Abstract
Right ventricular impairment is a predictor of cardiovascular outcomes in patients with degenerative mitral regurgitation. However, the time course of right ventricular functional changes post-surgical mitral valve repair remains largely unknown. Herein, using right ventricular-focused echocardiography, we aimed to investigate right ventricular reserve and its impact on hospitalization for heart failure after mitral valve repair. In this prospective study, we enrolled 108 patients scheduled to undergo surgical repair of degenerative mitral regurgitation. Echocardiography, including right ventricular strain analysis, was performed prior to, and one month and six months post mitral valve repair. Right ventricular strain that improved one month post-surgery was defined as reserved right ventricular. In addition, any cardiovascular outcomes comprising heart failure that required admission were recorded. The median follow-up duration is 31 months. Despite a significant improvement in mitral valve regurgitant volume post-operatively, left ventricular ejection fraction (LVEF) at six months was similar to LVEF at baseline. There was a transient decrease in LV longitudinal strain at one month that was recovered six months post mitral valve repair. Regarding the right ventricular, in contrast with conventional right ventricular parameters, including right ventricular tissue Doppler S′, fractional area change and tricuspid annular plane systolic excursion (TAPSE), only resolution of right ventricular strain at one month predicted the subsequent myocardial recovery. Furthermore, patients with reserved right ventricular had a lower risk of hospitalization for heart failure compared to those with non-reserved right ventricular. Collectively, the early resolution of right ventricular strain is associated with the improvement in right ventricular function (measured by TAPSE) and in heart failure hospitalization in patients who had undergone surgical mitral valve repair for degenerative mitral regurgitation.
Keywords: degenerative mitral regurgitation, mitral valve repair, right ventricular strain, heart failure
Introduction
In patients with severe, chronic mitral regurgitation (MR), the volume overload contributes to the increase of wedge pressure and the development of pulmonary hypertension.1 With the elevated afterload to the right ventricle (RV), it subsequently leads to RV dysfunction.2 Although left ventricular failure is the end point of decline in cardiac function in severe MR, RV dysfunction may represent an early and independent predictor of mortality.3 Most importantly, the high prevalence of RV dysfunction in patients with MR is probably frequently underestimated. Moreover, given that the severity of RV dysfunction is not absolutely reflected by pulmonary arterial systolic pressures or the degree of tricuspid regurgitation,2 a specific and reliable method for monitoring RV function turns out to be more crucial. Previous studies indicated that pre-operative RV ejection fraction predicted post-operative outcomes.2,4 However, the conventional echocardiographic parameters seem to be less sensitive in detecting the occult myocardial changes. Speckle-tracking echocardiography is sensitive in detecting early myocardial dysfunction and has been used to differentiate the outcomes of different phenotypes of cardiovascular diseases.5 Nevertheless, the time course of RV functional changes post MV repair remains largely unknown. Herein, using RV-focused echocardiography including RV strain, we aimed to study the frequency of RV recovery at an early stage and its impact on hospitalization for heart failure after surgical MV repair.
Method
Study design
The study was conducted in strict accordance with the Declaration of Helsinki on Biomedical Research involving human subjects and was approved by the local ethics committee (IRB: 10307-003). We prospectively included 80 patients preparing for surgical MV repair for severe degenerative MR in Chi-Mei Medical Center. The severity of MR is defined by the recommended guideline of American Society of Echocardiography in 2017.6 Patients who had concomitant tricuspid valve repair or received surgeries in an emergent condition were excluded. After excluding eight patients who lost follow-up and six patients who had a poor image window, we finally collected the medical records and echocardiography imaging results of 108 patients. The echocardiography was performed prior to, and one month and six months post MV repair. In addition to conventional echocardiographic parameters, both LV and RV longitudinal strain were measured. Patients with an improvement of RV strain one month post surgical MV repair compared with that pre-operatively were defined as having RV reserve. Conversely, patients with a declined RV strain post-operatively were defined as being without RV reserve. Finally, we identified 23 patients with no RV reserve and 85 patients with RV reserve. The diagnosis of heart failure was defined according to the ACC/AHA guideline including clinical presentation, echocardiography, biomarkers and response to medications. The end-point was hospitalization for heart failure during the follow-up period (the median follow-up duration: 31 months; 22–41 months).
Echocardiographic parameters
Echocardiography was performed with a 3.5-MHz multiphase-array probe and GE Vivid E9 system (Vingmed Ultrasound AS, Horten, Norway) by one cardiologist and two sonographers. The chamber dimensions and left ventricular mass index were measured using the two-dimensionally guided M-mode method. The right ventricular fractional area change (FAC) was measured with the apical 4-chamber view, while LV ejection fraction (LVEF) was measured by both apical 2- and 4- chamber Simpson method. Left atrial volume index (LAVi) was measured based on the maximal LA area in both apical 2- and 4-chamber views and the length from the posterior wall to the MV hinge, divided by body surface area. In addition, LV diastolic function-associated parameters including transmitral and tricuspid early filling velocity (E) to atrial velocity (A) ratio and tissue Doppler imaging were obtained from the apical four-chamber view. Peak systolic annular velocity (S′) and early (e′) annular diastolic velocities were measured in both ventricles. The effective regurgitant orifice (ERO), regurgitation volume (RVol) of MR as well as the vena contracta of tricuspid regurgitation (TR) were measured in accordance with the recommendations of the American Society of Echocardiography.6 Applying the Bernoulli equation, RV systolic pressure was estimated based on the maximum tricuspid regurgitation velocity. Tricuspid annular plane systolic excursion (TAPSE) represents the distance of systolic excursion of the RV annular plane towards the apex. The calculation of pulmonary vascular resistance is based on the equation of tricuspid regurgitant velocity/time velocity interval of right ventricular outflow tract × 10 + 0.16.7 The measurements were performed in a blinded fashion. Also, 20 subjects were randomly selected to assess intra- and inter-observer variability.
