Abstract
Inflammatory bowel diseases (IBD) comprise two major forms: Crohn’s disease and ulcerative colitis. The diagnosis of IBD is based on clinical symptoms combined with results found in endoscopic and radiological examinations. In addition, the discovery of biomarkers has significantly improved the diagnosis and management of IBD. Several potential genetic, serological, fecal, microbial, histological and immunological biomarkers have been proposed for IBD, and they have been evaluated for clinical routine and clinical trials. Ileocolonoscopy, especially with biopsy collection, has been considered the standard method to diagnose IBD and to assess clinical activity of the disease, but it is limited to the colon and terminal ileum and is considered invasive. For this reason, non-invasive biomarkers are necessary for this type of chronic inflammatory disease, which affects mostly young individuals, as they are expected to have a long follow-up.
Keywords: Inflammatory bowel diseases, Biomarkers, Endoscopic scores, Crohn's disease, Ulcerative colitis, Inflammation
Core Tip: Biomarkers are relevant for diagnostic support, differentiation between Crohn’s disease and ulcerative colitis, determination of disease activity, and in prediction of response to therapy. This review discusses the most recent studies that correlate non-invasive clinical biomarkers of disease activity with the endoscopic scores available in clinical practice.
INTRODUCTION
Inflammatory bowel diseases (IBD) comprise two major forms: Crohn’s disease (CD) and ulcerative colitis (UC). IBD affect over a million individuals in North America, and its prevalence rate is close to 200 per 100000[1]. Although the highest reported prevalence is in North America, Northern Europe, and Australia, incidence rates in developing countries are on the rise[2]. The cause of this increasing rate remains unknown, but environmental factors such as diet and microbial exposure are likely to be involved[3].
Although CD and UC may present similar clinical symptoms, such as abdominal pain, weight loss and diarrhea, hematochezia, tenesmus and defecatory urgency, they are different diseases with specific features. In CD, the inflammatory process most frequently affects the terminal ileum and colon in a discontinuous manner, but it can also affect any part of the gastrointestinal tract, and the inflammation may be transmural, which results in potential disease complications, including fibrosis and strictures in the bowel, and fistula formation. In UC, the disease is limited to the mucosa and submucosa of the colon, often involving the rectum. Both diseases present relapsing and remitting activity course. However, CD is typically progressive, and, because of its various complications, it requires surgical intervention besides medical therapy, more often than in UC patients[4].
The diagnosis of IBD is based on a combination of clinical symptoms with features found on endoscopy and radiology exams. In addition, the discovery of biomarkers has significantly enhanced our ability to diagnose and treat IBD. Biomarkers are relevant for supporting diagnosis, differentiating between CD and UC, determining disease activity, and in predicting response to therapy. Biomarkers indicate pathological processes such as inflammation, increased immune cell turnover, or acute phase reaction that may occur during the course of the condition. Several potential genetic, serological, fecal, microbial, and immunological biomarkers have been proposed for IBD, and their evaluation has been discussed for clinical routine and clinical trials. The latter present additional challenges regarding the ideal testing strategies to monitor disease activity[4-6].
Endoscopy also plays an integral role in estimating the severity of IBD as it makes it possible to evaluate mucosal healing, treatment efficacy and cancer surveillance[7]. Ileocolonoscopy, especially with biopsies, has been considered the standard method for diagnosing both UC (Figure 1) and CD (Figure 2), but it is limited to the colon and terminal ileum[7], and it is considered a rather invasive method to access the inflammatory status of the mucosa. Therefore, the aim of this review is to present the main endoscopic scores of IBD activity and to discuss their correlation to non-invasive biomarkers.
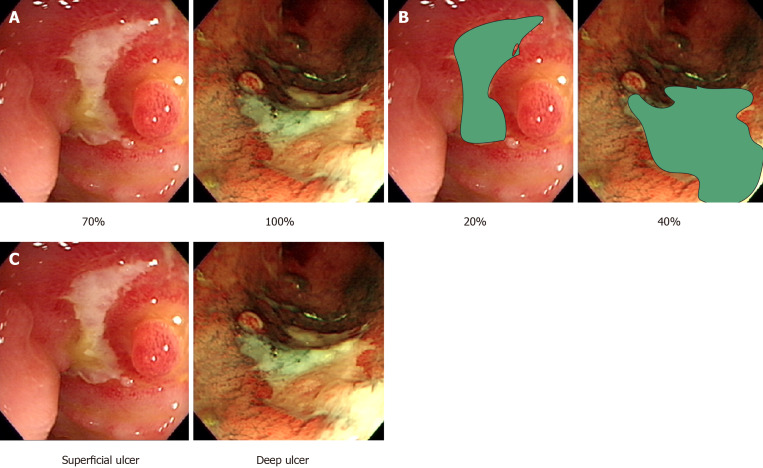
Figure 1.
Endoscopic parameters of lesion evaluation in ulcerative colitis endoscopic scores. A: Total lesion surface area; B: Total ulcer surface area; C: Depth of ulcers.
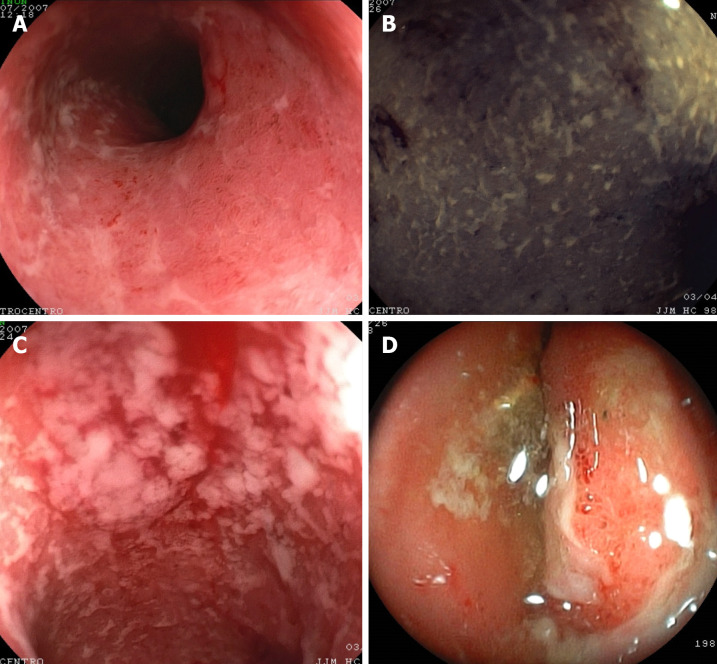
Figure 2.
Ulcerative colitis endoscopic findings. A: Erythema; B: Erosions and granularity; C: Mucopurulent exudate; D: Ulcers.
ENDOSCOPIC MONITORING OF DISEASE ACTIVITY
Since endoscopic healing has become an important tool to guide treatment in IBD, it has become necessary to evaluate intestinal mucosa lesions. In this way, scoring systems have been developed to help estimate the severity of the disease in both clinical practice and trial settings. Moreover, the use of well-validated scores decreases inter-observer variation caused by clinicians’ different interpretations.
Ulcerative colitis scoring systems
Back in 1955, Truelove and Witts made the first attempt to classify intestinal mucosa in patients with UC[8]. However, it distinguished only three categories: (1) Normal or near normal with slight hyperemia or granularity; (2) Improved; and (3) No changes or worse. As the classification lacked well-defined endoscopic characteristics, such as ulcers and lesions, other attempts were made, and the Mayo score and Ulcerative Colitis Endoscopic Index of Severity (UCEIS) are the most widely used nowadays.
Subsequently, several attempts were made to evaluate the endoscopic activity of UC. In 1964, Baron et al[9] suggested a score that took into account the presence of blood, and not ulceration, in the mucosa, using rigid sigmoidoscopy. UC activity is measured by a 4-point scale and remission is considered to occur when the score is ≤ 1 point[9]. Although the Baron Scale is a non-formal validated score, Feagan and colleagues modified it (Modified Baron Scale) in a placebo-controlled trial, and endoscopic activity was evaluated according to a 5-point scale in which the presence of ulceration requires a ≥ 3 points score[10].
The St. Mark’s Index (also known as Powell-Tuck Index), was developed by Powell-Tuck et al[11] in 1987, and it also focuses on mucosal bleeding as it grades the severity of inflammation in a 3-point scale, taking into account clinical features such as stool consistency, nausea/vomiting, general health and abdominal tenderness[11].
In a placebo-controlled trial published in 1987, Sutherland and colleagues developed the UC Disease Activity Index (UCDAI), also known as the Sutherland Index. The UCDAI score evaluates stool frequency, rectal bleeding, and the ranking of disease activity by a well-trained physician. It also takes into account mucosal appearance, which it classifies as normal, mild or moderate friability, and the presence of exudation and/or spontaneous bleeding[12].
Rachmilewitz et al[13] published the Rachmilewitz Endoscopic Index during a randomized trial in 1989. The score evaluates the main variables regarding mucosal appearance, such as vascular pattern, granularity, mucosal damage including erosions and ulcers, and bleeding. Endoscopic remission is considered to occur with a score ≤ 4 points[13].
In 1987, Schroeder and colleagues developed the Mayo score during a placebo-controlled trial to evaluate the efficacy of mesalamine in the treatment of UC[14]. The degree of endoscopic appearance was based on a 0-3 scale: (0) Normal mucosa; (1) Presence of erythema, decreased vascular pattern, mild friability; (2) Marked erythema, absent vascular pattern, friability, erosions; and (3) Ulceration, spontaneous bleeding. This score ranges from 0 to 12 points.
Studies have shown a correlation between the Mayo score and disease activity. Lewis and colleagues reported, in a placebo-controlled randomized trial of rosiglitazone for the treatment of 105 patients with mild to severe UC, that both the total and partial Mayo score were related to the patient’s assessment of disease activity. In addition, the score presented a maximal product of sensitivity and specificity (88% and 78%, respectively) to identify remission with a 4.5 cut-off point[15].
The Mayo score has also been associated with a lower risk of colectomy in UC patients under anti-tumor necrosis factor-alpha (TNF-α) therapy. In the Active Ulcerative Trial (ACT)-1 and -2 studies, early mucosal healing was scored as 0-1 according to the Mayo score, and correlated with a lower risk of colectomy, even up to 50 weeks of follow-up. In addition, it was associated with long-term clinical remission by week 8 of evaluation[16,17].
