Abstract
Hormonal contraceptives (HCs) affect various processes related to emotion processing, including emotional memory, fear extinction, and the cortisol response to stress. Despite the modulating role of HCs on the stress response in women and variance in synthetic hormone levels across the HC cycle, little is known about the phase-related effects of HCs on the brain's response to stress. We investigated the effect of HC cycle phase on functional connectivity of memory- and emotion-related regions at rest after exposure to a stressor. Twenty HC users completed two sessions of resting-state functional magnetic resonance imaging after exposure to the cold pressor test, one during the hormone-present HC phase (when synthetic hormones are taken) and one during the hormone-absent HC phase (when synthetic hormones are not taken). Women showed higher functional connectivity between left amygdala and ventromedial prefrontal cortex during the hormone-present phase. During the hormone-absent phase, women showed higher coupling between left parahippocampus and right superior lateral occipital cortex. Our results suggest that the synthetic hormones contained in HCs may protect against the negative effects of stress on functional connectivity of emotional processing regions.
Keywords: Hormonal contraceptives, Stress, Amygdala, Parahippocampus, vmPFC, Resting-state functional connectivity
1. Introduction
Stressful situations happen almost every day. Unhealthy levels of stress can lead to various physical and mental disorders (Bale, 2006; Schneiderman et al., 2005). Emotion regulation, “the process by which individuals influence which emotions they have, when they have them, and how they experience these emotions” (Gross, 1998), helps maintain physical and mental well-being in the presence of stressors. Emotion regulation and stress influence common neural circuits. Acute stress activates the neuroendocrine stress response system, the hypothalamic-pituitary-adrenal axis, which triggers the release of glucocorticoids (e.g., cortisol in humans). Glucocorticoids affect neural correlates of emotion regulation and stress reactivity by binding to specific receptors in regions associated with emotion regulation, including medial prefrontal cortex (mPFC), hippocampus, and amygdala (De Kloet et al., 2005; De Kloet, 2004). These brain regions, in turn, regulate glucocorticoid release (Dedovic et al., 2009; Herman et al., 2005). For instance, cortisol modulates amygdala functional connectivity (FC) after stress (Chang and Yu, 2018; Clewett et al., 2013; Fan et al., 2015; van Marle et al., 2010; Veer et al., 2011) and affects activation and metabolism of mPFC, amygdala, and hippocampus (Jentsch et al., 2019; Kern et al., 2008). Furthermore, changes in cortisol levels also affect functional connectivity of medial temporal lobe memory regions, such as hippocampus and parahippocampus (Shields et al., 2019).
Sex hormones exert influence over cortisol release and also modulate brain regions associated with stress and emotion regulation. Sex differences in the stress response and in affective processing have been previously demonstrated (Beckmann et al., 2005; Goldstein et al., 2010; Kajantie and Phillips, 2006). Men show higher cortisol responses to stressors and have lower recall of negative emotional memories (Buchanan and Tranel, 2008; Kajantie and Phillips, 2006). Moreover, higher baseline cortisol levels are associated with stronger amygdala-mPFC functional connectivity in men, while women show the opposite association (Kogler et al., 2016). Compared to non-stressful conditions, men also show altered resting-state functional connectivity of hippocampus and parahippocampus (Shields et al., 2019) in response to stress. Menstrual cycle phase and ovarian hormone levels also affect the stress response in women. During the high estradiol phases of the menstrual cycle, women show lower stress reactivity in mPFC, amygdala, and hippocampus compared to both men and low estradiol phases of the menstrual cycle (Goldstein et al, 2005, 2010; Jacobs et al., 2015). Higher estradiol levels within the same menstrual cycle phase is also associated with lower reported distress after stress (Albert et al., 2015).
Used to prevent pregnancy and suppress ovulation, hormonal contraceptives (HCs) modify the sex hormone fluctuations characteristic of the natural menstrual cycle. As with changes in hormone levels across the menstrual cycle, the different hormone levels induced by HC use are associated with differences in cognitive processes related to emotion processing, including emotional memory and fear extinction (Montoya and Bos, 2017; Nielson et al., 2011), and the cortisol response to stress (Mordecai et al., 2017; Rohleder et al., 2003). Specifically, HC users show blunted free cortisol responses to stress (Rohleder et al., 2003), suppressed amygdala reactivity to negative stimuli (Petersen and Cahill, 2015), and altered hippocampal reactivity to emotional information in stressful situations (Rohleder et al., 2003). Although research indicates that HCs modulate the stress response in women, little is known about the phase-related effects of HCs on stress. This is mainly because the majority of studies comparing HC-users to non-users rarely report HC cycle position, whereas menstrual cycle phase is controlled and reported.
Previous studies suggest HC cycle position affects brain activation patterns. For instance, resting state functional connectivity of the executive control network is reported to differ across the HC cycle (Petersen et al., 2014). In the current study, we investigated the effects of stress on resting state functional connectivity across the HC cycle. Specifically, we examined the effect of HC cycle phase on functional connectivity of brain regions important for emotional memory at rest after exposure to a stressor. Based on previous studies suggesting the influence of sex hormones on the stress response (Albert et al., 2015; Ossewaarde et al., 2010), we predicted resting state functional connectivity of monophasic HC users would differ after stress depending on the HC cycle phase. Due to the changes in emotional processing and emotional memory in HC users, we focused our analysis on the amygdala, hippocampus, and parahippocampus. We hypothesized that post-stress functional connectivity of the hippocampus, parahippocampal cortex, and amygdala would differ across the HC cycle. Based on previous findings in our lab (Clewett et al., 2013), we also hypothesized that functional connectivity of the amygdala would be associated with perceptions of pain and stress-related changes in mood in our participants.
2. Materials and methods
2.1. Participants
Twenty-four female participants aged 18-35 years-old were recruited for this study. Participants were using monophasic HCs for a minimum of 4 months before enrolling in the study. Accepted forms of monophasic HCs included combined oral contraceptives or vaginal rings containing seven hormone-absent days. Women being treated for any major chronic illness, using beta-blockers, corticosteroids, antidepressant, or other psychoactive drugs, who were pregnant or breastfeeding in the one year prior to participation, were left-handed, had known and reported claustrophobia, metal implants, or other contraindications for exposure to the MRI scanner, and any contraindications for cold pressor test exposure were excluded.
2.2. Cold pressor test and cortisol assays
A detailed description of cold pressor test application and cortisol measurement is provided in Herrera et al. (Herrera et al., 2020). In summary, sessions were conducted in the afternoons between 1200h and 1800h. Participants were instructed to avoid eating/drinking for 1 h, sleep for 3 h, and refrain from caffeine, alcohol and exercise for 24 h prior to their session. Multiple saliva samples were collected throughout each session. Here we report cortisol levels at baseline and at the time of the resting-state scan (39 min post-stress onset). Other hormonal results are discussed elsewhere (Herrera et al., 2020).
