Abstract
Objective
We aimed to perform a network meta-analysis that combined both direct and indirect evidence to compare the relative efficacy of interventional therapies to treat patients with postherpetic neuralgia (PHN) and to determine the treatments’ superiority and validity.
Method
A conventional paired meta-analysis was performed. This was followed by a network meta-analysis using the Bayesian framework.
Results
Botulinum toxin type A and pulsed radiofrequency (PRF) were the two most effective individual interventions. For combination therapy, PRF + nerve block (NB) was the best choice, followed by subcutaneous injection or local infiltration (SC) + NB + ozone (O3). However, the combination of PRF + NB + SC showed reduced the efficacy compared with each treatment and was highly invasive for patients. After a long-term follow-up, PRF was shown to be the most effective therapy for treating patients with PHN.
Conclusions
Regular anti-neuropathic drug administration that was accompanied by interventional therapies at an early stage is the best choice to treat patients with PHN. Appropriate combinations of different interventions show improved pain relief. Clinicians should manage therapeutic regimens on the basis of the patients specific condition and existing measures and strive to achieve personalized treatment.
Keywords: Interventional treatment, postherpetic neuralgia, radiofrequency, paravertebral block, dorsal root ganglion, botulinum toxin, stellate ganglion block
Introduction
Postherpetic neuralgia (PHN) refers to pain that does not disappear after vesicular acute herpes zoster heals. PHN is conventionally defined as lasting more than 3 months after the onset of herpes zoster rash eruption or more than 1 month after the vesicles have healed.1 PHN is the most common complication of shingles, with an incidence of 5% to 30% in patients with herpes zoster.2 It is a persistent, debilitating, and chronic pain condition that is characterized by spontaneous pain, hyperalgesia, allodynia, and paresthesia in the dermatomal areas where shingles was present. Patients consider these troublesome syndromes to be intolerable, and they interfere the sleep and cause depression, thereby decreasing a patient’s quality of life and leading to functional impairment and lost productivity.3 PHN causes an increase in the use of health care resources and an increased economic burden on society.2,4
The priority in PHN treatment is to provide effective pain control and relieve sleep and emotional disorders, thereby improving the patient’s quality of life. Currently, the primary therapy for PHN is early and sufficient administration of medication, including calcium channel regulators (such as pregabalin and gabapentin), antidepressants, lidocaine patches, tramadol, and opioids.5 However, medication alone does not always provide ideal efficacy, especially in patients with long-term PHN or with PHN in certain areas of the body. Moreover, some patients may experience intolerable side effects of drugs such as nausea, dizziness, and drug addiction. These patients might benefit from interventional therapies. An interventional therapy refers to the technique that alleviates pain by directly targeting the lesion tissue and performing physical, chemical, or mechanical treatments, including nerve block (NB), selective nerve destruction, intrathecal drug administration, radiofrequency, nerve stimulation, ozone (O3) therapy, and subcutaneous injection or local infiltration (SC).
Despite various interventions, there is no consensus on choosing an appropriate intervention or about each intervention’s efficacy. Many clinical trials have been performed to compare the efficacy between two therapies, or between a therapy and drug administration, but most of them were observational studies or random controlled trials with a small sample size, which leads to unconvincing conclusions. Thus, some researchers have performed paired comparisons or a conventional meta-analysis between two methods and systematic reviews to assess the efficacy of these therapies.6–9 These studies provide direct comparisons and limited information, which are not as valuable as they could be, and it is necessary to compare and evaluate the clinical efficacy of these interventions on the basis of an overall viewpoint and provide a quantitative comparison and visualized conclusion.
In our study, we aimed to perform a network meta-analysis (NMA) among all available PHN interventional therapies, to combine both direct and indirect evidence on PHN efficacy, to compare the relative efficacy of treatments for PHN, and to determine the PHN treatments’ superiority and validity.
Methods
This NMA was designed and conducted in accordance with the guidelines proposed by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and the PRISMA extension for NMAs.10,11
Literature search strategy
Databases including PubMed, Embase, and the Cochrane Library were searched for studies about PHN treatments. The search was limited to literature that was published in English and before January 2020. To increase the comprehensiveness of our analysis, manual searches were performed using the reference lists from crucial studies and reviews. We used the following combined MeSH terms and their synonyms for the search: “Neuralgia, Postherpetic” and “Postherpetic Neuralgia”. All searches were limited to human studies and clinical trial studies.
Inclusion and exclusion criteria
To be included in our NMA, studies fulfilled the inclusion criteria that were interpreted under the PICOS framework (P, patients; I, interventions; C, controls; O, outcomes; S, studies) as follows: 1) P: Patients with PHN in body areas that were innervated by spinal nerves; 2) I: Interventional treatments were applied to the treatment groups, as follows: NB (including epidural block, spinal NB, dorsal root ganglion block, intercostal NB, paravertebral block), SC (including subcutaneous injection of normal saline, local anesthetics, corticosteroids, MeB12 as well as local infiltration), stellate ganglion block (SGB), subcutaneous botulinum toxin type A (BTX-A) injection, and pulsed radiofrequency (PRF) with or without administration of drug therapy; 3) C: Another interventional therapy or sham procedure or blank control was used for the control groups; 4) O: At least one quantitative pain evaluation can be extracted in all enrolled studies; and 5) S: Randomized controlled clinical trials.
The exclusion criteria for the studies were as follows: 1) Studies conducted in patients who had other neuropathic pain; 2) Studies focusing on destructive methods; or 3) Studies without a clear description of the study design, specific interventions, or necessary information about the participants.
Studies identified by our search were independently selected by two authors, and disagreements were resolved by consensus or consulting with another author. Titles and abstracts were reviewed first to determine whether studies were related to the theme. Then, full articles were judged on the basis of the inclusion and exclusion criteria. If a study satisfied the inclusion criteria, it was included for data extraction and used in the detailed analysis.
Data extraction and quality assessment
Two authors independently performed the data extraction and quality assessment for the selected studies; all disagreements were resolved by consensus.
Data were extracted on the basis of a prespecified table including the following contents: 1) demographic data (total number of participants, age, sex, necessary information about PHN); 2) treatment protocols (methods, route, drug category, and dosage); 3) outcomes; and 4) any complications.
The quality of the included studies was assessed using the Cochrane Collaboration’s tool for assessing the risk of bias, which includes the following seven aspects: random sequence generation; allocation concealment; blinding of patients and personnel; blinding of outcome assessment; incomplete outcome data; selective outcome reporting; and other sources of bias (adequate description of sample size calculation and detailed disclosure of funding sources). RevMan5.3 (The Nordic Cochrane Centre for The Cochrane Collaboration, Copenhagen, Denmark) was used to judge bias, which was expressed as “high risk,” “low risk,” or “unclear risk.”
Statistical analysis
Pain intensity was the only outcome that was analyzed in this study, and it was evaluated using different pain scales such as the Visual Analogue Scale (VAS) and Numerical Rating Scale (NRS), which were described in the included studies. Thus, the mean difference (MD) was used to compare the relative differences between different groups. Because all of the included studies were RCTs, the baseline pain score was comparable between different groups within a trial, so we mainly compared the pain scores using the endpoints.
For the direct comparison, the results were described as the MD and 95% confidence intervals (CIs), and for the indirect comparison and mixed-treatment effect, MD and 95% credible intervals (CrIs) were used. Because a lower pain scale means a better effect, a negative MD showed if a particular therapy was more effective than another therapy.
We initially performed a conventional paired meta-analysis to compare the relative efficacy between two treatments. The I2 test was used to evaluate heterogeneity among the included studies.12,13 The heterogeneity was considered to be low, moderate, or high for I2 values <25%, 25% to 50%, or >50%, respectively.14 Because of the small number of studies that can be used for direct comparisons, the DerSimonian–Laird (DL) random effects (RE) model was used for the paired meta-analysis.