As the other scores do not consider the extent of mucosal inflammation, Lobatón and colleagues proposed, in 2015, a Modified Mayo Endoscopic Score (MMES). First of all, to calculate a modified score (MS), the colon should be divided into five segments (ascending, transverse, descending, sigmoid and rectum), and each segment should be given a score. After that, the maximal extent of inflammation should be multiplied by the MS value in order to obtain the extended modified score (EMS). Lastly, the EMS should be divided by the number of segments with active inflammation in order to obtain the MMES[18].
Based on the intra- and interindividual variation of endoscopic descriptors, Travis and colleagues developed a new tool for assessing disease activity in UC: The UCEIS score. This index evaluates precisely the following endoscopic findings: (1) Vascular pattern; (2) Bleeding; and (3) Lesions and ulcers. Reports in the literature suggest that the UCEIS is strongly correlated with symptoms that are self-reported by the patients and that it is more accurate than the Mayo score in predicting remission induced by the administration of tacrolimus, an immunosuppressant drug used in the treatment of UC[19].
A recent study reported that the UCEIS was more accurate in predicting the need for colectomy due to the lack of responsiveness to anti-TNF-α therapy, with a specificity of 94% and sensitivity of 44%, while the Mayo score showed a specificity and sensitivity of 50% and 65%, respectively[20].
Samuel et al[21] developed, in 2013, the Ulcerative Colitis Colonoscopic Index of Severity (UCCIS), and it has been recently reported to have a good-to-excellent inter-observer agreement by the ECCO-ESGAR guideline for the diagnostic and assessment of IBD (Sturm 2018). The UCCIS score is a 6-points scale that includes the evaluation of vascular pattern, granularity, ulceration, bleeding and friability, segmental and global assessment of endoscopic severity. However, a cut-off for endoscopic activity and remission is still lacking[21].
CD scoring systems
In CD, disease activity can be endoscopically assessed by two main reliable scores: Crohn’s Disease Endoscopic Index of Severity (CDEIS) and the Simple Endoscopic Score for Crohn’s Disease (SES-CD). Both scores are difficult to perform, so they are mostly used in clinical trials. However, they provide great intra-observer agreement and are reproducible[22,23].
CDEIS was developed in 1989 and it is not only used to guide treatment strategies, but it has also been shown to predict corticosteroid-free clinical remission[24]. CDEIS evaluates whether a given intestinal segment is explored. It also verifies the presence of deep and superficial ulcerations, ulcerated and non-ulcerated stenosis, and the extent of the disease and ulcerations. It presents good reliability and responsiveness, and the disease activity is considered when the CDEIS score is > 5. In addition, the presence of ulcers in the colon indicates an independent prediction of severe prognosis, and the presence of deep ulcers is associated with a high rate of colectomy[25]. In that way, CDEIS is an accurate tool for measuring CD severity.
Although CDEIS is a validated scoring system, it is considered complicated to perform and demands a post-procedure time to be scored. Therefore, it is not suitable for clinical practice. In this sense, SES-CD was developed to simplify CDEIS, as it is restricted to the amount of surface affected by ulcers, the size of such ulcers, and the presence of stenosis[23]. SES-CD has shown a correlation with CDEIS, and its results can be converted to CDEIS using the following equation: CDEIS = 0.76 × SES-CD + 0.29[26].
In order to evaluate damage location and reversibility, besides severity, extent and progression, Pariente et al[27] developed the Lémann score. This index takes into account cumulative digestive tissue damage based on imaging features, such as structuring and penetrating phenotype, as well as surgical resection history. Together with clinical findings, the Lémann score is helpful in assessing the patient at different disease stages, whether it is early or advanced, operated or non-operated CD, assisting the representation of the patient’s disease course[27].
In the post-surgical setting, activity recurrence can be measured using the Rutgeerts’ score. Its prognostic value has been validated over the past twenty years by different clinical trials[28-31]. In this score, patients scored as i1 are at minimal risk for subsequent recurrence, as they present minimal endoscopic recurrence of activity, such as less than five aphthous ulcers with interposed normal mucosa, whereas a score higher or equal to i2 predicts a more severe course of disease with high risk of recurrence[26]. The i3 score indicates the presence of aphthous ileitis with inflamed mucosa, and i4 indicates inflammation and the presence of large ulcers and/or stenosis[32].
CROSS-SECTIONAL IMAGING FOR DISEASE ACTIVITY MONITORING
As CD can also affect the small and large bowel, cross-sectional imaging (CSI) techniques, such as computed tomography enterography (CTE) and magnetic resonance enterography (MRE), are validated methods considered effective for evaluating these intestinal regions[33].
CTE and MRE are considered gold standard methods in the diagnosis of CD, as they are able to assess the location, severity of the disease, and they also identify some complications. CSI, in particular, is a useful tool to assess response to therapy and monitor disease progression. However, its capability to predict adverse outcomes is less well established[34,35]. The MRE method provides information on disease activity in the small intestine and colon in just one exam, and it also establishes the diagnosis of penetrating complications, including fistulas and abscesses, which may be difficult to identify clinically. Signs of inflammatory activity on MRE also correlate well with endoscopic lesion severity[36-38].
Previous research has produced a series of magnetic resonance imaging (MRI) features that are useful in assessing disease activity. Since then, several studies have developed and validated scoring systems, such as: Activity Magnetic Resonance Index (MaRIA)[37], Diffusion-weighted Images (DWI)-MaRIA or Clermont core[39,40], Crohn's Disease Magnetic Resonance Index (CDMI)[36], Nancy score[38], and The Global Magnetic Resonance Enterography Score (MEGS)[41].
The MaRIA score presents some MRE features of inflammation that correlate significantly (r = 0.82, P < 0.001) with characteristics observable in CDEIS, such as wall thickness, relative contrast enhancement, presence of edema, and presence of ulcers[37]. Ordás et al[42] validated this score in a prospective, multicenter trial in 48 patients with CD, showing it to be reliable and responsive in monitoring therapy response. For severe inflammatory lesions, the cut-off point for segmental MaRIA is ≥ 11 points (with 96% of accuracy), whereas < 7 points detects segmental mucosal healing[42].
The Clermont score is an index derived from the MaRIA score, which includes functional images, called DWI. This score makes it possible to predict ileal CD activity with a cut-off > 8.4 point while MaRIA is ≥ 7, and, for severe activity, the Clermont score is ≥ 12.5 points while MaRIA is ≥ 11 points. The overall sensitivity, specificity, and accuracy of this score were 93.7%, 98.7, and 97.6%, respectively[41].
CDMI was used in twenty-six CD patients and it correlated with histological inflammation scores in terminal ileal biopsy scores (transmural histopathological scoring of acute inflammation "eAIS" score 1-6). The data showed a significant correlation for predicting acute inflammation (eAIS ≥ 2) between the CDMI and eAIS (Kendall's tau = 0.40, 95%CI: 0.11-0.64, P = 0.02), with a sensitivity of 81%, specificity of 70% and accuracy of 77%[36].
The Nancy score (MRI-DWI-colonography) is a combination of MR colonography and DWI, without oral or rectal preparation, against a reference standard of SES-CD on colonoscopy. Oussalah and colleagues[38] observed, in 96 patients with IBD (35 with UC and 61 with CD), six radiological features in 5 colonic segments and ileum. The total score was calculated by the addition of the segment scores with values ranging from 0 to 36. Specific characteristics, such as the presence of bowel wall thickening and DWI hyperintensity, were able to independently predict endoscopic inflammation in the colon, while those scores were both correlated with the SES-CD[38].
To better evaluate the disease burden and to evaluate more parameters, such as longitudinal characteristics and extra-enteric findings, Makanyanga et al[43] developed a global MEGS using a standard surgical resection sample. MEGS is derived from a segmented magnetic resonance activity scoring system, and subsequently validated by comparison with endoscopic findings[41,43].
Other interesting indices, not yet validated, were described by Van Assche et al[44], and another by Rimola et al[35]. The Van Assche index[44] was developed to evaluate the therapy response of perianal fistulizing CD. This index is based on a previous classification of perianal fistulas created in 1976 by Parks et al[45]. Rimola et al[35] then included an evaluation of T1-weighted post-gadolinium hyperintensity, as well as the inflammatory process characterized by cell infiltration. Although Rimola did confirm the effectiveness of the Van Assche score in measuring therapy response, the small sample size limited the validation of individual index components.
In 2012, Steward et al[36] included more component items in the Van Assche index, such as extension, hyperintensity in T2-weighted images, rectal wall disability, inflammatory mass, and dominant feature of the tract and primary extension. The modified Van Assche index was considered consistent and reliable as the intra-class correlation coefficients for intra-rater and inter-rater reliability were 0.89 (0.85-0.92) and 0.67 (95%CI: 0.56-0.75), respectively[36].
As mentioned above, the Lémann index utilizes clinical, endoscopic, and MRE information measures of disease progression ranging from a minimum value, corresponding to the absence of damage, to a maximal theoretical value that corresponds to the complete resection of the digestive tract. This index is used mainly to evaluate damage in the bowel and, therefore, to assess disease progression. The entire digestive tract was divided into four organs and subsequently into segments with disease damage graded per segment. Information on previous surgery, presence of strictures and/or penetrating lesions (grades 1-3) were collected, and damage evaluations ranging from 0.0 (no lesion) to 10.0 (complete resection) were provided[46].
SEROLOGICAL BIOMARKERS
Although they are not IBD-specific, some non-invasive serological biomarkers are used in clinical practice to correlate with disease activity, besides serving as accessible and lower cost tests in the follow-up of IBD.
C-reactive protein
C-reactive protein (CRP) is produced in the liver in response to an inflammatory process, whether it is acute or chronic. High levels of cytokines associated with active IBD, such as interleukin (IL)-6 and -1β, and TNF-α stimulate the production of CRP. As these cytokines are more numerous during various microbial infections and other autoimmune disorders, CPR is not considered an exclusive marker of IBD activity[4]. It has been, however, mostly investigated in IBD to detect and differentiate IBD from other bowel disorders, to monitor disease activity, and to evaluate treatment response[47,48].
