Abstract
BACKGROUND
The expression of jumonji domain-containing 3 (Jmjd3) and trimethylated H3 lysine 27 (H3K27me3) in active ulcerative colitis (UC) and the correlation between vitamin D receptor (VDR) and the Jmjd3 pathway are unknown.
AIM
To study the relationship between VDR, Jmjd3 and H3K27me3 in patients with active UC.
METHODS
One hundred patients with active UC and 56 healthy controls were enrolled in this study. The patients with active UC were divided into groups according to mild (n = 29), moderate (n = 32) and severe (n = 29) disease activity based on the modified Mayo score. Vitamin D levels were measured by radioimmunoassay. Colonic mucosal tissues from UC patients and controls were collected by colonoscopy. The expression of VDR, Jmjd3 and H3K27me3 in the intestinal mucosa was determined by immunohistochemistry staining.
RESULTS
Patients with active UC had lower levels of serum vitamin D (13.7 ± 2.8 ng/mL, P < 0.001) than the controls (16.2 ± 2.5 ng/mL). In the UC cohort, serum vitamin D level was negatively correlated with disease activity (r = -0.323, P = 0.001). VDR expression in the mucosa of UC patients was reduced compared to that in normal tissues (P < 0.001) and negatively correlated with disease activity (r = -0.868, P < 0.001). Similar results for VDR expression were noted in the most serious lesion (defined as UC diseased) and 20 cm proximal to the anus (defined as UC normal) (P < 0.05). Simultaneously, Jmjd3 expression significantly increased in UC patients (P < 0.001), but no difference was found between the different sites in UC patients. H3K27me3 expression in UC patients was significantly down-regulated when compared with normal tissues (P < 0.001), but up-regulated in the mild disease activity group in comparison with the moderate disease activity group of UC patients (P < 0.05). Jmjd3 Level was negatively correlated with the level of VDR (r = -0.342, P = 0.002) and H3K27me3 (r = -0.341, P = 0.002), while VDR level was positively correlated with H3K27me3 (r = 0.473, P < 0.001).
CONCLUSION
Serum vitamin D and VDR were inversely correlated with disease activity in active UC. Jmjd3 expression increased in the colonic mucosa of active UC patients and was negatively associated with VDR and H3K27me3 level.
Keywords: Vitamin D, Ulcerative colitis, Disease activity, Vitamin D receptor, Jumonji domain-containing 3, Trimethylated H3 lysine 27
Core Tip: This is the first report of the relationship between serum vitamin D, vitamin D receptor (VDR), Jumonji domain-containing 3 (Jmjd3) and trimethylated H3 lysine 27 expression and pathological activity in patients with active ulcerative colitis (UC). In active UC, vitamin D level was negatively correlated with disease activity and positively correlated with VDR expression. Furthermore, colonic Jmjd3 expression was significantly increased, while trimethylated H3 lysine 27 was decreased in UC patients compared to controls. These findings indicate that VDR expression was inversely related to Jmjd3 expression and disease activity in the colonic mucosa of patients with UC.
INTRODUCTION
Inflammatory bowel disease (IBD) is a multifactorial disease characterized by alternating periods of remission interspersed with relapses, and includes Crohn’s disease and ulcerative colitis (UC)[1]. Although the pathological mechanism has not been fully elucidated, most studies have provided evidence that genetic susceptibility, intestinal antigens, overactive immune responses and various environmental triggers can contribute to intestinal inflammation[2,3].
In the last decades, emerging evidence has demonstrated the fundamental role of vitamin D in the development and pathogenesis of IBD[4]. Vitamin D is metabolized in the liver to 25 (OH) D and is then converted to the active form of 1, 25-dihydroxy vitamin D by 1α-hydroxylase in the kidney[5]. Vitamin D receptor (VDR) is widely distributed in intestinal mucosal epithelial cells in human small intestine and colon[6]. Studies have suggested that VDR not only participates in the regulation of calcium and phosphorus metabolism, but also plays an important role in cell proliferation, differentiation and immune responses. Vitamin D exerts its anti-inflammatory effect mainly by acting on VDR and has been proven to be associated with IBD[7]. Low vitamin D status has been observed in patients with IBD and was shown to be inversely associated with disease activity[8-10]. Loss of VDR in intestinal epithelial or myeloid cells can increase the expression of mucosal pro-inflammatory cytokines and exacerbate experimental colitis[11]. In addition, the expression of VDR in patients with IBD is significantly decreased[12-14]. Thus, the vitamin D pathway may play an important role in immune homeostasis in patients with IBD.
The Jumonji domain-containing 3 (Jmjd3) is a histone demethylase that specifically demethylates trimethylated H3 lysine 27 (H3K27me3), which is a conventionally “repressive” histone modification[15,16]. Increased Jmjd3 can enhance pro-inflammatory responses by demethylating the repressive H3K27me3 epigenetic mark in distinct transcription factors[17]. New research shows that Jmjd3 can induce the nucleotide-binding domain-like receptors family pyrin domain-containing 3 inflammasome activation by decreasing the enrichment of H3K27me3 on the promotors of nuclear factor-erythroid 2-related factor 2 (Nrf2), thereby inducing the occurrence of colitis[18]. GSKJ4 is a small-molecule Jmjd3 inhibitor and can alleviate experimental dextran sulfate sodium-induced colitis[18]. However, a large number of studies have shown that nuclear factor-kappa B (NF-κB) signaling and signal transducer and activator of transcription (STAT) signaling promote the expression of pro-inflammatory genes by activating Jmjd3[19]. Interestingly, vitamin D has been shown to reduce intestinal inflammation by negatively regulating NF-κB and STAT signals by acting on the VDR[20,21]. Importantly, a previous study revealed that vitamin D regulates the ribonucleic acid (RNA) expression of genes encoding for Jmjd3, suggesting a potential role of VDR in Jmjd3 activation[22,23]. However, the expression of Jmjd3 and H3K27me3 in clinical patients with UC and the correlation between VDR and Jmjd3 pathway remain unknown.
In this study, we investigated serum vitamin D level and the differential expression of VDR, Jmjd3 and H3K27me3 between healthy and UC patients with different disease activity to determine the correlation between VDR and the Jmjd3 signaling pathway.