Speckle-tracking echocardiography analysis
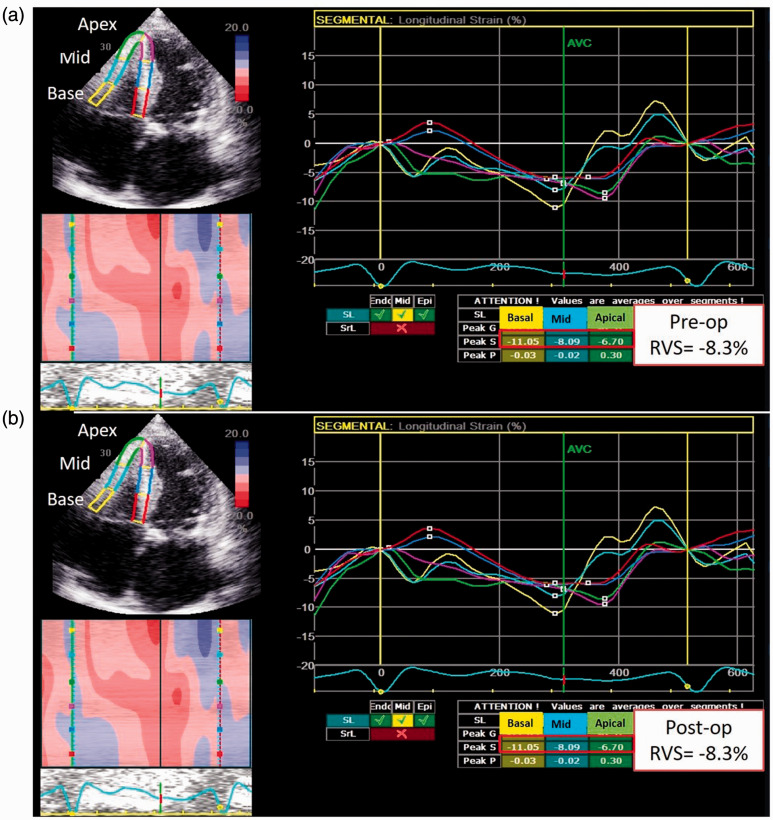
Standard apical 4-, 2- and 3-chamber views were recorded in digital loops for deformation analysis of the LV, and an apical 4-chamber view focusing on the RV was used for RV deformation. The images were acquired with frame rates of 70–90 frames/s and stored for three cycles. The images were analyzed off-line using computer software (EchoPAC, GE-Vingmed Ultrasound AS, Horten, Norway). As described previously, left ventricular peak systolic global longitudinal strain (LVGLS) was calculated after defining the timing of aortic valve closure.5 RV deformation was measured by two-dimensional speckle tracking in the apical 4-chamber view. RV longitudinal strain was derived from the average of three regional strains comprising the lateral wall. Fig. 1 shows an example of improved RV strain in a patient post surgical MV repair.
Fig. 1.
An illustration of RV strain measurement prior to and post MV repair.
Statistical analysis
Continuous data are presented as mean ± standard deviation or median (interquartile range), depending on the distribution. Dichotomous data are presented as numbers and percentages. The Chi-squared test or Fisher’s exact test was used to compare categorical variables as appropriate. The Kaplan–Meier method with log-rank test was used to compare event-free rates between groups. A multivariate Cox regression analysis was performed to evaluate factors associated with hospitalization for heart failure. All analyses were performed using SPSS, version 18 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Baseline clinical characteristics of patients who had MV repair
The majority of the enrolled patients were male and the average age was similar between patients without and with RV reserve. There was no significant difference in other comorbidities including diabetes, coronary artery disease, atrial fibrillation, and renal failure between the groups (Table 1). Moreover, the pre-operative New York Functional classification (NYFc), N-terminal-pro hormone B-type natriuretic peptide level, medical prescriptions, operation, aortic clamping and cardiopulmonary bypass time were similar between the two groups.
Table 1.
The baseline clinical characteristics and medical therapies of patients with non-reserved and reserved right ventricular (RV) function post mitral valve (MV) repair.