Solem et al[49] correlated CRP measurements with endoscopic and histological activity in one hundred and forty-seven patients with IBD (104 with CD and 43 with UC). Although the activity was measured by endoscopical global assessment and not by validated scores, CRP levels were associated with CD activity: Sixty-three percent of patients with elevated CRP levels presented inflammation at colonoscopy, whereas sixty-seven percent of patients with normal CRP levels did not present any sign of disease activity. The same result was observed with UC. However, for histologic evaluation, this correlation was not statistically significant (P = 0.09)[49].
Iaculli and colleagues published the first study associating serum CRP levels at the time of ileo-cecal resection and endoscopic recurrence in patients with CD, and they observed that postoperative CRP levels are statistically related to mucosal inflammation (Rutgeerts’ score ≥ i2) and recurrence rate[50]. In addition, elevated CRP level was associated with an increased risk of CD-related hospitalization and subsequent CD-related intestinal resection[51].
These findings that correlate CRP levels and endoscopic findings were corroborated by other studies: Jones et al[52] also reported that hsCRP (high sensitivity CRP) was associated with endoscopic activity classified by the SES-CD score as > 7, although it was not correlated with the Crohn’s disease activity index (CDAI), a clinical activity index[52].
However, other studies have shown controversial results regarding this correlation. Denis et al[53] observed that patients with normal CRP levels presented mild ulceration in the intestinal mucosa (CDEIS > 6)[53]. This finding was supported by Schoepfer and colleagues, who observed that fecal calprotectin presented a better accuracy than CRP in predicting endoscopic activity (SES-CD > 4)[54].
Erythrocyte sedimentation rate
Erythrocyte sedimentation rate (ESR) is a common test performed in the laboratory setting and it shows the variance in proteins in the acute phase of inflammation. It is, therefore, influenced by various conditions and it is not IBD-specific. This test determines the rate at which red blood cells migrate through the plasma over the period of one hour. When an inflammatory process is initiated, pro-sedimentary factors, i.e., fibrinogen, cause the red blood cells to stick together and consequently settle faster[55].
ESR has been widely used as one of the biomarkers of IBD activity. Alper and colleagues noticed the correlation between ESR and disease activity in pediatric patients with CD. It was observed that higher ESR values were found in CD than in UC, and this abnormal rate was correlated with endoscopic and histologic findings of disease activity[56].
Platelet count
Platelet count is also associated with disease activity in IBD. High platelet counts indicate inflammation, and it has been investigated whether these counts correlate with either CD or UC. Nakarai and colleagues observed that platelet count was statistically elevated in patients with relapsed UC and mucosal healing (Mayo score = 0)[57]. In addition, platelet activation factors, especially platelet factor-4 and β-thromboglobulin, were correlated with clinical activity (CDAI = 219.5)[58].
FECAL BIOMARKERS
Fecal biomarkers have become a valuable tool in assessing disease activity in IBD as they are specific to the gastrointestinal tract. The most frequently used fecal markers are calprotectin and lactoferrin. The cost of these markers is low and their use has been beneficial in diagnosing IBD, evaluating disease activity, predicting disease relapse, and assessing therapy response[59]. S100A12 is a pro-inflammatory protein that has recently been studied as a fecal biomarker for IBD[60].
Calprotectin
The initial identification of calprotectin as a biomarker of IBD was received with great enthusiasm. It is a relevant noninvasive tool that has had a positive impact on clinical care, and there are several studies in the literature that correlate calprotectin with endoscopic activity (Table 1).
Table 1.
Diagnostic accuracy of fecal calprotectin in inflammatory bowel diseases
|
Ref.
|
Patients number
|
Calprotectin cut-off (μg/g)
|
Sensitivity (%)
|
Specificity (%)
|
Correlation with clinical, endoscopic and/or histological data
|
| Kennedy et al[98], 2019 | 918 | 115 | N/A | N/A | disease location |
| Monteiro et al[99], 2018 | 75 | 100 | 78, 6 | 87 | SBEC-Lewis score |
| Pendsé et al[100], 2017 | 69 | 120 | 83 | 52 | MRI, DWI, MEGS |
| Kopylov et al[101], 2016 | 463 | 50 | 83 | 53 | endoscopic capsule |
| Chung-Faye et al[102], 2014 | 109 | 250 | 90 | 82 | histology score |
| Fascì-Spurio et al[103], 2014 | 114 | 110 | 76 | 70 | SBMRI |
| Makanyanga et al[43], 2014 | 71 | 100 | 70 | 63 | MEGS, CDAI |
| Lobatón et al[104], 2013 | 115 | 272 | 79 | 97 | CDEIS |
| D'Haens et al[65], 2012 | 87 | 250 | 60 | 79 | CDEIS e SES-CD |
| Koulaouzidis et al[105], 2011 | 67 | 100 | 66 | 78 | SBEC |
| Jensen et al[106], 2011 | 83 | 50 | 95 | 56 | Ileo-colonoscopy, SBEC and surgery |
| Schoepfer et al[54], 2010 | 140 | 70 | 87 | 72 | SES-CD, CDAI |
| Vieira et al[66], 2009 | 78 | 200 | 88,6 | 97,1 | CDAI, MDAI |
| Xiang et al[64], 2008 | 66 | 50 | 79,4 | 91,9 | DAI (Sutherland criteria) |
| Sipponen et al[107], 2008 | 77 | 200 | 70 | 92 | CDEIS |
SBEC: Small bowel enteroclysis; MRI: Magnetic resonance imaging; DWI: Diffusion-weighted imaging; MEGS: Global magnetic resonance enterography score; SBMRI: Small bowel magnetic resonance imaging; CDAI: Crohn’s disease activity index; CDEIS: Crohn's disease endoscopic activity index of severity; SES-CD: Simple endoscopic score for Crohn's disease; MDAI: Mayo disease activity index; DAI: Disease activity index.
Calprotectin is a 36-kilodalton glycoprotein with affinity for calcium and zinc. It is found in the cytoplasm of neutrophils as well as other inflammatory cells, including activated monocytes and macrophages. Therefore, calprotectin levels are directly proportional to the presence and grade of the inflammatory process in the gastrointestinal tract[61]. In addition, calprotectin is considered a stable marker: Its concentration in stool samples remained unaltered for three days at room temperature[62].
Fecal calprotectin concentration has been correlated with clinical activity using the CDAI for CD and Mayo score for UC[63]. In a study by Xiang et al[64], the levels of calprotectin were useful in discriminating the activity of UC (whether it is active or inactive), with a cut-off point of 50 μg/g and a high sensitivity and specificity (91.9% and 79.4%, respectively)[64].
Concerning the correlation with endoscopic findings, D’Haens and colleagues reported that a cut-off value of 250 μg/g suggests the presence of large ulcers in CD, with a sensitivity of 60.4%, and specificity of 79.5%, whereas in UC, a calprotectin concentration > 250 μg/g was related to mucosal disease activity (Mayo > 0)[65].
Vieira and colleagues also observed a correlation between fecal calprotectin and endoscopic activity in CD (CDEIS > 3), as they reported that the patients who presented with IBD (83.3% with CD and 71.4% with UC) and histological inflammation also had positive fecal calprotectin (> 200 ng/mL)[66]. In addition, a meta-analysis supported the use of fecal calprotectin as a predictor of recurrence in CD patients who had undergone intestinal resection previously[67].
In pediatric patients, fecal calprotectin levels were also correlated with disease activity and became a predictive marker of relapse for both UC and CD[68]. A recent study showed that a calprotectin cut-off < 300 μg/g predicts mucosal healing in CD[69].
Lactoferrin
Lactoferrin is an iron binding glycoprotein also found in neutrophil granules, and possesses antimicrobial properties. It can easily be quantified by the ELISA method, and it is resistant to proteolysis, which makes it a useful marker in feces as an indicator of intestinal inflammation[55].
The dosage of lactoferrin in stool samples was correlated with disease activity in IBD. In the same calprotectin study by Vieira and colleagues, it was also reported that positive lactoferrin was present in 92% of CD patients with histological inflammation, and in 83.3% of UC patients[66].
S100A12
S100A12 activates the nuclear factor-κB signal transduction pathway, which induces pro-inflammatory cytokine release, such as TNF-α. Although S1000A12 is detectable in serum, the fecal assay is more sensitive and specific for IBD[60].
There are still few studies regarding the correlation between S100A12 and endoscopic activity in IBD. Most of the studies with this protein are related to investigation and response to therapy. Fecal S100A12 correlates to intestinal inflammation and can distinguish chronic IBD from other bowel disorders with high sensitivity and specificity (86% and 96%, respectively)[60]. In addition, the literature suggests that a concentration of S100A12 > 82 μg/g predicts a sustained clinical response to anti-TNF-α therapy[70].
HISTOLOGICAL EVALUATION
IBD are characterized by the extent and the histological distribution of mucosal architectural abnormality, the lamina propria cellularity, and the immune cell subtypes. An accurate histological examination of endoscopic biopsies is essential for the diagnosis, sub-classification, and management of IBD. Moreover, the diagnosis should be carried out before beginning treatment given that pharmacological therapy can induce changes in the morphologic features[71].
Histopathological evaluation is able to assess disease activity, as it describes the extent of neutrophil granulocyte infiltration and epithelial damage. Given different disease stages and phenotypes seen in IBD patients, the histological report should also indicate crypt distortion, and granulomas present at the biopsy collection site[72]. In this way, it is recommended that multiple sections from each tissue sample should be examined. Serial sectioning of biopsy specimens increases diagnostic accuracy by increasing the ability to detect more focal abnormalities[73].
Most of the systems described in the literature are developed for UC, but only a few are validated and reproducible. Walsh et al[74] described twenty-six histological activity indices in a recent review paper, but only two are validated[74].
To evaluate UC activity, the Geboes score (GS)[75] and the modified Riley score (MRS)[76,77] are the two most frequently used histological indices. Both have been shown to predict clinical relapse in patients with endoscopic disease. However, histological parameters are partially validated, and they lack reproducibility[78].