MATERIALS AND METHODS
Study design and patient data
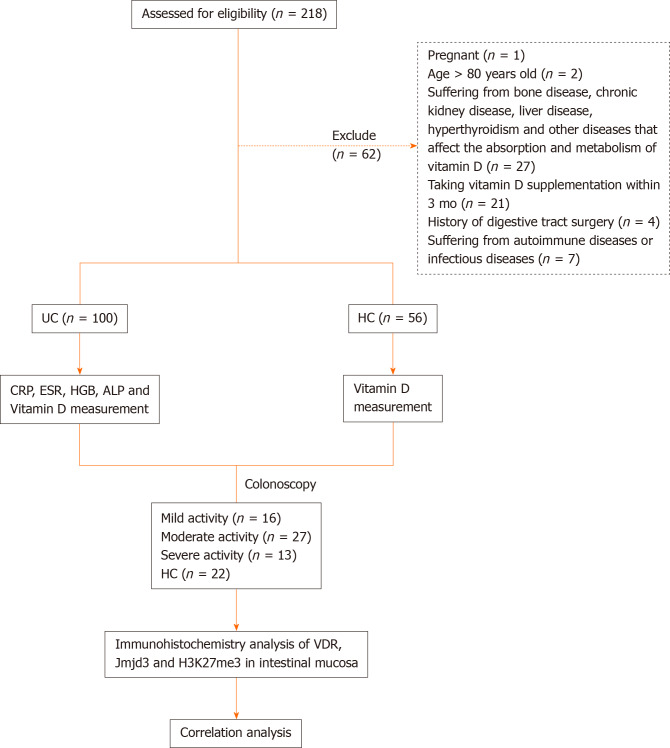
A total of 100 patients with UC (57 females and 43 males) were enrolled in this study in the First Affiliated Hospital of Anhui Medical University from December 2019 to June 2020. The patients with active UC were divided into groups according to mild, moderate and severe disease activity based on the modified Mayo score. In addition, 56 healthy individuals (32 females and 24 males) with normal physical examination were included as controls (Figure 1). The median age of UC patients and healthy controls was 45.5 and 45.8 years, respectively. In order to exclude the effects of light exposure and geographical location on serum vitamin D level, all UC patients and controls were permanent residents in Anhui. Patient gender, age, body mass index (BMI), history of medication and duration of disease were recorded. White blood cell counts, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), hemoglobin (HGB), alkaline phosphatase (ALP) and vitamin D levels were measured in all UC patients. The study was approved by the Ethics Committee of the First Affiliated Hospital of Anhui Medical University (No. PJ2019-14-23), and written informed consent was obtained from all participants.
Figure 1.
Study overview. ALP: Alkaline phosphatase; CRP: C-reactive protein; ESR: Erythrocyte sedimentation rate; H3K27me3: Trimethylated H3 lysine 27; HGB: Hemoglobin; Jmjd3: Jumonji domain-containing 3; UC: Ulcerative colitis; VDR: Vitamin D receptor.
Data collection
Clinical data were obtained from case data and medical history questionnaires and included age, sex, BMI, disease duration, family history of UC, smoking status, history of medication and vitamin D supplementation. The Mayo disease activity index was scored based on the clinical manifestations, endoscopic findings and histological scores of the patients. The UC patients were classified into rectal type, left colon type and total colon type based on the Montreal classification.
Collection of blood and tissue samples
Blood samples from UC patients and healthy subjects were collected and sera were separated by centrifugation at 3000 rpm for 10 min at 37 °C and then stored at -80 °C until analysis. Colonic mucosal biopsies were obtained from 16 patients with mild, 27 with moderate and 13 with severe active UC. At the time of endoscopy, two colonic mucosal biopsies were obtained from the most serious lesion (defined as UC diseased) and 20 cm proximal to the anus (defined as UC normal), which were placed in 10% buffered formalin for immunohistochemistry staining.
Vitamin D measurement
The DIAsource 25 (OH) Vitamin D total-RIA-CT kit (Louvain-la-Neuve, Belgium) was used to measure the concentration of serum vitamin D according to the manufacturer’s instructions. Serum CRP and ALP were determined by immunoturbidimetry. ESR was measured using the Westergren method. White blood cells and HGB were detected using a Sysmex XN-9000 automatic hematology analyzer (Kobe, Japan).
Immunohistochemistry
Fresh colon biopsies were obtained and embedded in paraffin wax after fixation with 10% neutral buffered formalin. Tissues were cut into 5-μm thick sections. The sections were subjected to routine deparaffinization and rehydration, incubated with 3% hydrogen peroxide for 15 min to inhibit endogenous peroxidase activity and microwaved in 0.01 mol/L citrate buffer (pH 9.0) for 30 min to achieve antigen retrieval. After that, rabbit monoclonal antibody against VDR (Ab3508; dilution 1:2000; Abcam Inc., Cambridge, United Kingdom), anti-Jmjd3 rabbit monoclonal antibody (Ab38113; 1:400 Abcam Inc.) or anti-H3K27me3 mouse monoclonal antibody (Ab6002; 1:200; Abcam Inc.) were added to the tissue specimens and incubated at 4 ˚C overnight, followed by staining with mouse anti-rabbit immunoglobulin G (1:100; Dako, Glostrup, Denmark) or a biotinylated goat anti-mouse immunoglobulin G (1:100; Dako) as secondary antibodies for 1 h at 37 °C. The sections were then incubated with 3,3’-diaminobenzidine and lightly counterstained with hematoxylin to demonstrate binding. Finally, the above sections were observed under a light microscope after dehydration. Ten fields of view per section were observed blindly at × 40 as a semiquantitative assessment of immunohistochemical staining. VDR, Jmjd3 and H3K27me3 staining was evaluated with Image-Pro Plus analysis software (version 6.0; Dallas, TX, United States). Positive signals were quantified as the mean optical density.
Statistical analysis
Statistical analyses were performed using Statistic Package for Social Science Statistical Software (Ver. 22.0; IBM Corp., Armonk, NY, United States), and figures were produced with GraphPad Prism (Ver. 6.02; La Jolla, CA, United States). All data were expressed as the mean ± standard deviation or median (minimum and maximum) for continuous variables and as number (percent) for categorical variables. The Shapiro-Wilk test was used to assess the normality of numeric variables. For continuous variables, differences were analyzed by the Student’s t test or the Mann–Whitney U test. For categorical variables, the c2 or Fisher’s exact test was used to compare frequencies. The correlations between serum vitamin D, VDR, Jmjd3, H3K27me3 and other indices (ESR, CRP, HGB, ALP and the Mayo score) were analyzed using Pearson’s correlation analysis or Spearman’s correlation analysis. P < 0.05 was considered statistically significant.
RESULTS
Participant characteristics
In total, 100 UC patients and 56 healthy controls were included in the study according to the inclusion and exclusion criteria. The subjects’ demographics and clinical characteristics are shown in Table 1. The mean age of UC patients and healthy controls was 45.5 years and 45.8 years, respectively, and the mean disease duration was 5.4 years. Women accounted for 57.0% and 57.1% of UC patients and healthy controls, respectively. Forty-five percent of UC patients had a history of smoking, compared with 37.5% in the control group. The BMI of UC patients and controls was 21.2 kg/m2 and 20.4 kg/m2, respectively. The level of serum vitamin D in the UC group was significantly lower than that in healthy control group (P < 0.001). Eight percent of patients had pancolitis, and 38% had extensive colitis. Eighty percent of patients were taking sulfasalazine or 5-aminosalcylate, 2% were treated with immunosuppressants, 31% with corticosteroids, 4% with biological agents and the remaining 22% with antibiotics, probiotics or Traditional Chinese Medicine at the time of enrollment. In addition, comparisons of vitamin D and laboratory indicators among the different groups of UC patients are shown in Table 2.