Then, we performed NMA among all treatments using the Bayesian framework. The node-splitting method and the inconsistency factor were used to analyze the inconsistency between direct and indirect comparisons. The publication bias was assessed using funnel plots. Network plots, funnel plots, and cumulative probability plots were obtained using Stata 14.0 (StataCorp LLC, College Station, TX, USA), and NMA and the paired meta-analysis were performed using ADDIS (Department of Epidemiology, University Medical Center, Groningen, The Netherlands). P < 0.05 was considered to be statistically significant.
Results
Study selection
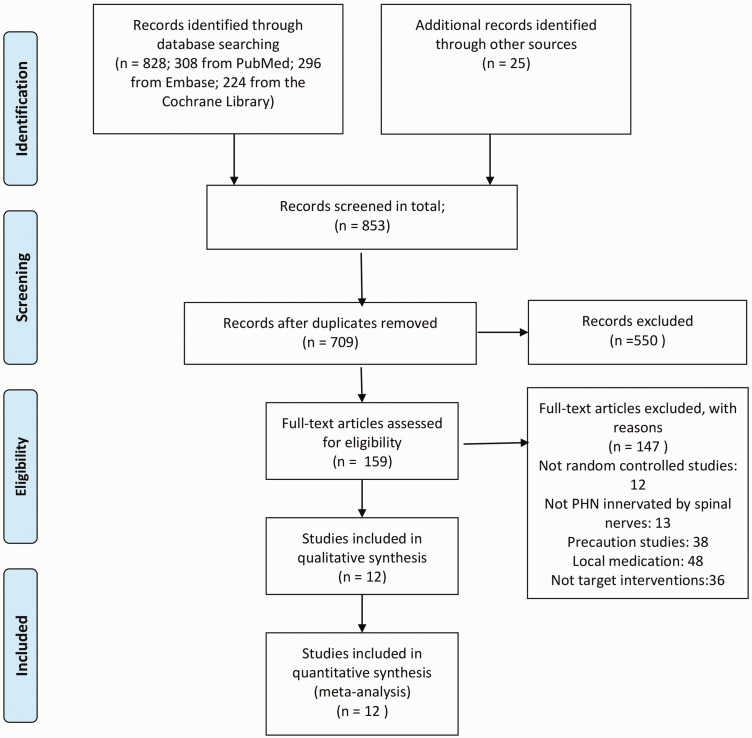
As shown in Figure 1, 828 studies were identified from the above-mentioned databases, and 25 studies were obtained via a manual search. After removing duplicates, 709 studies remained in the primary selection, and 550 of them were excluded based on their title and abstracts, leaving 159 studies for full-text evaluation on the basis of the inclusion and exclusion criteria. After reading the full text, 147 studies were excluded with explanations, and 12 studies were included in our NMA.
Figure 1.
Flowchart for study selection.
Study characteristics
Table 1 describes the summary of the 12 included studies that were published between 2010 and 2017.15–26 There were 702 participants with an age range from 57.06 to 73.53 years. Among these included studies, two focused on the efficiency of subcutaneous BTX-A,21,26 three focused on NB,15–17 five studies focused on PRF therapy,15,16,19–21 one study focused on PRF + NB,17 one study focused on PRF + NB + SC,19 three studies focused on SC,23,24,26 one study focused on SC + NB,24 one study focused on SC + NB + O3 injection,24 and one study focused on SGB.25 The detailed parameters, methods, and drug dosages are listed in Table 1. For oral medication, among nine studies,16–24 all patients regularly took oral calcium channel regulators (first-line drugs for PHN treatment); however, in one study,25 only some patients took calcium channel regulators, while in two studies,26,27 no patients took these medications.
Table 1.
Characteristics of the included studies.
|
Autdor, Year |
Study Design |
Mean Age (years) |
Female (%) |
Duration of PHN |
Pain Intensity |
Body Area |
Follow-up |
Pain Scale |
| Ashok, 201622 | Double-blind RCT | 60.17 | 43.30 | >3 months | VAS ≥30 mm | T3–T11 | 8 weeks | VAS |
| Dan, 201817 | RCT | 67 | – | >1 month | – | Cervical or thoracolumbar | 12 hours | VAS |
| Dong, 201719 | Single-blind RCT | 69.98 | 69.70 | >3 months | – | Abdominal and thoracic back | 48 hours | VAS |
| Gang, 201323 | RCT | 66.93 | 52.04 | 1 month–3 months | NRS ≥4 | – | 4 weeks | NRS |
| Guo, 201324 | RCT | 57.06 | 47.06 | >1 month | – | – | 4 weeks | VAS |
| Ji, 201225 | RCT | 63.5 | 40 | – | – | Chest and back | 8 weeks | VAS |
| Lizu, 201026 | Double-blind RCT | 67.33 | 53.33 | >3 months | VAS ≥5 | Orofacial, cervical and upper extremity, lumbar and lower extremity | 3 months | VAS |
| Ma, 201320 | Double-blind RCT | 72.16 | 50.00 | ≥6 months | VAS >30 mm | T2–T11 | 6 months | VAS |
| Mohamed, 201821 | Double-blind RCT | 58.74 | 44.19 | >6 months | VAS >30 mm | T2–T11 | 1 year | VAS |
| Wang, 201218 | RCT | 67.2 | 44.23 | >3 months | VAS >6 | Thoracic and back | 12 weeks | VAS |
| Xia, 201816 | RCT | 66.47 | 50.0 | 4 months–3 years | – | Abdominal and thoracic area | 12 weeks | NRS |
| Zoe, 201327 | Double-blind RCT | 75.35 | 40% | ≥3 months | VAS ≥7 | Thoracic, sciatic, brachial plexus | 20 weeks | VAS |
|
Autdor, Year |
Evaluation time point |
Basic Drugs |
N |
Groups, n |
Dose |
Route |
Adverse events related to interventional treatment |
|
| Ashok, 201622 | Basic, 1 week, 2 weeks, 4 weeks, 8 weeks | Pregabalin; tramadol as rescue | 60 | PRF, 30; sham, 30 | 42°, 180 s; - | Intercostal | 2 local site reactions; 2 local site reactions | |
| Dan, 201817 | Basic, 12 hours after treatment | Gabapentin; tramadol | 60 | Conventional puncture, 15; NB, 15; PRF, 15; PRF+NB, 15 | -; mecobalamin 1 mg/mL, extract from rabbit skin inflamed by vaccinia virus for injection 6 mL, 1% ropivacaine 2 mL; 0.9% normal saline 11 mL; 42°, 100 s × 3; PRF followed by NB | Paravertebral | None | |
| Dong, 201719 | Basic, 48 hours after treatment | Pregabalin; diclofenac; cobamamide | 32 | Control, 18; PRF + NB + SC, 15 | -; 42°, 120 s × 2 + 5 mL (lidocaine + ropivacaine + betamethasone + cobamamide + NS) | DRG+SC | - | |