The Geboes index is composed of five features: (1) Architectural change; (2) Presence of lamina propria neutrophils and eosinophils; (3) Presence of neutrophils in the epithelium; (4) Crypt destruction; and (5) Presence of erosion or ulceration. On the other hand, the MRS is a four-point scale (none, mild, moderate, or severe) that includes six items, grading them on a score ranging from 0 (no inflammation) to 7 (severe acute inflammation). This score excludes the items of architectural distortion found in the original Riley score[76].
Two other indices have been developed for UC: The Nancy Index[77,79], and Robarts Histopathology Index (RHI)[80]. Both are validated, reproducible, and responsive. The Nancy Index comprises three histological characteristics that divide disease activity into five grades: (0) Absence of significant histological disease; (1) Chronic inflammatory infiltrate with no acute inflammatory infiltrate; (2) Mildly active disease; (3) Moderately active disease; and (4) Severely active disease. The advantages of this index are its simplicity, ease of use, and its excellent intra- and inter-observer reliability[74].
RHI is considered a useful score for clinical trials due to its good response to changes in disease activity. RHI incorporates four histological features each of which are graded between 0 and 3: Severity of chronic inflammatory infiltrate, the number of lamina propria neutrophils, the number of neutrophils in the epithelium, and the severity of erosions or ulceration[77].
Some other indices have been developed, such as those by Gramlich et al[81] and Gupta et al[82]. However, they were not developed through a formal validation process, and their operational properties remain poorly understood. Bresseno et al[78] compared the Goebe Score (GS) with Riley, Gupta, and Gramlich scores and with global visual evaluation, and their results show that the five classification systems are strongly correlated and that intra-observer reproducibility and inter-observer agreement are very good for all indices.
The design of histological scores for CD presents a challenge once the inflammatory process in this disease occurs in a discontinuous and transmural way, and it can exist beyond the reach of the endoscope[83]. There is a need for a scoring index to assess histologic inflammation objectively as well as histologic mucosal healing to assist the evaluation for a treatment target in clinical trials[84].
The most commonly used histologic disease activity index in clinical studies is the Global Histological Activity Score[85], which consists of eight items that evaluate acute and chronic inflammatory changes, epithelial damage and the extent of inflammation as it assesses architectural changes, infiltration of mononuclear cells within the lamina propria, neutrophils within the epithelium, presence of erosions/ulcers, presence of granulomas, and the number of biopsy specimens affected.
The study by Naini et al[86] had the specific objective of developing and validating a histological score index. However, the Naini and Corina Score is not specific to CD, and it was developed to diagnose IBD and to standardize histological assessment of chronic ileocolitis, and not to assess the histological activity of the disease. From a histological point of view, this score is likely to be useful in measuring disease activity in CD.
Significant recent literature on histological healing and the new histological scoring systems have added knowledge regarding the disease’s activity. There is a considerable improvement in the evaluation of the histological disease activity given that the reduction of intestinal inflammation, in addition to endoscopic healing, can provide additional clinical benefit.
A few studies have correlated non-invasive biomarkers with histological activity, mostly from fecal samples, with histological evaluations of disease activity. As mentioned above, fecal calprotectin (FC) levels can be used to monitor IBD activity and response to therapy, and to predict relapses, the need for colectomy and postoperative recurrence[52,87]. Zittan et al[88] investigated whether there was a correlation between histologic activity, using the GS and FC levels. Although the GS is designed only for UC, it was used to define active (GS ≥ 3.1) vs inactive (GS < 3.1) disease in 27 IBD patients. The area under the curve (AUC) for FC and the presence of histological remission (GS < 3.1) was 0.95. They found that FC < 100 μg/g was highly correlated with histological remission both in UC and in colonic CD and with focal (r = 0.77, P < 0.01) or diffuse (r = 0.80, P < 0.01) presence of basal plasmacytosis.
In a recent observational study, Magro and colleagues[89] correlated three of the most used histological scoring systems in UC (GS, and Nancy Index or RHI), with endoscopic outcomes and FC cut-off. Normally, patients who have an FC level below 150 μg/g are considered to have an inactive form of the disease. This study corroborated this view as they showed that most of the patients with FC < 150 μg/g were in histological remission according to the scores. In addition, these scores were able to predict the Mayo endoscopic score and FC levels, but their sensitivity and specificity levels depended on the cut-off used.
There is no formal recommendation for the use of scoring systems that correlate the histological activity with disease outcomes in CD, especially for use in clinical studies. However, the presence of histological inflammation in endoscopically quiescent disease should warn against the de-escalation of therapy[90]. Histology may be more effective in assessing the benefit of therapy or in predicting clinical recurrences[91].
TRANSCRIPTIONAL BIOMARKERS
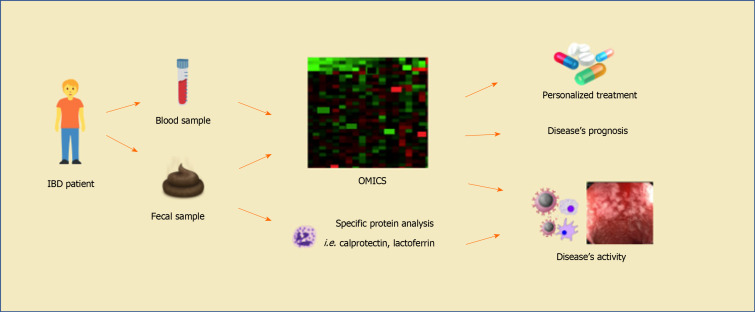
Recently, some techniques have made it possible to discover new non-invasive biomarkers that may be associated with disease activity in IBD (Figure 3).
Figure 3.
Prospect of the future usefulness of transcriptional biomarkers in the monitoring of activity and treatment efficacy in patients with inflammatory bowel diseases. OMICS analysis includes transcriptomics, genomics, metabolomics, lipidomics, and epigenomics studies. Fecal biomarkers of disease activity such as calprotectin and lactoferrin are already available in current clinical practice. Moreover, serological exams, such as C-reactive protein, erythrocyte sedimentation rate and platelet count, are commonly used to assess disease activity, despite being nonspecific. IBD: Inflammatory bowel diseases.
Whole blood transcriptional analysis has revealed that a set of 122 genes that were significantly upregulated in UC patients was correlated with endoscopic lesions. However, the association of these gene expressions was considered modest: Only 15 genes were regulated up to 2-fold in active patients when compared with remission of even non-IBD individuals. Also, gene expression changes were associated with endoscopic Mayo score in these patients, as were other biomarkers, such as CRP, ESR and platelet count[92]. One of these upregulated genes in UC patients that best correlated with mucosal lesions was haptoglobin (HP). The authors observed that serological HP dosage also presented a significant correlation with endoscopic activity. In addition, specific transcriptional biomarkers, including HP and S100A12, but also CD117 and GPR84, were associated with endoscopic changes in response to anti-TNF-α treatment[92].
MicroRNA (miRNA) has also been associated with IBD[93]. miRNA regulates gene expression and, consequently, a number of cellular behaviors, including proliferation, differentiation and apoptosis. High levels of four different types of miRNA (miR-18a, miR-629, let-7b, and miR-140-3p) and lower levels of three miRNAs (miR-422a, miR-885-5p, and miR-328) were identified in intestinal mucosa of active CD when compared with inactive CD. With regard to UC, it was observed that two miRNAs (miR-650 and miR-548a-3p) were higher, and three (miR-630, miR-489, and miR-196b) were lower in active UC when compared with inactive UC[94].
Altered levels of neutrophil gelatinase-associated lipocalin (NGAL) and metalloproteinase-9 (MMP9) have also been associated with endoscopic activity in UC. NGAL and MMP9 are granules within neutrophils. They bond to each other and thus protect themselves from autodegradation[95]. De Bruyn and colleagues reported a correlation between these proteins and the Mayo score in UC patients: Higher levels of the NGAL-MMP9 complex were associated with a Mayo subscore of 2 and 3 points. The concentration of this complex decreased as the patients underwent anti-TNF-α therapy, and this lowered level was related to mucosal healing[96].
There are only a few studies that intend to correlate endoscopic activity with a transcriptional biomarker. Recently, a transcriptomic analysis was performed on purified CD8 T cells and whole blood cells from patients with IBD. This study provided a validated tool by relying on this cell population as a biomarker to individually predict the IBD course after diagnosis, but it has not yet been correlated with endoscopic activity[97].. Therefore, more studies must be carried out to evaluate the role of transcriptional biomarkers in IBD clinical practice.
CONCLUSION
Available serological markers of IBD activity such as CRP, ERS and platelet count are usually insufficiently specific. Colonoscopy assessment is the gold standard to establish the presence of inflammation in IBD. However, emerging non-invasive biomarkers, such as fecal calprotectin, have been considered an attractive tool to determine the activity of the disease due to its positive correlation with the available endoscopic scores. In fact, reliable, non-invasive and widely available biomarkers are sorely needed to facilitate clinical management of IBD, and results from blood transcriptome may be possible biomarkers in the future.
ACKNOWLEDGEMENTS
We thank Professor Tristan Torriani for revising the English version of our manuscript.
Footnotes
Conflict-of-interest statement: The authors declare that they have no competing interests.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: Brazilian Study Group in Inflammatory Bowel Diseases (GEDIIB); European Crohn’s Colitis Organisation (ECCO); Pan American Crohn’s Colitis Organisation (PANCCO); International Society of University Colon and Rectal Surgeons (ISUCRS); and Coloproctology Brazilian Society (SBCP).
Peer-review started: July 1, 2020
First decision: September 21, 2020
Article in press: November 5, 2020
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chiu CT, Ju SQ, Romano M S-Editor: Fan JR L-Editor: Webster JR P-Editor: Wang LL
Contributor Information
Bruno Lima Rodrigues, Inflammatory Bowel Disease Research Laboratory, Gastrocenter, Colorectal Surgery Unit, Department of Surgery, School of Medical Sciences, University of Campinas (UNICAMP), Campinas 13083-878, São Paulo, Brazil.
Márcia Carolina Mazzaro, Inflammatory Bowel Disease Research Laboratory, Gastrocenter, Colorectal Surgery Unit, Department of Surgery, School of Medical Sciences, University of Campinas (UNICAMP), Campinas 13083-878, São Paulo, Brazil.