Table 1.
Demographic characteristics of ulcerative colitis patients and controls
|
Variable
|
UC patients, n = 100
|
HC, n = 56
|
P
value
|
| Age [mean (SD), yr] | 45.5 (14.2) | 45.8 (10.0) | 0.8731 |
| Sex (female: male) | 57:43 | 32:24 | 0.9862 |
| Smoker, n (%) | 45 (45.0) | 21 (37.5) | 0.3632 |
| BMI [mean (SD), kg/m2] | 21.2 (3.4) | 20.4 (2.1) | 0.1011 |
| 25 (OH) D [mean (SD), nmol/L] | 13.7 (2.8) | 16.2 (2.5) | < 0.0011 |
| Disease Location, n (%) | |||
| Ulcerative proctitis (E1) | 8 (8.0) | - | - |
| Left-sided colitis (E2) | 54 (54.0) | - | - |
| Extensive colitis (E3) | 38 (38.0) | - | - |
| Severity of UC, n (%) | |||
| Mild | 29 (29.0) | - | - |
| Moderate | 32 (32.0) | - | - |
| Severe | 39 (39.0) | - | - |
| Current medications, n (%) | |||
| SASP/5-ASA | 80 (80.0) | - | - |
| Immunosuppressants | 2 (2.0) | - | - |
| Steroids | 31 (31.0) | - | - |
| Biologics | 4 (4.0) | - | - |
| Others | 22 (22.0) | - | - |
| Duration of disease mean (SD), yr | 5.4 (6.0) | - | - |
Independent sample t tests.
χ 2 test. 5-ASA: 5-Aminosalicylic acid; SASP: Sulfasalazine; SD: Standard deviation; UC: Ulcerative colitis.
Table 2.
Laboratory indexes characteristics of ulcerative colitis patients with different disease activity
|
Indexes
|
Mild activity, n = 29
|
Moderate activity, n = 32
|
Severe activity, n = 39
|
| Serum vitamin D (ng/mL) | 14.8 ± 2.4 | 14.2 ± 2.4 | 11.7 ± 3.1 |
| WBC (109/L) | 6.90 (4.53-12.81) | 7.13 (0.32-6.02) | 7.13 (3.28-17.59) |
| CRP (mg/L) | 1.51 (0.38-66.23) | 9.90 (0.30-168.78) | 70.67 (1.44-190.07) |
| ESR (mm/h) | 8 (3-34) | 23 (3-95) | 41 (7-127) |
| ALP (U/L) | 81 (49-140) | 85 (40-602) | 77.50 (53-109) |
| PLT (109/L) | 222 (113-363) | 260 (130-518) | 286 (125-500) |
| HGB (g/L) | 137 (74-155) | 115 (59-164) | 115 (55-149) |
| ALB (g/L) | 42.11 ± 4.97 | 37.25 ± 6.13 | 31.31 ± 4.79 |
| Ca (mmol/L) | 2.31 (1.01-2.63) | 2.23 (1.67-2.49) | 2.06 (1.82-2.31) |
| P (mmol/L) | 1.12 ± 0.20 | 1.17 ± 0.21 | 1.11 ± 0.27 |
| Mayo score | 5 (3-5) | 8 (6-10) | 11.5 (11.0-12.0) |
Values are given as the mean ± SD if they follow a normal distribution, otherwise given as medians (minimum and maximum). ALB: Albumin; ALP: Alkaline phosphatase; Ca: Calcium; CRP: C-reactive protein; ESR: Erythrocyte sedimentation rate; HGB: Hemoglobin; P: Phosphorus; PLT: Platelet; UC: Ulcerative colitis.
Serum 25 (OH) D level was associated with UC disease activity
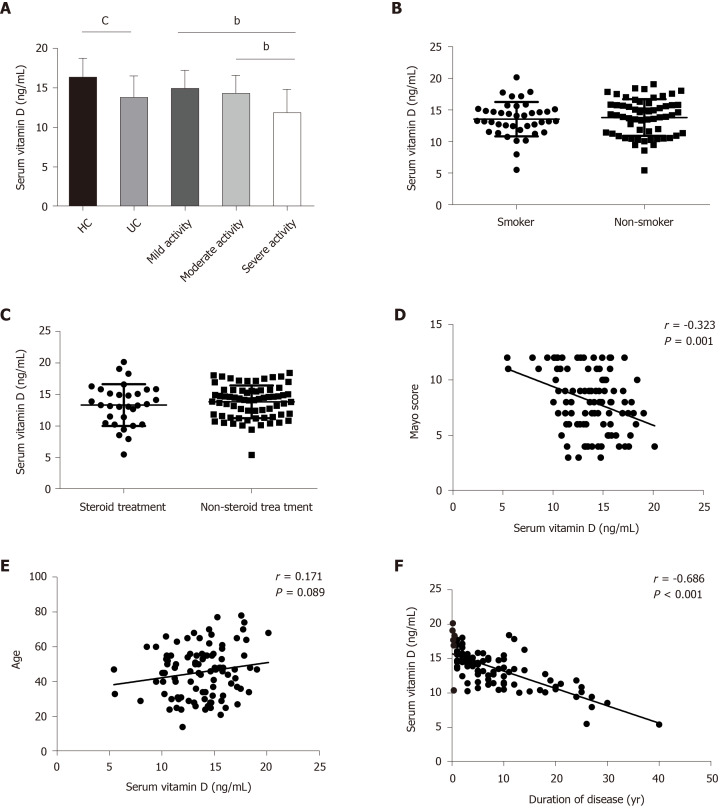
Mean serum vitamin D level in UC patients was decreased (13.7 ± 2.8 ng/mL) compared with that in healthy controls (16.2 ± 2.5 ng/mL) (P < 0.001; Figure 2A). UC patients with mild disease activity had a significantly higher vitamin D level than those with severe disease activity (14.8 ± 2.4 ng/mL vs 11.7 ± 3.1 ng/mL, P = 0.001), and this relationship was also found between those with moderate and severe disease activity (14.4 ± 2.4 ng/mL vs 11.7 ± 3.1 ng/mL, P = 0.003; Figure 2A). UC patients who were smokers, showed a similar vitamin D level to non-smokers (13.5 ± 2.7 ng/mL vs 13.8 ± 2.9 ng/mL; Figure 2B). In addition, we noted that serum vitamin D level in UC patients was not associated with steroid or non-steroid therapy (13.3 ± 3.3 ng/mL vs 13.8 ± 2.6 ng/mL; Figure 2C). Furthermore, serum vitamin D level was inversely correlated with disease activity as defined by the total Mayo score in UC patients (r = -0.323, P = 0.001; Figure 2D). No close relationship between serum vitamin D and the age of UC patients was observed (r = 0.171, P = 0.089; Figure 2E), in contrast to the negative correlation observed between serum vitamin D and duration of disease (r = -0.686, P < 0.001; Figure 2F).
Figure 2.