Free cortisol responses were induced at each session using the cold pressor test (CPT; Lovallo, 1975), which reliably increases free cortisol levels (Herrera et al., 2019; Nielsen et al., 2013, 2014). During CPT, participants were instructed to keep their dominant hand in ice water (0°–3 °C) for at least 1 minute and up to 3 minutes. Baseline free cortisol levels were assessed via passive drool outside of the MRI scanner. The post-CPT sample was collected using Salimetrics, LLC (State College, PA) oral collection swabs while in the MRI scanner. All samples were assayed in duplicate. Passive drool samples were processed using Salimetrics, LLC ELISA kits, without modifications to the manufacturers' protocol and measured optically using Molecular Devices, LLC SpectraMax M3 Multi-mode Microplate Reader (Sunnyvale, CA). Swab samples were frozen and shipped to Salimetrics' SalivaLab (Carlsbad, CA) and processed using Salimetrics Salivary Cortisol Assay Kit (Cat. No. 1–3002), without modifications to the manufacturers’ protocol. The inter- and intra-assay variations of cortisol were 7.9% and 6.9% for passive drools and 6.0% and 4.6% for oral collection swabs.
2.3. Image acquisition
Resting-state pulsed arterial spin labeling (PASL) perfusion images were acquired on a MAGNETOM PrismaFIT Scanner with Tim using a 32-channel matrix head coil at the Dana and David Dornsife Neuroscience Institute at the University of Southern California. To ensure high labeling efficiency, “proximal inversion with a control for off-resonance effects” (PICORE) mode was used (Luh et al., 1999). A total of 88 interleaved tag and control volumes along with one M0 image were acquired (axial slices = 20, TR/TE/TI = 4000/30/1800 ms; voxel size: 3.0 × 3.0 × 5.0 mm; 23 slices using a matrix size 64 × 64; flip angle = 90°; in-plane resolution = 3 × 3 mm2, slice thickness = 5 mm, bolus duration = 1675 ms; saturation thickness = 100 mm; imaging slab gap = 100 mm, acquisition time = 6 min and 10 s).
High-resolution anatomical images were collected using a high-resolution 3D MPRAGE sequence (slices = 176 coronal; TR/TE = 2,300/2.26 ms; FOV = 256 × 256 mm; in-plane resolution = 1 mm2; slice thickness = 1 mm with no gap; bandwidth = 200 Hz/Px, acquisition time = 4 min and 44 s).
2.4. Procedure
Participants attended two sessions. One session occurred during the hormone-present phase (Days 8–21; Day 1 = first day of new oral contraceptive pack or insertion of new vaginal ring) and one session occurred during the hormone-absent phase (Days 24–28; Day 22 = first hormone-absent day). Upon arrival, participants consumed an 8-oz bottle of water. The baseline saliva sample was collected after at least 10 min had elapsed since participants finished drinking the 8 oz of water (approximately 50 min prior to the stress task). Before starting the CPT, participants completed the Positive and Negative Affective Scale (PANAS; Watson et al., 1988) and the state measure of the State-Trait Anxiety Inventory (STAI; Beckler, 2010) questionnaires. Participants also rated the amount of pain they were currently feeling from none to the “worst possible pain” on a visual analog scale using 10-cm long lines immediately before immersing their hand in the ice water. Immediately after hand immersion, participants were again asked to rate the maximum amount of pain they felt while their “hand was in the water”. Participants provided another saliva sample during the resting-state ASL scan (39 min post-stress onset). After the scans, participants were taken out of the scanner and completed the PANAS and STAI questionnaires again. Throughout the session, participants provided additional saliva samples and completed n-back working memory and auditory oddball tasks that are not reported here.
2.5. Statistical analyses
We selected a within-subject design selected to reduce the effects of individual variability and also other factors such as synthetic hormone content; which can affect the magnitude of the cortisol response to stress (Herrera et al., 2019). The only previous work examining resting state functional connectivity across the HC cycle found a large effect (Petersen et al., 2014). Therefore, we selected a sample size of N = 20 to ensure sufficient power to detect moderate-to-large (d= .67) differences in brain activation patterns across the HC cycle (Faul et al., 2007).
Twenty women were included in the analyses (Mage = 23.45, SDage = 3.05; Myears of education = 15.90, SDyears of education = 2.10; MBMI = 22.83, SDBMI = 4.11). Four women were excluded from analyses: one for use of an antidepressant, one for early termination due to experiencing claustrophobia in the MRI bore, one for failing to continue beyond session 1, and one for missing too many days of her oral contraceptive requiring stopping the current pack and starting a new pack between sessions 1 and 2.
2.5.1. Cortisol, pain, and stress changes analyses
We conducted a 2 (HC cycle phase) x 2 (time: baseline and 39 min post CPT) repeated measure ANOVA to investigate the effect of CPT on the free cortisol response. Three participants were excluded from cortisol analysis. Two women didn't provide enough saliva in one of the time-points. The third woman was excluded from cortisol-related analyses because her baseline salivary cortisol levels exceeded 1.0 μg/dL.
2.5.2. Anxiety and mood changes analyses
In order to examine the effects of stress and HC cycle on anxiety and affect reports, we conducted two 2 (HC cycle phase) × 2 (rating time) repeated-measures ANOVAs on STAI and PANAS ratings. Post-hoc t-tests were performed to investigate the main effects of cycle phase and rating time.
2.6. Image processing
2.6.1. Preprocessing
FSL 6.0.1 (FMRIB's Software Library, www.fmrib.ox.ac.uk/fsl) was used to preprocess PASL images. The methods described below for preprocessing and data analysis were adapted and modified from Clewett et al. (2013).
The first volume (M0) was discarded and preprocessing steps were conducted on the 88 interleaved tag and control images. Preprocessing steps consisted of: motion correction using FMRIB's Linear Registration Tool (MCFLIRT) (Jenkinson et al., 2002a), exclusion of non-brain areas using FMRIB's Brain Extraction Tool (BET) (Jenkinson et al., 2002b), spatial smoothing of data using a 5 mm full-width-half-maximum Gaussian kernel, grand-mean intensity normalization of the entire 4-D data set by a single multiplicative factor, temporal highpass (sigma = 90.0s) and lowpass filtering (Gaussian filter = 1.4s). In addition to these steps, single-session independent component analysis (ICA) was performed to identify noise components (Beckmann et al., 2005). Components for which, signals were mainly located in ventricles or white matter, had erratic fluctuations in their power spectra, had rings of activation around the edge of the brain, or spikes in their principal eigenvector timeseries exceeding six scale units, were identified as noise and removed from the data. PASL images were registered to the individual T1-weighted structural images and then into the standard MNI space using a 12 degrees of freedom linear affine transformation in FLIRT (Jenkinson and Smith, 2001).