| Variables | RV non-reserved (RV strain declined) N = 23 | RV reserved (RV strain improved) N = 85 | p value |
|---|---|---|---|
| Clinical | |||
| Age (y/o) | 59.2 ± 12.9 | 55.7 ± 26.4 | 0.65 |
| Male (%) | 9 (39.1) | 38 (44.7) | 0.12 |
| Body weight (kg) | 58.6 ± 17.7 | 62.1 ± 10.4 | 0.71 |
| Body height (cm) | 159.6 ± 8.7 | 161.8 ± 20 | 0.35 |
| NYFc, n (%) | |||
| II | 10 (46.1) | 38 (44.7) | 0.72 |
| III | 7 (30.7) | 29 (34.1) | 0.51 |
| IV | 6 (23) | 18 (21.2) | 0.12 |
| Hypertension, n (%) | 9 (39.1) | 44 (51.7) | 0.09 |
| Diabetes, n (%) | 3(13) | 10 (11.7) | 0.46 |
| CAD, n (%) | 3(13) | 10 (11.7) | 0.65 |
| Af (baseline) | 11(47.8) | 44 (51.7) | 0.52 |
| Creatinine (mg/dl) | 0.9 ± 0.2 | 1.3 ± 1.5 | 0.34 |
| NT-proBNP (pg/ml) | 4938.4 ± 2979.4 | 4677.1 ± 6998.6 | 0.3 |
| Surgeries | |||
| MV annular ring | 19 (82.6) | 74 (87.1) | 0.52 |
| Repair leaflet | |||
| Anterior leaflet | 6 (26) | 22 (25.8) | 0.71 |
| Posterior leaflet | 9 (39.1) | 34 (40) | 0.64 |
| Both leaflets | 4 (17.4) | 18 (21.2) | 0.28 |
| Operation time (hr) | 5.4 ± 0.7 | 5.9 ± 0.9 | 0.08 |
| Aortic clamping time (min) | 103.1 ± 29.3 | 116 ± 35.5 | 0.23 |
| CPB time (min) | 159 ± 47.3 | 179.5 ± 47.9 | 0.16 |
| ICU stay (days) | 3.1 ± 2.7 | 2.8 ± 3.6 | 0.8 |
| Treatment | |||
| β-blocker, n (%) | 6 (26) | 9 (10.6) | 0.67 |
| ACEi/ARB, n (%) | 12 (52.2) | 22 (25.8) | 0.28 |
| Diuretics, n (%) | 6 (26) | 18 (21.2) | 0.28 |
| Anti-coagulants, n (%) | 9 (39.1) | 18 (21.2) | 0.55 |
| Anti-platelet, n (%) | 9 (39.1) | 34 (40) | 0.48 |
| Amiodarone, n (%) | 9 (39.1) | 20 (23.5) | 0.55 |
| Digoxin, n (%) | 2 (8.6) | 4 (4.7) | 0.31 |
Note: Data are expressed as mean ± SD or number (%). p < 0.05 as significance.
BMI: body mass index; CAD: coronary artery disease; Af: atrial fibrillation; NT-proBNP : NT-pro B-type natriuretic peptite; CPB : cardiopulmonary bypass; ICU: intensive care unit; ACEi/ARB: angiotensin converting enzyme inhibitors/ angiotensin II receptor blocker.
Among the total of 108 patients receiving mitral valve (MV) repair, there was a trend of improvement of the pre- and post-operative (during intensive care unit; ICU stay) hemodynamic characteristics (Supplement Table 1). Regarding the use of inotropics, more than half of the studied patients received post-operative Dobutamine, while a small portion of patients received Milrinone for a short period of time. The use of inotropics did not specifically prolong the ICU stay (Supplement Table 2).
Table 2.
The baseline and followed left heart echocardiographic parameters of patients with non-reserved and reserved right ventricular (RV) function post mitral valve (MV) repair.
| Variables | RV non-reserved (RV strain declined) N = 23 | RV reserved (RV strain improved) N = 85 | p value |
|---|---|---|---|
| LV | |||
| Medium follow-up (months) | 29 (19–41) | 35 (26–45) | |
| LVEDV (baseline) (ml) | 62.6 ± 37.7 | 69.5 ± 29.1 | 0.48 |
| LVESV(baseline) (ml) | 23.7 ± 18.4 | 21 ± 10.7 | 0.49 |
| LVMI (baseline) (g/M2) | 99.6 ± 31.1 | 127.9 ± 39.8 | 0.02 |
| E/A(baseline) | 2.14 ± 1.92 | 2.39 ± 1.76 | 0.05 |
| e′ (baseline) (cm/s) | 12.8 ± 7.2 | 12.9 ± 7.6 | 0.97 |
| E/e′ (baseline) | 13.3 ± 7.3 | 11.3 ± 4.7 | 0.24 |
| LVEF (baseline) (%) | 62.7 ± 10.3 | 65.9 ± 10.1 | 0.32 |
| LVEF (post 1M) (%) | 66 ± 9.8 | 66.1 ± 8.7 | 0.97 |
| LVEF (post 6M) (%) | 63.8 ± 8.7 | 65.6 ± 7.2 | 0.64 |
| LVEF change (baseline to 1M) (%) | 2.1 ± 10.2 | −1.6 ± 7.9 | 0.37 |
| LV GLS (baseline) (%) | −19.7 ± 7.4 | −19.8 ± 5.9 | 0.92 |
| LV GLS (post 1M) (%) | −16.9 ± 4.8 | −16.3 ± 2.9 | 0.69 |
| LV GLS (post 6M) (%) | −17.1 ± 4.7 | −16.1 ± 4.4 | 0.62 |
| LV GLS change (baseline to 1M) (%) | 2.5 ± 9.5 | 4.4 ± 3.9 | 0.57 |
| MV | |||
| ERO (baseline) (%) | 0.42 ± 0.14 | 0.47 ± 0.18 | 0.21 |
| ERO (post 1M) (%) | 0.21 ± 0.06 | 0.18 ± 0.08 | 0.51 |
| ERO (post 6M) (%) | 0.18 ± 0.1 | 0.15 ± 0.04 | 0.12 |
| ERO change (baseline to 1M) (%) | −0.28 ± 0.16 | −0.36 ± 0.19 | 0.14 |
| RVol (baseline) (ml) | 57.63 ± 11.85 | 75.86 ± 30.09 | 0.05 |
| RVol (post 1M) (ml) | 21.73 ± 24 | 15.97 ± 7.6 | 0.1 |
| RVol (post 6M) (ml) | 10.3 ± 7.4 | 9.1 ± 10.8 | 0.88 |
| RVol change (baseline to 1M) (ml) | −38.44 ± 1.57 | −60.56 ± 35.17 | 0.05 |
| LA | |||
| LAVi (baseline) (ml/m2) | 60.4 ± 25.9 | 55.5 ± 53.1 | 0.75 |
| LAVi (post 1M) (ml/m2) | 40 ± 22.2 | 30.2 ± 10 | 0.19 |
| LAVi (post 6M) (ml/m2) | 27.7 ± 14.7 | 29.6 ± 8.9 | 0.8 |
| LAVi change (baseline to 1M) (ml/m2) | 17.5 ± 41.6 | −28.6 ± 21.6 | 0.49 |
LVEDV: left ventricular end diastolic volume; LVESV: left ventricular end systolic volume; LVMI: left ventricular mass index; E/A: trans-mitral valve E to A velocity ratio; e′: early diastolic mitral annular velocity; LVEF: left ventricular ejection fraction; LV GLS: left ventricular global longitudinal strain; ERO: effective regurgitant orifice; RVol: regurgitation volume; LAVi: left atrial volume index.