Vitamin D status in healthy controls and ulcerative colitis patients and its relationship with disease activity, age and duration of disease. A: Serum levels of vitamin D in control and ulcerative colitis (UC) patients (control: 56; UC 100; mild activity: 29; moderate activity: 32; severe activity: 39); B: Serum vitamin D levels in smokers (13.5 ± 2.7 ng/mL, n = 40) and non-smokers (13. 8 ± 2.9 ng/mL, n = 60); C: Serum vitamin D levels in patients receiving steroids (13.3 ± 3.3 ng/mL, n = 31) and non-steroids (13.8 ± 2.6 ng/mL, n = 69); D: Correlation between vitamin D level and the Mayo score in patients with UC; E: Correlation between vitamin D level and age in patients with UC; F: Correlation between vitamin D level and duration of disease in patients with UC. bP < 0.01 and cP < 0.001.
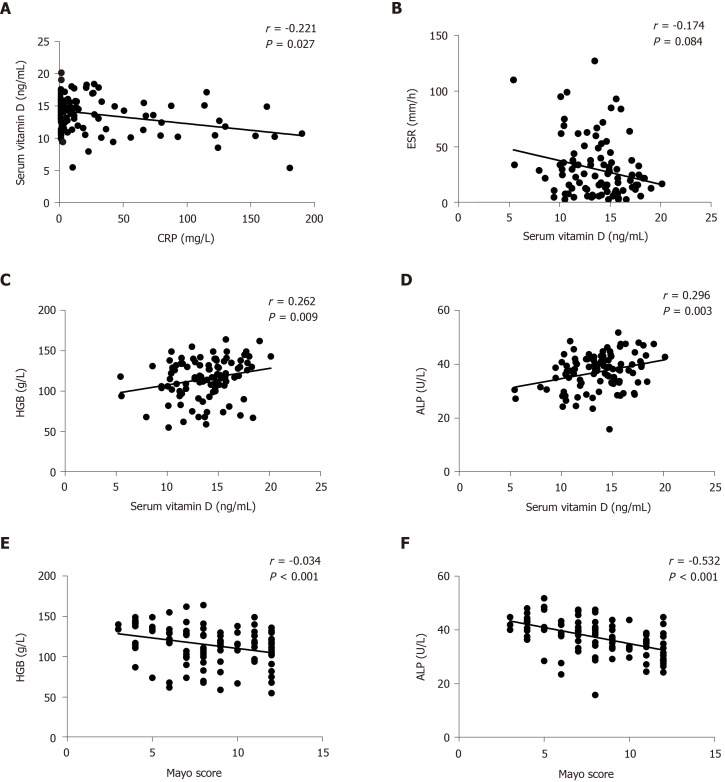
Serum vitamin D level was negatively correlated with CRP but positively correlated with HGB and ALP
We applied Spearman’s r to investigate the relationship between serum vitamin D level and inflammatory markers such as CRP, ESR, HGB and ALP. As expected, vitamin D level was inversely correlated with CRP (r = -0.221, P = 0.027; Figure 3A) but not with ESR in UC patients (r = -0.174, P = 0.084; Figure 3B). In contrast, vitamin D level was positively correlated with HGB (r = 0.262, P = 0.009; Figure 3C) and ALP (r = 0.296, P = 0.003; Figure 3D) levels. Furthermore, the Mayo score was negatively correlated with HGB (r = -0.334, P < 0.001; Figure 3E) and ALP (r = -0.532, P < 0.001; Figure 3F) levels.
Figure 3.
Correlation between vitamin D level and C-reactive protein, erythrocyte sedimentation rate, hemoglobin and alkaline phosphatase. A: Correlation between vitamin D level and C-reactive protein; B: Correlation between vitamin D level and erythrocyte sedimentation rate; C: Correlation between vitamin D level and hemoglobin; D: Correlation between vitamin D level and alkaline phosphatase; E: Correlation between hemoglobin and the Mayo score; and F: Correlation between alkaline phosphatase and the Mayo score. ALP: Alkaline phosphatase; CRP: C-reactive protein; ESR: Erythrocyte sedimentation rate; HGB: Hemoglobin.
VDR expression was inversely correlated with disease activity in colonic epithelial cells of UC patients
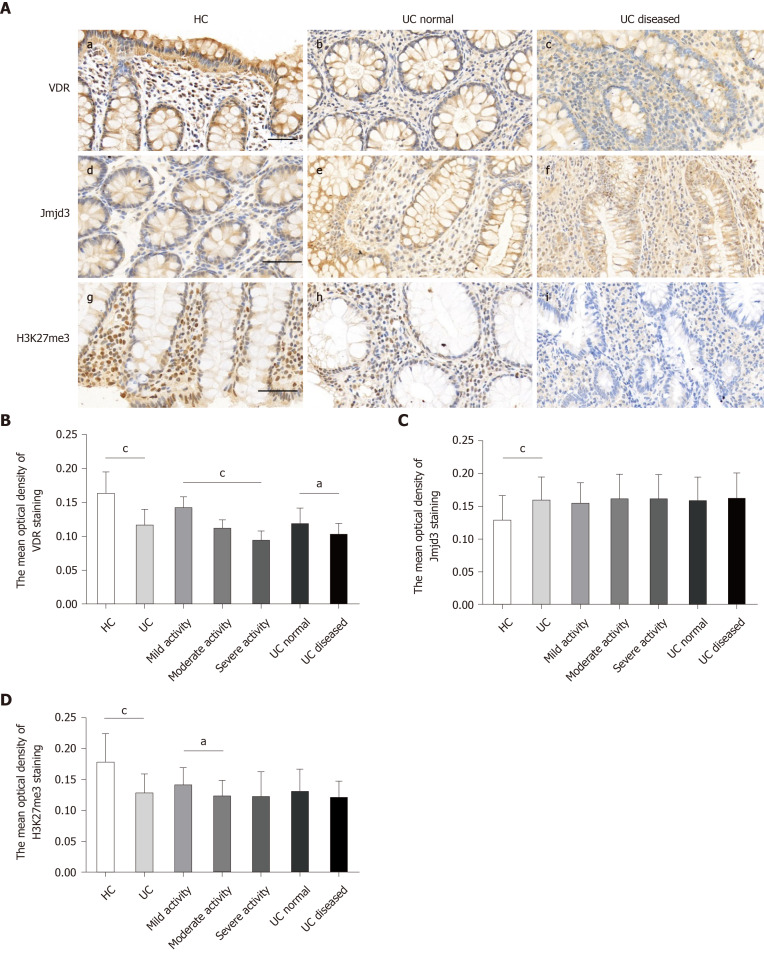
Immunohistochemical examination of VDR was performed on paraffin sections of colon biopsy specimens from 56 UC patients (16 cases with mild, 27 with moderate and 13 with severe disease activity) and 22 healthy controls. The results showed that VDR was abundantly expressed in the mucosa, glandular ducts and inflammatory cells in all samples (Figure 4A). VDR expression in UC diseased (c) was lower than that in the control (a) and UC normal (b). The mean optical density of VDR staining in UC diseased markedly decreased in comparison with the UC normal and control (Figure 4B). The abundance of VDR expression was found to decrease in patients with mild, moderate and severe disease activity in turn (Figure 4B).
