating these results into the classification of Vermeij-Keers et al. (2018; Table 1), this should imply that OFC in females is more frequent (in)complete clefts of the hard and/or soft palate or (in)complete clefts of the uvula. In addition, we found out that OFC in males more often occur in the EL-periods are more frequent clefts of PS-palates, and are less frequently the result of an F-defect only. This should imply that males more frequently have combinations of a(n) (in)complete or submucous cleft lip and/or alveolus or hypoplastic lip and/or alveolus and a(n) (in)complete or submucous cleft of the hard and/or soft palate or hypoplastic hard and/or soft palate including the uvula.
Freitas et al. (2004) already noted predominance for isolated CP among females, while there was male predominance for all other cleft types. This is in accordance with our results: (in)complete clefts of hard and/or soft palate occur in the L-period are S-palatal clefts, and are the result of an F-defect, all 3 found out to be significantly more frequent in females (Figure 1). In addition, other subphenotypes that are distinguished in our study (ie, (in)complete or submucous clefts of lip and/or alveolus, hypoplastic lip and/or alveolus, a(n) (in)complete or submucous clefts of hard and/or soft palate, and hypoplastic hard and/or soft palates including the uvula) occur in the EL-period are PS-palatal clefts and are the result of both F- and D-defects, and appeared significantly more frequent in males.
In contrast to the unanimity of the literature on the female dominance in the prevalence of CP, there is still a contradiction about the gender distribution and severity in CL and cleft lip with palate (CLP). Carroll and Mossey (2012) concluded that in isolated unilateral CL, females were significantly more likely to have a cleft involving the full height of the lip, that is, complete CL, compared to males (39% vs 25%). In unilateral CLP, on the other hand, the opposite was the case: males were significantly more likely to have a complete CL compared to females (90% vs 85%). Using the classification of Vermeij-Keers et al. (2018), we arrive at the following conclusions. The complete CLs only arise in the E-period (females 7% and males 5%; Tables 1 and 3; Figure 1). P-palate defects include complete CLs, and incomplete, submucous and hypoplastic CLs, with 28% for females and 33% for males, of which 21% and 28% for incomplete/submucous/hypoplastic CLs, respectively. The percentage of complete clefts in females with an isolated CL is now 24% and for males 16%. These percentages are much lower but endorse the result of Carroll and Mossey (2012) regarding gender difference in isolated complete unilateral CLs.
The CLPs with a complete CL only develop during the EL-periods (females 27% and males 40%; Tables 1 and 3; Figure 1) and concern PS-palates (females 30% and males 44%). Cleft lip with palates with an incomplete/submucous/hypoplastic CL develop in the L-period and also concern PS-palates. Then incomplete/submucous/hypoplastic CLs in CLP patients were found to occur in 3% of females and 4% of males. The percentage of complete CLs in CLPs for both females and males is then 90%. These data contradict the result of Carroll and Mossey (2012) that—in cases of unilateral CLP—males were more likely to have a complete CL.
Carroll and Mossey (2012) used the “LAHSAL” documentation coding system for the OFC cases included in their study and then classified them in the 3 categories: CL, CLP, and CP. If we compare this coding system and the study design of these authors with ours, the following discrepancies can be noted. Encoding hypoplasia of lip, alveolus, and/or hard/soft palate and isolated bifid uvula was not possible in the LAHSAL system, submucous CPs were grouped as an F-defect (CRANE Database, Data Dictionary), bilateral CL and CLP cases were excluded from their analysis and children with OFC syndromes were included in their study. By including OFC syndromes whose embryogenesis (and associated classification) and genetics can be totally different from nonsyndromic OFC children, the results can be significantly influenced. Furthermore, we excluded children with non-Caucasian or consanguineous parents and adoptive or foster children. Therefore, the above shortcomings and selections might have caused some of the conflicting results.
In this study, we investigated gender differences in the prevalence of OFC subphenotypes using an embryologically based classification. Herewith, we have obtained more detailed information about the appearance and distribution of the subphenotypes among males and females. Orofacial cleft in females are mostly (in)complete clefts of the hard and/or soft palate, or (in)complete clefts of the uvula, while males mainly have combinations of a(n) (in)complete or submucous cleft lip and/or alveolus or hypoplastic lip and/or alveolus, and a(n) (in)complete or submucous cleft of the hard and/or soft palate, or hypoplastic hard and/or soft palate including the uvula. This more specified information can further help us to find out the etiology of OFC and hopefully preventive treatment in the future.