Baseline and sequential echocardiographic characteristics of patients treated by MV repair
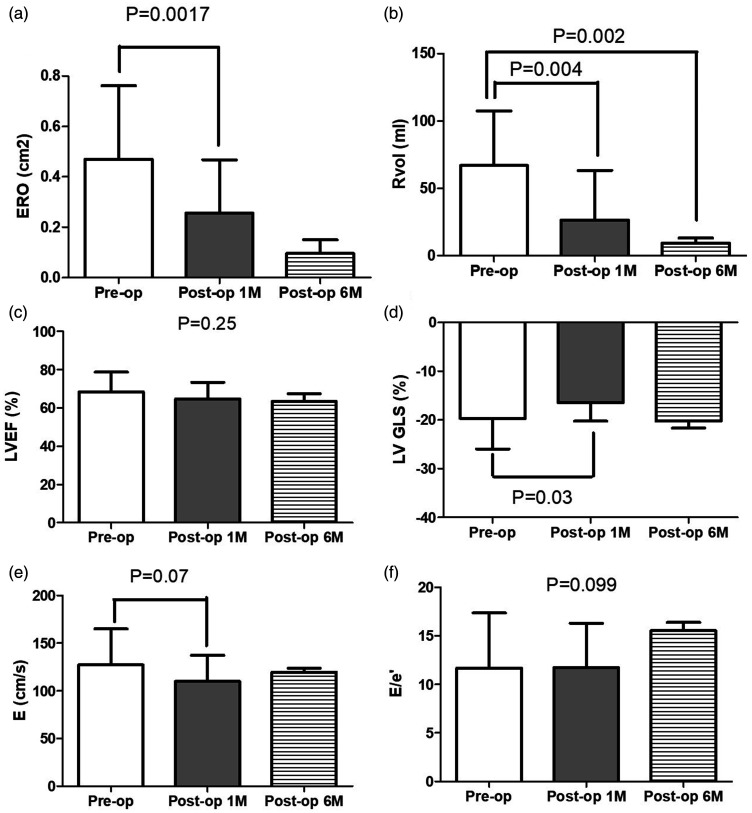
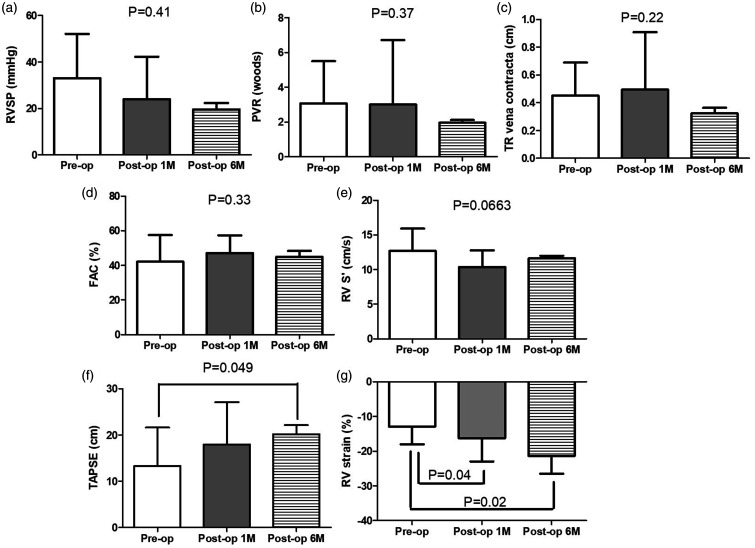
In a prospective manner, we monitored both left and right heart echocardiographic parameters comprehensively. Among all enrolled patients, there were significant reductions of ERO and RVol (Fig. 2a and b). The changes persisted to the six-month follow-up visits. Despite no significant changes in LVEF (Fig. 2c), LV strain declined at the first month and returned to the baseline six months post MV repair (Fig. 2d), while the LV diastolic function (Fig. 2e) and filling pressure-associated parameters (Fig. 2f) showed no significant changes. Interestingly, although both right ventricular systolic pressure (Fig. 3a) and pulmonary vascular resistance insignificantly decreased (Fig. 3b), the severity of TR became worse transiently at the post-operative acute stage and recovered after six months (Fig. 3c). Similarly, despite non-significant changes of RV FAC and tissue Doppler-derived S′, TAPSE displayed a continuous recovery postoperatively (Fig. 3d–f). Conversely, a consistent improvement was observed on only RV strain post MV repair (Fig. 3g).
Fig. 2.
Echocardiographic characteristics of mitral regurgitation severity, left heart systolic and diastolic functions in patients prior to, one month and six months post MV repair. ERO: effective regurgitant orifice; RVol: regurgitation volume, LVEF: left ventricular ejection fraction; LVGLS: left ventricular global longitudinal strain; E: transmitral early filling velocity; e′: tissue Doppler derived early mitral annular diastolic velocity.
Fig. 3.