| Gang, 201323 | Basic, 1 week, 2 weeks, 4 weeks | Gabapentin or pregabalin | 98 | MeB12 injection, 33; oral MeB12, 33; SC lidocaine injection, 32 | 1 mg MeB12; 0.5 mg MeB12 t.i.d.; 1% lidocaine | SC; oral; SC | - | |
| Guo, 201324 | Basic, 4 weeks | Gabapentin, ibuprofen codeine, Vitb12 | 80 | SC + oral MeB12, 20; SC + IM MeB12, 19; paravertebral block + SC, 20; paravertebral block + O3 injection + SC, 21; | Ropivacaine 10 mg + betamethasone 1 mg + NS 20 mL; ropivacaine 10 mg+ betamethasone 1 mg + NS 20 mL; Ropivacaine 10 mg + betamethasone 1 mg + NS 20 mL; ropivacaine 10 mg + betamethasone 1 mg + NS 20 mL + O3 35 μg/mL | SC; SC; paravertebral; paravertebral | - | |
| Ji, 201225 | Basic, 1 week, 2 weeks, 3 weeks, 4 weeks, 8 weeks | Pregabalin, celecoxib as rescue | 120 | SGB, 40; Pregabalin, 40; SGB + pregabalin, 40 | 0.5% lidocaine 10 mL; -; 0.5% lidocaine 10 mL | SGB | 1 hoarseness, 1 hematoma; -; 2 hoarseness, 1 hematoma | |
| Lizu, 201026 | Basic, 1 day, 7 days, 3 months | Opioid as rescue | 60 | BTX-A, 20; Lidocaine, 20; placebo, 20 | 5 IU/mL (100 IU in total) BTX-A; 0.5% lidocaine; NS | SC | None | |
| Ma, 201320 | Basic, 3 days, 1 weeks, 2 weeks, 1 month, 2 months, 3 months, 6 months | Gabapentin; antidepressants; tramadol | 92 | PRF, 46; sham, 46 | 42°, 120 s × 2; - | Intercostal | 1 bradycardia; 1 needle injury | |
| Mohamed, 201821 | Basic, 2 weeks, 4 weeks, 6 weeks, 8 weeks, 10 weeks, 12 weeks, 14 weeks, 16 weeks, 18 weeks, 20 weeks, 22 weeks, 6 months, 9 months, 12 months | Pregabalin; acetaminophen as rescue | 43 | PRF, 21; sham, 22 | 42°, 120 s × 2; - | Intercostal | None | |
| Wang, 201218 | Basic, 1 weeks, 2 weeks, 3 weeks, 4 weeks, 8 weeks, 12 weeks | Pregabalin | 52 | Thoracic paravertebral nerve block, 27; only drug, 25 | 0.3% lidocaine 10 mL + triamcinolone 10 mg; - | Paravertebral | None | |
| Xia, 201816 | Basic, 2 weeks, 4 weeks, 12 weeks | Gabapentin | 60 | Acupuncture trigger point, 20; continuous epidural nerve block, 20; selective DRG PRF, 20 | -; 2 mL/hour (ropivacaine 225 mg + betamethasone + cobamamide + NS); 42° 10 minutes with an interval of 15 s | -; epidural; DRG | - | |
| Zoe, 201327 | Basic, every day in the first 2 weeks, 4 weeks, 6 weeks, 8 weeks, 12 weeks, 16 weeks, 20 weeks, 24 weeks | Paracetamol | 30 | BTX-A, 15; NS, 15 | 100 IU + 4 mL NS; 4 mL NS only | SC; SC | None | |
RCT, random controlled trial; PRF, pulsed radiofrequency; NB, nerve block; SC, subcutaneous injection or local infiltration; O3, ozone injection; SGB, stellate ganglion block; BTX-A, botulinum toxin type A; DRG, dorsal root ganglion; NS, normal saline; T, thoracic nerve; VAS, visual analog scale; NRS, numerical rating scale; IM, intramuscular; t.i.d., three times daily; -, data not available from the original article.
Quality of the included studies
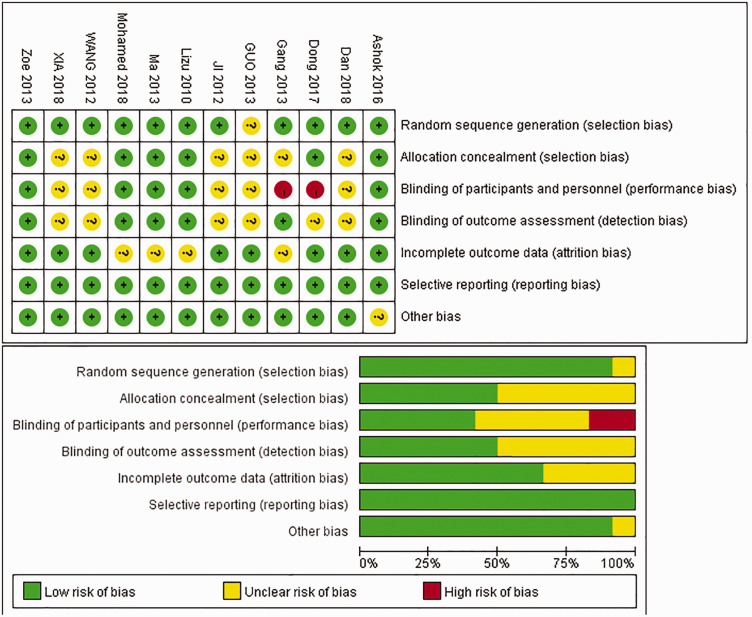
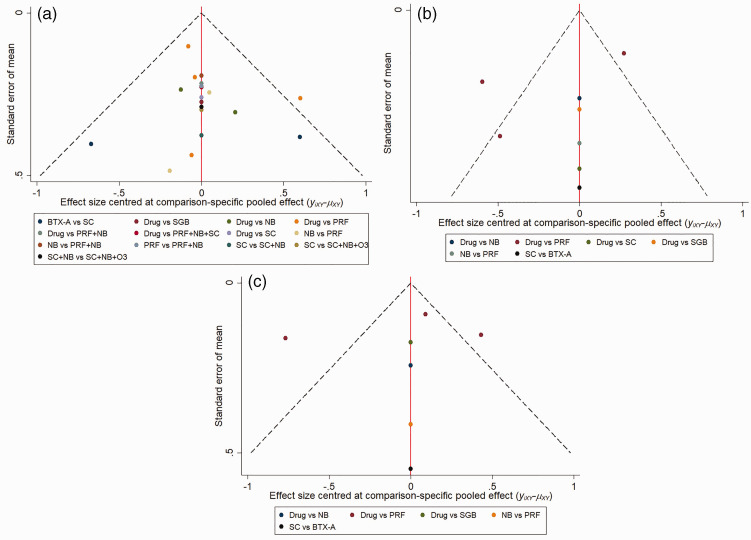
Studies included in our NMA were assessed using the Cochrane Collaboration’s tool for assessing the risk of bias (Figure 2). All 12 of the included studies reported all of the results, and most studies16–22,24–27 described how the random sequence was generated and showed all of the outcome data. Half of the included studies19–22,26,27 showed their allocation concealment method and outcome concealment strategy, while two studies19,23 did not perform blinding for their participants and personnel. The publication bias for the included studies is shown in Figure 3. The data points were grouped in the middle and upper parts of the funnel plot, and most of the data points were contained within the 95% CI lines, indicating that the publication bias was relatively low.
Figure 2.
Bias assessment graph.
Figure 3.
Comparison-adjusted funnel plot. (a) Comparison-adjusted funnel plot for 1 week after treatment, (b) Comparison-adjusted funnel plot for 1 month after treatment, (c) Comparison-adjusted funnel plot for 3 months after treatment.
Drug, oral drug therapy; PRF, pulsed radiofrequency; NB, nerve block; SC, subcutaneous injection or local infiltration; O3, ozone injection; SGB, stellate ganglion block; BTX-A, botulinum toxin type A.
Pairwise comparison
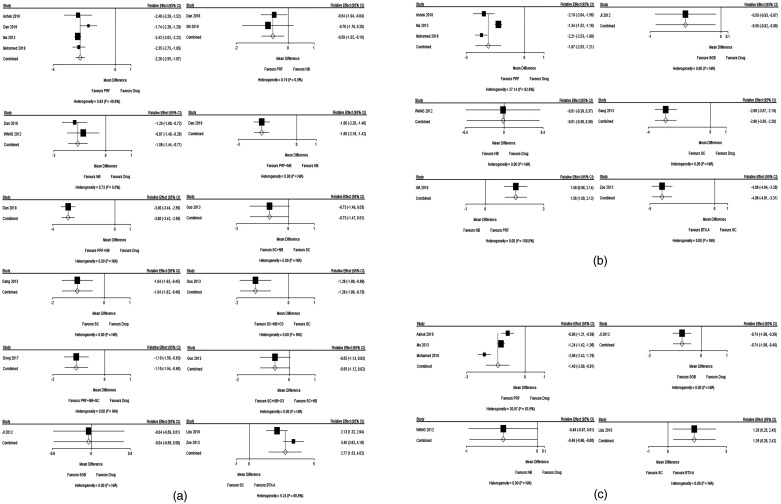
As shown in Figure 4, we chose the three most common time points (1 week, 1 month, and 3 months after treatment) to conduct 22 paired meta-analyses between each group of two interventions, and the results are presented as the MD and 95% CI. The analyzed outcome was pain intensity, and a negative MD meant that the former intervention was better than the later intervention.