Figure 4.
Vitamin D receptor and trimethylated H3 lysine 27 expression significantly decreased and Jumonji domain-containing 3 increased in the inflamed mucosa of ulcerative colitis patients. A: Vitamin D receptor was determined by immunostaining for control mucosa (a), for ulcerative colitis (UC) normal (b), and for UC diseased (c). Jumonji domain-containing 3 was determined by immunostaining for control mucosa (d), for UC normal (e) and for UC diseased (f). Trimethylated H3 lysine 27 was determined by immunostaining for control mucosa (g), for UC normal (h), and for UC diseased (i); B: The mean optical density of vitamin D receptor staining in colonic mucosal specimens from controls (n = 22) and UC (n = 56), including UC with mild activity (n = 16), moderate activity (n = 27) and severe activity (n = 13) and UC normal (n = 18) and UC diseased (n = 18); C: The mean optical density of Jumonji domain-containing 3 staining; D: The mean optical density of trimethylated H3 lysine 27 staining. Data, mean ± standard deviation. Original magnification, ´400. Scale bars = 50 μm. aP < 0.05 and cP < 0.001. H3K27me3: Trimethylated H3 lysine 27; Jmjd3: Jumonji domain-containing 3; UC: Ulcerative colitis; VDR: Vitamin D receptor.
Jmjd3 expression increased while H3K27me3 decreased in UC patients
As seen in the immunohistochemical detection of VDR, the expression of Jmjd3 and H3K27me3 was examined in UC patients and healthy controls. Jmjd3 and H3K27me3 were specifically expressed in the mucosal surface, glandular ducts and inflammatory cells in all biopsies. Jmjd3 expression was elevated in UC patients (e, f) compared with healthy controls (d) (Figure 4C). However, there were no significant differences in Jmjd3 expression among the mild, moderate and severe disease activity groups (Figure 4C). H3K27me3 expression in UC patients distinctly declined in comparison with normal controls (g) (Figure 4D) but was notably higher in the mild disease activity group than that in the moderate disease activity group (Figure 4D). However, significant high expression of H3K27me3 was not seen in UC normal (h) and in UC diseased (i).
VDR expression was negatively correlated with Jmjd3 but positively correlated with H3K27me3
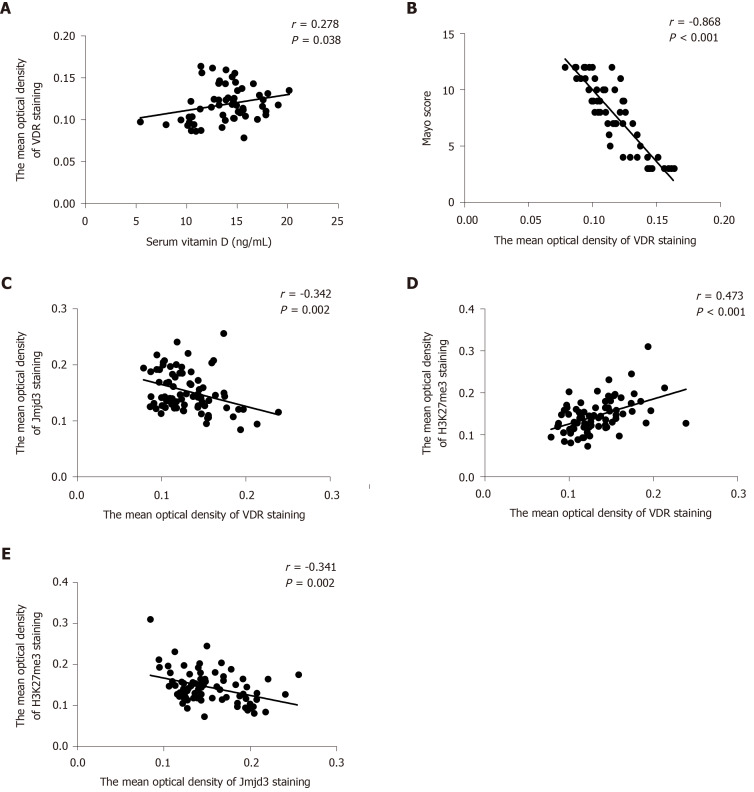
To analyze the correlation of VDR with Jmjd3 and H3K27me3, we compared the expression levels of the three molecules. It was found that VDR expression in colonic mucosa was positively related to the serum vitamin D level in all UC patients and healthy controls (r = 0.278, P = 0.038; Figure 5A) but negatively correlated with the Mayo score in all participants (r = -0.868, P < 0.001; Figure 5B). Statistical analysis showed that elevated expression of VDR was observed with decreased Jmjd3 expression (r = -0.342, P = 0.002) and with increased H3K27me3 staining (r = 0.473, P < 0.001; Figure 5C and D). Accordingly, a significant negative correlation was found between Jmjd3 and H3K27me3 (r = -0.341, P = 0.002; Figure 5E).
Figure 5.
Vitamin D receptor expression was negatively correlated with Jumonji domain-containing 3 and positively correlated with trimethylated H3 lysine 27 Levels. A: Correlation between vitamin D level and vitamin D receptor (VDR); B Correlation between the Mayo score and VDR; C: Correlation between VDR and Jumonji domain-containing 3; D: Correlation between VDR and trimethylated H3 lysine 27; and E: Correlation between trimethylated H3 lysine 27 and Jumonji domain-containing 3 H3K27me3: Trimethylated H3 lysine 27; Jmjd3: Jumonji domain-containing 3; VDR: Vitamin D receptor.
DISCUSSION
In this study, we demonstrated a lower level of serum vitamin D in patients with UC compared with healthy controls, and the level of serum vitamin D was inversely associated with disease activity. Our results also showed that the expression of VDR and H3K27me3 in the colonic mucosa of UC patients was synchronously decreased, while Jmjd3 expression was increased. Importantly, a significant negative correlation was noted between VDR and Jmjd3 expression, and this relationship was also observed between Jmjd3 and H3K27me3. These findings indicated that vitamin D and VDR were inversely associated with disease activity in active UC in which both Jmjd3 and H3K27me3 were probably involved.