Echocardiographic characteristics of right ventricular pressure, pulmonary vascular resistance, tricuspid regurgitation severity, right functions in patients prior to, one month and six months post MV repair. RVSP: right ventricular systolic pressure; PVR: pulmonary vascular resistance; TR: tricuspid regurgitation; FAC: fractional area change; S′: tissue Doppler-derived peak systolic tricuspid annular velocity; TAPSE: peak systolic tricuspid annular velocity.
Compared with patients with no RV reserve, those with RV reserve (RV strain improved post MV repair) had a higher left ventricular mass index, while the LV function including the systolic, diastolic and LV strains showed no significant differences (Table 2). Likewise, the conventional echocardiography-derived RV pressure and function parameters, including FAC, TAPSE and S′ were similar at baseline between the two groups (Table 3). Compared with patients without RV reserve, those with RV reserve presented a similar RV strain at Post MV repair, although the ERO and RVol specifically decreased in both groups, the degree of reduction from baseline to one month post-operatively was insignificant. Correspondingly, despite a reduction of LAVi in both groups, the change was more significant in patients with RV reserve. In an attempt to identify an early parameter that might reflect the recovery of RV function, we compared the sequential changes in FAC, TAPSE, S′ and RV strain prior to and post MV repair. Except for significant improvements of RV strain (−5.6 ± 6.4% vs. 2.1 ± 3.5, p = 0.001) and TAPSE (0.4 ± 2.1 vs. 2.7 ± 6.6, p = 0.04) in patients with RV reserve compared with that in patients with no RV reserve, the other conventional echocardiography-derived RV parameters showed insignificant changes.
Table 3.
The baseline and followed right heart echocardiographic parameters of patients with non-reserved and reserved right ventricular (RV) function post mitral valve (MV) repair.
| Variables | RV non-reserved (RV strain declined) N = 23 | RV reserved (RV strain improved) N = 85 | p value |
|---|---|---|---|
| RV | |||
| TR VC (baseline) (cm) | 0.4 ± 0.1 | 0.4 ± 0.2 | 0.77 |
| TR VC (post 1M) (cm) | 0.52 ± 0.24 | 0.59 ± 0.13 | 0.12 |
| TR VC (post 6M) (cm) | 0.39 ± 0.25 | 0.33 ± 0.16 | 0.07 |
| TR VC change (baseline to 1M) (cm) | 0.08 ± 0.32 | 0.19 ± 0.27 | 0.12 |
| RVSP (baseline) (mmHg) | 27.3 ± 14.7 | 34.9 ± 21.3 | 0.23 |
| RVSP (post 1M) (mmHg) | 21.57 ± 9.73 | 23.2 ± 9.23 | 0.65 |
| RVSP (post 6M) (mmHg) | 20.62 ± 8.7 | 23.02 ± 9.17 | 0.57 |
| RVSP change (baseline to 1M) (mmHg) | −7.95 ± 15.98 | −12.19 ± 17.33 | 0.53 |
| PVR (baseline) (woods) | 3.1 ± 0.7 | 3.4 ± 2.7 | 0.12 |
| PVR (post 1M) (woods) | 3.2 ± 0.77 | 4.08 ± 5.7 | 0.36 |
| PVR (post 6M) (woods) | 2.01 ± 0.37 | 1.93 ± 0.66 | 0.83 |
| PVR change (baseline to 1M) (woods) | 0.07 ± 1.04 | 0.6 ± 2.02 | 0.57 |
| FAC (baseline) (%) | 47.7 ± 9.9 | 40.4 ± 16.3 | 0.13 |
| FAC (post 1M) (%) | 47.1 ± 10.6 | 47.1 ± 10.2 | 0.98 |
| FAC (post 6M) (%) | 47.1 ± 12.6 | 43.9 ± 15 | 0.66 |
| FAC change (baseline to 1M) (%) | 18.1 ± 0.6 | 11.1 ± 19.1 | 0.22 |
| TAPSE (baseline) (cm) | 18.1 ± 4.7 | 18.2 ± 6.8 | 0.64 |
| TAPSE (post 1M) (cm) | 18.8 ± 5.8 | 20.7 ± 4.3 | 0.8 |
| TAPSE (post 6M) (cm) | 18.2 ± 6.8 | 22.8 ± 4.2 | 0.05 |
| TAPSE change (baseline to 1M) (cm) | 0.4 ± 2.1 | 2.7 ± 6.6 | 0.04 |
| S′ (baseline) (cm/s) | 13.2 ± 3.4 | 12.8 ± 3.1 | 0.69 |
| S′ (post 1M) (cm/s) | 10.3 ± 1.9 | 11.1 ± 2.4 | 0.37 |
| S′ (post 6M) (cm/s) | 10.5 ± 2.2 | 11.9 ± 3.1 | 0.3 |
| S′ change (baseline to 1M) (cm/s) | −3.3 ± 3.2 | −1.5 ± 2.8 | 0.12 |
| RV strain (baseline) (%) | −17.2 ± 5.3 | −15.1 ± 8.1 | 0.05 |
| RV strain (post 1M) (%) | −16.8 ± 4.3 | −20.1 ± 3.7 | 0.08 |
| RV strain (post 6M) (%) | −18.2 ± 2.8 | −22.9 ± 3.8 | 0.34 |
| RV strain change (baseline to 1M) (%) | 2.1 ± 3.5 | −5.6 ± 6.4 | 0.001 |
TR VC : tricuspid regurgitation vena contracta; RVSP: right ventricular systolic pressure; FAC: fractional area change; TAPSE: tricuspid annular plane systolic excursion; S′: systolic tricuspid annular velocity.