2.6.2. Functional connectivity analysis
Whole brain analysis. A seed-based correlation analysis was performed to assess the effects of HC phase on resting state functional connectivity of six seed regions: left and right amygdala, left and right parahippocampus, and left and right hippocampus. Left and right amygdala ROIs were defined using the Harvard–Oxford Atlas in FSL and masks were thresholded at 80%. The ROIs used for left and right parahippocampus and hippocampus were developed by Ritchey et al. (2015) and were used by Shields et al. (2019) to investigate the effect of stress on emotional memory. Since hippocampal analyses were exploratory, we decided to use masks of the entire hippocampus, rather than the individual hippocampal segments for our analysis to increase our analysis power to detect differences in functional connectivity across the HC cycle. Therefore, we combined masks of the hippocampus head, tail, and body for each hemisphere and created a single left/right hippocampus ROI. Mean timeseries for each of these seeds were then obtained for each participant's two sessions. FMRIB's Automated Segmentation Tool (FAST) (Zhang et al., 2001) was used to run segmentation on structural images and produce white matter (WM) and cerebrospinal fluid (CSF) masks. These CSF and WM masks were registered to functional space resolution (2 mm3) using the inverse transformation matrix created from registration of each structural T1 image to the related mean perfusion image. Mean timeseries then were extracted from these co-registered masks.
For each of the selected ROIs, a general linear model was created for each session of participants, using three regressors: the timeseries of each ROI, and WM and CSF timeseries as the nuisance signals. We used a modified version of FSL's full perfusion signal modelling to extract perfusion-only signal from control-tag image pairs (Mumford et al., 2006). In this model, the label and control image are considered the “ON” and “OFF” periods of a square waveform. The duration of each of these periods is equal to the duration of a TR. This model also includes BOLD nuisance signal as a regressor. BOLD signal is modeled as the mean of the tag and control volumes. The BOLD signal regressor is convolved with the square perfusion waveform using a standard hemodynamic response function (HRF) convolution. The first-level analysis results were then added to a second-level paired t-test group analysis. To control for multiple comparisons of the six seed regions, spatial maps were thresholded at a cluster size of Z > 3.1 and a whole-brain cluster-level FWE-corrected significance of p < 0.008.
2.6.3. Characterizing directionality of amygdala and parahippocampus connectivity
Post-hoc ROI analyses were conducted in order to determine the direction of functional connectivity between left amygdala/parahippocampus and significant clusters in the group level analyses for these seed regions. Small ROIs were created as 8 mm-diameter spheres in MNI spaces around the peak coordinates found in group level analyses. For amygdala analyses, the ROI sphere was centered on vmPFC (x = 0 y = 40 z = -20). For parahippocampus analyses, the ROI sphere was centered on right superior lateral occipital cortex (x = 12 y = -74 z = 54).
To measure the strength of connectivity between regions represented by beta values, we applied the binarized masks of ROIs to connectivity maps from first-level analyses. Significance of functional connectivity between these regions was determined using two-tailed single-sample t-tests of the beta values against zero within each HC cycle condition.
2.6.4. Associations between amygdala functional connectivity, pain, and mood outcomes
Pearson's correlations were used to investigate the association between amygdala functional connectivity values and changes in mood measures.
3. Results
3.1. Cortisol and behavioral results
CPT exposure significantly increased free cortisol levels from baseline to 39-min post-stress onset, F(1,16) = 11.79, p < 0.01. There was no effect of HC phase and the time × phase interaction was not significant.
Across the HC cycle, participants reported higher levels of state anxiety before the CPT compared to after MRI scanning, F(1,19) = 15.26, p = 0.01. There was no significant effect of phase or a phase × time interaction on reported state anxiety scores.
Negative and positive affect were not significantly affected by time, HC phase, or an HC phase × time interaction.
3.2. Amygdala functional connectivity analysis results
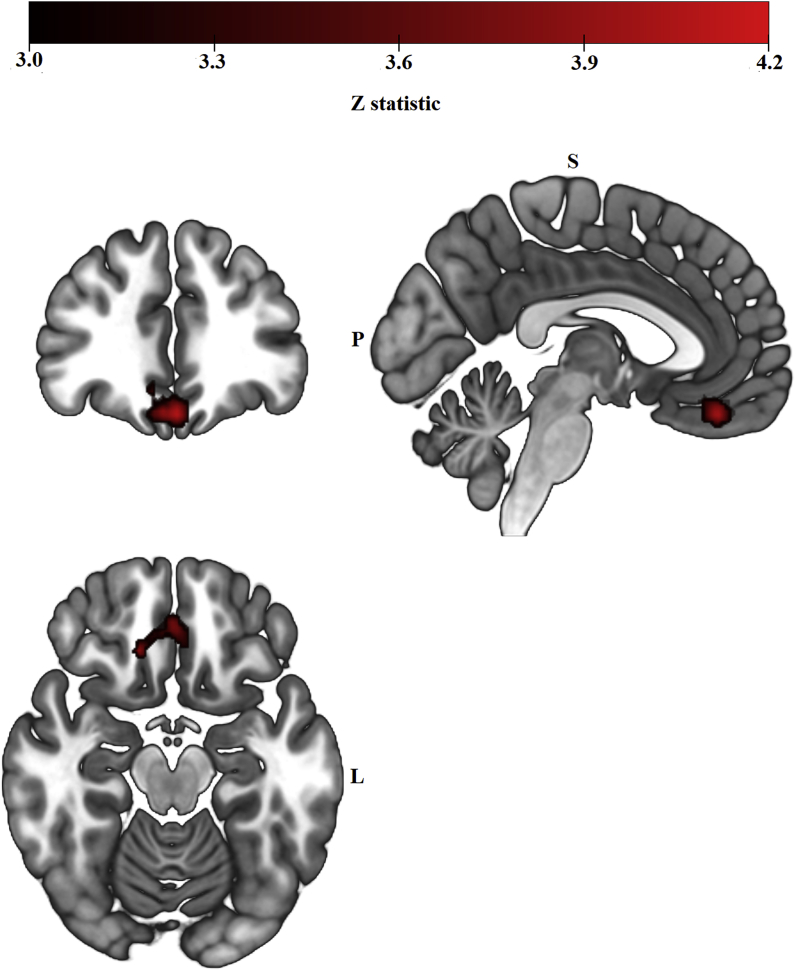
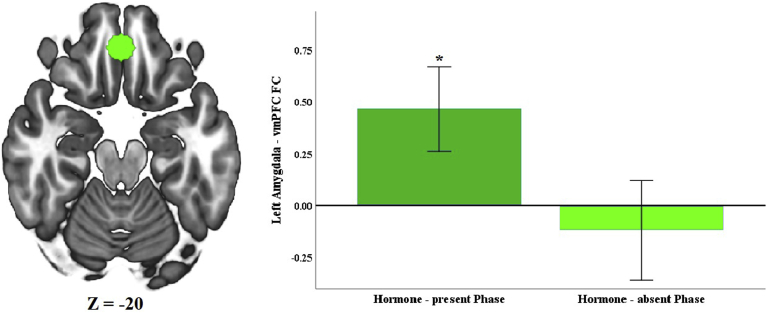
As hypothesized, post-stress left amygdala functional connectivity differed between the hormone-present and hormone-absent HC phases. Women showed greater post-stress left amygdala functional connectivity with a cluster of voxels with a peak in right vmPFC during the hormone-present versus hormone-absent HC phase (Fig. 1 and Table 1). Follow-up one sample t-tests demonstrated that in the hormone-present phase, women showed a significant positive coupling between left amygdala and right vmPFC (Fig. 2), t(19) = 2.782 p = 0.010). Women did not show a significant coupling during the hormone-absent phase. Right amygdala functional connectivity did not significantly differ between the two HC phases.