Figure 4.
Forest plot for the direct comparison. (a) Forest plot for the direct comparison of the pain score 1 week after treatment, (b) Forest plot for the direct comparison of the pain score 1 month after treatment, (c) Forest plot for the direct comparison of the pain score 3 months after treatment. A negative MD means that the efficacy of the former therapy is better than that of the latter therapy
CI, confidence interval; Drug, oral drug therapy; PRF, pulsed radiofrequency; NB, nerve block; SC, subcutaneous injection or local infiltration; O3, ozone injection; SGB, stellate ganglion block; BTX-A, botulinum toxin type A; MD, mean difference.
After 1 week of treatment, there were 12 pairwise comparisons, and the results are shown in Figure 4A. In contrast to oral medications, five treatment classes showed a significant effect on pain relief, including NB, PRF, PRF + NB, PRF + NB + SC, and SC. Additionally, SGB was not superior to oral medications. Compared with SC, subcutaneous BTX-A injection and SC + NB + O3, but not SC + NB, showed significant efficacy. Compared with NB, neither PRF nor PRF + NB was more effective, while PRF + NB showed the best effectiveness.
After 1 month of treatment, six paired comparisons were performed, and the results are shown in Figure 4B. The efficacy of PRF, SC, and SGB was better than that of oral medications, and NB showed no significant difference from oral medications. PRF was also more effective for pain relief than NB.
Three months after treatment, paired comparisons showed that PRF and SGB, but not NB, were more effective than oral medications. Subcutaneous BTX-A injection was still more effective than SC (Figure 4C).
Network meta-analysis
Figure 5 shows network plots of 1 week, 1 month, and 3 months after treatments. The NMA outcomes showing the relative effects and 95% CrIs are presented in Table 2. A negative number indicates better efficacy, a 95% CrI including zero means that there is no significant difference between two interventions. The NMA was accomplished using Bayesian analysis methods, which are based on the Markov chain–Monte Carlo method. NMA results were similar to the direct paired comparisons above, but they offered more information, as described below.
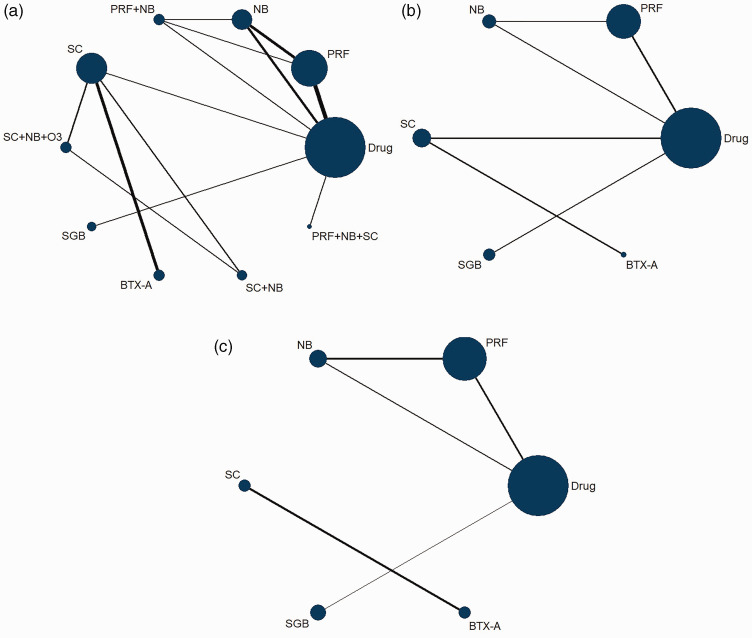
Figure 5.
Network evidence plot. (a) Network evidence plot 1 week after treatment, (b) Network evidence plot 1 month after treatment, (c) Network evidence plot 3 months after treatment. The size of the blue circles describes the total number of participants in each treatment class, and the thickness of the black lines between two circles describes the total number of studies that compared the two treatment classes
Drug, oral drug therapy; PRF, pulsed radiofrequency; NB, nerve block; SC, subcutaneous injection or local infiltration; O3, ozone injection; SGB, stellate ganglion block; BTX-A, botulinum toxin type A.
Table 2A.
The league table of NMA results for pain score 1 week after treatment.
| BTX-A | 3.81 (2.35, 5.28) | 2.55 (0.98, 4.21) | 1.61 (0.05, 3.22) | 0.64 (−1.08, 2.46) | 2.73 (0.85, 4.52) | 2.77 (1.88, 3.66) | 2.03 (0.48, 3.60) | 1.49 (−0.05, 2.94) | 3.78 (1.86, 5.67) |
|---|---|---|---|---|---|---|---|---|---|
| −3.81 (−5.28, −2.35) | Drug | −1.27 (−1.93, −0.53) | −2.21 (−2.73, −1.63) | −3.17 (−4.13, −2.17) | −1.10 (−2.24, −0.02) | −1.05 (−2.22, 0.12) | −1.77 (−3.58, −0.07) | −2.31 (−4.04, −0.64) | −0.04 (−1.19, 1.15) |
| −2.55 (−4.21, −0.98) | 1.27 (0.53, 1.93) | NB | −0.94 (−1.66, −0.24) | −1.90 (−2.94, −0.90) | 0.16 (−1.20, 1.45) | 0.24 (−1.17, 1.53) | −0.49 (−2.42, 1.26) | −1.04 (−2.96, 0.70) | 1.23 (−0.18, 2.59) |
| −1.61 (−3.22, −0.05) | 2.21 (1.63, 2.73) | 0.94 (0.24, 1.66) | PRF | −0.96 (−1.97, −0.01) | 1.12 (−0.23, 2.30) | 1.17 (−0.19, 2.43) | 0.44 (−1.48, 2.17) | −0.10 (−1.94, 1.62) | 2.16 (0.82, 3.42) |
| −0.64 (−2.46, 1.08) | 3.17 (2.17, 4.13) | 1.90 (0.90, 2.94) | 0.96 (0.01, 1.97) | PRF+NB | 2.08 (0.50, 3.49) | 2.13 (0.55, 3.61) | 1.41 (−0.71, 3.34) | 0.85 (−1.21, 2.79) | 3.13 (1.57, 4.69) |
| −2.73 (−4.52, −0.85) | 1.10 (0.02, 2.24) | −0.16 (−1.45, 1.20) | −1.12 (−2.30, 0.23) | −2.08 (−3.49, −0.50) | PRF+NB+SC | 0.05 (−1.53, 1.72) | −0.68 (−2.80, 1.40) | −1.23 (−3.31, 0.77) | 1.07 (−0.52, 2.74) |
| −2.77 (−3.66, −1.88) | 1.05 (−0.12, 2.22) | −0.24 (−1.53, 1.17) | −1.17 (−2.43, 0.19) | −2.13 (−3.61, −0.55) | −0.05 (−1.72, 1.53) | SC | −0.72 (−2.06, 0.51) | −1.27 (−2.49, −0.09) | 1.00 (−0.63, 2.65) |
| −2.03 (−3.60, −0.48) | 1.77 (0.07, 3.58) | 0.49 (−1.26, 2.42) | −0.44 (−2.17, 1.48) | −1.41 (−3.34, 0.71) | 0.68 (−1.40, 2.80) | 0.72 (−0.51, 2.06) | SC+NB | −0.56 (−1.73, 0.66) | 1.72 (−0.36, 3.91) |
| −1.49 (−2.94, 0.05) | 2.31 (0.64, 4.04) | 1.04 (−0.70, 2.96) | 0.10 (−1.62, 1.94) | −0.85 (−2.79, 1.21) | 1.23 (−0.77, 3.31) | 1.27 (0.09, 2.49) | 0.56 (−0.66, 1.73) | SC+NB+O3 | 2.27 (0.28, 4.37) |
| −3.78 (−5.67, −1.86) | 0.04 (−1.15, 1.19) | −1.23 (−2.59, 0.18) | −2.16 (−3.42, −0.82) | −3.13 (−4.69, −1.57) | −1.07 (−2.74, 0.52) | −1.00 (−2.65, 0.63) | −1.72 (−3.91, 0.36) | −2.27x(−4.37, −0.28) | SGB |
Table 2B.