Vitamin D deficiency is common in patients with IBD, probably due to malabsorption and intestinal inflammation that influence vitamin D absorption. Therefore, vitamin D deficiency may be closely associated with the occurrence of IBD, although the causal relationship between them is not fully clarified. A potential link between the pathogenesis of IBD and vitamin D deficiency is supported by laboratory studies. For example, vitamin D has been shown to induce the production of antimicrobial peptides by activating Toll-like receptors[24]. Other studies have reported that vitamin D promotes the proliferation of T-helper 2 cells and inhibits T-helper 1 cells[25,26]. In experimental colitis models, vitamin D or VDR deficiency leads to increased severity of colitis[27,28]. In addition, increasing evidence has demonstrated that low vitamin D level is inversely correlated with disease activity in patients with IBD, even in newly diagnosed patients[12,29,30]. A multiyear cohort study showed that low serum vitamin D level increased disease activity in IBD patients[8]. A cohort study of 70 UC patients also indicated that vitamin D level was negatively associated with endoscopic and histologic inflammation severity during clinical remission[9]. Taken together, these findings indicate that low vitamin D may increase the risk of IBD. Consistent with previous studies, our study also indicated that there is an inverse correlation between vitamin D level and disease activity in UC. Furthermore, we observed that serum vitamin D level was inversely related to CRP level in patients with active UC. Interestingly, it was observed that HGB and ALP levels were positively correlated with vitamin D level and inversely correlated with disease activity, suggesting that HGB and ALP might contribute to the amelioration of disease activity. However, it is still unknown how the age of the patient and duration of UC change the mechanisms of vitamin D in the body. Our findings suggested that disease duration was directly correlated with lower vitamin D level in UC, which may be caused by the decrease of absorption. In addition, UC patients who smoked had a similar vitamin D level to UC patients who did not smoke. These data suggest that vitamin D may not be a risk factor for UC, which is consistent with a previous study[31].
VDR is widely expressed in intestinal mucosal cells[6]. Vitamin D exerts its biological effect mainly by acting on VDR. Although the expression of VDR has been reported to be decreased in patients with UC, the association between VDR and disease activity is still controversial. Wada et al[32] found that VDR expression in UC was significantly lower than that in healthy controls. An additional study also showed that the expression of VDR in the colonic mucosa of UC patients was attenuated but not associated with disease duration and disease activity[33]. A negative correlation between VDR expression and disease activity was observed, but there was no significant variation in different intestinal segments in UC[13]. Our results showed that the expression of VDR in patients with UC significantly decreased in comparison with healthy controls. However, the role of VDR in the pathogenesis of IBD remains unclear. Of note, we found that the expression of VDR was negatively correlated with disease activity, and was higher in UC normal than in UC diseased lesions. In addition, the level of serum vitamin D was positively correlated with VDR. These results suggested that patients with active UC might have local intestinal VDR deficiency, resulting in intestinal inflammation, or reduced expression of VDR may be due to UC progression. VDR is believed to be a protective factor for UC.
In recent years, Jmjd3 has been found to play an important role in inflammation. Increased Jmjd3 can demethylate the repressive H3K27me3 epigenetic mark in pro-inflammatory genes and induced inflammatory responses[34,35]. Previous research indicated that Jmjd3 could be induced by NF-κB in response to lipopolysaccharide or other inflammatory stimuli in macrophages[36]. Jmjd3 knockdown can suppress the expression of inflammatory genes regulated by NF-κB[37]. In addition, STAT1 and STAT3 can facilitate inflammatory responses by promoting the expression of Jmjd3 in microglia[38]. A recent study indicated that blockage of Jmjd3 inhibited Nrf2 expression by increasing the enrichment of H3K27me3 on promotors of Nrf2, which disrupted nucleotide-binding domain-like receptors family pyrin domain-containing 3 inflammasome assembly and ameliorated the severity of dextran sulfate sodium-induced acute colitis in mice[18]. These studies suggest the potential role of Jmjd3 in pro-inflammatory responses and colitis through specific demethylation of H3K27me3. Interestingly, VDR was found to negatively regulate bacteria-stimulated NF-κB activity in the intestine[39]. Activating the vitamin D pathway could inhibit the activation of STAT1 and STAT3 and the production of inflammatory cytokines[21]. Therefore, the expression of Jmjd3 may be related to disease activity in patients with UC. The association between VDR and the Jmjd3 pathway in clinical patients with UC requires further investigation.
The association of Jmjd3 and H3K27me3 with disease activity in UC patients has not been elucidated. The relationship between VDR and Jmjd3 in the colonic mucosa of UC patients is still unknown. In this context, the relationship between VDR and Jmjd3 was examined in the present study. We found that Jmjd3 was significantly lower in healthy controls than in UC patients, while H3K27me3 was decreased in UC patients. However, there was no correlation between the expression of Jmjd3 and H3K27me3 and disease activity. Jmjd3 and H3K27me3 expression was not significantly different between UC diseased and UC normal. Furthermore, the levels of VDR and H3K27me3 in intestinal mucosa were inversely correlated with Jmjd3. In summary, Jmjd3 and H3K27me3 may be related to the occurrence of UC but not to disease activity. The expression of Jmjd3 and H3K27me3 may be affected by VDR. However, further research on the relationship between VDR and the Jmjd3 pathway is necessary.
The purpose of this study was to investigate the relationship between VDR, Jmjd3 and H3K27me3. However, there are several limitations in the present study. Firstly, although all patients were local residents, confounders such as season, current therapy and sunlight time affect the level of serum vitamin D. In addition, due to the limitation of sample size, VDR, Jmjd3 and H3K27me3 were measured only by immunohistochemistry, which may not be sufficient to provide a strong causality.
CONCLUSION
Vitamin D level and VDR expression decreased in active ulcerative colitis, and the patients with UC were found to have increased expression of Jmjd3 and decreased expression of H3K27me3. The results strongly suggest a negative correlation of vitamin D and VDR with UC in which both Jmjd3 and H3K27me3 are involved.
ARTICLE HIGHLIGHTS
Research background
Vitamin D has been proved to be associated with the pathogenesis of ulcerative colitis (UC) due to its role in regulating immunity. Increased Jumonji domain-containing 3 (Jmjd3) can aggravate colitis in mice by demethylating repressive trimethylated H3 lysine 27 (H3K27me3). However, it is unknown whether Jmjd3 and H3K27me3 are associated with the pathogenesis of UC, and the association between vitamin D receptor (VDR) and the Jmjd3 pathway remains unclear.
Research motivation
There is limited evidence on the influence of vitamin D on disease activity in Chinese patients with active UC. The expression of Jmjd3 and H3K27me3 in the intestinal mucosa of patients with UC has not been studied. Research on the relationship between VDR and the Jmjd3 is helpful to understand the possible pathogenesis of UC.
Research objectives
To investigate the expression of serum vitamin D, VDR, Jmjd3 and H3K27me3 in UC patients and controls, and to determine the correlation between VDR and the Jmjd3 pathway.
Research methods
In this study, 100 patients with active UC and 56 healthy controls were enrolled. Serum C-reactive protein and alkaline phosphatase levels were determined by immunoturbidimetry. White blood cell and hemoglobin were detected using a Sysmex XN-9000 automatic hematology analyzer. The content of serum vitamin D was determined by radioimmunoassay. The expression of VDR, Jmjd3 and H3K27me3 in intestinal mucosa was detected by immunohistochemistry.
Research results
Patients with active UC had lower levels of vitamin D than controls. Serum vitamin D level was negatively correlated with disease activity in the UC cohort. VDR expression in the mucosa of UC patients was reduced compared with normal tissues and negatively correlated with disease activity. Simultaneously, Jmjd3 expression increased, while H3K27me3 decreased in UC patients. The Jmjd3 level was negatively correlated with the level of VDR and H3K27me3, while the VDR level was positively correlated with H3K27me3 in all subjects.