Fig. 1.
Left amygdala resting-state functional connectivity clusters from the group-level analysis (Hormone-present > Hormone-absent) contrast, cluster-level FWE-corrected at Z > 3.1, p < 0.008). During the hormone-present phase, women showed greater functional connectivity between left amygdala and a cluster of 283 voxels with a peak in right vmPFC.
Table 1.
List of significant clusters from the group-level seed-based resting-state functional connectivity analysis for each of the ROIs.
| Left Amygdala Functional Connectivity | ||||||
| Hormone-present > Hormone-absent | P-value | Z-Stat | X | Y | Z | Voxels |
| R Ventromedial Prefrontal Cortex | 0.00462 | 4.17 | 0 | 40 | −20 | 283 |
| Hormone-absent > Hormone-present | No significant results | |||||
| Left Amygdala Functional Connectivity | No significant results | |||||
| Left Hippocampus Functional Connectivity | No significant results | |||||
| Right Hippocampus Functional Connectivity | No significant results | |||||
| Left Parahippocampus Functional connectivity | ||||||
| Hormone-absent > Hormone-present | P-value | Z-Stat | X | Y | Z | Voxels |
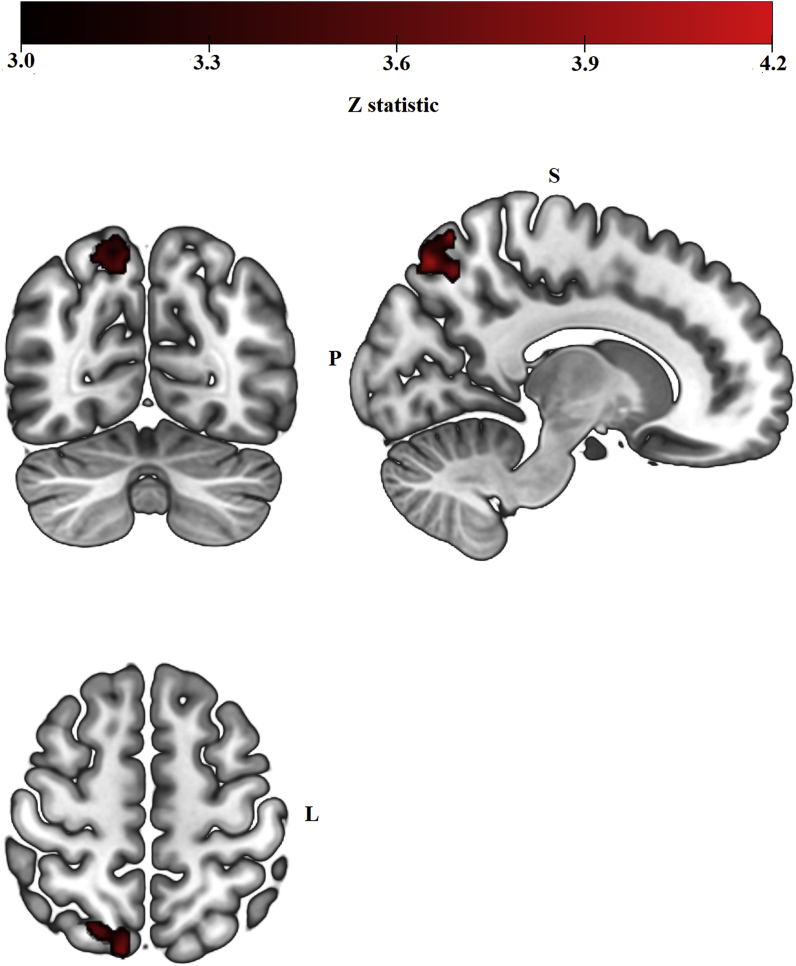
| R Superior Lateral Occipital Cortex | 0.00157 | 4.01 | 12 | −74 | 54 | 312 |
| Hormone-present > Hormone-absent | No significant results | |||||
| Right Parahippocampus Functional Connectivity | No significant results | |||||
Fig. 2.
vmPFC ROI was created around peak of the cluster. Mean connectivity values were extracted from lower-level spatial maps. Two-tailed, single-sample t-tests against zero were performed to determine whether amygdala coupling with connectivity-defined target region was significantly positive or negative for each phase. Error bars represent 95% confidence intervals. *p < 0.001.
3.3. Parahippocampus and hippocampus functional connectivity results
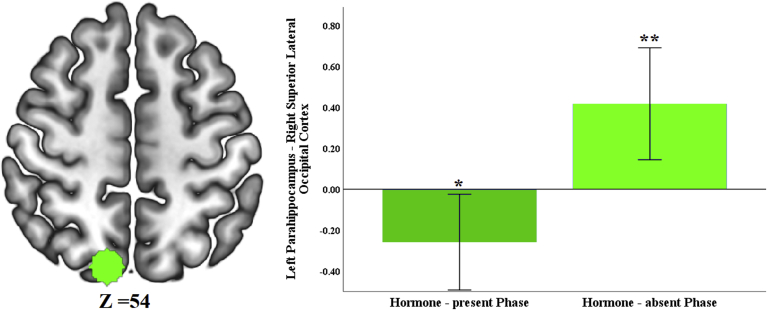
Functional connectivity between left parahippocampus and a cluster with peak in the right superior lateral occipital cortex differed between HC phases (Fig. 3 and Table 1). Follow-up one-sample t-test analyses revealed a significant negative coupling between left parahippocampus and right superior lateral occipital cortex during the hormone-present phase, t(19) = −2.313, p = 0.032; and a significant positive coupling between these two regions during the hormone-absent phase t(19) = 3.191, p = 0.005 (Fig. 4).
Fig. 3.
Left parahippocampus resting-state functional connectivity clusters from the group-level analysis (Hormone-absent > Hormone-present) contrast, cluster-level FWE-corrected at Z > 3.1, p < 0.008). During the hormone-absent phase, women showed positive functional connectivity between left parahippocampus and a cluster of 312 voxels with a peak in right superior lateral occipital cortex, while women in the hormone-present phase showed a negative functional connectivity between these two regions.
Fig. 4.
Right superior lateral occipital cortex ROI was created around peak of the cluster. Mean connectivity values were extracted from lower-level spatial maps. Two-tailed, single-sample t-tests were performed against zero to determine whether parahippocampus coupling with connectivity-defined target region was significantly positive or negative for each phase. Error bars represent 95% confidence intervals. *p < 0.05, **p < 0.01.