The league table of NMA results for pain score 1 month after treatment.
| BTX-A | 6.92 (3.79, 9.96) | 6.81 (3.35, 10.18) | 5.10 (1.82, 8.35) | 4.05 (1.96, 6.14) | 6.43 (2.73, 10.06) |
|---|---|---|---|---|---|
| −6.92 (−9.96, −3.79) | Drug | −0.12 (−1.74, 1.51) | −1.83 (−3.00, −0.70) | −2.88 (−5.02, −0.73) | −0.51 (−2.58, 1.57) |
| −6.81 (−10.18, −3.35) | 0.12 (−1.51, 1.74) | NB | −1.70 (−3.33, −0.12) | −2.77 (−5.50, 0.03) | −0.37 (−3.05, 2.31) |
| −5.10 (−8.35, −1.82) | 1.83 (0.70, 3.00) | 1.70 (0.12, 3.33) | PRF | −1.06 (−3.46, 1.42) | 1.32 (−1.15, 3.73) |
| −4.05 (−6.14, −1.96) | 2.88 (0.73, 5.02) | 2.77 (−0.03, 5.50) | 1.06 (−1.42, 3.46) | SC | 2.38 (−0.60, 5.36) |
| −6.43 (−10.06, −2.73) | 0.51 (−1.57, 2.58) | 0.37 (−2.31, 3.05) | −1.32 (−3.73, 1.15) | −2.38 (−5.36, 0.60) | SGB |
After 1 week of treatment, as shown in Figure 5A and Table 2A, comparisons were made among six independent interventions and four combined therapies. Among all of these therapies, subcutaneous BTX-A injection was more effective than the other treatments except for PRF + NB and SC + NB + O3. Oral medication alone was less effective than other treatments except for SC and SGB. For NB, SC, PRF, and their combinations, PRF + NB exhibited a significant improvement in pain relief compared with the other treatments, while PRF + NB + SC was not significantly effective compared with the other treatments, and NB was less effective than PRF.
After 1 month of treatment, outcomes between the comparison of six therapies are shown in Figure 5B and Table 3B. BTX-A was more effective than the other treatments. Thus, PRF was significantly better than oral medications and NB for alleviating pain.
Table 3B.
Inconsistency factor.
| Time Point | Cycle | Inconsistency Factor, median (95%CrI) |
|---|---|---|
| 1 week | Drug, NB, PRF | 0.06 (−3.03, 2.20) |
| 1 month | Drug, NB, PRF | 0.10 (−2.07, 2.31) |
| 2 months | Drug, NB, PRF | −0.17 (−2.06, 1.39) |
Data were described as MD and 95% CI or 95% CrI. P≤0.05 indicates a statistically significant difference between direct and indirect effects. An inconsistency factor close to zero means that there was no significant inconsistency between the direct and indirect therapy.
Drug; drug therapy; PRF, pulsed radiofrequency; NB, nerve block; MD, mean difference; CI, confidence interval; CrI, credible interval; N.S., not significant.
After 3 months of treatment, Figure 5C and Table 2C show that PRF was significantly better than oral medications. For all other comparisons, the 95% CrI included zero.
Table 2C.
League table of NMA results for pain score 3 months after treatment.
| Drug | −0.17 (−1.72, 1.42) | −1.51 (−2.62, −0.42) | −0.74 (−2.80, 1.27) |
|---|---|---|---|
| 0.17 (−1.42, 1.72) | NB | −1.34 (−2.98, 0.26) | −0.58 (−3.09, 2.08) |
| 1.51 (0.42, 2.62) | 1.34 (−0.26, 2.98) | PRF | 0.76 (−1.56, 3.08) |
| 0.74 (−1.27, 2.80) | 0.58 (−2.08, 3.09) | −0.76 (−3.08, 1.56) | SGB |
Therapies are reported in alphabetical order. Data are described as the MD and 95% CrI. A 95% CrI that includes zero means that there is no significant difference between two therapies. A negative MD favors the column-defining therapy. Significant results are in bold and underscored.
Drug, drug therapy; PRF, pulsed radiofrequency; NB, nerve block; SC, subcutaneous injection or local infiltration; O3, ozone injection; SGB, stellate ganglion block; BTX-A, botulinum toxin type A; NMA, network meta-analysis; MD, mean difference; CrI, credible interval.
Consistency assessment
We performed this NMA based on the consistency model. To evaluate its validity, we used the following two methods: node-splitting method and inconsistency factors.27,28 The results are shown in Table 3.
Table 3A.
Results of node-split analysis.
| Time | Name | Direct Effect | Indirect Effect | Overall | P-Value |
|---|---|---|---|---|---|
| 1 week | Drug, NB | −1.12 (−2.00, −0.14) | −1.43 (−3.13, 0.20) | −1.27 (−1.93, −0.53) | N.S. |
| Drug, PRF | −2.24 (−2.93, −1.56) | −1.86 (−3.68, −0.02) | −2.21 (−2.73, −1.63) | N.S. | |
| NB, PRF | −0.63 (−1.36, 0.10) | −1.33 (−2.18, −0.47) | −0.94 (−1.66, −0.24) | N.S. | |
| 1 month | Drug, NB | −0.01 (−3.13, 3.15) | −0.31 (−3.81, 3.40) | −0.12 (−1.74, 1.51) | N.S. |
| Drug, PRF | −1.85 (−3.59, −0.10) | −1.58 (−5.88, 2.52) | −1.83 (−3.00, −0.70) | N.S. | |
| NB, PRF | −1.59 (−4.80, 1.70) | −1.86 (−5.83, 1.85) | −1.70 (−3.33, −0.12) | N.S. | |
| 2 months | Drug, NB | −0.50 (−2.74, 1.84) | 0.37 (−2.28, 3.08) | −0.17 (−1.72, 1.42) | N.S. |
| Drug, PRF | −1.41 (−2.75, −0.10) | −2.24 (−5.62, 1.01) | −1.51 (−2.62, −0.42) | N.S. | |
| NB, PRF | −1.74 (−4.14, 0.60) | −0.94 (−3.62, 1.72) | −1.34 (−2.98, 0.26) | N.S. |
The node-splitting analysis assessed whether indirect and direct evidence were in agreement on a split node.29 Table 3A shows the node-splitting analysis results at different time points after treatment, including direct, indirect, and combined evidence. The P-value was also shown to indicate whether there was significant inconsistency. P ≤ 0.05 was considered to be statistically significant. Table 3A suggests that no significant inconsistency was present in the NMA. Moreover, it is meaningful to assess the consistency using the node-splitting analysis only when there is concurrent direct and indirect evidence. Thus, the consistency could not be assessed for comparisons using only an indirect comparison.
If closed loops are present in the evidence structure, the inconsistency factor can be used to detect the inconsistency. The closer the inconsistency factor is to zero, the smaller the amount of inconsistency that is present.28 The results are summarized in Table 3B, which indicates that there is no significant inconsistency. This is in agreement with the results of the node-splitting analysis.