Cristiane Kibune Nagasako, Department of Gastroenterology, Gastrocenter, School of Medical Sciences, University of Campinas (UNICAMP), Campinas 13083-878, São Paulo, Brazil.
Maria de Lourdes Setsuko Ayrizono, Inflammatory Bowel Disease Research Laboratory, Gastrocenter, Colorectal Surgery Unit, Department of Surgery, School of Medical Sciences, University of Campinas (UNICAMP), Campinas 13083-878, São Paulo, Brazil.
João José Fagundes, Inflammatory Bowel Disease Research Laboratory, Gastrocenter, Colorectal Surgery Unit, Department of Surgery, School of Medical Sciences, University of Campinas (UNICAMP), Campinas 13083-878, São Paulo, Brazil.
Raquel Franco Leal, Inflammatory Bowel Disease Research Laboratory, Gastrocenter, Colorectal Surgery Unit, Department of Surgery, School of Medical Sciences, University of Campinas (UNICAMP), Campinas 13083-878, São Paulo, Brazil. rafranco.unicamp@gmail.com.
References
- 1.Shivashankar R, Tremaine WJ, Harmsen WS, Loftus EV Jr. Incidence and Prevalence of Crohn's Disease and Ulcerative Colitis in Olmsted County, Minnesota From 1970 Through 2010. Clin Gastroenterol Hepatol. 2017;15:857–863. doi: 10.1016/j.cgh.2016.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, Kaplan GG. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012; 142: 46-54. :quiz e30. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein CN. Assessing environmental risk factors affecting the inflammatory bowel diseases: a joint workshop of the Crohn's & Colitis Foundations of Canada and the USA. Inflamm Bowel Dis. 2008;14:1139–1146. doi: 10.1002/ibd.20494. [DOI] [PubMed] [Google Scholar]
- 4.Iskandar HN, Ciorba MA. Biomarkers in inflammatory bowel disease: current practices and recent advances. Transl Res. 2012;159:313–325. doi: 10.1016/j.trsl.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ricanek P, Brackmann S, Perminow G, Lyckander LG, Sponheim J, Holme O, Høie O, Rydning A, Vatn MH IBSEN II Study Group. Evaluation of disease activity in IBD at the time of diagnosis by the use of clinical, biochemical, and fecal markers. Scand J Gastroenterol. 2011;46:1081–1091. doi: 10.3109/00365521.2011.584897. [DOI] [PubMed] [Google Scholar]
- 6.Rogler G, Biedermann L. Clinical Utility of Biomarkers in IBD. Curr Gastroenterol Rep. 2015;17:26. doi: 10.1007/s11894-015-0449-x. [DOI] [PubMed] [Google Scholar]
- 7.Leighton JA, Shen B, Baron TH, Adler DG, Davila R, Egan JV, Faigel DO, Gan SI, Hirota WK, Lichtenstein D, Qureshi WA, Rajan E, Zuckerman MJ, VanGuilder T, Fanelli RD Standards of Practice Committee; American Society for Gastrointestinal Endoscopy. ASGE guideline: endoscopy in the diagnosis and treatment of inflammatory bowel disease. Gastrointest Endosc. 2006;63:558–565. doi: 10.1016/j.gie.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Truelove SC, Witts LJ. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J. 1955;2:1041–1048. doi: 10.1136/bmj.2.4947.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baron JH, Connell AM, Lennard-Jones JE. Variation between observers in describing mucosal appearances in proctocolitis. Br Med J. 1964;1:89–92. doi: 10.1136/bmj.1.5375.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feagan BG, Greenberg GR, Wild G, Fedorak RN, Paré P, McDonald JW, Dubé R, Cohen A, Steinhart AH, Landau S, Aguzzi RA, Fox IH, Vandervoort MK. Treatment of ulcerative colitis with a humanized antibody to the alpha4beta7 integrin. N Engl J Med. 2005;352:2499–2507. doi: 10.1056/NEJMoa042982. [DOI] [PubMed] [Google Scholar]
- 11.Powell-Tuck J, Bown RL, Lennard-Jones JE. A comparison of oral prednisolone given as single or multiple daily doses for active proctocolitis. Scand J Gastroenterol. 1978;13:833–837. doi: 10.3109/00365527809182199. [DOI] [PubMed] [Google Scholar]
- 12.Sutherland LR, Martin F. 5-Aminosalicylic acid enemas in treatment of distal ulcerative colitis and proctitis in Canada. Dig Dis Sci. 1987;32:64S–66S. doi: 10.1007/BF01312466. [DOI] [PubMed] [Google Scholar]
- 13.Rachmilewitz D. Coated mesalazine (5-aminosalicylic acid) versus sulphasalazine in the treatment of active ulcerative colitis: a randomised trial. BMJ. 1989;298:82–86. doi: 10.1136/bmj.298.6666.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625–1629. doi: 10.1056/NEJM198712243172603. [DOI] [PubMed] [Google Scholar]
- 15.Lewis JD, Chuai S, Nessel L, Lichtenstein GR, Aberra FN, Ellenberg JH. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis. 2008;14:1660–1666. doi: 10.1002/ibd.20520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joossens S, Colombel JF, Landers C, Poulain D, Geboes K, Bossuyt X, Targan S, Rutgeerts P, Reinisch W. Anti-outer membrane of porin C and anti-I2 antibodies in indeterminate colitis. Gut. 2006;55:1667–1669. doi: 10.1136/gut.2005.089623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colombel JF, Rutgeerts P, Reinisch W, Esser D, Wang Y, Lang Y, Marano CW, Strauss R, Oddens BJ, Feagan BG, Hanauer SB, Lichtenstein GR, Present D, Sands BE, Sandborn WJ. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology. 2011;141:1194–1201. doi: 10.1053/j.gastro.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 18.Lobatón T, Bessissow T, De Hertogh G, Lemmens B, Maedler C, Van Assche G, Vermeire S, Bisschops R, Rutgeerts P, Bitton A, Afif W, Marcus V, Ferrante M. The Modified Mayo Endoscopic Score (MMES): A New Index for the Assessment of Extension and Severity of Endoscopic Activity in Ulcerative Colitis Patients. J Crohns Colitis. 2015;9:846–852. doi: 10.1093/ecco-jcc/jjv111. [DOI] [PubMed] [Google Scholar]
- 19.Ikeya K, Hanai H, Sugimoto K, Osawa S, Kawasaki S, Iida T, Maruyama Y, Watanabe F. The Ulcerative Colitis Endoscopic Index of Severity More Accurately Reflects Clinical Outcomes and Long-term Prognosis than the Mayo Endoscopic Score. J Crohns Colitis. 2016;10:286–295. doi: 10.1093/ecco-jcc/jjv210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Ruscio M, Variola A, Vernia F, Lunardi G, Castelli P, Bocus P, Geccherle A. Role of Ulcerative Colitis Endoscopic Index of Severity (UCEIS) versus Mayo Endoscopic Subscore (MES) in Predicting Patients' Response to Biological Therapy and the Need for Colectomy. Digestion. 2020:1–12. doi: 10.1159/000509512. [DOI] [PubMed] [Google Scholar]
- 21.Samuel S, Bruining DH, Loftus EV Jr, Thia KT, Schroeder KW, Tremaine WJ, Faubion WA, Kane SV, Pardi DS, de Groen PC, Harmsen WS, Zinsmeister AR, Sandborn WJ. Validation of the ulcerative colitis colonoscopic index of severity and its correlation with disease activity measures. Clin Gastroenterol Hepatol 2013; 11: 49-54. :e1. doi: 10.1016/j.cgh.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mary JY, Modigliani R. Development and validation of an endoscopic index of the severity for Crohn's disease: a prospective multicentre study. Groupe d'Etudes Thérapeutiques des Affections Inflammatoires du Tube Digestif (GETAID) Gut. 1989;30:983–989. doi: 10.1136/gut.30.7.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daperno M, D'Haens G, Van Assche G, Baert F, Bulois P, Maunoury V, Sostegni R, Rocca R, Pera A, Gevers A, Mary JY, Colombel JF, Rutgeerts P. Development and validation of a new, simplified endoscopic activity score for Crohn's disease: the SES-CD. Gastrointest Endosc. 2004;60:505–512. doi: 10.1016/s0016-5107(04)01878-4. [DOI] [PubMed] [Google Scholar]
- 24.Ferrante M, Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D'Haens GR, van der Woude CJ, Danese S, Diamond RH, Oortwijn AF, Tang KL, Miller M, Cornillie F, Rutgeerts PJ International Organization for the Study of Inflammatory Bowel Diseases. Validation of endoscopic activity scores in patients with Crohn's disease based on a post hoc analysis of data from SONIC. Gastroenterology 2013; 145: 978-986. :e5. doi: 10.1053/j.gastro.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 25.Allez M, Lemann M, Bonnet J, Cattan P, Jian R, Modigliani R. Long term outcome of patients with active Crohn's disease exhibiting extensive and deep ulcerations at colonoscopy. Am J Gastroenterol. 2002;97:947–953. doi: 10.1111/j.1572-0241.2002.05614.x. [DOI] [PubMed] [Google Scholar]