Contrary to predictions, hippocampal functional connectivity did not significantly differ between HC phases.
3.4. Relationship between amygdala functional connectivity, mood, and changes in subjective pain reports
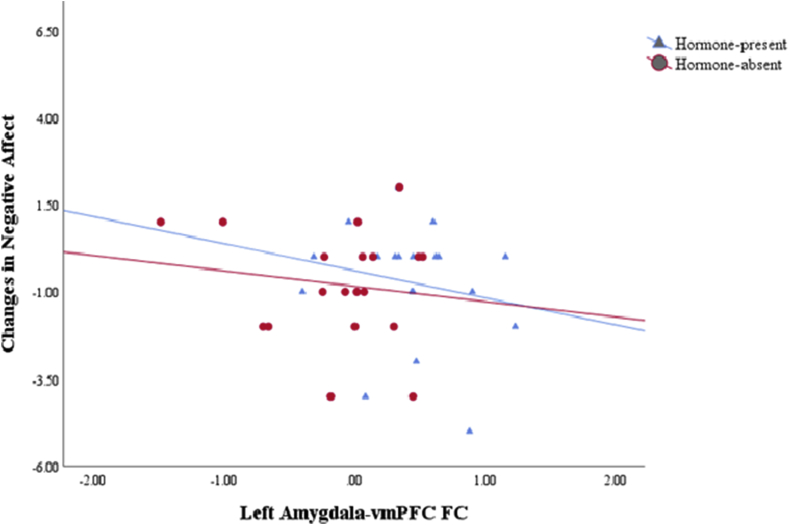
Two participants’ negative mood data were excluded from this analysis as they were identified as outliers. While a negative coupling trend was observed between changes in negative affect and amygdala-vmPFC connectivity across the HC cycle, this correlation was not statistically significant (Fig. 5).
Fig. 5.
In both phases of HC cycle, a negative coupling trend was observed between changes in reported negative emotions and resting-state functional connectivity between left amygdala and vmPFC.
We found no association between changes in subjective pain and amygdala functional connectivity with prefrontal regions.
4. Discussion
Hormonal contraceptives affect the cortisol response to stress and emotional memory (Nielsen et al., 2011, 2013). It has been previously reported that HC users show altered patterns of functional connectivity in different phases of the HC cycle (Petersen et al., 2014), but little is known about how the HC cycle affects functional connectivity of brain regions in response to stress. In order to address this gap, we investigated how functional connectivity of emotion-related regions after stress differs across the HC cycle.
We found that effects of stress on left amygdala-vmPFC functional connectivity differs across the HC cycle. Women showed higher left amygdala-vmPFC coupling during the hormone-present phase of the HC cycle compared to the hormone-absent phase. vmPFC and amygdala are both involved in affective processing. vmPFC appears to regulate emotional responses to negative events through top-down inhibition of the amygdala (Hiser and Koenigs, 2018, Phelps et al., 2004; Quirck and Gehlert, 2006). Previous resting-state functional connectivity studies supported this claim by reporting higher amygdala-vmPFC coupling after fear extinction and in individuals with low trait anxiety (Feng et al., 2016; Kim et al., 2011) and reduced amygdala-vmPFC functional connectivity in patients with post-traumatic stress disorder (Koch et al., 2016). Across the HC cycle in our study we also observed a negative coupling trend between amygdala-vmPFC functional connectivity and changes in negative emotion intensity due to stress.
There were no significant differences in the free cortisol response to stress across the HC cycle in our sample. This finding is consistent with previous work from our lab (Herrera et al., 2019). Despite exhibiting similar free cortisol responses across HC phases, we found higher amygdala-vmPFC functional connectivity after stress exposure during the hormone-present HC phase only. This finding points toward a possible role of synthetic hormones on emotion regulation after stress in HC users. Estradiol appears to modulate the stress response in women (Herrera and Mather, 2015). Naturally cycling women show reduced amygdala reactivity to stress during higher estradiol phases of menstrual cycle (Goldstein et al., 2005; Jacobs et al., 2015). These findings along with lower reported anxiety and negative affect during the higher estradiol phases (Gonda et al., 2008) suggest estradiol affects the emotion-regulation circuit in response to negative events. Endogenous levels of estradiol and progesterone are suppressed during both phases of HC cycle; but exposure to ethinyl estradiol and progestins, which act as synthetic agonists for estradiol and progesterone receptors, is higher during the hormone-present phase of HC cycle. Previous findings regarding effects of HCs on mood changes and anxiety symptoms are inconsistent, ranging from reduction (Freeman et al., 2012; Toffol et al., 2012; Young et al., 2007) to no change (Rapkin et al., 2006) and increase (Skovlund et al., 2016) in symptoms. Some of these contradictory results may be related to genetic predispositions to mood disorders among some HC users and different formulations of HC (Mitchell and Welling, 2020; Porcu et al., 2019). Nevertheless, it has been shown that depending on the dose given to the animals, synthetic contraceptive hormones can mimic effects of ovarian hormones on anxiety-related behaviors (Simone et al., 2015). Furthermore, it has been shown that among women with major depressive disorder and compared to naturally cycling women and progestin-only HC users, women who use combined HCs, show ameliorated anxiety and depressive symptoms (Young et al., 2007). The observed changes in vmPFC-amygdala functional connectivity in our study can stem from the changes in levels of synthetic hormones across the HC cycle. Therefore, these changes in synthetic hormones levels may influence the vulnerability of vmPFC-amygdala functional connectivity to the negative consequences of stress. Participants in this study used combined hormonal contraceptive formulations; therefore, we cannot isolate the effect of either of the individual synthetic hormones on resting state functional connectivity after stress, as one of them may influence the effect of other one and vice versa. Furthermore, as previously stated, brands of combined HC differ in synthetic hormone dosage and progestin generation used in their formulations. We previously showed in our lab that progestin generation can differentially affect the free steroid response to cold pressor test stress (Herrera et al., 2019), which in turn can modulate the brain's emotion network differently. Future work should be done to separate the influence of ethinyl estradiol and different progestin generation by assigning participants to individual hormone components.