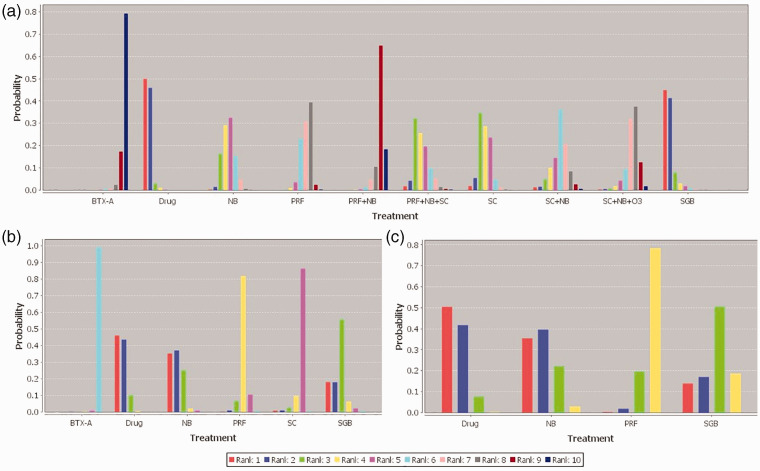
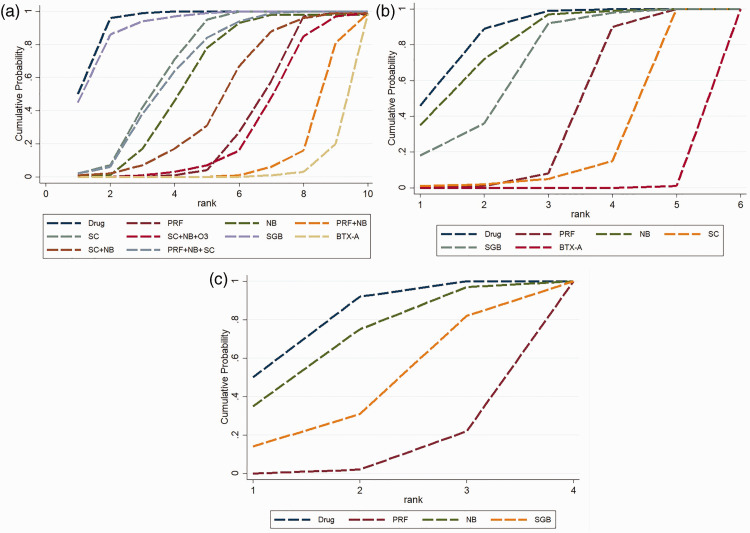
Ranking of treatment classes
The Bayesian approach allows estimation of the probability, provides the different previous therapies, and the data so that the best treatment can be identified, followed by the second-best treatment and so on. The results are shown in Figure 6 and Figure 7. Figure 6 shows the rank probabilities for each treatment. The sum of the rank probabilities is 1, whether it is within a rank over treatments or within a treatment over ranks. Rank 10 indicates the best treatment for pain reduction. Figure 7 shows the cumulative probability of different therapies, and larger areas under the curve mean a smaller rank and worse pain relief efficacy. After 1 week of treatments, subcutaneous BTX-A ranked the best and PRF + NB ranked the second best, followed by SC + NB + O3 and PRF; SC + NB, NB, PRF + NB + SC, and SC; and the last two were SGB and oral medication. After 1 month of treatment, the ranking sequence from the most to least effective was as follows: BTX-A, SC, PRF, SGB, NB, and oral medication. After 3 months of treatment, the sequence was as follows: PRF, SGB, NB, and oral medication.
Figure 6.
Rank probability plot. (a) Rank probability plot for pain score 1 week after treatment, (b) Rank probability plot for pain score 1 month after treatment, (c) Rank probability plot for pain score 3 months after treatment. Rank 1 is the worst and rank 10 is the best rank.
Drug, oral drug therapy; PRF, pulsed radiofrequency; NB, nerve block; SC, subcutaneous injection or local infiltration; O3, ozone injection; SGB, stellate ganglion block; BTX-A, botulinum toxin type A.
Figure 7.
Cumulative probability plot. (a) Cumulative probability plot for the pain score 1 week after treatment, (b) Cumulative probability plot for the pain score 1 month after treatment, (c) Cumulative probability plot for pain score 3 months after treatment. The larger the area under each curve, the less effective the treatment is for pain reduction.
Drug, oral drug therapy; PRF, pulsed radiofrequency; NB, nerve block; SC, subcutaneous injection or local infiltration; O3, ozone injection; SGB, stellate ganglion block; BTX-A, botulinum toxin type A.
Discussion
Currently, there are various interventions to treat patients with PHN. For a pain physician, choosing a suitable treatment for a particular patient is vital. Although many studies have been performed to explore each measure’s roles, there was still a lack of overall and quantitative studies among all treatments. To evaluate the efficiency of different interventions, we performed this NMA (including direct and indirect comparisons and mixed treatment comparisons). Twelve RCT studies, including 702 patients and ten treatments, were analyzed. Each included study had compared one, two, or three types of interventions at different time points after therapy. To simplify our NMA, we chose the three most common time points (1 week, 1 month, and 3 months) to analyze.
Through direct pairwise comparison at 1 week, interventions other than SGB all had a better therapeutic effect than oral drug therapy, indicating the requirement for early intervention in addition to the correct oral medication. For PRF and NB, which are the two most commonly used therapies, the efficacy of PRF was better than that of NB, while the combination of these therapies was better than using them alone. For SC, SC + NB had no significant advantages over SC, but if O3 was injected with NB + SC, the efficacy was better than either of these treatments alone. Additionally, subcutaneous injection of BTX-A was better than subcutaneous injection of saline or lidocaine. After 1 to 3 months of treatment, almost all of the interventions had better efficacy than that of oral drug therapy.
The results of this NMA were similar to those of the direct comparison, but the NMA provided more information for treatments without direct comparisons. After 1 week of treatment, PRF + NB + SC (ranked forth) was not significantly better than NB (ranked fifth), PRF (ranked seventh), or NB + PRF (ranked ninth), but it was better than SC (ranked third). Additionally, subcutaneous injection of BTX-A (ranked tenth) was significantly more effective than other therapies, except PRF + NB and SC + NB + O3. Furthermore, combination therapies, including PRF + NB, SC + NB, and SC + NB + O3, showed comparable efficacy and were superior to each therapy alone except for BTX-A. After 1 month of treatment, BTX-A was still ranked the best, while SC (ranked fifth) was better than PRF (ranked fourth), but the difference was not significant. SGB (ranked third) was better than NB (ranked as the second) and oral medication (ranked first). After 3 months of treatment, the order of priority was PRF, SGB, NB, and oral medication.
PRF is a commonly used neuromodulation therapy in pain management, and it is characterized as highly selective and minimally invasive.30 Compared with continuous radiofrequency therapy, PRF produces a rapidly changing electric field and lower temperature; thus, it is safer and easier to use, and it causes fewer complications such as neural damage and postoperative soreness. PRF can block pain transmission by interrupting signals in unmyelinated C fibers and myelinated A-delta fibers without influencing myelinated A-alpha and A-beta fibers.31 Moreover, PRF appears to change c-fos mRNA expression in the dorsal horn of spinal cord.32 In our NMA, PRF was highly effective for pain reduction in PHN patients at all time points compared among all the analyzed interventions. It can alleviate pain for a long time and promote curing patients with PHN. Overall, considering its strong safety profile, minimal invasiveness, fewer adverse effects, and nondestructive nature, PRF is easily accepted by both patients and doctors, and it is an appropriate intervention for PHN.
NB is a minimally invasive therapy, which means injecting mainly local anesthetics or drugs including local anesthetics (sometimes steroids may be added) into the nerve root, trunk, ganglion, or epidural space. In our analysis, NB includes epidural block, dorsal root ganglion block, intercostal NB, and paravertebral block. NB can temporally block nerve transduction without damaging the nerves. Our NMA showed that NB was valid at all the analyzed time points, but at 1 month and 3 months, the efficacy rank of NB decreased. This phenomenon indicates that the effect of NB is transient, persisting for a short period. However, NB is beneficial to for the PHN patient’s prognosis over the long term.