- 26.Annese V, Daperno M, Rutter MD, Amiot A, Bossuyt P, East J, Ferrante M, Götz M, Katsanos KH, Kießlich R, Ordás I, Repici A, Rosa B, Sebastian S, Kucharzik T, Eliakim R European Crohn's and Colitis Organisation. European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohns Colitis. 2013;7:982–1018. doi: 10.1016/j.crohns.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 27.Pariente B, Cosnes J, Danese S, Sandborn WJ, Lewin M, Fletcher JG, Chowers Y, D'Haens G, Feagan BG, Hibi T, Hommes DW, Irvine EJ, Kamm MA, Loftus EV Jr, Louis E, Michetti P, Munkholm P, Oresland T, Panés J, Peyrin-Biroulet L, Reinisch W, Sands BE, Schoelmerich J, Schreiber S, Tilg H, Travis S, van Assche G, Vecchi M, Mary JY, Colombel JF, Lémann M. Development of the Crohn's disease digestive damage score, the Lémann score. Inflamm Bowel Dis. 2011;17:1415–1422. doi: 10.1002/ibd.21506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marteau P, Lémann M, Seksik P, Laharie D, Colombel JF, Bouhnik Y, Cadiot G, Soulé JC, Bourreille A, Metman E, Lerebours E, Carbonnel F, Dupas JL, Veyrac M, Coffin B, Moreau J, Abitbol V, Blum-Sperisen S, Mary JY. Ineffectiveness of Lactobacillus johnsonii LA1 for prophylaxis of postoperative recurrence in Crohn's disease: a randomised, double blind, placebo controlled GETAID trial. Gut. 2006;55:842–847. doi: 10.1136/gut.2005.076604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calabrese E, Petruzziello C, Onali S, Condino G, Zorzi F, Pallone F, Biancone L. Severity of postoperative recurrence in Crohn's disease: correlation between endoscopic and sonographic findings. Inflamm Bowel Dis. 2009;15:1635–1642. doi: 10.1002/ibd.20948. [DOI] [PubMed] [Google Scholar]
- 30.Sailer J, Peloschek P, Reinisch W, Vogelsang H, Turetschek K, Schima W. Anastomotic recurrence of Crohn's disease after ileocolic resection: comparison of MR enteroclysis with endoscopy. Eur Radiol. 2008;18:2512–2521. doi: 10.1007/s00330-008-1034-6. [DOI] [PubMed] [Google Scholar]
- 31.Reinisch W, Angelberger S, Petritsch W, Shonova O, Lukas M, Bar-Meir S, Teml A, Schaeffeler E, Schwab M, Dilger K, Greinwald R, Mueller R, Stange EF, Herrlinger KR International AZT-2 Study Group. Azathioprine versus mesalazine for prevention of postoperative clinical recurrence in patients with Crohn's disease with endoscopic recurrence: efficacy and safety results of a randomised, double-blind, double-dummy, multicentre trial. Gut. 2010;59:752–759. doi: 10.1136/gut.2009.194159. [DOI] [PubMed] [Google Scholar]
- 32.Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn's disease. Gastroenterology. 1990;99:956–963. doi: 10.1016/0016-5085(90)90613-6. [DOI] [PubMed] [Google Scholar]
- 33.Deepak P, Fletcher JG, Fidler JL, Barlow JM, Sheedy SP, Kolbe AB, Harmsen WS, Loftus EV, Hansel SL, Becker BD, Bruining DH. Radiological Response Is Associated With Better Long-Term Outcomes and Is a Potential Treatment Target in Patients With Small Bowel Crohn's Disease. Am J Gastroenterol. 2016;111:997–1006. doi: 10.1038/ajg.2016.177. [DOI] [PubMed] [Google Scholar]
- 34.Deepak P, Fletcher JG, Fidler JL, Bruining DH. Computed Tomography and Magnetic Resonance Enterography in Crohn's Disease: Assessment of Radiologic Criteria and Endpoints for Clinical Practice and Trials. Inflamm Bowel Dis. 2016;22:2280–2288. doi: 10.1097/MIB.0000000000000845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rimola J, Planell N, Rodríguez S, Delgado S, Ordás I, Ramírez-Morros A, Ayuso C, Aceituno M, Ricart E, Jauregui-Amezaga A, Panés J, Cuatrecasas M. Characterization of inflammation and fibrosis in Crohn's disease lesions by magnetic resonance imaging. Am J Gastroenterol. 2015;110:432–440. doi: 10.1038/ajg.2014.424. [DOI] [PubMed] [Google Scholar]
- 36.Steward MJ, Punwani S, Proctor I, Adjei-Gyamfi Y, Chatterjee F, Bloom S, Novelli M, Halligan S, Rodriguez-Justo M, Taylor SA. Non-perforating small bowel Crohn's disease assessed by MRI enterography: derivation and histopathological validation of an MR-based activity index. Eur J Radiol. 2012;81:2080–2088. doi: 10.1016/j.ejrad.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 37.Rimola J, Rodriguez S, García-Bosch O, Ordás I, Ayala E, Aceituno M, Pellisé M, Ayuso C, Ricart E, Donoso L, Panés J. Magnetic resonance for assessment of disease activity and severity in ileocolonic Crohn's disease. Gut. 2009;58:1113–1120. doi: 10.1136/gut.2008.167957. [DOI] [PubMed] [Google Scholar]
- 38.Oussalah A, Laurent V, Bruot O, Bressenot A, Bigard MA, Régent D, Peyrin-Biroulet L. Diffusion-weighted magnetic resonance without bowel preparation for detecting colonic inflammation in inflammatory bowel disease. Gut. 2010;59:1056–1065. doi: 10.1136/gut.2009.197665. [DOI] [PubMed] [Google Scholar]
- 39.Buisson A, Joubert A, Montoriol PF, Da Ines D, Hordonneau C, Pereira B, Garcier JM, Bommelaer G, Petitcolin V. Diffusion-weighted magnetic resonance imaging for detecting and assessing ileal inflammation in Crohn's disease. Aliment Pharmacol Ther. 2013;37:537–545. doi: 10.1111/apt.12201. [DOI] [PubMed] [Google Scholar]
- 40.Hordonneau C, Buisson A, Scanzi J, Goutorbe F, Pereira B, Borderon C, Da Ines D, Montoriol PF, Garcier JM, Boyer L, Bommelaer G, Petitcolin V. Diffusion-weighted magnetic resonance imaging in ileocolonic Crohn's disease: validation of quantitative index of activity. Am J Gastroenterol. 2014;109:89–98. doi: 10.1038/ajg.2013.385. [DOI] [PubMed] [Google Scholar]
- 41.Prezzi D, Bhatnagar G, Vega R, Makanyanga J, Halligan S, Taylor SA. Monitoring Crohn's disease during anti-TNF-α therapy: validation of the magnetic resonance enterography global score (MEGS) against a combined clinical reference standard. Eur Radiol. 2016;26:2107–2117. doi: 10.1007/s00330-015-4036-1. [DOI] [PubMed] [Google Scholar]
- 42.Ordás I, Rimola J, Rodríguez S, Paredes JM, Martínez-Pérez MJ, Blanc E, Arévalo JA, Aduna M, Andreu M, Radosevic A, Ramírez-Morros AM, Pinó S, Gallego M, Jauregui-Amezaga A, Ricart E, Panés J. Accuracy of magnetic resonance enterography in assessing response to therapy and mucosal healing in patients with Crohn's disease. Gastroenterology 2014; 146: 374-82. :e1. doi: 10.1053/j.gastro.2013.10.055. [DOI] [PubMed] [Google Scholar]
- 43.Makanyanga JC, Pendsé D, Dikaios N, Bloom S, McCartney S, Helbren E, Atkins E, Cuthbertson T, Punwani S, Forbes A, Halligan S, Taylor SA. Evaluation of Crohn's disease activity: initial validation of a magnetic resonance enterography global score (MEGS) against faecal calprotectin. Eur Radiol. 2014;24:277–287. doi: 10.1007/s00330-013-3010-z. [DOI] [PubMed] [Google Scholar]
- 44.Van Assche G, Vermeire S, Rutgeerts P. The potential for disease modification in Crohn's disease. Nat Rev Gastroenterol Hepatol. 2010;7:79–85. doi: 10.1038/nrgastro.2009.220. [DOI] [PubMed] [Google Scholar]
- 45.Parks AG, Gordon PH, Hardcastle JD. A classification of fistula-in-ano. Br J Surg. 1976;63:1–12. doi: 10.1002/bjs.1800630102. [DOI] [PubMed] [Google Scholar]
- 46.Pariente B, Mary JY, Danese S, Chowers Y, De Cruz P, D'Haens G, Loftus EV Jr, Louis E, Panés J, Schölmerich J, Schreiber S, Vecchi M, Branche J, Bruining D, Fiorino G, Herzog M, Kamm MA, Klein A, Lewin M, Meunier P, Ordas I, Strauch U, Tontini GE, Zagdanski AM, Bonifacio C, Rimola J, Nachury M, Leroy C, Sandborn W, Colombel JF, Cosnes J. Development of the Lémann index to assess digestive tract damage in patients with Crohn's disease. Gastroenterology 2015; 148: 52-63. :e3. doi: 10.1053/j.gastro.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 47.Dubinsky MC, Mei L, Friedman M, Dhere T, Haritunians T, Hakonarson H, Kim C, Glessner J, Targan SR, McGovern DP, Taylor KD, Rotter JI. Genome wide association (GWA) predictors of anti-TNFalpha therapeutic responsiveness in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2010;16:1357–1366. doi: 10.1002/ibd.21174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Consigny Y, Modigliani R, Colombel JF, Dupas JL, Lémann M, Mary JY Groupe d'Etudes Thérapeutiques des Affections Inflammatoires Digestives (GETAID) A simple biological score for predicting low risk of short-term relapse in Crohn's disease. Inflamm Bowel Dis. 2006;12:551–557. doi: 10.1097/01.ibd.0000225334.60990.5b. [DOI] [PubMed] [Google Scholar]
- 49.Solem CA, Loftus EV Jr, Tremaine WJ, Harmsen WS, Zinsmeister AR, Sandborn WJ. Correlation of C-reactive protein with clinical, endoscopic, histologic, and radiographic activity in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:707–712. doi: 10.1097/01.mib.0000173271.18319.53. [DOI] [PubMed] [Google Scholar]