While we did not find any differences in hippocampal functional connectivity across the HC cycle, we found differences in parahippocampal functional connectivity. Specifically, we found significant negative left parahippocampus-right superior lateral occipital gyrus functional connectivity during the hormone-present phase, while women showed significant positive functional connectivity between these two regions during the hormone-absent phase. HC users show altered emotional memory compared with naturally cycling women (Nielsen et al., 2011; Spalek et al., 2019). HC use is also associated with altered emotional memory in response to stress (Nielsen et al., 2013). Parahippocampus, along with hippocampus and amygdala, is part of the emotional memory processing circuit and is important for encoding and retrieval of emotional episodic memories (Aminoff et al., 2013; Murty et al., 2010). Furthermore, left posterior parahippocampus has been implicated in perceptual processing, encoding, and retrieval of emotional memory for scenes and locations (Murty et al., 2010). Interestingly, behavioral evidence suggest that HC use may modulate these memory processes. In non-stressful conditions and compared to naturally cycling women, HC users appear to have worse memory for details of emotional stories under non-stressful conditions (Nielsen et al., 2011). Differences in memory pattern are also observed under stressful conditions, where naturally cycling women show enhanced recall for overall gist and details of emotional stories, while HC users’ emotional memory is not affected by stress (Nielsen et al., 2014). Furthermore, in women with post-traumatic stress disorder, the intake of emergency HC after traumatic events has been shown to attenuate post-traumatic stress symptoms (Ferree et al., 2012), and both HC use and emergency use at the time of trauma has been associated with fewer intrusive post-traumatic stress symptoms (Ferree et al., 2012). Emergency HCs have similar components to combined HCs, albeit at much higher doses than daily HC. Given previous finding regarding altered emotional memory performance after stress exposure in HC users and our current finding regarding differences in parahippocampal functional connectivity between HC phases, more studies are needed to investigate the relationship between parahippocampal functional connectivity across the HC cycle and performance in emotional memory tasks after exposure to stress.
HC, mainly in the form of oral contraceptives is commonly used around the world by women. For example, more than 150 million women worldwide, and more than 10 million women in the U.S., use HC (Jones et al., 2012; United Nations Department of Economic and Social Affair Population Division, 2019). HCs suppress endogenous sex hormones, while introducing low levels of exogenous, synthetic hormones. These alterations in sex hormones' profile may change women's vulnerability to affective disorders by modulating the brain structure and function. Given the prevalence of HC usage, its influence on the stress response and emotional memory, and the higher rate of affective disorders in women than men (Bijl et al., 2002; Faravelli et al., 2013), our results have important implications. While HC users show stable cortisol response to stress, they may have different levels of vulnerability to affective disorders due to variation in functional connectivity of affective processing regions after stress.
Our study has some limitations that should be addressed in future research. Although our within-subject design increased the power of our study to detect HC cycle effects, we had a relatively small sample size. Additional research with bigger sample sizes would be able to shed more light onto the effect of the HC cycle on resting state brain activation patterns after stress. Also, due to the lack of a no-stress comparison condition, we could not compare functional connectivity of emotional processing regions under normal versus stressful conditions.
This study is unique in that it is the first study to investigate the effect of HC cycle on resting state functional connectivity of affective regions after stress. We found that despite showing stable free cortisol responses to stress, HC cycle phase affects functional connectivity of amygdala and parahippocampus after stress. Changes in functional connectivity of these affective processing regions in HC users across the HC cycle point toward possible modulating role of exogenous synthetic hormones on the stress response of emotional processing regions.
CRediT authorship contribution statement
Padideh Nasseri: Software, Formal analysis, Data curation, Visualization, Writing - original draft, Writing - review & editing. Alexandra Ycaza Herrera: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing - review & editing, Supervision, Project administration. Katherine Gillette: Formal analysis, Data curation. Sophia Faude: Investigation, Data curation. Jessica D. White: Investigation, Data curation. Ricardo Velasco: Software, Data curation. Mara Mather: Conceptualization, Methodology, Formal analysis, Resources, Writing - review & editing, Supervision, Funding acquisition.
Declaration of competing interest
None.
Acknowledgement
This work was supported by grants from the National Institute on Aging awarded to MM (R21AG-048463 and R01AG-025340).
Footnotes
This research was funded by the National Institute on Aging grants R21AG048463 and 31 R01AG025340.
References
- Albert K., Pruessner J., Newhouse P. Estradiol levels modulate brain activity and negative responses to psychosocial stress across the menstrual cycle. Psychoneuroendocrinology. 2015;59:14–24. doi: 10.1016/j.psyneuen.2015.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminoff E.M., Kveraga K., Bar M. The role of the parahippocampal cortex in cognition. Trends Cognit. Sci. 2013 doi: 10.1016/j.tics.2013.06.009. NIH Public Access. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale T.L. Stress sensitivity and the development of affective disorders. Horm. Behav. 2006;50(4):529–533. doi: 10.1016/j.yhbeh.2006.06.033. [DOI] [PubMed] [Google Scholar]
- Beckler K. State-Trait anxiety inventory for adults sampler set manual, instrument and scoring guide. 2010. 1983 Consulting Psychologists Press, Inc. Mind Garden, Inc., 0–78. [DOI]
- Beckmann C.F., DeLuca M., Devlin J.T., Smith S.M. Investigations into resting-state connectivity using independent component analysis. Phil. Trans. Biol. Sci. 2005;360(1457):1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijl R.V., de Graaf R., Ravelli A., Smit F., Vollebergh W.A.M. Gender and age-specific first incidence of DSM-III-R psychiatric disorders in the general population. Results from The Netherlands mental health survey and incidence study (NEMESIS) Soc. Psychiatr. Psychiatr. Epidemiol. 2002;37(8):372–379. doi: 10.1007/s00127-002-0566-3. [DOI] [PubMed] [Google Scholar]
- Buchanan T.W., Tranel D. Stress and emotional memory retrieval: effects of sex and cortisol response. Neurobiol. Learn. Mem. 2008;89(2):134–141. doi: 10.1016/j.nlm.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J., Yu R. Alternations in functional connectivity of amygdalar subregions under acute social stress. Neurobiology of Stress. 2018;9:264–270. doi: 10.1016/j.ynstr.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewett D., Schoeke A., Mather M. Amygdala functional connectivity is reduced after the cold pressor task. Cognit. Affect Behav. Neurosci. 2013;13(3):501–518. doi: 10.3758/s13415-013-0162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet E.R., Joëls M., Holsboer F. Stress and the brain: from adaptation to disease. Nat. Rev. Neurosci. 2005;6(6):463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- de Kloet E.R. Hormones and the stressed brain. Ann. N. Y. Acad. Sci. 2004;1018(1):1–15. doi: 10.1196/annals.1296.001. [DOI] [PubMed] [Google Scholar]