NB is also synergistic with PRF and SC, and their combination was better than each treatment alone. Thus, NB is safe, convenient, and easy to perform, it is good for temporary anesthesia, and application of NB in the early stage of PHN can promote pain relief for a long time. If permitted, continuous blockade or the combination of NB with PRF or SC are good choices.
SC, in our study, includes subcutaneous injection of lidocaine, saline, or methylcobalamin and local infiltration of lidocaine or glucocorticoids. For development of PHN including central and peripheral sensitization,33 SC mainly acts on peripheral sensitization. Reactivation of Varicella zoster virus causes neural damage, inflammation, and tissue edema in the affected tissue. Inflammatory mediators are then released by the injured tissue, which reduces the nociception threshold and activates local nociceptors.34 All these processes promote the development of peripheral sensitization. SC can ameliorate PHN by alleviating local inflammation, blocking peripheral nociceptors, and nourishing injured nerves. In our NMA, after 1 week of treatment, the efficacy of SC was limited, but after 1 month, its efficacy was better than that of PRF. This may be because SC is a long-term treatment that requires frequent injections. The combination of SC and NB was better than either of them alone, or if O3 injection was added, the benefit became significant. However, if PRF, NB, and SC were used together, the efficacy was reduced, and it was worse than that of PRF and PRF + NB. This indicates that too many interventions at the same time is not advisable. Thus, to treat patients with PHN, SC is effective especially when applied with NB and O3 injection. However, all injected medications were combined in this study, so further studies should be performed to explore the difference among various drugs for long periods.
BTX-A is a neurotoxin that is purified from the bacterium Clostridium botulinum, and it can inhibit the release of neurotransmitters (including acetylcholine, glutamate, calcitonin gene-related peptide, and substance P) from both motor and sensory neurons.35,36 Subcutaneous injection of BTX-A reduces the peripheral nociceptive input, prevents sensitization of nociceptors, and relieves neurogenic inflammation.36 In our study, subcutaneous injection of BTX-A was the most effective measure after 1 week and 1 month of treatment. Furthermore, in the two studies related to BTX-A, subcutaneous injection of BTX-A showed better pain relief than SC after 2 weeks and 3 months.26,27 However, there was no data to compare its long-term effect with other treatments and to evaluate their combined efficacy.
SGB is widely used in pain management, and it changes the function of the sympathetic nervous system. There was only one study that was related to SGB in our analysis. The NMA showed that SGB was worse than NB 1 week after treatment, but its effect was better than NB after 1 month and 2 months. This may be because regulation of sympathetic function is a long-term process and its effect occurs slowly. Thus, to treat patients with PHN, SGB is not a good choice for multiple treatments, and a long course of treatment is required.
PHN treatment must involve interventional therapies at an early stage and include regular anti-neuropathic drug administration. After 1 week of treatment, BTX-A and PRF were the two most effective treatments among all individual interventions. However, if combined therapy was allowed, PRF + NB was the best choice, followed by SC + NB + O3. Too many therapies at the same time was not recommended, because PRF + NB + SC seemed to reduce the efficacy of each treatment, and it was highly invasive for patients. After long-term follow-up, PRF was the most effective therapy to treat patients with PHN.
This study has some limitations. First, only literature published in English was searched for the analysis. Second, participants were patients who had PHN in areas that were innervated by spinal nerves, so this conclusion may not be applicable to PHN in other body areas. Third, many interventions lacked data after the long-term follow-up, making it difficult to compare the patients’ long-term efficacy. Finally, because there were no RCTs, many new interventions, such as spinal cord stimulation and autologous fat grafting were not analyzed in our NMA.37 Therefore, more studies involving these new therapies are needed to develop more effective and safe methods to treat patients with PHN.
Conclusions
Regular anti-neuropathic drug administration that was accompanied by interventional therapies at an early stage is the best choice to treat patients with PHN. BTX-A and PRF are the two most effective individual interventions after 1 week of treatment. However, after long-term follow-up, PRF was the most effective treatment. For combined therapy, PRF + NB was the best choice, followed by SC + NB + O3. Additionally, too many therapies at the same time is not recommended. Clinicians should manage therapeutic regimens in accordance with the patients’ specific condition and existing measures to achieve personalized treatment.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_0300060520977416 for Efficacy of different interventions for the treatment of postherpetic neuralgia: a Bayesian network meta-analysis by Bei Wen, Yajie Wang, Cong Zhang, Weicheng Xu and Zhijian Fu in Journal of International Medical Research
Supplemental material, sj-pdf-2-imr-10.1177_0300060520977416 for Efficacy of different interventions for the treatment of postherpetic neuralgia: a Bayesian network meta-analysis by Bei Wen, Yajie Wang, Cong Zhang, Weicheng Xu and Zhijian Fu in Journal of International Medical Research
Supplemental material, sj-pdf-3-imr-10.1177_0300060520977416 for Efficacy of different interventions for the treatment of postherpetic neuralgia: a Bayesian network meta-analysis by Bei Wen, Yajie Wang, Cong Zhang, Weicheng Xu and Zhijian Fu in Journal of International Medical Research
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the National Natural Science Foundation of China (81771199; 81271346).
ORCID iDs: Bei Wen https://orcid.org/0000-0002-3387-5191
Yajie Wang https://orcid.org/0000-0002-9793-8956
Zhijian Fu https://orcid.org/0000-0002-9387-9768
References
- 1.Johnson RW, Rice AS. Clinical practice. Postherpetic neuralgia. N Engl J Med 2014; 371: 1526–1533. DOI: 10.1056/NEJMcp1403062. [DOI] [PubMed] [Google Scholar]
- 2.Yu SY, Fan BF, Yang F, et al. Patient and economic burdens of postherpetic neuralgia in China. Clinicoecon Outcomes Res 2019; 11: 539–550. DOI: 10.2147/ceor.S203920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson RW, Bouhassira D, Kassianos G, et al. The impact of herpes zoster and post-herpetic neuralgia on quality-of-life. BMC Med 2010; 8: 37. DOI: 10.1186/1741-7015-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friesen KJ, Falk J, Alessi-Severini S, et al. Price of pain: population-based cohort burden of disease analysis of medication cost of herpes zoster and postherpetic neuralgia. J Pain Res 2016; 9: 543–550. DOI: 10.2147/jpr.S107944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 2015; 14: 162–173. DOI: 10.1016/s1474-4422(14)70251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo S, Shen M, Zhang L, et al. The effect of interventional pain management on treating postherpetic neuralgia. Indian J Dermatol 2019; 64: 251. DOI: 10.4103/ijd.IJD_130_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin CS, Lin YC, Lao HC, et al. Interventional treatments for postherpetic neuralgia: a systematic review. Pain Physician 2019; 22: 209–228. [PubMed] [Google Scholar]
- 8.Shi Y, Wu W. Treatment of neuropathic pain using pulsed radiofrequency: a meta-analysis. Pain Physician 2016; 19: 429–444. [PubMed] [Google Scholar]