- 50.Iaculli E, Agostini M, Biancone L, Fiorani C, Di Vizia A, Montagnese F, Sibio S, Manzelli A, Tesauro M, Rufini A, Sica GS. C-reactive protein levels in the perioperative period as a predictive marker of endoscopic recurrence after ileo-colonic resection for Crohn's disease. Cell Death Discov. 2016;2:16032. doi: 10.1038/cddiscovery.2016.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oh K, Oh EH, Baek S, Song EM, Kim GU, Seo M, Hwang SW, Park SH, Yang DH, Kim KJ, Byeon JS, Myung SJ, Yang SK, Ye BD. Elevated C-reactive protein level during clinical remission can predict poor outcomes in patients with Crohn's disease. PLoS One. 2017;12:e0179266. doi: 10.1371/journal.pone.0179266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jones J, Loftus EV Jr, Panaccione R, Chen LS, Peterson S, McConnell J, Baudhuin L, Hanson K, Feagan BG, Harmsen SW, Zinsmeister AR, Helou E, Sandborn WJ. Relationships between disease activity and serum and fecal biomarkers in patients with Crohn's disease. Clin Gastroenterol Hepatol. 2008;6:1218–1224. doi: 10.1016/j.cgh.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 53.Denis MA, Reenaers C, Fontaine F, Belaïche J, Louis E. Assessment of endoscopic activity index and biological inflammatory markers in clinically active Crohn's disease with normal C-reactive protein serum level. Inflamm Bowel Dis. 2007;13:1100–1105. doi: 10.1002/ibd.20178. [DOI] [PubMed] [Google Scholar]
- 54.Schoepfer AM, Beglinger C, Straumann A, Trummler M, Vavricka SR, Bruegger LE, Seibold F. Fecal calprotectin correlates more closely with the Simple Endoscopic Score for Crohn's disease (SES-CD) than CRP, blood leukocytes, and the CDAI. Am J Gastroenterol. 2010;105:162–169. doi: 10.1038/ajg.2009.545. [DOI] [PubMed] [Google Scholar]
- 55.Mendoza JL, Abreu MT. Biological markers in inflammatory bowel disease: practical consideration for clinicians. Gastroenterol Clin Biol. 2009;33 Suppl 3:S158–S173. doi: 10.1016/S0399-8320(09)73151-3. [DOI] [PubMed] [Google Scholar]
- 56.Alper A, Zhang L, Pashankar DS. Correlation of Erythrocyte Sedimentation Rate and C-Reactive Protein With Pediatric Inflammatory Bowel Disease Activity. J Pediatr Gastroenterol Nutr. 2017;65:e25–e27. doi: 10.1097/MPG.0000000000001444. [DOI] [PubMed] [Google Scholar]
- 57.Nakarai A, Kato J, Hiraoka S, Takashima S, Inokuchi T, Takahara M, Sugihara Y, Harada K, Okada H. An Elevated Platelet Count Increases the Risk of Relapse in Ulcerative Colitis Patients with Mucosal Healing. Gut Liver. 2018;12:420–425. doi: 10.5009/gnl17236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takeyama H, Mizushima T, Iijima H, Shinichiro S, Uemura M, Nishimura J, Hata T, Takemasa I, Yamamoto H, Doki Y, Mori M. Platelet Activation Markers Are Associated with Crohn's Disease Activity in Patients with Low C-Reactive Protein. Dig Dis Sci. 2015;60:3418–3423. doi: 10.1007/s10620-015-3745-2. [DOI] [PubMed] [Google Scholar]
- 59.Lewis JD. The utility of biomarkers in the diagnosis and therapy of inflammatory bowel disease. Gastroenterology 2011; 140: 1817-1826. :e2. doi: 10.1053/j.gastro.2010.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaiser T, Langhorst J, Wittkowski H, Becker K, Friedrich AW, Rueffer A, Dobos GJ, Roth J, Foell D. Faecal S100A12 as a non-invasive marker distinguishing inflammatory bowel disease from irritable bowel syndrome. Gut. 2007;56:1706–1713. doi: 10.1136/gut.2006.113431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut. 2006;55:426–431. doi: 10.1136/gut.2005.069476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lasson A, Stotzer PO, Öhman L, Isaksson S, Sapnara M, Strid H. The intra-individual variability of faecal calprotectin: a prospective study in patients with active ulcerative colitis. J Crohns Colitis. 2015;9:26–32. doi: 10.1016/j.crohns.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 63.Lee YW, Lee KM, Lee JM, Chung YY, Kim DB, Kim YJ, Chung WC, Paik CN. The usefulness of fecal calprotectin in assessing inflammatory bowel disease activity. Korean J Intern Med. 2019;34:72–80. doi: 10.3904/kjim.2016.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xiang JY, Ouyang Q, Li GD, Xiao NP. Clinical value of fecal calprotectin in determining disease activity of ulcerative colitis. World J Gastroenterol. 2008;14:53–57. doi: 10.3748/wjg.14.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.D'Haens G, Ferrante M, Vermeire S, Baert F, Noman M, Moortgat L, Geens P, Iwens D, Aerden I, Van Assche G, Van Olmen G, Rutgeerts P. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2218–2224. doi: 10.1002/ibd.22917. [DOI] [PubMed] [Google Scholar]
- 66.Vieira A, Fang CB, Rolim EG, Klug WA, Steinwurz F, Rossini LG, Candelária PA. Inflammatory bowel disease activity assessed by fecal calprotectin and lactoferrin: correlation with laboratory parameters, clinical, endoscopic and histological indexes. BMC Res Notes. 2009;2:221. doi: 10.1186/1756-0500-2-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qiu Y, Mao R, Chen BL, He Y, Zeng ZR, Xue L, Song XM, Li ZP, Chen MH. Fecal calprotectin for evaluating postoperative recurrence of Crohn's disease: a meta-analysis of prospective studies. Inflamm Bowel Dis. 2015;21:315–322. doi: 10.1097/MIB.0000000000000262. [DOI] [PubMed] [Google Scholar]
- 68.Costa F, Mumolo MG, Ceccarelli L, Bellini M, Romano MR, Sterpi C, Ricchiuti A, Marchi S, Bottai M. Calprotectin is a stronger predictive marker of relapse in ulcerative colitis than in Crohn's disease. Gut. 2005;54:364–368. doi: 10.1136/gut.2004.043406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weinstein-Nakar I, Focht G, Church P, Walters TD, Abitbol G, Anupindi S, Berteloot L, Hulst JM, Ruemmele F, Lemberg DA, Leach ST, Cytter R, Greer ML, Griffiths AM, Turner D ImageKids study group. Associations Among Mucosal and Transmural Healing and Fecal Level of Calprotectin in Children With Crohn's Disease. Clin Gastroenterol Hepatol 2018; 16: 1089-1097. :e4. doi: 10.1016/j.cgh.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 70.Boschetti G, Garnero P, Moussata D, Cuerq C, Préaudat C, Duclaux-Loras R, Mialon A, Drai J, Flourié B, Nancey S. Accuracies of serum and fecal S100 proteins (calprotectin and calgranulin C) to predict the response to TNF antagonists in patients with Crohn's disease. Inflamm Bowel Dis. 2015;21:331–336. doi: 10.1097/MIB.0000000000000273. [DOI] [PubMed] [Google Scholar]
- 71.Stange EF, Travis SP, Vermeire S, Reinisch W, Geboes K, Barakauskiene A, Feakins R, Fléjou JF, Herfarth H, Hommes DW, Kupcinskas L, Lakatos PL, Mantzaris GJ, Schreiber S, Villanacci V, Warren BF European Crohn's and Colitis Organisation (ECCO) European evidence-based Consensus on the diagnosis and management of ulcerative colitis: Definitions and diagnosis. J Crohns Colitis. 2008;2:1–23. doi: 10.1016/j.crohns.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 72.Langner C, Magro F, Driessen A, Ensari A, Mantzaris GJ, Villanacci V, Becheanu G, Borralho Nunes P, Cathomas G, Fries W, Jouret-Mourin A, Mescoli C, de Petris G, Rubio CA, Shepherd NA, Vieth M, Eliakim R, Geboes K European Society of Pathology; European Crohn's and Colitis Foundation. The histopathological approach to inflammatory bowel disease: a practice guide. Virchows Arch. 2014;464:511–527. doi: 10.1007/s00428-014-1543-4. [DOI] [PubMed] [Google Scholar]
- 73.Surawicz CM. Serial sectioning of a portion of a rectal biopsy detects more focal abnormalities: a prospective study of patients with inflammatory bowel disease. Dig Dis Sci. 1982;27:434–436. doi: 10.1007/BF01295652. [DOI] [PubMed] [Google Scholar]
- 74.Walsh AJ, Bryant RV, Travis SP. Current best practice for disease activity assessment in IBD. Nat Rev Gastroenterol Hepatol. 2016;13:567–579. doi: 10.1038/nrgastro.2016.128. [DOI] [PubMed] [Google Scholar]
- 75.Geboes K, Riddell R, Ost A, Jensfelt B, Persson T, Löfberg R. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut. 2000;47:404–409. doi: 10.1136/gut.47.3.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Riley SA, Mani V, Goodman MJ, Dutt S, Herd ME. Microscopic activity in ulcerative colitis: what does it mean? Gut. 1991;32:174–178. doi: 10.1136/gut.32.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Feagan BG, Sandborn WJ, D'Haens G, Lee SD, Allez M, Fedorak RN, Seidler U, Vermeire S, Lawrance IC, Maroney AC, Jurgensen CH, Heath A, Chang DJ. Randomised clinical trial: vercirnon, an oral CCR9 antagonist, vs. placebo as induction therapy in active Crohn's disease. Aliment Pharmacol Ther. 2015;42:1170–1181. doi: 10.1111/apt.13398. [DOI] [PubMed] [Google Scholar]
- 78.Bressenot A, Salleron J, Bastien C, Danese S, Boulagnon-Rombi C, Peyrin-Biroulet L. Comparing histological activity indexes in UC. Gut. 2015;64:1412–1418. doi: 10.1136/gutjnl-2014-307477. [DOI] [PubMed] [Google Scholar]
- 79.Marchal-Bressenot A, Salleron J, Boulagnon-Rombi C, Bastien C, Cahn V, Cadiot G, Diebold MD, Danese S, Reinisch W, Schreiber S, Travis S, Peyrin-Biroulet L. Development and validation of the Nancy histological index for UC. Gut. 2017;66:43–49. doi: 10.1136/gutjnl-2015-310187. [DOI] [PubMed] [Google Scholar]
- 80.Mosli MH, Feagan BG, Zou G, Sandborn WJ, D'Haens G, Khanna R, Shackelton LM, Walker CW, Nelson S, Vandervoort MK, Frisbie V, Samaan MA, Jairath V, Driman DK, Geboes K, Valasek MA, Pai RK, Lauwers GY, Riddell R, Stitt LW, Levesque BG. Development and validation of a histological index for UC. Gut. 2017;66:50–58. doi: 10.1136/gutjnl-2015-310393. [DOI] [PubMed] [Google Scholar]