- Dedovic K., Duchesne A., Andrews J., Engert V., Pruessner J.C. The brain and the stress axis: the neural correlates of cortisol regulation in response to stress. Neuroimage. 2009;47(3):864–871. doi: 10.1016/j.neuroimage.2009.05.074. [DOI] [PubMed] [Google Scholar]
- Fan Y., Pestke K., Feeser M., Aust S., Pruessner J.C., Böker H. Amygdala-hippocampal connectivity changes during acute psychosocial stress: joint effect of early life stress and oxytocin. Neuropsychopharmacology. 2015;40(12):2736–2744. doi: 10.1038/npp.2015.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faravelli C., Alessandra Scarpato M., Castellini G., Lo Sauro C. Gender differences in depression and anxiety: the role of age. Psychiatr. Res. 2013;210(3):1301–1303. doi: 10.1016/j.psychres.2013.09.027. [DOI] [PubMed] [Google Scholar]
- Faul F., Erdfelder E., Lang A.-G., Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. 2007;39(2):175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Feng P., Zheng Y., Feng T. Resting-state functional connectivity between amygdala and the ventromedial prefrontal cortex following fear reminder predicts fear extinction. Soc. Cognit. Affect Neurosci. 2016;11(6):991–1001. doi: 10.1093/scan/nsw031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferree N.K., Wheeler M., Cahill L. The influence of emergency contraception on post-traumatic stress symptoms following sexual assault. J. Forensic Nurs. 2012;8(3):122–130. doi: 10.1111/j.1939-3938.2012.01134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman E.W., Halbreich U., Grubb G.S., Rapkin A.J., Skouby S.O., Smith L. An overview of four studies of a continuous oral contraceptive (levonorgestrel 90 mcg/ethinyl estradiol 20 mcg) on premenstrual dysphoric disorder and premenstrual syndrome. Contraception. 2012;85(5):437–445. doi: 10.1016/j.contraception.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Goldstein J.M., Jerram M., Abbs B., Whitfield-Gabrieli S., Makris N. Sex differences in stress response circuitry activation dependent on female hormonal cycle. J. Neurosci. 2010;30(2):431–438. doi: 10.1523/JNEUROSCI.3021-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J.M., Jerram M., Poldrack R., Ahern T., Kennedy D.N., Seidman L.J., Makris N. Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. J. Neurosci. 2005;25(40):9309–9316. doi: 10.1523/JNEUROSCI.2239-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda X., Telek T., Juhász G., Lazary J., Vargha A., Bagdy G. Patterns of mood changes throughout the reproductive cycle in healthy women without premenstrual dysphoric disorders. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2008;32(8):1782–1788. doi: 10.1016/j.pnpbp.2008.07.016. [DOI] [PubMed] [Google Scholar]
- Gross J.J. The emerging field of emotion regulation: an integrative review. Rev. Gen. Psychol. 1998;2(3):271–299. doi: 10.1037/1089-2680.2.3.271. [DOI] [Google Scholar]
- Herman J.P., Ostrander M.M., Mueller N.K., Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2005;29(8):1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Herrera A.Y., Faude S., Nielsen S.E., Locke M., Mather M. Effects of hormonal contraceptive phase and progestin generation on stress-induced cortisol and progesterone release. Neurobiology of Stress. 2019;10:100151. doi: 10.1016/j.ynstr.2019.100151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera A.Y., Mather M. Actions and interactions of estradiol and glucocorticoids in cognition and the brain: implications for aging women. Neurosci. Biobehav. Rev. 2015;55:36–52. doi: 10.1016/j.neubiorev.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera A.Y., Velasco R., Faude S., White J.D., Opitz P.C., Huang R. Brain activity during a post-stress working memory task differs between the hormone-present and hormone-absent phase of hormonal contraception. Neurobiology of Stress. 2020;13:100248. doi: 10.1016/j.ynstr.2020.100248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiser J., Koenigs M. The multifaceted role of the ventromedial prefrontal cortex in emotion, decision making, social cognition, and psychopathology. Biol. Psychiatr. 2018;83(8):638–647. doi: 10.1016/j.biopsych.2017.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs E.G., Holsen L.M., Lancaster K., Makris N., Whitfield-Gabrieli S., Remington A. 17β-Estradiol differentially regulates stress circuitry activity in healthy and depressed women. Neuropsychopharmacology. 2015;40(3):566–576. doi: 10.1038/npp.2014.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1016/S1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Pechaud M., Smith S. vol. 17. 2002. BET2-MR-Based estimation of brain, skull and scalp surfaces.www.fmrib.ox.ac.uk/analysis/research/bet (Human Brain Mapping). Retrieved from. [Google Scholar]
- Jenkinson M., Smith S. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 2001;5(2):143–156. doi: 10.1016/S1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jentsch V.L., Merz C.J., Wolf O.T. Restoring emotional stability: cortisol effects on the neural network of cognitive emotion regulation. Behav. Brain Res. 2019;374:111880. doi: 10.1016/j.bbr.2019.03.049. [DOI] [PubMed] [Google Scholar]
- Jones J., Mosher W., Daniels K. vol. 18. 2012. (Current Contraceptive Use in the United States, 2006-2010, and Changes in Patterns of Use since 1995). [PubMed] [Google Scholar]
- Kajantie E., Phillips D.I.W. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006, February doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Kern S., Oakes T.R., Stone C.K., McAuliff E.M., Kirschbaum C., Davidson R.J. Glucose metabolic changes in the prefrontal cortex are associated with HPA axis response to a psychosocial stressor. Psychoneuroendocrinology. 2008;33(4):517–529. doi: 10.1016/j.psyneuen.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.J., Gee D.G., Loucks R.A., Davis F.C., Whalen P.J. Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cerebr. Cortex. 2011;21(7):1667–1673. doi: 10.1093/cercor/bhq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch S.B.J., van Zuiden M., Nawijn L., Frijling J.L., Veltman D.J., Olff M. Aberrant resting-state brain activity in posttraumatic stress disorder: a meta-analysis and systematic review. Depress. Anxiety. 2016;33(7):592–605. doi: 10.1002/da.22478. [DOI] [PubMed] [Google Scholar]
- Kogler L., Müller V.I., Seidel E.-M., Boubela R., Kalcher K., Moser E. Sex differences in the functional connectivity of the amygdalae in association with cortisol. Neuroimage. 2016;134:410–423. doi: 10.1016/j.neuroimage.2016.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo W. The cold pressor test and autonomic Function: a review and integration. Psychophysiology. 1975;12(3):268–282. doi: 10.1111/j.1469-8986.1975.tb01289.x. [DOI] [PubMed] [Google Scholar]
- Luh W.M., Wong E.C., Bandettini P.A., Hyde J.S. QUIPSS II with thin-slice TI1 periodic saturation: a method for improving accuracy of quantitative perfusion imaging using pulsed arterial spin labeling. Magn. Reson. Med. 1999;41(6):1246–1254. doi: 10.1002/(sici)1522-2594(199906)41:6<1246::aid-mrm22>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Mitchell V.E., Welling L.L.M. Not all progestins are created equally: considering unique progestins individually in psychobehavioral research. Adaptive Human Behavior and Physiology. 2020;6(3):381–412. doi: 10.1007/s40750-020-00137-1. [DOI] [Google Scholar]