Research conclusions
This report outlines an inverse correlation of vitamin D and VDR with disease activity in active UC patients in which both Jmjd3 and H3K27me3 are presumedly participated.
Research perspectives
More quantitative measures will be performed for detecting VDR, Jmjd3 and H3K27me3 in UC patients. Clinical trials of vitamin D supplementation should be carried out, which will contribute to a better understanding of the effect of vitamin D on UC.
ACKNOWLEDGEMENTS
We are grateful to the experimental platform of the Key Laboratory of Environmental Toxicology of Anhui Higher Education Institution. We are grateful to the Digestive Endoscope Center of the First Affiliated Hospital of Anhui Medical University for providing a facility for specimen collection.
Footnotes
Institutional review board statement: The study was approved by the ethics committee of the First Affiliated Hospital of Anhui Medical University (No. PJ2019-14-23).
Informed consent statement: All patients gave informed consent.
Conflict-of-interest statement: None of the authors has any conflicts of interest to declare.
STROBE statement: The authors have read the STROBE Statement-checklist of items, and the manuscript was prepared and revised according to the STROBE Statement-checklist of items.
Manuscript source: Unsolicited manuscript
Peer-review started: September 27, 2020
First decision: October 17, 2020
Article in press: November 4, 2020
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Eyles D, Pallav K S-Editor: Zhang L L-Editor: Filipodia P-Editor: Li JH
Contributor Information
Hong-Qian Wang, Department of Gastroenterology, The First Affiliated Hospital of Anhui Medical University, Hefei 230022, Anhui Province, China.
Wen-Hui Zhang, Department of Gastroenterology, The First Affiliated Hospital of Anhui Medical University, Hefei 230022, Anhui Province, China.
Ya-Qi Wang, Department of Gastroenterology, The First Affiliated Hospital of Anhui Medical University, Hefei 230022, Anhui Province, China.
Xiao-Pan Geng, Department of Gastroenterology, The First Affiliated Hospital of Anhui Medical University, Hefei 230022, Anhui Province, China.
Ming-Wei Wang, Department of Gastroenterology, The First Affiliated Hospital of Anhui Medical University, Hefei 230022, Anhui Province, China.
Yuan-Yuan Fan, Department of Gastroenterology, The First Affiliated Hospital of Anhui Medical University, Hefei 230022, Anhui Province, China.
Jing Guan, Department of Gastroenterology, The First Affiliated Hospital of Anhui Medical University, Hefei 230022, Anhui Province, China.
Ji-Long Shen, Department of Pathogen Biology, Anhui Medical University, Hefei 230032, Anhui Province, China.
Xi Chen, Department of Gastroenterology, The First Affiliated Hospital of Anhui Medical University, Hefei 230022, Anhui Province, China. ayfychenxi@163.com.
Data sharing statement
No additional data are available.
References
- 1.Carter MJ, Lobo AJ, Travis SP IBD Section; British Society of Gastroenterology. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2004;53 Suppl 5:V1–V16. doi: 10.1136/gut.2004.043372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–317. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atreya R, Neurath MF. IBD pathogenesis in 2014: Molecular pathways controlling barrier function in IBD. Nat Rev Gastroenterol Hepatol. 2015;12:67–68. doi: 10.1038/nrgastro.2014.201. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen OH, Rejnmark L, Moss AC. Role of Vitamin D in the Natural History of Inflammatory Bowel Disease. J Crohns Colitis. 2018;12:742–752. doi: 10.1093/ecco-jcc/jjy025. [DOI] [PubMed] [Google Scholar]
- 5.Cava RC, Javier AN. Vitamin D deficiency. N Engl J Med. 2007;357:1981; author reply 1981–1981; author reply 1982. [PubMed] [Google Scholar]
- 6.Wang Y, Zhu J, DeLuca HF. Where is the vitamin D receptor? Arch Biochem Biophys. 2012;523:123–133. doi: 10.1016/j.abb.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto E, Jørgensen TN. Immunological effects of vitamin D and their relations to autoimmunity. J Autoimmun. 2019;100:7–16. doi: 10.1016/j.jaut.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Kabbani TA, Koutroubakis IE, Schoen RE, Ramos-Rivers C, Shah N, Swoger J, Regueiro M, Barrie A, Schwartz M, Hashash JG, Baidoo L, Dunn MA, Binion DG. Association of Vitamin D Level With Clinical Status in Inflammatory Bowel Disease: A 5-Year Longitudinal Study. Am J Gastroenterol. 2016;111:712–719. doi: 10.1038/ajg.2016.53. [DOI] [PubMed] [Google Scholar]
- 9.Gubatan J, Mitsuhashi S, Zenlea T, Rosenberg L, Robson S, Moss AC. Low Serum Vitamin D During Remission Increases Risk of Clinical Relapse in Patients With Ulcerative Colitis. Clin Gastroenterol Hepatol 2017; 15: 240-246. :e1. doi: 10.1016/j.cgh.2016.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wigg AJ, Chin JK, Muller KR, Ramachandran J, Woodman RJ, Kaambwa B. Cost-effectiveness of a chronic disease management model for cirrhosis: Analysis of a randomized controlled trial. J Gastroenterol Hepatol. 2018::. doi: 10.1111/jgh.14127. [DOI] [PubMed] [Google Scholar]
- 11.Leyssens C, Verlinden L, De Hertogh G, Kato S, Gysemans C, Mathieu C, Carmeliet G, Verstuyf A. Impact on Experimental Colitis of Vitamin D Receptor Deletion in Intestinal Epithelial or Myeloid Cells. Endocrinology. 2017;158:2354–2366. doi: 10.1210/en.2017-00139. [DOI] [PubMed] [Google Scholar]
- 12.Abreu-Delgado Y, Isidro RA, Torres EA, González A, Cruz ML, Isidro AA, González-Keelan CI, Medero P, Appleyard CB. Serum vitamin D and colonic vitamin D receptor in inflammatory bowel disease. World J Gastroenterol. 2016;22:3581–3591. doi: 10.3748/wjg.v22.i13.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garg M, Royce SG, Tikellis C, Shallue C, Sluka P, Wardan H, Hosking P, Monagle S, Thomas M, Lubel JS, Gibson PR. The intestinal vitamin D receptor in inflammatory bowel disease: inverse correlation with inflammation but no relationship with circulating vitamin D status. Therap Adv Gastroenterol. 2019;12:1756284818822566. doi: 10.1177/1756284818822566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang YG, Lu R, Xia Y, Zhou D, Petrof E, Claud EC, Sun J. Lack of Vitamin D Receptor Leads to Hyperfunction of Claudin-2 in Intestinal Inflammatory Responses. Inflamm Bowel Dis. 2019;25:97–110. doi: 10.1093/ibd/izy292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Z, Zang C, Rosenfeld JA, Schones DE, Barski A, Cuddapah S, Cui K, Roh TY, Peng W, Zhang MQ, Zhao K. Combinatorial patterns of histone acetylations and methylations in the human genome. Nat Genet. 2008;40:897–903. doi: 10.1038/ng.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou VW, Goren A, Bernstein BE. Charting histone modifications and the functional organization of mammalian genomes. Nat Rev Genet. 2011;12:7–18. doi: 10.1038/nrg2905. [DOI] [PubMed] [Google Scholar]