The limitation of our study is that gender differences in laterality of the clefts was not analyzed. Cleft lip tends to be unilateral (around 90%) and approximately two-thirds occur on the left side regardless of sex, ethnic group, and severity of defect (Fraser & Calnan, 1961; Bonaiti et al., 1982; Tolarova, 1987; Jensen et al., 1988). Using the recently published validated classification system could also reveal gender differences in laterality for the subphenotypes of OFC and should, therefore, be subject to further investigation.
Conclusions
Orofacial cleft in females mainly occur in the late embryonic period are mostly clefts of the secondary palate only, and are usually the result of a fusion defect. Compared to females, clefts in males more commonly occur in both the early and late embryonic periods are therefore more often combined clefts of the primary and secondary palate, and are more frequently the result of differentiation defects and combined fusion and differentiation defects.
These more specified results about gender differences in the prevalence of OFC subphenotypes using an embryologically based classification can be important in future clinical genetic research and may bring us closer to the etiology and preventive treatment of OFC.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Shariselle M. W. Pool, MD, PhD  https://orcid.org/0000-0003-1415-103X
https://orcid.org/0000-0003-1415-103X
Lisanne M. van der Lek, MD  https://orcid.org/0000-0003-3745-4316
https://orcid.org/0000-0003-3745-4316
Kim de Jong, PhD  https://orcid.org/0000-0002-3948-1046
https://orcid.org/0000-0002-3948-1046
References
- Allori AC, Mulliken JB, Meara JG, Shusterman S, Marcus JR. Classification of cleft lip/palate: then and now. Cleft Palate Craniofac J. 2017;54(2):175–188. [DOI] [PubMed] [Google Scholar]
- Beasley TM, Schumacker RE. Multiple regression approach to analyzing contingency tables: post hoc and planned comparison procedures. J Exp Educ. 1995;64(1):79–93. [Google Scholar]
- Bonaiti C, Briard ML, Feingold J, Pavy B, Psaume J, Migne-Tufferaud G, Kaplan J. An epidemiological and genetic study of facial clefting in France: epidemiology and frequency in relatives. J Med Genet. 1982;19(2):8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdi AR, Silvey RG. Sexual differences in closure of the human palatal shelves. Cleft Palate J. 1969;6:1–7. [PubMed] [Google Scholar]
- Carlson JC, Nidey NL, Butali A, Buxo CJ, Christensen K, Deleyiannis FW, Hecht JT, Field LL, Moreno-Uribe LM, Orioli IM, et al. Genome-wide interaction studies identify sex-specific risk alleles for nonsyndromic orofacial clefts. Genet Epidemiol. 2018;42(7):664–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll K, Mossey PA. Anatomical variations in clefts of the lip with or without cleft palate. Plast Surg Int. 2012;2012(1):542078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRANE Database. Information for Cleft Teams—Data Collection Forms—patient registration (Form 1; Data Dictionary). Published 2018. Accessed August 8, 2019 https://www.crane-database.org.uk/?!.iD=etQ
- Dixon MJ, Marazita ML, Beaty TH, Murray JC. Cleft lip and palate: understanding genetic and environmental influences. Nat Rev Genet. 2011;12(3):167–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser GR, Calnan JS. Cleft lip and palate: seasonal incidence, birth weight, birth rank, sex, site, associated malformation and parental age. Arch Dis Child. 1961;36:420–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas JA, Dalben Gda S, Santamaria M, Jr, Freitas PZ. Current data on the characterization of oral clefts in Brazil. Braz Oral Res. 2004;18(2):128–133. [DOI] [PubMed] [Google Scholar]
- Garcia-Perez MA, Nunez-Anton V. Cellwise residual analysis in two-way contingency tables. Educ Psychol Meas. 2003;63(5):825–839. [Google Scholar]
- Hammond M, Stassen L. Do you CARE? A national register for cleft lip and palate patients. Br J Plast Surg. 1999;52(3):12–17. [DOI] [PubMed] [Google Scholar]
- Härtel J, Kriens O, Kundt G. Incidence of cleft lip, alveolus and palate forms. J Craniomaxillofac Surg. 1991;19(4):144–146. [DOI] [PubMed] [Google Scholar]