Clinical and echocardiographic characteristics of patients subsequently developing HF
Further, we divided patients according to the development of post-operative HF (Supplement Table 3). There was no significant difference between groups regarding clinical and operational characteristics, while patients who had Af as baseline were prone to readmission for HF. Compared with patients free from HF, those developing HF presented an increasing E/e′ at baseline and RVol one month post-operatively, while the other parameters of LV systolic function and strains showed no significant differences. Regarding RV parameters, there were more significant improvements of post-operative tricuspid regurgitation severity and RV strain in patients free from hospitalization for HF.
Presence or absence of RV reserve and frequency of hospitalization for cardiac failure
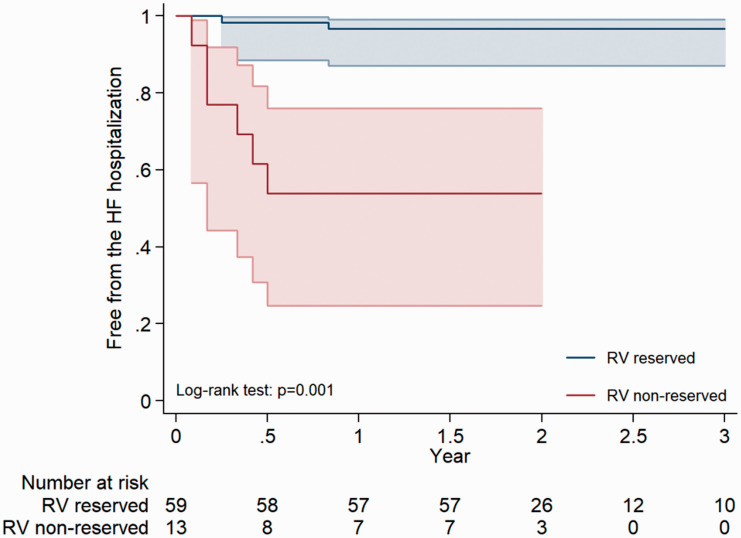
Among 85 patients of RV reserve and 23 patients of RV non-reserve, there were 4 and 13 patients reached the end-point of hospitalization for HF. To further investigate whether RV reserve is associated with the outcomes post MV repair, the Kaplan–Meier analysis showed a consistently lower risk of hospitalization for heart failure in patients with RV reserve (Fig. 4). In the Cox regression analysis, only RV strain one month post-operatively and RV reserve (HR: 1.33; CI: 1.07–1.66, p = 0.009 and HR: 12.8; CI: 3.5–28.9, p = 0.001, respectively) predicted the risks of hospitalization for heart failure in patients post MV repair (Table 4).
Fig. 4.
The Kaplan–Meier analysis showing a consistently lower risk of hospitalization for heart failure in patients with RV reserve.
Table 4.
Hazard ratios of hospitalization for heart failure in patients post mitral valve (MV) repair for degenerative mitral regurgitation.
| Variables | HR | p |
|---|---|---|
| Male | 2.1 (0.24–19.2) | 0.49 |
| Hypertension | 3.1 (0.6–16.3) | 0.16 |
| ERO (baseline) | 0.11 (0.002–8.41) | 0.32 |
| ERO change | 21.02 (0.45–98.1) | 0.12 |
| RVol (baseline) | 0.98 (0.95–1.01) | 0.27 |
| RVol change | 1.04 (0.92–1.17) | 0.47 |
| LVEF (baseline) | 1.01 (0.82–1.27) | 0.72 |
| LV GLS (baseline) | 1.06 (0.94–1.19) | 0.31 |
| LAVi (baseline) | 0.99 (0.98–1.01) | 0.89 |
| FAC (baseline) | 1.03 (0.97–1.09) | 0.22 |
| TAPSE (baseline) | 1.25 (0.74–1.39) | 0.62 |
| TAPSE (post 6M) | 1.05 (0.93–1.19) | 0.08 |
| TAPSE changes | 1.12 (0.82–1.45) | 0.23 |
| S′ | 1.02 (0.79–1.31) | 0.85 |
| RV strain (baseline) | 0.95 (0.86–1.05) | 0.34 |
| RV strain (post 1 month) | 1.33 (1.07–1.66) | 0.009 |
| RV reserve | 12.8 (3.5–28.9) | 0.001 |
Abbreviation as Table 2.
Regional differences in RV strain between patients with and without RV reserve post MV repair
For the assessment of the regional changes of RV strain in patients post MV repair, we separately investigated the RV free wall in the basal, middle and apical regions prior to and one month post MV repair (Supplement Table 4). Prior to MV repair, patients with no RV reserve had greater residual RV strain at the basal region compared with those with RV reserve. In contrast, one month post MV repair, the strain declined in all three regions in patients with no RV reserve. In contrast, patients with RV reserve represented with a significant improvement of the basal RV strain.
Reproducibility of LV and RV strain
The echocardiographic images of 20 randomly selected subjects were analyzed by two readers for a total of three times each. The intra- and inter-observer interclass correlation coefficients for RV strain were 0.94 (0.82–0.97) and 0.93 (0.8–0.95), respectively. For LV strain, the intra- and inter-observer interclass correlation coefficients were 0.94 (0.78–0.97) and 0.93 (0.82–0.97).
Discussion
TR and RV dysfunction are both frequently observed in patients with significant MR.8,9 The phenomenon is regarded as being due to a complex inter-ventricular remodeling and an increasing RV afterload from the post-capillary pulmonary hypertension.2 Although isolated TR has been reported to be associated with a poor prognosis, independent of RV dimension and function,10 Abello et al.11 suggested that RV dysfunction is as important as TR in their respective contribution to the negative prognostic impact in patients with severe MR. Also, a recently published article indicated that deterioration of RV contraction is associated with enhanced RV functional recovery at one year but not to preoperative levels.12 However, the potential clinical impact of RV recovery has not yet been studied. In our study, despite a significant reduction of mitral regurgitant volume post MV repair, LV filling pressure and RVSP failed to change significantly, which implied that the subsequent changes in RV performance were not mainly caused by the relief of afterload. Most importantly, although the severity of TR declined six months post MV repair, at the post-operative acute stage it became worse compared with its severity at the baseline. Conversely, RV function measured by RV strain manifested an immediate and consistent improvement post MV repair. In addition, in the Cox regression analysis, RV reserve was significantly associated with subsequent heart failure and could be a more optimal parameter to predict the outcomes in patients undergoing MV repair. The rapid recovery of RV function instead of pulmonary pressure implied that RV reserve is the key factor of heart failure hospitalization in addition to the pure reduction of afterload.