- 81.Gramlich T, Petras RE. Pathology of inflammatory bowel disease. Semin Pediatr Surg. 2007;16:154–163. doi: 10.1053/j.sempedsurg.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 82.Gupta RB, Harpaz N, Itzkowitz S, Hossain S, Matula S, Kornbluth A, Bodian C, Ullman T. Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: a cohort study. Gastroenterology. 2007;133:1099–105; quiz 1340. doi: 10.1053/j.gastro.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mojtahed A, Khanna R, Sandborn WJ, D'Haens GR, Feagan BG, Shackelton LM, Baker KA, Dubcenco E, Valasek MA, Geboes K, Levesque BG. Assessment of histologic disease activity in Crohn's disease: a systematic review. Inflamm Bowel Dis. 2014;20:2092–2103. doi: 10.1097/MIB.0000000000000155. [DOI] [PubMed] [Google Scholar]
- 84.Kevans D, Kirsch R, Dargavel C, Kabakchiev B, Riddell R, Silverberg MS. Histological Markers of Clinical Relapse in Endoscopically Quiescent Ulcerative Colitis. Inflamm Bowel Dis. 2020;26:1722–1729. doi: 10.1093/ibd/izz308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Geboes K, Rutgeerts P, Opdenakker G, Olson A, Patel K, Wagner CL, Marano CW. Endoscopic and histologic evidence of persistent mucosal healing and correlation with clinical improvement following sustained infliximab treatment for Crohn's disease. Curr Med Res Opin. 2005;21:1741–1754. doi: 10.1185/030079905x65457. [DOI] [PubMed] [Google Scholar]
- 86.Naini BV, Cortina G. A histopathologic scoring system as a tool for standardized reporting of chronic (ileo)colitis and independent risk assessment for inflammatory bowel disease. Hum Pathol. 2012;43:2187–2196. doi: 10.1016/j.humpath.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 87.Rutgeerts P, Van Assche G, Sandborn WJ, Wolf DC, Geboes K, Colombel JF, Reinisch W EXTEND Investigators; Kumar A; Lazar A; Camez A; Lomax KG; Pollack PF; D'Haens G. Adalimumab induces and maintains mucosal healing in patients with Crohn's disease: data from the EXTEND trial. Gastroenterology 2012; 142: 1102-1111. :e2. doi: 10.1053/j.gastro.2012.01.035. [DOI] [PubMed] [Google Scholar]
- 88.Zittan E, Kelly OB, Kirsch R, Milgrom R, Burns J, Nguyen GC, Croitoru K, Van Assche G, Silverberg MS, Steinhart AH. Low Fecal Calprotectin Correlates with Histological Remission and Mucosal Healing in Ulcerative Colitis and Colonic Crohn's Disease. Inflamm Bowel Dis. 2016;22:623–630. doi: 10.1097/MIB.0000000000000652. [DOI] [PubMed] [Google Scholar]
- 89.Magro F, Lopes J, Borralho P, Lopes S, Coelho R, Cotter J, Castro FD, Sousa HT, Salgado M, Andrade P, Vieira AI, Figueiredo P, Caldeira P, Sousa A, Duarte MA, Ávila F, Silva J, Moleiro J, Mendes S, Giestas S, Ministro P, Sousa P, Gonçalves R, Gonçalves B, Oliveira A, Rosa I, Rodrigues M, Chagas C, Dias CC, Afonso J, Geboes K, Carneiro F Portuguese IBD Study Group (GEDII) Comparison of different histological indexes in the assessment of UC activity and their accuracy regarding endoscopic outcomes and faecal calprotectin levels. Gut. 2019;68:594–603. doi: 10.1136/gutjnl-2017-315545. [DOI] [PubMed] [Google Scholar]
- 90.Bryant RV, Winer S, Travis SP, Riddell RH. Systematic review: histological remission in inflammatory bowel disease. Is 'complete' remission the new treatment paradigm? J Crohns Colitis. 2014;8:1582–1597. doi: 10.1016/j.crohns.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 91.Best WR, Becktel JM, Singleton JW, Kern F Jr. Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. 1976;70:439–444. [PubMed] [Google Scholar]
- 92.Planell N, Masamunt MC, Leal RF, Rodríguez L, Esteller M, Lozano JJ, Ramírez A, Ayrizono MLS, Coy CSR, Alfaro I, Ordás I, Visvanathan S, Ricart E, Guardiola J, Panés J, Salas A. Usefulness of Transcriptional Blood Biomarkers as a Non-invasive Surrogate Marker of Mucosal Healing and Endoscopic Response in Ulcerative Colitis. J Crohns Colitis. 2017;11:1335–1346. doi: 10.1093/ecco-jcc/jjx091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dalal SR, Kwon JH. The Role of MicroRNA in Inflammatory Bowel Disease. Gastroenterol Hepatol (N Y) 2010;6:714–722. [PMC free article] [PubMed] [Google Scholar]
- 94.Iborra M, Bernuzzi F, Correale C, Vetrano S, Fiorino G, Beltrán B, Marabita F, Locati M, Spinelli A, Nos P, Invernizzi P, Danese S. Identification of serum and tissue micro-RNA expression profiles in different stages of inflammatory bowel disease. Clin Exp Immunol. 2013;173:250–258. doi: 10.1111/cei.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kubben FJ, Sier CF, Hawinkels LJ, Tschesche H, van Duijn W, Zuidwijk K, van der Reijden JJ, Hanemaaijer R, Griffioen G, Lamers CB, Verspaget HW. Clinical evidence for a protective role of lipocalin-2 against MMP-9 autodegradation and the impact for gastric cancer. Eur J Cancer. 2007;43:1869–1876. doi: 10.1016/j.ejca.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 96.de Bruyn M, Arijs I, Wollants WJ, Machiels K, Van Steen K, Van Assche G, Ferrante M, Rutgeerts P, Vermeire S, Opdenakker G. Neutrophil gelatinase B-associated lipocalin and matrix metalloproteinase-9 complex as a surrogate serum marker of mucosal healing in ulcerative colitis. Inflamm Bowel Dis. 2014;20:1198–1207. doi: 10.1097/MIB.0000000000000068. [DOI] [PubMed] [Google Scholar]
- 97.Biasci D, Lee JC, Noor NM, Pombal DR, Hou M, Lewis N, Ahmad T, Hart A, Parkes M, McKinney EF, Lyons PA, Smith KGC. A blood-based prognostic biomarker in IBD. Gut. 2019;68:1386–1395. doi: 10.1136/gutjnl-2019-318343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kennedy NA, Jones GR, Plevris N, Patenden R, Arnott ID, Lees CW. Association Between Level of Fecal Calprotectin and Progression of Crohn's Disease. Clin Gastroenterol Hepatol 2019; 17: 2269-2276. :e4. doi: 10.1016/j.cgh.2019.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Monteiro S, Barbosa M, Cúrdia Gonçalves T, Boal Carvalho P, Moreira MJ, Rosa B, Cotter J. Fecal Calprotectin as a Selection Tool for Small Bowel Capsule Endoscopy in Suspected Crohn's Disease. Inflamm Bowel Dis. 2018;24:2033–2038. doi: 10.1093/ibd/izy098. [DOI] [PubMed] [Google Scholar]
- 100.Pendsé DA, Makanyanga JC, Plumb AA, Bhatnagar G, Atkinson D, Rodriguez-Justo M, Halligan S, Taylor SA. Diffusion-weighted imaging for evaluating inflammatory activity in Crohn's disease: comparison with histopathology, conventional MRI activity scores, and faecal calprotectin. Abdom Radiol (NY) 2017;42:115–123. doi: 10.1007/s00261-016-0863-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kopylov U, Nemeth A, Koulaouzidis A, Makins R, Wild G, Afif W, Bitton A, Johansson GW, Bessissow T, Eliakim R, Toth E, Seidman EG. Small bowel capsule endoscopy in the management of established Crohn's disease: clinical impact, safety, and correlation with inflammatory biomarkers. Inflamm Bowel Dis. 2015;21:93–100. doi: 10.1097/MIB.0000000000000255. [DOI] [PubMed] [Google Scholar]
- 102.Chung-Faye G, Rahman A, Sandhu K, Hayee B, Tumova J, Sherwood R. P219 Faecal calprotectin is an accurate predictor of endoscopic and histological disease activity in IBD. J Crohn’s Colitis. 2014;8: :S153–S154. [Google Scholar]
- 103.Fascì-Spurio F, Kennedy NA, Wong L, MacLean P, Satsangi J, Glancy S, Lees CW. P220 Faecal calprotectin and ileal Crohn's disease: correlation with a small bowel MRI score for disease activity. J Crohn’s Colitis . 2014:S154. doi: 10.1093/ecco-jcc/jjy187. [DOI] [PubMed] [Google Scholar]
- 104.Lobatón T, López-García A, Rodríguez-Moranta F, Ruiz A, Rodríguez L, Guardiola J. A new rapid test for fecal calprotectin predicts endoscopic remission and postoperative recurrence in Crohn's disease. J Crohns Colitis. 2013;7:e641–e651. doi: 10.1016/j.crohns.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 105.Koulaouzidis A, Douglas S, Rogers MA, Arnott ID, Plevris JN. Fecal calprotectin: a selection tool for small bowel capsule endoscopy in suspected IBD with prior negative bi-directional endoscopy. Scand J Gastroenterol. 2011;46:561–566. doi: 10.3109/00365521.2011.551835. [DOI] [PubMed] [Google Scholar]
- 106.Jensen MD, Kjeldsen J, Nathan T. Fecal calprotectin is equally sensitive in Crohn's disease affecting the small bowel and colon. Scand J Gastroenterol. 2011;46:694–700. doi: 10.3109/00365521.2011.560680. [DOI] [PubMed] [Google Scholar]
- 107.Sipponen T, Kärkkäinen P, Savilahti E, Kolho KL, Nuutinen H, Turunen U, Färkkilä M. Correlation of faecal calprotectin and lactoferrin with an endoscopic score for Crohn's disease and histological findings. Aliment Pharmacol Ther. 2008;28:1221–1229. doi: 10.1111/j.1365-2036.2008.03835.x. [DOI] [PubMed] [Google Scholar]