- Montoya E.R., Bos P.A. Elsevier Ltd; 2017. How oral contraceptives impact social-emotional behavior and brain function. Trends Cognit. Sci. [DOI] [PubMed] [Google Scholar]
- Mordecai K.L., Rubin L.H., Eatough E., Sundermann E., Drogos L., Savarese A., Maki P.M. Cortisol reactivity and emotional memory after psychosocial stress in oral contraceptive users. J. Neurosci. Res. 2017;95(1–2):126–135. doi: 10.1002/jnr.23904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumford J.A., Hernandez-Garcia L., Lee G.R., Nichols T.E. Estimation efficiency and statistical power in arterial spin labeling fMRI. Neuroimage. 2006;33(1):103–114. doi: 10.1016/j.neuroimage.2006.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty V.P., Ritchey M., Adcock R.A., LaBar K.S. FMRI studies of successful emotional memory encoding: a quantitative meta-analysis. Neuropsychologia. 2010;48(12):3459–3469. doi: 10.1016/j.neuropsychologia.2010.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S.E., Ahmed I., Cahill L. Postlearning stress differentially affects memory for emotional gist and detail in naturally cycling women and women on hormonal contraceptives. Behav. Neurosci. 2014;128(4):482–493. doi: 10.1037/a0036687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S.E., Ertman N., Lakhani Y.S., Cahill L. Hormonal contraception usage is associated with altered memory for an emotional story. Neurobiol. Learn. Mem. 2011;96(2):378–384. doi: 10.1016/j.nlm.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S.E., Segal S.K., Worden I.V., Yim I.S., Cahill L. Hormonal contraception use alters stress responses and emotional memory. Biol. Psychol. 2013;92(2):257–266. doi: 10.1016/j.biopsycho.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S.E., Segal S.K., Worden I.V., Yim I.S., Cahill L. Hormonal contraception use alters stress responses and emotional memory. Biol. Psychol. 2013;92(2):257–266. doi: 10.1016/j.biopsycho.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossewaarde L., Hermans E.J., van Wingen G.A., Kooijman S.C., Johansson I.-M., Bäckström T., Fernández G. Neural mechanisms underlying changes in stress-sensitivity across the menstrual cycle. Psychoneuroendocrinology. 2010;35(1):47–55. doi: 10.1016/j.psyneuen.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Petersen N., Cahill L. Amygdala reactivity to negative stimuli is influenced by oral contraceptive use. Soc. Cognit. Affect Neurosci. 2015;10(9):1266–1272. doi: 10.1093/scan/nsv010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen N., Kilpatrick L.A., Goharzad A., Cahill L. Oral contraceptive pill use and menstrual cycle phase are associated with altered resting state functional connectivity. Neuroimage. 2014;90:24–32. doi: 10.1016/j.neuroimage.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps E.A., Delgado M.R., Nearing K.I., Ledoux J.E. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43(6):897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Porcu P., Serra M., Concas A. The brain as a target of hormonal contraceptives: evidence from animal studies. Front. Neuroendocrinol. 2019 doi: 10.1016/j.yfrne.2019.100799. Academic Press Inc. [DOI] [PubMed] [Google Scholar]
- Quirk G.J., Gehlert D.R. Inhibition of the amygdala: key to pathological states? Ann. N. Y. Acad. Sci. 2006;985(1):263–272. doi: 10.1111/j.1749-6632.2003.tb07087.x. [DOI] [PubMed] [Google Scholar]
- Rapkin A.J., Morgan M., Sogliano C., Biggio G., Concas A. Decreased neuroactive steroids induced by combined oral contraceptive pills are not associated with mood changes. Fertil. Steril. 2006;85(5):1371–1378. doi: 10.1016/j.fertnstert.2005.10.031. [DOI] [PubMed] [Google Scholar]
- Ritchey M., Montchal M.E., Yonelinas A.P., Ranganath C. Delay-dependent contributions of medial temporal lobe regions to episodic memory retrieval. ELife. 2015;4(4) doi: 10.7554/eLife.05025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohleder N., Wolf J.M., Piel M., Kirschbaum C. Impact of oral contraceptive use on glucocorticoid sensitivity of pro-inflammatory cytokine production after psychosocial stress. Psychoneuroendocrinology. 2003;28(3):261–273. doi: 10.1016/S0306-4530(02)00019-7. [DOI] [PubMed] [Google Scholar]
- Schneiderman N., Ironson G., Siegel S.D. Stress and health: psychological, behavioral, and biological determinants. Annu. Rev. Clin. Psychol. 2005;1(1):607–628. doi: 10.1146/annurev.clinpsy.1.102803.144141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields G.S., McCullough A.M., Ritchey M., Ranganath C., Yonelinas A.P. Stress and the medial temporal lobe at rest: functional connectivity is associated with both memory and cortisol. Psychoneuroendocrinology. 2019;106:138–146. doi: 10.1016/j.psyneuen.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone J., Bogue E.A., Bhatti D.L., Day L.E., Farr N.A., Grossman A.M., Holmes P.V. Ethinyl estradiol and levonorgestrel alter cognition and anxiety in rats concurrent with a decrease in tyrosine hydroxylase expression in the locus coeruleus and brain-derived neurotrophic factor expression in the hippocampus. Psychoneuroendocrinology. 2015;62:265–278. doi: 10.1016/j.psyneuen.2015.08.015. [DOI] [PubMed] [Google Scholar]
- Skovlund C.W., Mørch L.S., Kessing L.V., Lidegaard O. Association of hormonal contraception with depression. JAMA Psychiatry. 2016;73(11):1154–1162. doi: 10.1001/jamapsychiatry.2016.2387. [DOI] [PubMed] [Google Scholar]
- Spalek K., Loos E., Schicktanz N., Hartmann F., de Quervain D., Stier C., Milnik A. Women using hormonal contraceptives show increased valence ratings and memory performance for emotional information. Neuropsychopharmacology. 2019;44(7):1258–1264. doi: 10.1038/s41386-019-0362-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toffol E., Heikinheimo O., Koponen P., Luoto R., Partonen T. Further evidence for lack of negative associations between hormonal contraception and mental health. Contraception. 2012;86(5):470–480. doi: 10.1016/j.contraception.2012.02.014. [DOI] [PubMed] [Google Scholar]
- United Nations Department of Economic and Social Affair Population Division . 2019. Contraceptive Use by Method 2019: Data Booklet. [Google Scholar]
- van Marle H.J.F., Hermans E.J., Qin S., Fernández G. Enhanced resting-state connectivity of amygdala in the immediate aftermath of acute psychological stress. Neuroimage. 2010;53(1):348–354. doi: 10.1016/j.neuroimage.2010.05.070. [DOI] [PubMed] [Google Scholar]
- Veer I.M., Oei N.Y.L., Spinhoven P., van Buchem M.A., Elzinga B.M., Rombouts S.A.R.B. Beyond acute social stress: increased functional connectivity between amygdala and cortical midline structures. Neuroimage. 2011;57(4):1534–1541. doi: 10.1016/j.neuroimage.2011.05.074. [DOI] [PubMed] [Google Scholar]
- Watson D., Clark L.A., Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J. Pers. Soc. Psychol. 1988;54(6):1063–1070. doi: 10.1037/0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Young E.A., Kornstein S.G., Harvey A.T., Wisniewski S.R., Barkin J., Fava M. Influences of hormone-based contraception on depressive symptoms in premenopausal women with major depression. Psychoneuroendocrinology. 2007;32(7):843–853. doi: 10.1016/j.psyneuen.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Brady M., Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans. Med. Imag. 2001;20(1):45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]