Despite RV strain being reported as an important diagnostic and prognostic predictor in congenital heart disease, pulmonary hypertension and various types of cardiomyopathy,13 its clinical application remains limited owing to the lack of reference values and standardization.14 Instead of a binary cutoff value for RV strains, we suggested a continuous parameter to define RV reserve such that changes in right ventricular afterload and valvular function. Conversely, a longitudinal follow-up of RV dysfunction is meaningful based on the interpretation of serial changes. In line with our findings, previous publications reported that TAPSE was reduced immediately after MV surgery and then recovered during follow-up assessment.15 In contrast, RV strain was found to improve during the post-operative period as well as on follow-up but the values of RV strain varied between individuals.16 This meant that despite the fact that most studies focused on pre-operative RV strain categorization in patients undergoing MV surgery, instead of setting an absolute cut-off point of RV strain and applying it to all patients, we elected to longitudinally follow the changes of RV strain in the same patient to monitor RV reserve. Most importantly, in the clinical setting, patients with early recovery of RV strain (effectively those with RV reserve) had a lower risk of hospitalization for heart failure compared to those with no RV reserve.
Regarding the regional changes of RV strain post MV repair, our findings indicated that compared with patients with no RV reserve, those with RV reserve represented with a significant improvement of RV strain especially at the basal region. In a 4D blood flow study, the impact of right atrial inflow was primarily focus on the basal region of the RV.17 Previous studies showed that in pulmonary hypertension the RV becomes more spherical and cardiomyocyte fiber orientation changes with longitudinal fibers arranging in a more circumferential fashion.18 Interestingly, the changes mainly happened at the basal RV where encountered most impulse of the flow from the right atrium.19 Corresponding to our study, the significant value of basal RV strain was also observed in patients with mitral stenosis and heart failure post cardiac resynchronization therapy.20
Limitations
There are some limitations of this study. First, the limited number of patients may attenuate statistical power. To compensate for this flaw, all enrolled patients were followed prospectively and longitudinally. This dual approach more comprehensively delineated the impact of MV repair on individual changes in RV strain. Second, regarding the outcomes of hospitalizations for heart failure, although the events were evaluated by cardiologists based on the ACC/AHA guidelines, it remains possible that patients presented to other outside hospitals. Third, although strain imaging is a relatively load- and angle-independent technique, it may still be influenced by the pre- and afterload of the RV. Moreover, a cut-off value for pre-operative RV strain was not defined in this study to sensitively predict subsequent changes to RV function after MV repair surgery. Fourth, patients who received concomitant tricuspid valve surgeries or were operated in an emergent condition were excluded. Excluding these patients may limit the evaluation of the true real-world population. Fifth, in this study, the maximal duration of echo follow-up was up to six months, while the long-term follow-up will further expand our understanding regarding the post-operative cardiac remodeling.
Conclusions
Postoperative RV reserve is associated with the subsequent changes in RV function (measured by TAPSE) and the risks of decompensated heart failure in patients treated with surgical MV repair for severe MR. The feasibility and value of quantification of RV strain during MV surgery require further investigation.notepad
Supplemental Material
Supplemental material, sj-jpg-1-pul-10.1177_2045894020943858 for Right ventricular reserve post mitral valve repair is associated with heart failure hospitalization by Wei-Ting Chang, Nan-Chun Wu, Jhih-Yuan Shih, Chih-Hsin Hsu, Zhih-Cherng Chen and Bor-Chih Cheng in Pulmonary Circulation
Supplemental material, sj-pdf-2-pul-10.1177_2045894020943858 for Right ventricular reserve post mitral valve repair is associated with heart failure hospitalization by Wei-Ting Chang, Nan-Chun Wu, Jhih-Yuan Shih, Chih-Hsin Hsu, Zhih-Cherng Chen and Bor-Chih Cheng in Pulmonary Circulation
Footnotes
Authors’ contributions: WT Chang, NC Wu, JY Shih, CH Hsu, ZC Chen and BC Cheng contributed to the conception and design of the study. WT Chang, NC Wu, JY Shih, CH Hsu, ZC Chen and BC Cheng contributed to the acquisition, analyses, and interpretation of data. WT Chang drafted the manuscript and all authors critically revised it. All authors gave final approval and agreed to be accountable for all aspects of the work ensuring integrity and accuracy.
Conflict of interest: The author(s) declare that there is no conflict of interest.
Data sharing: The datasets of this study are available from the corresponding author on reasonable request.
Ethical approval: Our study was complied with the 1964 Declaration of Helsinki and its later amendments, and approved by the Ethics Committee of Chi-Mei Medical Center (IRB: 10307-003).
Funding: This study was supported by Ministry of Science and Technology (MOST; 108-2628-B-384 -002) and Chi-Mei Medical Center.
Supplemental material: Supplemental material for this article is available online.