- 17.Burchfield JS, Li Q, Wang HY, Wang RF. JMJD3 as an epigenetic regulator in development and disease. Int J Biochem Cell Biol. 2015;67:148–157. doi: 10.1016/j.biocel.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang M, Wang Q, Long F, Di Y, Wang J, Zhun Zhu Y, Liu X. Jmjd3 regulates inflammasome activation and aggravates DSS-induced colitis in mice. FASEB J. 2020;34:4107–4119. doi: 10.1096/fj.201902200RR. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, Liu L, Yuan X, Wei Y, Wei X. JMJD3 in the regulation of human diseases. Protein Cell. 2019;10:864–882. doi: 10.1007/s13238-019-0653-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdo J, Rai V, Agrawal DK. Interplay of Immunity and Vitamin D: Interactions and Implications with Current IBD Therapy. Curr Med Chem. 2017;24:852–867. doi: 10.2174/0929867323666161026124951. [DOI] [PubMed] [Google Scholar]
- 21.Olson KC, Kulling Larkin PM, Signorelli R, Hamele CE, Olson TL, Conaway MR, Feith DJ, Loughran TP Jr. Vitamin D pathway activation selectively deactivates signal transducer and activator of transcription (STAT) proteins and inflammatory cytokine production in natural killer leukemic large granular lymphocytes. Cytokine. 2018;111:551–562. doi: 10.1016/j.cyto.2018.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pereira F, Barbáchano A, Singh PK, Campbell MJ, Muñoz A, Larriba MJ. Vitamin D has wide regulatory effects on histone demethylase genes. Cell Cycle. 2012;11:1081–1089. doi: 10.4161/cc.11.6.19508. [DOI] [PubMed] [Google Scholar]
- 23.Pereira F, Barbáchano A, Silva J, Bonilla F, Campbell MJ, Muñoz A, Larriba MJ. KDM6B/JMJD3 histone demethylase is induced by vitamin D and modulates its effects in colon cancer cells. Hum Mol Genet. 2011;20:4655–4665. doi: 10.1093/hmg/ddr399. [DOI] [PubMed] [Google Scholar]
- 24.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zügel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 25.Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O'Garra A. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol. 2001;167:4974–4980. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- 26.Sloka S, Silva C, Wang J, Yong VW. Predominance of Th2 polarization by vitamin D through a STAT6-dependent mechanism. J Neuroinflammation. 2011;8:56. doi: 10.1186/1742-2094-8-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palmer MT, Weaver CT. Linking vitamin d deficiency to inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:2245–2256. doi: 10.1097/MIB.0b013e31828a3b6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Froicu M, Zhu Y, Cantorna MT. Vitamin D receptor is required to control gastrointestinal immunity in IL-10 knockout mice. Immunology. 2006;117:310–318. doi: 10.1111/j.1365-2567.2005.02290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jørgensen SP, Hvas CL, Agnholt J, Christensen LA, Heickendorff L, Dahlerup JF. Active Crohn's disease is associated with low vitamin D levels. J Crohns Colitis. 2013;7:e407–e413. doi: 10.1016/j.crohns.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 30.Leslie WD, Miller N, Rogala L, Bernstein CN. Vitamin D status and bone density in recently diagnosed inflammatory bowel disease: the Manitoba IBD Cohort Study. Am J Gastroenterol. 2008;103:1451–1459. doi: 10.1111/j.1572-0241.2007.01753.x. [DOI] [PubMed] [Google Scholar]
- 31.Piovani D, Danese S, Peyrin-Biroulet L, Nikolopoulos GK, Lytras T, Bonovas S. Environmental Risk Factors for Inflammatory Bowel Diseases: An Umbrella Review of Meta-analyses. Gastroenterology 2019; 157: 647-659. :e4. doi: 10.1053/j.gastro.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 32.Wada K, Tanaka H, Maeda K, Inoue T, Noda E, Amano R, Kubo N, Muguruma K, Yamada N, Yashiro M, Sawada T, Nakata B, Ohira M, Hirakawa K. Vitamin D receptor expression is associated with colon cancer in ulcerative colitis. Oncol Rep. 2009;22:1021–1025. doi: 10.3892/or_00000530. [DOI] [PubMed] [Google Scholar]
- 33.Coskun A, Yavasoglu I, Meteoglu I, Unubol M, Yasar B, Borazan S, Omurlu IK, Yukselen V, Yasa MH. Vitamin D Receptor Level in Biopsy Specimen of Patients with Ulcerative Colitis: Results from a Center in Western Anatolia. J Natl Med Assoc. 2018;110:276–280. doi: 10.1016/j.jnma.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Bosselut R. Pleiotropic Functions of H3K27Me3 Demethylases in Immune Cell Differentiation. Trends Immunol. 2016;37:102–113. doi: 10.1016/j.it.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agger K, Cloos PA, Christensen J, Pasini D, Rose S, Rappsilber J, Issaeva I, Canaani E, Salcini AE, Helin K. UTX and JMJD3 are histone H3K27 demethylases involved in HOX gene regulation and development. Nature. 2007;449:731–734. doi: 10.1038/nature06145. [DOI] [PubMed] [Google Scholar]
- 36.De Santa F, Totaro MG, Prosperini E, Notarbartolo S, Testa G, Natoli G. The histone H3 Lysine-27 demethylase Jmjd3 Links inflammation to inhibition of polycomb-mediated gene silencing. Cell. 2007;130:1083–1094. doi: 10.1016/j.cell.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 37.Das ND, Jung KH, Choi MR, Yoon HS, Kim SH, Chai YG. Gene networking and inflammatory pathway analysis in a JMJD3 knockdown human monocytic cell line. Cell Biochem Funct. 2012;30:224–232. doi: 10.1002/cbf.1839. [DOI] [PubMed] [Google Scholar]
- 38.Przanowski P, Dabrowski M, Ellert-Miklaszewska A, Kloss M, Mieczkowski J, Kaza B, Ronowicz A, Hu F, Piotrowski A, Kettenmann H, Komorowski J, Kaminska B. The signal transducers Stat1 and Stat3 and their novel target Jmjd3 drive the expression of inflammatory genes in microglia. J Mol Med (Berl) 2014;92:239–254. doi: 10.1007/s00109-013-1090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu S, Liao AP, Xia Y, Li YC, Li JD, Sartor RB, Sun J. Vitamin D receptor negatively regulates bacterial-stimulated NF-kappaB activity in intestine. Am J Pathol. 2010;177:686–697. doi: 10.2353/ajpath.2010.090998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.