- Hashmi SS, Waller DK, Langlois P, Canfield M, Hecht JT. Prevalence of nonsyndromic oral clefts in Texas: 1995-1999. Am J Med Genet A. 2005;134(10):368–372. [DOI] [PubMed] [Google Scholar]
- Jensen BL, Kreiborg S, Dahl E, Fogh-Andersen P. Cleft lip and palate in Denmark 1976–1981: epidemiology variability and early somatic development. Cleft Palate J. 1988;25(6):258–269. [PubMed] [Google Scholar]
- Kernahan DA. The striped Y—a symbolic classification for cleft lip and palate. Plast Reconstr Surg. 1971;47(5):469–470. [DOI] [PubMed] [Google Scholar]
- Koul R. Describing cleft lip and palate using a new expression system. Cleft Palate Craniofac J. 2007;44(6):585–589. [DOI] [PubMed] [Google Scholar]
- Kriens O. LAHSHAL, a concise documentation system for CLP diagnoses In: Kriens O, ed. What Is a Cleft Lip and Palate? A Multidisciplinary Update: Proceedings of an Advanced Workshop, Bremen 1987. Thieme; 1989:30–34. [Google Scholar]
- Kubon C, Sivertsen A, Vindenes HA, Abyholm F, Wilcox A, Lie RT. Completeness of registration of oral clefts in a medical birth registry: a population-based study. Acta Obstet Gynecol Scand. 2007;86(12):1453–1457. [DOI] [PubMed] [Google Scholar]
- Liu Q, Yang ML, Li ZJ, Bai XF, Wang XK, Lu L, Wang YX. A simple and precise classification for cleft lip and palate: a five-digit numerical recording system. Cleft Palate Craniofac J. 2007; 44(5):465–468. [DOI] [PubMed] [Google Scholar]
- Luijsterburg AJ, Rozendaal AM, Vermeij-Keers C. Classifying common oral clefts: a new approach after descriptive registration. Cleft Palate Craniofac J. 2014;51(4):381–391. [DOI] [PubMed] [Google Scholar]
- Luijsterburg AJ, Vermeij-Keers C. Ten years recording common oral clefts with a new descriptive system. Cleft Palate Craniofac J. 2011;48(2):173–182. [DOI] [PubMed] [Google Scholar]
- McBride WA, McIntyre GT, Carroll K, Mossey PA. Subphenotyping and classification of orofacial clefts: need for orofacial cleft subphenotyping calls for revised classification. Cleft Palate Craniofac J. 2016;53(5):539–549. [DOI] [PubMed] [Google Scholar]
- Mossey PA, Modell B. Epidemiology of oral clefts 2012: an international perspective. Front Oral Biol. 2012;16(4):1–18. [DOI] [PubMed] [Google Scholar]
- Ortiz-Posadas MR, Vega-Alvarado L, Maya-Behar J. A new approach to classify cleft lip and palate. Cleft Palate Craniofac J. 2001;38(6):545–550. [DOI] [PubMed] [Google Scholar]
- Rozendaal AM, Luijsterburg AJ, Mohangoo AD, Ongkosuwito EM, de Vries E, Vermeij-Keers C. Validation of the Dutch registry of common oral clefts: quality of recording specific oral cleft features. Cleft Palate Craniofac J. 2012;49(5):609–617. [DOI] [PubMed] [Google Scholar]
- Schwartz S, Kapala JT, Rajchgot H, Roberts GL. Accurate and systematic numerical recording system for the identification of various types of lip and maxillary clefts (RPL system). Cleft Palate Craniofac J. 1993;30(2):330–332. [DOI] [PubMed] [Google Scholar]
- Shaw GM, Croen LA, Curry CJ. Isolated oral cleft malformations: associations with maternal and infant characteristics in a California population. Teratology. 1991;43(3):225–228. [DOI] [PubMed] [Google Scholar]
- Smith AW, Khoo AK, Jackson IT. A modification of the Kernahan “Y” classification in cleft lip and palate deformities. Plast Reconstr Surg. 1998;102(6):1842–1847. [DOI] [PubMed] [Google Scholar]
- Tolarova MM. Orofacial clefts in Czechoslovakia: incidence, genetics and prevention of cleft lip and palate over a 19- year period. Scand J Plast Reconstr Surg. 1987;21(1):19–25. [DOI] [PubMed] [Google Scholar]
- Vermeij-Keers C, Rozendaal AM, Luijsterburg AJM, Latief BS, Lekkas C, Kragt L, Ongkosuwito EM. Subphenotyping and classification of cleft lip and alveolus in adult unoperated patients: a new embryological approach. Cleft Palate Craniofac J. 2018;55(9):1267–1276. [DOI] [PubMed] [Google Scholar]
- Watkins SE, Meyer RE, Strauss RP, Aylsworth AS. Classification, epidemiology, and genetics of orofacial clefts. Clin Plast Surg. 2014;41(2):149–163. [DOI] [PubMed] [Google Scholar]
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10) Version for 2010; 2010. Accessed May 20, 2019 https://icd.who.int/browse10/2010/en#/Q35-Q37