References
- 1.Patel H, Desai M, Tuzcu EM, et al. Pulmonary hypertension in mitral regurgitation. J Am Heart Assoc 2014; 3: e000748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Le Tourneau T, Deswarte G, Lamblin N, et al. Right ventricular systolic function in organic mitral regurgitation: impact of biventricular impairment. Circulation 2013; 127: 1597–1608. [DOI] [PubMed] [Google Scholar]
- 3.Dini FL, Conti U, Fontanive P, et al. Right ventricular dysfunction is a major predictor of outcome in patients with moderate to severe mitral regurgitation and left ventricular dysfunction. Am Heart J 2007; 154: 172–179. [DOI] [PubMed] [Google Scholar]
- 4.Kammerlander AA, Marzluf BA, Graf A, et al. Right ventricular dysfunction, but not tricuspid regurgitation, is associated with outcome late after left heart valve procedure. J Am Coll Cardiol 2014; 64: 2633–2642. [DOI] [PubMed] [Google Scholar]
- 5.Mor-Avi V, Lang RM, Badano LP, et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the japanese society of echocardiography. J Am Soc Echocardiogr 2011; 24: 277–313. [DOI] [PubMed] [Google Scholar]
- 6.Zoghbi WA, Adams D, Bonow RO, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the american society of echocardiography developed in collaboration with the society for cardiovascular magnetic resonance. J Am Soc Echocardiogr 2017; 30: 303–371. [DOI] [PubMed] [Google Scholar]
- 7.Abbas AE, Franey LM, Marwick T, et al. Noninvasive assessment of pulmonary vascular resistance by doppler echocardiography. J Am Soc Echocardiogr 2013; 26: 1170–1177. [DOI] [PubMed] [Google Scholar]
- 8.Shiran A, Sagie A. Tricuspid regurgitation in mitral valve disease incidence, prognostic implications, mechanism, and management. J Am Coll Cardiol 2009; 53: 401–408. [DOI] [PubMed] [Google Scholar]
- 9.Chipeta P, Shim CY, Hong GR, et al. Time course of left atrial reverse remodelling after mitral valve surgery and the impact of left ventricular global longitudinal strain in patients with chronic severe mitral regurgitation. Interactive Cardiovasc Thorac Surg 2016; 23: 876–882. [DOI] [PubMed] [Google Scholar]
- 10.Dahou A, Magne J, Clavel MA, et al. Tricuspid regurgitation is associated with increased risk of mortality in patients with low-flow low-gradient aortic stenosis and reduced ejection fraction: results of the multicenter Topas study (true or pseudo-severe aortic stenosis). JACC Cardiovasc Intervent 2015; 8: 588–596. [DOI] [PubMed] [Google Scholar]
- 11.Vargas Abello LM, Klein AL, Marwick TH, et al. Understanding right ventricular dysfunction and functional tricuspid regurgitation accompanying mitral valve disease. J Thorac Cardiovasc Surg 2013; 145: 1234–1241.e1235. [DOI] [PubMed] [Google Scholar]
- 12.Orde SR, Chung SY, Pulido JN, et al. Changes in right ventricle function after mitral valve repair surgery. Heart Lung Circul 2019; 29: 785--792. [DOI] [PubMed] [Google Scholar]
- 13.Shukla M, Park JH, Thomas JD, et al. Prognostic value of right ventricular strain using speckle-tracking echocardiography in pulmonary hypertension: a systematic review and meta-analysis. Canad J Cardiol 2018; 34: 1069–1078. [DOI] [PubMed] [Google Scholar]
- 14.Muraru D, Onciul S, Peluso D, et al. Sex- and method-specific reference values for right ventricular strain by 2-dimensional speckle-tracking echocardiography. Circul Cardiovasc Imag 2016; 9: e003866. [DOI] [PubMed] [Google Scholar]
- 15.Desai RR, Vargas Abello LM, Klein AL, et al. Tricuspid regurgitation and right ventricular function after mitral valve surgery with or without concomitant tricuspid valve procedure. J Thorac Cardiovasc Surg 2013; 146: 1126–1132.e1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hyllen S, Nozohoor S, Ingvarsson A, et al. Right ventricular performance after valve repair for chronic degenerative mitral regurgitation. Ann Thorac Surg 2014; 98: 2023–2030. [DOI] [PubMed] [Google Scholar]
- 17.Fredriksson AG, Zajac J, Eriksson J, et al. 4-d blood flow in the human right ventricle. Am J Physiol Heart Circul Physiol 2011; 301: H2344–2350. [DOI] [PubMed] [Google Scholar]
- 18.Hill MR, Simon MA, Valdez-Jasso D, et al. Structural and mechanical adaptations of right ventricle free wall myocardium to pressure overload. Ann Biomed Eng 2014; 42: 2451–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rich S. Right ventricular adaptation and maladaptation in chronic pulmonary arterial hypertension. Cardiol Clin 2012; 30: 257–269. [DOI] [PubMed] [Google Scholar]
- 20.Kumar V, Jose VJ, Pati PK, et al. Assessment of right ventricular strain and strain rate in patients with severe mitral stenosis before and after balloon mitral valvuloplasty. Indian Heart J 2014; 66: 176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-jpg-1-pul-10.1177_2045894020943858 for Right ventricular reserve post mitral valve repair is associated with heart failure hospitalization by Wei-Ting Chang, Nan-Chun Wu, Jhih-Yuan Shih, Chih-Hsin Hsu, Zhih-Cherng Chen and Bor-Chih Cheng in Pulmonary Circulation
Supplemental material, sj-pdf-2-pul-10.1177_2045894020943858 for Right ventricular reserve post mitral valve repair is associated with heart failure hospitalization by Wei-Ting Chang, Nan-Chun Wu, Jhih-Yuan Shih, Chih-Hsin Hsu, Zhih-Cherng Chen and Bor-Chih Cheng in Pulmonary Circulation