- 9.Pei W, Zeng J, Lu L, et al. Is acupuncture an effective postherpetic neuralgia treatment? A systematic review and meta-analysis. J Pain Res 2019; 12: 2155–2165. DOI: 10.2147/JPR.S199950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornell JE. The PRISMA extension for network meta-analysis: bringing clarity and guidance to the reporting of systematic reviews incorporating network meta-analyses. Ann Intern Med 2015; 162: 797–798. DOI: 10.7326/m15-0930. [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. DOI: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin CP, Chang KV, Huang YK, et al. Regenerative injections including 5% dextrose and platelet-rich plasma for the treatment of carpal tunnel syndrome: a systematic review and network meta-analysis. Pharmaceuticals (Basel) 2020; 13: 49. DOI: 10.3390/ph13030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiu YH, Chang KV, Chen IJ, et al. Utility of sonoelastography for the evaluation of rotator cuff tendon and pertinent disorders: a systematic review and meta-analysis. Eur Radiol 2020; 30: 6663–6672. DOI: 10.1007/s00330-020-07059-2. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. DOI: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saxena AK, Lakshman K, Sharma T, et al. Modulation of serum BDNF levels in postherpetic neuralgia following pulsed radiofrequency of intercostal nerve and pregabalin. Pain Manag 2016; 6: 217–227. DOI: 10.2217/pmt.16.3. [DOI] [PubMed] [Google Scholar]
- 16.Li D, Sun G, Sun H, et al. Combined therapy of pulsed radiofrequency and nerve block in postherpetic neuralgia patients: a randomized clinical trial. PeerJ 2018; 6: e4852. DOI: 10.7717/peerj.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang D, Zhang K, Han S, et al. PainVision(R) apparatus for assessment of efficacy of pulsed radiofrequency combined with pharmacological therapy in the treatment of postherpetic neuralgia and correlations with measurements. Biomed Res Int 2017; 2017: 5670219. DOI: 10.1155/2017/5670219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu G, Lv ZW, Feng Y, et al. A single-center randomized controlled trial of local methylcobalamin injection for subacute herpetic neuralgia. Pain Med 2013; 14: 884–894. DOI: 10.1111/pme.12081. [DOI] [PubMed] [Google Scholar]
- 19.Guo XF, Liu YG, Huo YS, et al . Clinical observation on thoracic paravertebral nerve block with ozone treatment in patients with postherpetic neuralgia. Chinese Journal of Contemporary Neurology and Neurosurgery 2013; 13: 863–867. DOI: 10.3969/j.issn.1672-6731.2013.10.009. available from: https://schlr.cnki.net/Detail/index/SJDJLAST/SJDJA651D12896E7238F0C81BD31304D412E [Google Scholar]
- 20.Ji CM, Li XM, Sun DH, et al . Clinical study on stellate ganglion block combined with pregabalin for treating postherpetic neuralgia at chest and back. Chinese Journal of New Drugs 2012; 21: 1503–1506. Available from: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD2012&filename=ZXYZ201213016&v=oKIvWw0PYVc2ySbBnYMBK2WuI86J357yaEfOA1UNKJVt8atxZV3Eo52uNC6x9o7i [Google Scholar]
- 21.Xiao L, Mackey S, Hui H, et al. Subcutaneous injection of botulinum toxin a is beneficial in postherpetic neuralgia. Pain Med 2010; 11: 1827–1833. DOI: 10.1111/j.1526-4637.2010.01003.x. [DOI] [PubMed] [Google Scholar]
- 22.Ke M, Yinghui F, Yi J, et al. Efficacy of pulsed radiofrequency in the treatment of thoracic postherpetic neuralgia from the angulus costae: a randomized, double-blinded, controlled trial. Pain Physician 2013; 16: 15–25. [PubMed] [Google Scholar]
- 23.Makharita MY, El Bendary HM, Sonbul ZM, et al. Ultrasound-guided pulsed radiofrequency in the management of thoracic postherpetic neuralgia: a randomized, double-blinded, controlled trial. Clin J Pain 2018; 34: 1017–1024. DOI: 10.1097/ajp.0000000000000629. [DOI] [PubMed] [Google Scholar]
- 24.Wang Q, He MW, Ni JX. Effect of pregabalin combined with ultrasound-guided thoracic paravertebral nerve block on postherpetic neuralgia. Chinese Journal of New Drugs 2012; 21: 2932–2935. Available from: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD2012&filename=ZXYZ201224022&v=oKIvWw0PYVcaBabI3SLf8JVRo3iyuPqeosebkstyfSHAkKQndFniOcOxX%25mmd2B%25mmd2B%25mmd2BrLrc [Google Scholar]
- 25.Xia YZ, Zha J, Chen JM, et al. Clinical study on three treatment methods of postherpetic neuralgia. Chinese Journal of Contemporary Neurology and Neurosurgery 2018; 18: 674–677. DOI: 10.3969/j.issn.1672-6731.2018.09.009. [Google Scholar]
- 26.Apalla Z, Sotiriou E, Lallas A, et al. Botulinum toxin A in postherpetic neuralgia: a parallel, randomized, double-blind, single-dose, placebo-controlled trial. Clin J Pain 2013; 29: 857–864. DOI: 10.1097/AJP.0b013e31827a72d2. [DOI] [PubMed] [Google Scholar]
- 27.Yu-Kang T. Node-splitting generalized linear mixed models for evaluation of inconsistency in network meta-analysis. Value Health 2016; 19: 957–963. DOI: 10.1016/j.jval.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Lu G, Ades AE. Assessing evidence inconsistency in mixed treatment comparisons. J Am Stat Assoc 2006; 101: 447–459. DOI: 10.1198/016214505000001302. Available from: https://amstat.tandfonline.com/doi/abs/10.1198/016214505000001302 [Google Scholar]
- 29.Dias S, Welton NJ, Caldwell DM, et al. Checking consistency in mixed treatment comparison meta-analysis. Stat Med 2010; 29: 932–944. [DOI] [PubMed] [Google Scholar]
- 30.Racz GB, Ruiz-Lopez R. Radiofrequency procedures. Pain Pract 2006; 6: 46–50. DOI: 10.1111/j.1533-2500.2006.00058.x. [DOI] [PubMed] [Google Scholar]
- 31.Ding Y, Hong T, Li H, et al. Efficacy of CT guided pulsed radiofrequency treatment for trigeminal postherpetic neuralgia. Front Neurosci 2019; 13: 708. DOI: 10.3389/fnins.2019.00708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Higuchi Y, Nashold BS, Jr, Sluijter M, et al. Exposure of the dorsal root ganglion in rats to pulsed radiofrequency currents activates dorsal horn lamina I and II neurons. Neurosurgery 2002; 50: 850–855; discussion 856. DOI: 10.1097/00006123-200204000-00030. [DOI] [PubMed] [Google Scholar]
- 33.Jones J. Postherpetic neuralgia. J Pain Palliat Care Pharmacother 2015; 29: 180–181. DOI: 10.3109/15360288.2015.1037520. [DOI] [PubMed] [Google Scholar]
- 34.Kidd BL, Urban LA. Mechanisms of inflammatory pain. Br J Anaesth 2001; 87: 3–11. DOI: 10.1093/bja/87.1.3. [DOI] [PubMed] [Google Scholar]
- 35.Blasi J, Chapman ER, Link E, et al. Botulinum neurotoxin A selectively cleaves the synaptic protein SNAP-25. Nature 1993; 365: 160–163. DOI: 10.1038/365160a0. [DOI] [PubMed] [Google Scholar]
- 36.Cui M, Khanijou S, Rubino J, et al. Subcutaneous administration of botulinum toxin A reduces formalin-induced pain. Pain 2004; 107: 125–133. DOI: 10.1016/j.pain.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 37.Sollie M, Thomsen JB, Sørensen JA. Autologous fat grafting seems to alleviate postherpetic neuralgia – a feasibility study investigating patient-reported levels of pain. J Plast Reconstr Aesthet Surg 2020: S1748-6815(20)30368-5. DOI: 10.1016/j.bjps.2020.08.038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_0300060520977416 for Efficacy of different interventions for the treatment of postherpetic neuralgia: a Bayesian network meta-analysis by Bei Wen, Yajie Wang, Cong Zhang, Weicheng Xu and Zhijian Fu in Journal of International Medical Research
Supplemental material, sj-pdf-2-imr-10.1177_0300060520977416 for Efficacy of different interventions for the treatment of postherpetic neuralgia: a Bayesian network meta-analysis by Bei Wen, Yajie Wang, Cong Zhang, Weicheng Xu and Zhijian Fu in Journal of International Medical Research
Supplemental material, sj-pdf-3-imr-10.1177_0300060520977416 for Efficacy of different interventions for the treatment of postherpetic neuralgia: a Bayesian network meta-analysis by Bei Wen, Yajie Wang, Cong Zhang, Weicheng Xu and Zhijian Fu in Journal of International Medical Research